Exploring the Effect of Resveratrol, Tyrosol, and Their Derivatives on Platelet-Activating Factor Biosynthesis in U937 Cells
Abstract
1. Introduction
2. Results
2.1. Effect of Phenolic Compounds, of Their Derivatives, and of Signal Transduction Inhibitors on Cell Viability
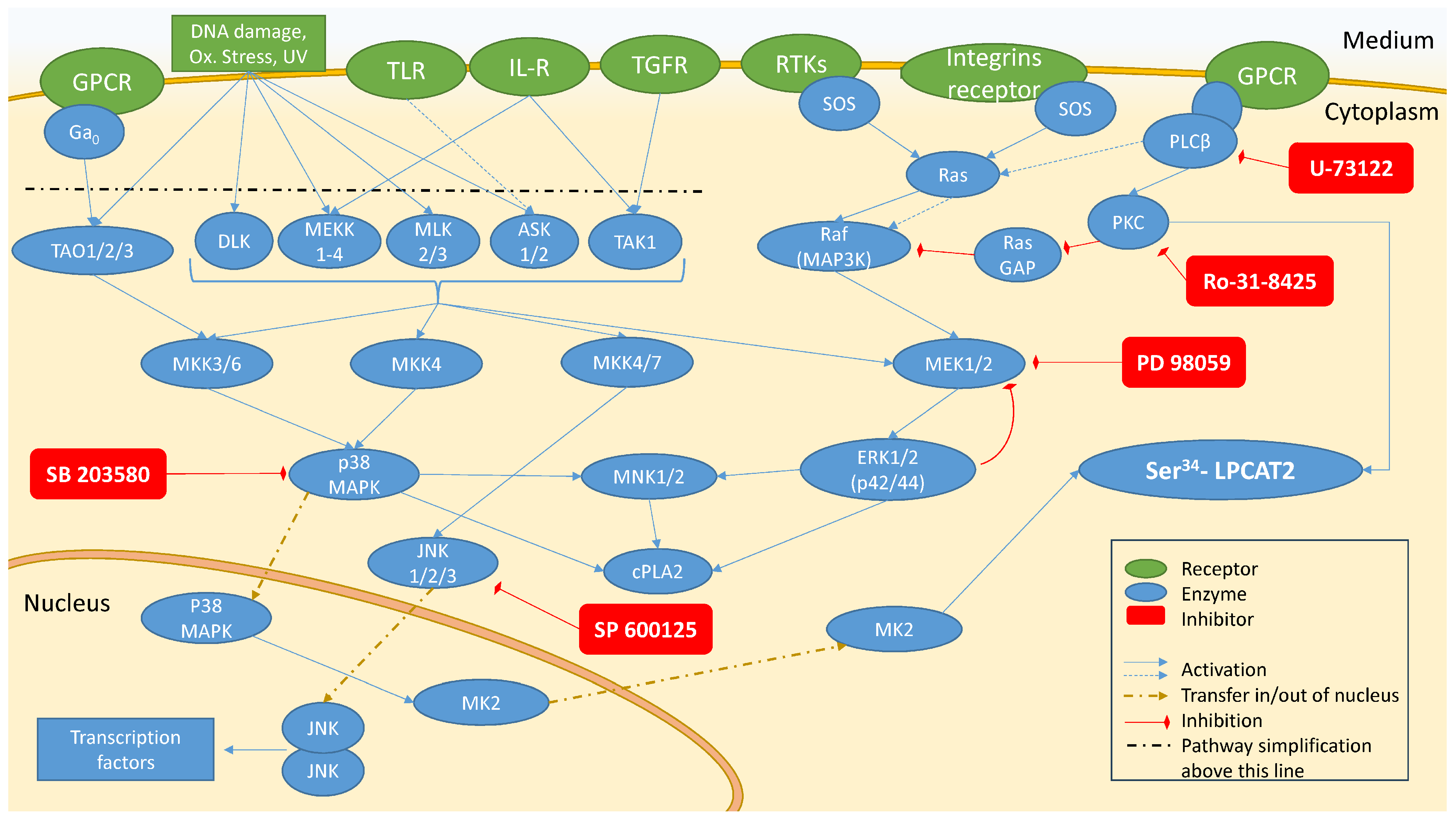
2.2. Investigation of Signal Transduction Involved in Activation of PAF Biosynthetic Enzymes in U937 Cells
2.2.1. Enzymes’ Activity in Unstimulated Cells (Basal Conditions) and IL-1β-Stimulated Cells
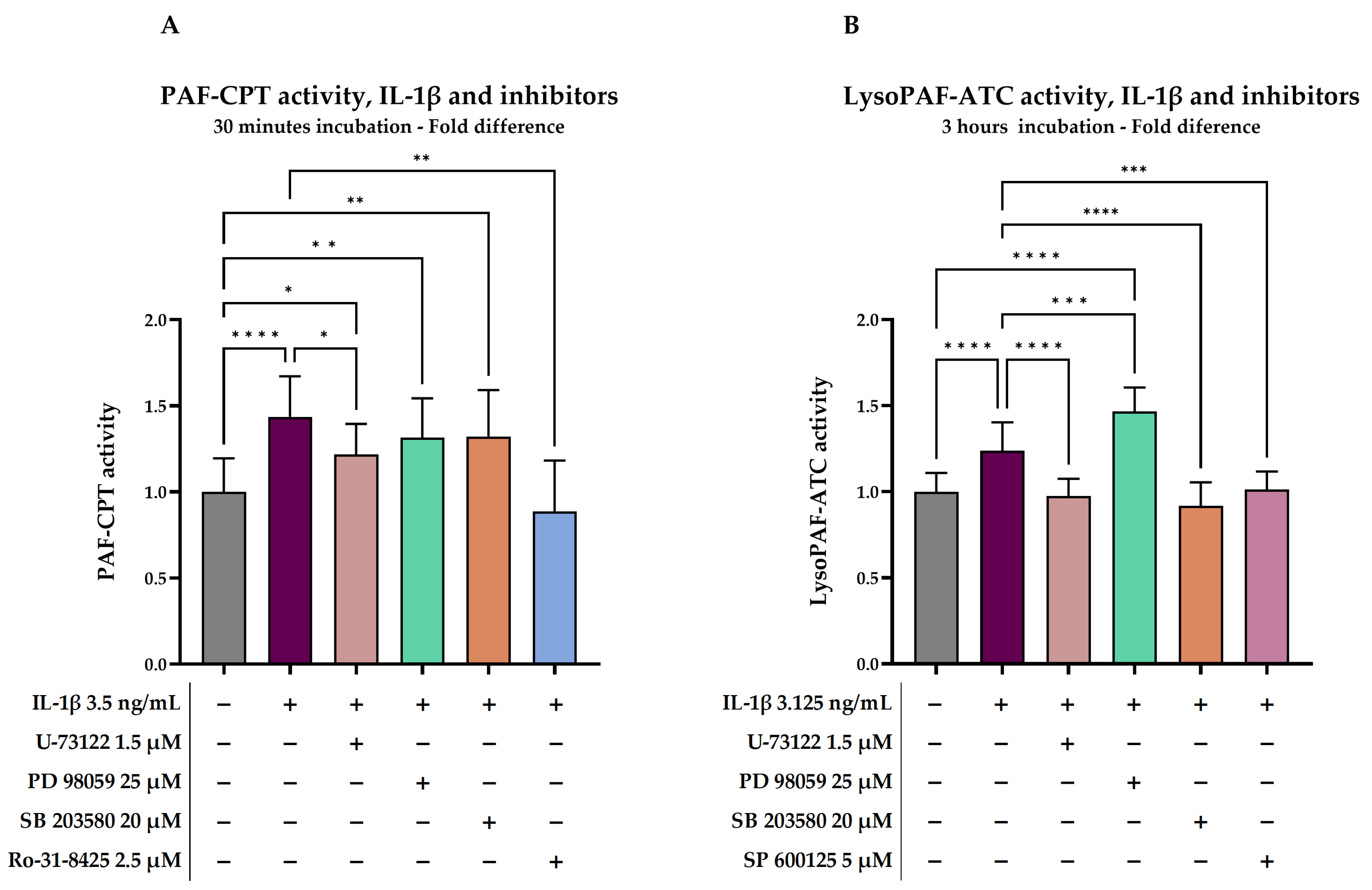
2.2.2. Investigation of Signal Transduction on IL-1β-Stimulated Cells
2.3. Short-Time Effect of Resveratrol and Its Derivatives on PAF-CPT and LysoPAF-ATC in U937 Cells
2.3.1. Enzymes’ Activity in Unstimulated Cells (Basal Conditions)
2.3.2. Effect of Resveratrol and Derivatives on PAF-CPT and LysoPAF-ATC
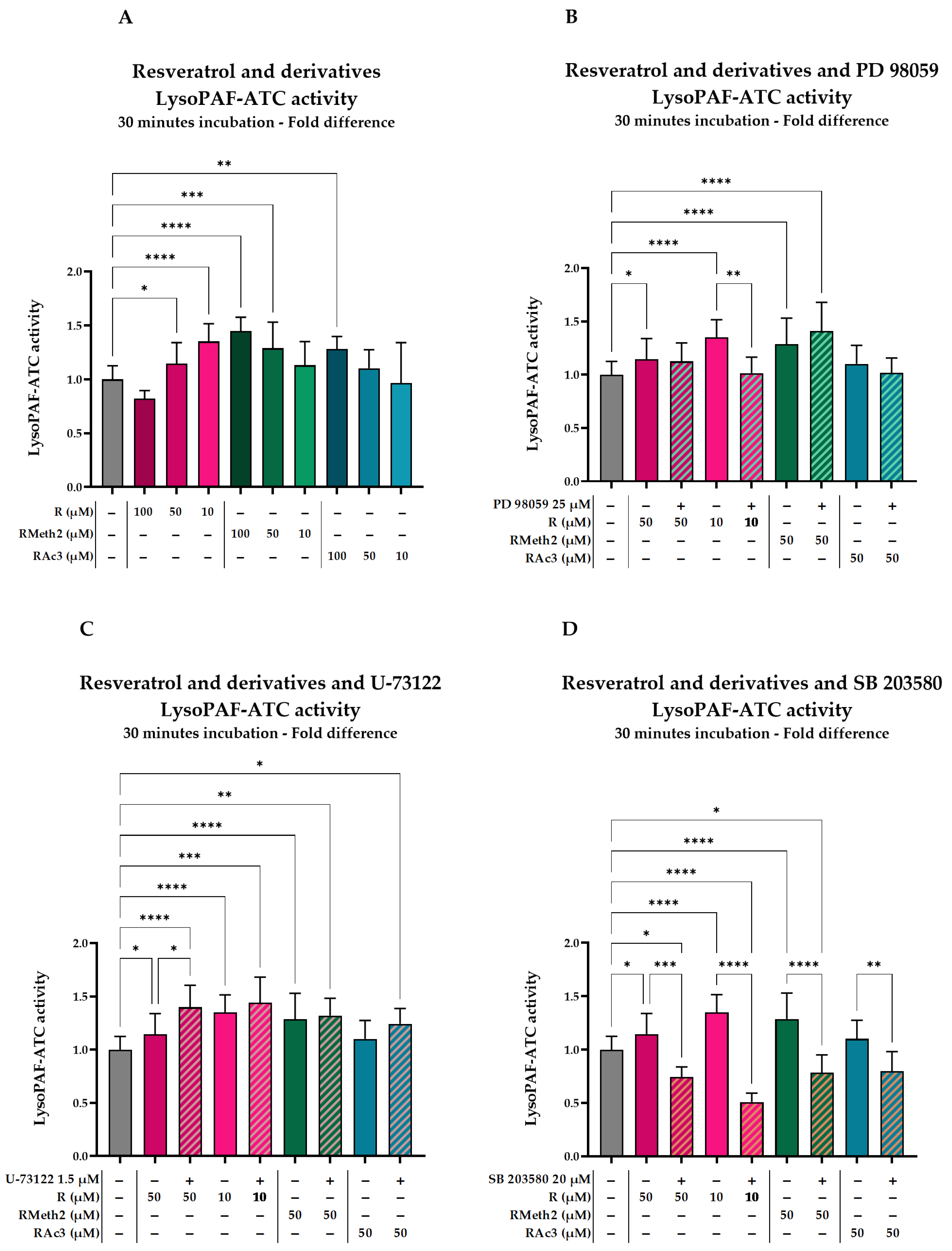
2.3.3. Effect of Inhibitors on the LysoPAF-ATC Activation by Resveratrol and Its Derivatives in U937 Cells After 30 min
2.4. Long-Term Effect of Resveratrol, Tyrosol, and Their Derivatives on PAF-CPT, LysoPAF-ATC and LysoPAF-ATE
2.4.1. Enzymes’ Activity in Unstimulated Cells (Basal Conditions)
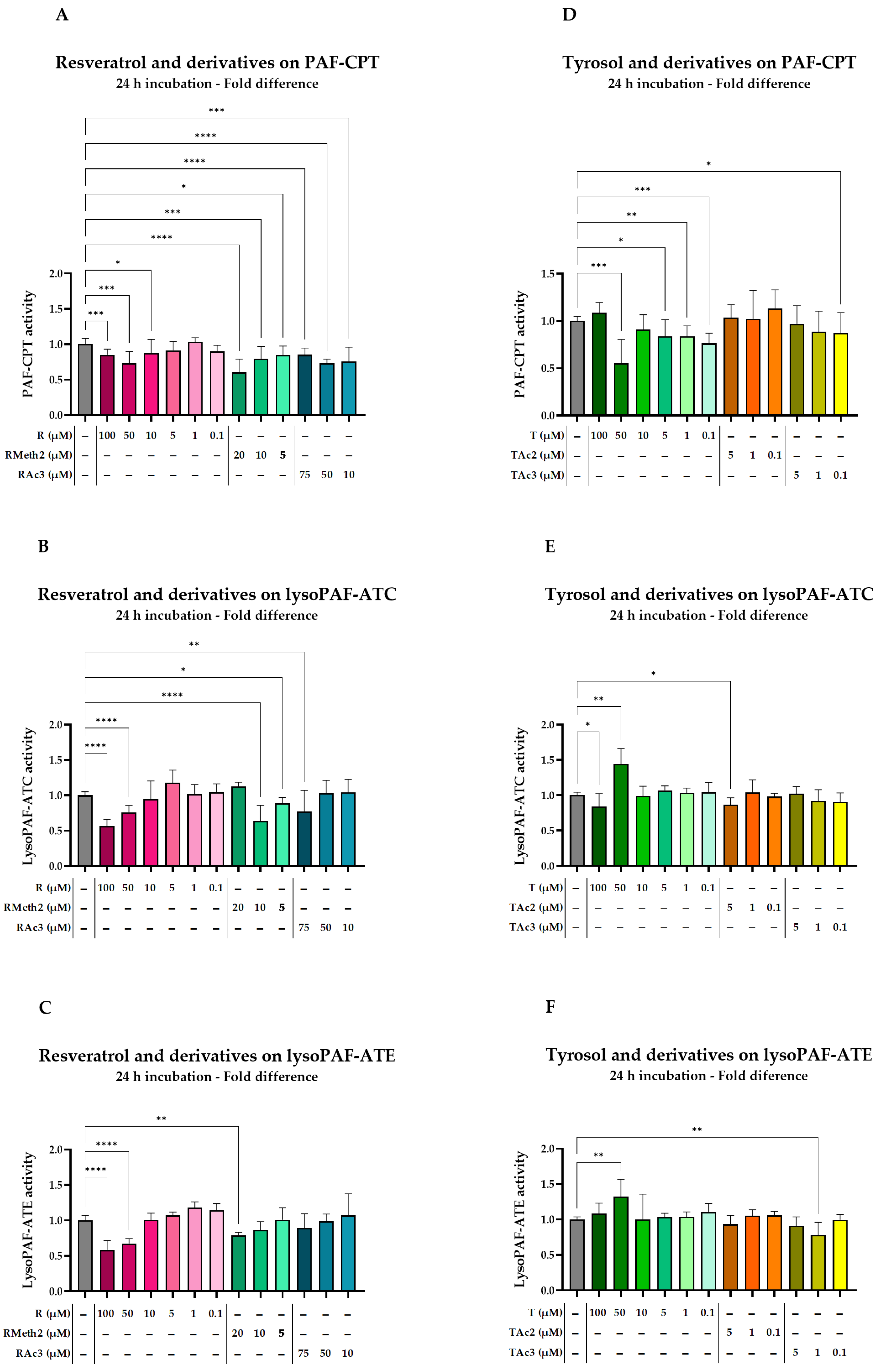
2.4.2. Effect of Resveratrol and Derivatives on Enzymes
2.4.3. Effect of Tyrosol and Derivatives on Enzymes
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of the Phenolic Compounds
4.3. Cell Culture
4.4. Cell Viability
4.5. Effect of Inhibitors on PAF Biosynthesis in Stimulated U937 Cells
4.6. Effect of Resveratrol and Its Derivatives on PAF-CPT and LysoPAF-ATC
4.7. Effect of Inhibitors on the LysoPAF-ATC Activation by Resveratrol and Its Derivatives
4.8. Effect of Resveratrol, Tyrosol and Their Derivatives in U937 Cells After 24 h
4.9. Enzymatic Activity Assay
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mori, T.A. Marine OMEGA-3 Fatty Acids in the Prevention of Cardiovascular Disease. Fitoterapia 2017, 123, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Dubois, V.; Breton, S.; Linder, M.; Fanni, J.; Parmentier, M. Fatty Acid Profiles of 80 Vegetable Oils with Regard to Their Nutritional Potential. Eur. J. Lipid Sci. Technol. 2007, 109, 710–732. [Google Scholar] [CrossRef]
- Karantonis, H.C.; Tsantila, N.; Stamatakis, G.; Samiotaki, M.; Panayotou, G.; Antonopoulou, S.; Demopoulos, C.A. Bioactive Polar Lipids in Olive Oil, Pomace and Waste Byproducts. J. Food Biochem. 2008, 32, 443–459. [Google Scholar] [CrossRef]
- Karantonis, H.C.; Antonopoulou, S.; Demopoulos, C.A. Antithrombotic Lipid Minor Constituents from Vegetable Oils. Comparison between Olive Oils and Others. J. Agric. Food Chem. 2002, 50, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Vijakumaran, U.; Goh, N.-Y.; Razali, R.A.; Abdullah, N.A.H.; Yazid, M.D.; Sulaiman, N. Role of Olive Bioactive Compounds in Respiratory Diseases. Antioxidants 2023, 12, 1140. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.S.; Gómez, B.; Barba, F.J.; Mora, L.; Pérez-Santaescolástica, C.; Toldrá, F. Bioactive Peptides as Natural Antioxidants in Food Products—A Review. Trends Food Sci. Technol. 2018, 79, 136–147. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-Derived Bioactive Peptides in Human Health: Challenges and Opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef]
- Réhault-Godbert, S.; Guyot, N.; Nys, Y. The Golden Egg: Nutritional Value, Bioactivities, and Emerging Benefits for Human Health. Nutrients 2019, 11, 684. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Wyss, A. Carotenoids in Human Nutrition and Health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef]
- Borneo, R.; León, A.E. Whole Grain Cereals: Functional Components and Health Benefits. Food Funct 2012, 3, 110–119. [Google Scholar] [CrossRef]
- Adlercreutz, H. Lignans and Human Health. Crit. Rev. Clin. Lab. Sci. 2007, 44, 483–525. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A Concise Overview on the Chemistry, Occurrence, and Human Health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, D.; Rajora, A.K.; Greco, F.; Osborn, H.M.I. Flavonoids as Prospective Compounds for Anti-Cancer Therapy. Int. J. Biochem. Cell Biol. 2013, 45, 2821–2831. [Google Scholar] [CrossRef]
- Angeloni, C.; Malaguti, M.; Barbalace, M.; Hrelia, S. Bioactivity of Olive Oil Phenols in Neuroprotection. Int. J. Mol. Sci. 2017, 18, 2230. [Google Scholar] [CrossRef]
- Goyal, R.; Mittal, P.; Gautam, R.K.; Kamal, M.A.; Perveen, A.; Garg, V.; Alexiou, A.; Saboor, M.; Haque, S.; Farhana, A.; et al. Natural Products in the Management of Neurodegenerative Diseases. Nutr. Metab. 2024, 21, 26. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Hecker, K.D.; Bonanome, A.; Coval, S.M.; Binkoski, A.E.; Hilpert, K.F.; Griel, A.E.; Etherton, T.D. Bioactive Compounds in Foods: Their Role in the Prevention of Cardiovascular Disease and Cancer. Am. J. Med. 2002, 113, 71–88. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The Effects of Polyphenols and Other Bioactives on Human Health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Mehmood, A.; Usman, M.; Patil, P.; Zhao, L.; Wang, C. A Review on Management of Cardiovascular Diseases by Olive Polyphenols. Food Sci. Nutr. 2020, 8, 4639–4655. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Rimm, E.B.; Medina-Remón, A.; Martínez-González, M.A.; De La Torre, R.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; Arós, F.; et al. Inverse Association between Habitual Polyphenol Intake and Incidence of Cardiovascular Events in the PREDIMED Study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 639–647. [Google Scholar] [CrossRef]
- Zordoky, B.N.M.; Robertson, I.M.; Dyck, J.R.B. Preclinical and Clinical Evidence for the Role of Resveratrol in the Treatment of Cardiovascular Diseases. Biochim. Biophys. Acta BBA—Mol. Basis Dis. 2015, 1852, 1155–1177. [Google Scholar] [CrossRef] [PubMed]
- Wiciński, M.; Socha, M.; Walczak, M.; Wódkiewicz, E.; Malinowski, B.; Rewerski, S.; Górski, K.; Pawlak-Osińska, K. Beneficial Effects of Resveratrol Administration—Focus on Potential Biochemical Mechanisms in Cardiovascular Conditions. Nutrients 2018, 10, 1813. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an Anti-Cancer Agent: A Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1428–1447. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Tiwari, R.; Tiwari, G.; Ramachandran, V. Resveratrol: A Vital Therapeutic Agent with Multiple Health Benefits. Drug Res. 2022, 72, 5–17. [Google Scholar] [CrossRef]
- Kulkarni, S.S.; Cantó, C. The Molecular Targets of Resveratrol. Biochim. Biophys. Acta BBA—Mol. Basis Dis. 2015, 1852, 1114–1123. [Google Scholar] [CrossRef]
- Rahman, I.; Biswas, S.K.; Kirkham, P.A. Regulation of Inflammation and Redox Signaling by Dietary Polyphenols. Biochem. Pharmacol. 2006, 72, 1439–1452. [Google Scholar] [CrossRef]
- Miloso, M.; Bertelli, A.A.E.; Nicolini, G.; Tredici, G. Resveratrol-Induced Activation of the Mitogen-Activated Protein Kinases, ERK1 and ERK2, in Human Neuroblastoma SH-SY5Y Cells. Neurosci. Lett. 1999, 264, 141–144. [Google Scholar] [CrossRef]
- Das, S.; Tosaki, A.; Bagchi, D.; Maulik, N.; Das, D.K. Potentiation of a Survival Signal in the Ischemic Heart by Resveratrol through P38 Mitogen-Activated Protein Kinase/Mitogen- and Stress-Activated Protein Kinase 1/cAMP Response Element-Binding Protein Signaling. J. Pharmacol. Exp. Ther. 2006, 317, 980–988. [Google Scholar] [CrossRef]
- El-Mowafy, A.M.; White, R.E. Resveratrol Inhibits MAPK Activity and Nuclear Translocation in Coronary Artery Smooth Muscle: Reversal of Endothelin-1 Stimulatory Effects. FEBS Lett. 1999, 451, 63–67. [Google Scholar] [CrossRef]
- Schwager, J.; Richard, N.; Widmer, F.; Raederstorff, D. Resveratrol Distinctively Modulates the Inflammatory Profiles of Immune and Endothelial Cells. BMC Complement. Altern. Med. 2017, 17, 309. [Google Scholar] [CrossRef]
- Cignarella, A.; Minici, C.; Bolego, C.; Pinna, C.; Sanvito, P.; Gaion, R.M.; Puglisi, L. Potential Pro-Inflammatory Action of Resveratrol in Vascular Smooth Muscle Cells from Normal and Diabetic Rats. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Salla, M.; Karaki, N.; El Kaderi, B.; Ayoub, A.J.; Younes, S.; Abou Chahla, M.N.; Baksh, S.; El Khatib, S. Enhancing the Bioavailability of Resveratrol: Combine It, Derivatize It, or Encapsulate It? Pharmaceutics 2024, 16, 569. [Google Scholar] [CrossRef]
- Tuck, K.L.; Hayball, P.J. Major Phenolic Compounds in Olive Oil: Metabolism and Health Effects. J. Nutr. Biochem. 2002, 13, 636–644. [Google Scholar] [CrossRef]
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Huang, G.; Wang, Z.; Zhuang, S.; Xu, L.; Song, B.; Xiong, Y.; Guan, S. Tyrosol Exhibits Negative Regulatory Effects on LPS Response and Endotoxemia. Food Chem. Toxicol. 2013, 62, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Mihara, Y.; Kanai, K.; Yamashita, Y.; Kimura, Y.; Itoh, N. Tyrosol Ameliorates Lipopolysaccharide-Induced Ocular Inflammation in Rats via Inhibition of Nuclear Factor (NF)-κB Activation. J. Vet. Med. Sci. 2016, 78, 1429–1438. [Google Scholar] [CrossRef]
- Demopoulos, C.A.; Karantonis, H.C.; Antonopoulou, S. Platelet Activating Factor—A Molecular Link between Atherosclerosis Theories. Eur. J. Lipid Sci. Technol. 2003, 105, 705–716. [Google Scholar] [CrossRef]
- Upton, J.E.M.; Grunebaum, E.; Sussman, G.; Vadas, P. Platelet Activating Factor (PAF): A Mediator of Inflammation. BioFactors 2022, 48, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Palur Ramakrishnan, A.V.K.; Varghese, T.P.; Vanapalli, S.; Nair, N.K.; Mingate, M.D. Platelet Activating Factor: A Potential Biomarker in Acute Coronary Syndrome? Cardiovasc. Ther. 2017, 35, 64–70. [Google Scholar] [CrossRef]
- Fragopoulou, E.; Antonopoulou, S.; Demopoulos, C.A. Biologically Active Lipids with Antiatherogenic Properties from White Wine and Must. J. Agric. Food Chem. 2002, 50, 2684–2694. [Google Scholar] [CrossRef]
- Antonopoulou, S.; Demopoulos, C.A. Protective Effect of Olive Oil Microconstituents in Atherosclerosis: Emphasis on PAF Implicated Atherosclerosis Theory. Biomolecules 2023, 13, 700. [Google Scholar] [CrossRef] [PubMed]
- Nomikos, T.; Fragopoulou, E.; Antonopoulou, S.; Panagiotakos, D.B. Mediterranean Diet and Platelet-Activating Factor; a Systematic Review. Clin. Biochem. 2018, 60, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Argyrou, C.; Vlachogianni, I.; Stamatakis, G.; Demopoulos, C.A.; Antonopoulou, S.; Fragopoulou, E. Postprandial Effects of Wine Consumption on Platelet Activating Factor Metabolic Enzymes. Prostaglandins Other Lipid Mediat. 2017, 130, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Detopoulou, M.; Ntzouvani, A.; Petsini, F.; Gavriil, L.; Fragopoulou, E.; Antonopoulou, S. Consumption of Enriched Yogurt with PAF Inhibitors from Olive Pomace Affects the Major Enzymes of PAF Metabolism: A Randomized, Double Blind, Three Arm Trial. Biomolecules 2021, 11, 801. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulou, S.; Nomikos, T.; Karantonis, H.C.; Fragopoulou, E.; Demopoulos, C.A. PAF, a Potent Lipid Mediator. In Bioactive Phospholipids. Role in Inflammation and Atherosclerosis; Tselepis, A.D., Ed.; Transworld Research Network: Kerala, India, 2008; pp. 85–134. ISBN 978-81-7895-323-6. [Google Scholar]
- Shindou, H.; Hishikawa, D.; Nakanishi, H.; Harayama, T.; Ishii, S.; Taguchi, R.; Shimizu, T. A Single Enzyme Catalyzes Both Platelet-Activating Factor Production and Membrane Biogenesis of Inflammatory Cells. J. Biol. Chem. 2007, 282, 6532–6539. [Google Scholar] [CrossRef]
- Harayama, T.; Shindou, H.; Ogasawara, R.; Suwabe, A.; Shimizu, T. Identification of a Novel Noninflammatory Biosynthetic Pathway of Platelet-Activating Factor. J. Biol. Chem. 2008, 283, 11097–11106. [Google Scholar] [CrossRef]
- Sánchez Crespo, M.; Montero, O.; Fernandez, N. The Role of PAF in Immunopathology: From Immediate Hypersensitivity Reactions to Fungal Defense. BioFactors 2022, 48, 1217–1225. [Google Scholar] [CrossRef]
- Morimoto, R.; Shindou, H.; Oda, Y.; Shimizu, T. Phosphorylation of Lysophosphatidylcholine Acyltransferase 2 at Ser34 Enhances Platelet-Activating Factor Production in Endotoxin-Stimulated Macrophages. J. Biol. Chem. 2010, 285, 29857–29862. [Google Scholar] [CrossRef]
- Morimoto, R.; Shindou, H.; Tarui, M.; Shimizu, T. Rapid Production of Platelet-Activating Factor Is Induced by Protein Kinase Cα-Mediated Phosphorylation of Lysophosphatidylcholine Acyltransferase 2 Protein. J. Biol. Chem. 2014, 289, 15566–15576. [Google Scholar] [CrossRef]
- Woodard, D.S.; Lee, T.C.; Snyder, F. The Final Step in the de Novo Biosynthesis of Platelet-Activating Factor. Properties of a Unique CDP-Choline:1-Alkyl-2-Acetyl-Sn-Glycerol Choline-Phosphotransferase in Microsomes from the Renal Inner Medulla of Rats. J. Biol. Chem. 1987, 262, 2520–2527. [Google Scholar] [CrossRef]
- Snyder, F. CDP-Choline:Alkylacetylglycerol Cholinephosphotransferase Catalyzes the Final Step in the de Novo Synthesis of Platelet-Activating Factor. Biochim. Biophys. Acta 1997, 1348, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Satoh, N.; Harada, A.; Yokoyama, K.; Karasawa, K.; Inoue, K.; Setaka, M. Regulation of Activities of Cytidine 5’-Diphospho-Choline: 1-O-Alkyl-2-Acetyl-Sn-Glycerol Cholinephosphotransferase, an Enzyme Responsible for de Novo Synthesis of Platelet-Activating Factor, by Membrane Phospholipids. J. Health Sci. 2003, 49, 13–21. [Google Scholar] [CrossRef]
- Vlachogianni, I.C.; Nomikos, T.; Fragopoulou, E.; Stamatakis, G.M.; Karantonis, H.C.; Antonopoulou, S.; Demopoulos, C.A. Interleukin-1beta Stimulates Platelet-Activating Factor Production in U-937 Cells Modulating Both Its Biosynthetic and Catabolic Enzymes. Cytokine 2013, 63, 97–104. [Google Scholar] [CrossRef]
- Weber, A.; Wasiliew, P.; Kracht, M. Interleukin-1 (IL-1) Pathway. Sci. Signal. 2010, 3, cm1. [Google Scholar] [CrossRef]
- Nixon, A.B.; O’Flaherty, J.T.; Salyer, J.K.; Wykle, R.L. Acetyl-CoA:1-O-Alkyl-2-Lyso-Sn-Glycero-3-Phosphocholine Acetyltransferase Is Directly Activated by P38 Kinase. J. Biol. Chem. 1999, 274, 5469–5473. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.R.S.; Owen, J.S.; Nixon, A.B.; Thomas, L.N.; Wooten, R.; Daniel, L.W.; O’Flaherty, J.T.; Wykle, R.L. Regulation of Platelet-Activating Factor Synthesis in Human Neutrophils by MAP Kinases. Biochim. Biophys. Acta BBA—Mol. Cell Res. 2002, 1592, 175–184. [Google Scholar] [CrossRef]
- Fragopoulou, E.; Nomikos, T.; Karantonis, H.C.; Apostolakis, C.; Pliakis, E.; Samiotaki, M.; Panayotou, G.; Antonopoulou, S. Biological Activity of Acetylated Phenolic Compounds. J. Agric. Food Chem. 2007, 55, 80–89. [Google Scholar] [CrossRef]
- Fragopoulou, E.; Gkotsi, K.; Petsini, F.; Gioti, K.; Kalampaliki, A.D.; Lambrinidis, G.; Kostakis, I.K.; Tenta, R. Synthesis and Biological Evaluation of Resveratrol Methoxy Derivatives. Molecules 2023, 28, 5547. [Google Scholar] [CrossRef] [PubMed]
- Xanthopoulou, M.N.; Asimakopoulos, D.; Antonopoulou, S.; Demopoulos, C.A.; Fragopoulou, E. Effect of Robola and Cabernet Sauvignon Extracts on Platelet Activating Factor Enzymes Activity on U937 Cells. Food Chem. 2014, 165, 50–59. [Google Scholar] [CrossRef]
- Petsini, F.; Detopoulou, M.; Kostakis, I.K.; Fragopoulou, E.; Antonopoulou, S. Resveratrol, Tyrosol and Their Derivatives Inhibit Platelet Activating Factor Biosynthetic Enzymes in Homogenized U-937 Cells. Pharm. Chem. J. 2024, 58, 216–226. [Google Scholar] [CrossRef]
- Vlachogianni, I. Study of the Effect of Phenolic Compounds and Their Derivatives in Cell Functions Under Inflammatory Conditions. Ph.D. Thesis, Harokopio University, Athens, Greek, 2015. [Google Scholar]
- Vlachogianni, I.C.; Fragopoulou, E.; Stamatakis, G.M.; Kostakis, I.K.; Antonopoulou, S. Platelet Activating Factor (PAF) Biosynthesis Is Inhibited by Phenolic Compounds in U-937 Cells under Inflammatory Conditions. Prostaglandins Other Lipid Mediat. 2015, 121, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Segeritz, C.-P.; Vallier, L. Cell Culture. In Basic Science Methods for Clinical Researchers; Elsevier: Amsterdam, The Netherlands, 2017; pp. 151–172. ISBN 978-0-12-803077-6. [Google Scholar]
- Tremblay, M.-È.; Almsherqi, Z.A.; Deng, Y. Plasmalogens and Platelet-Activating Factor Roles in Chronic Inflammatory Diseases. BioFactors Oxf. Engl. 2022, 48, 1203–1216. [Google Scholar] [CrossRef]
- Krishnan, J.; Selvarajoo, K.; Tsuchiya, M.; Lee, G.; Choi, S. Toll-like Receptor Signal Transduction. Exp. Mol. Med. 2007, 39, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Interleukin-1 in the Pathogenesis and Treatment of Inflammatory Diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Tsoupras, A.; Zabetakis, I.; Demopoulos, C.A. Forty Years Since the Structural Elucidation of Platelet-Activating Factor (PAF): Historical, Current, and Future Research Perspectives. Molecules 2019, 24, 4414. [Google Scholar] [CrossRef]
- Deo, D.D.; Bazan, N.G.; Hunt, J.D. Activation of Platelet-Activating Factor Receptor-Coupled Gαq Leads to Stimulation of Src and Focal Adhesion Kinase via Two Separate Pathways in Human Umbilical Vein Endothelial Cells. J. Biol. Chem. 2004, 279, 3497–3508. [Google Scholar] [CrossRef]
- Pelech, S.L.; Charest, D.L.; Howard, S.L.; Paddon, H.B.; Salari, H. Protein Kinase C Activation by Platelet-Activating Factor Is Independent of Enzyme Translocation. Biochim. Biophys. Acta BBA—Mol. Cell Res. 1990, 1051, 100–107. [Google Scholar] [CrossRef]
- Shimo, T.; Matsumura, S.; Ibaragi, S.; Isowa, S.; Kishimoto, K.; Mese, H.; Nishiyama, A.; Sasaki, A. Specific Inhibitor of MEK-Mediated Cross-Talk between ERK and P38 MAPK during Differentiation of Human Osteosarcoma Cells. J. Cell Commun. Signal. 2007, 1, 103–111. [Google Scholar] [CrossRef]
- Xiao, Y.Q.; Malcolm, K.; Worthen, G.S.; Gardai, S.; Schiemann, W.P.; Fadok, V.A.; Bratton, D.L.; Henson, P.M. Cross-Talk between ERK and P38 MAPK Mediates Selective Suppression of Pro-Inflammatory Cytokines by Transforming Growth Factor-β. J. Biol. Chem. 2002, 277, 14884–14893. [Google Scholar] [CrossRef]
- Wauson, E.M.; Guerra, M.L.; Barylko, B.; Albanesi, J.P.; Cobb, M.H. Off-Target Effects of MEK Inhibitors. Biochemistry 2013, 52, 5164–5166. [Google Scholar] [CrossRef]
- Malaguarnera, L. Influence of Resveratrol on the Immune Response. Nutrients 2019, 11, 946. [Google Scholar] [CrossRef] [PubMed]
- Alesci, A.; Nicosia, N.; Fumia, A.; Giorgianni, F.; Santini, A.; Cicero, N. Resveratrol and Immune Cells: A Link to Improve Human Health. Molecules 2022, 27, 424. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-H.; Mustafa, F.B.; Pervaiz, S.; Ng, F.S.P.; Lim, L.H.K. ERK1/2 Activation Is Required for Resveratrol-Induced Apoptosis in MDA-MB-231 Cells. Int. J. Oncol. 2008, 33, 81–92. [Google Scholar] [CrossRef]
- Jakus, P.B.; Kalman, N.; Antus, C.; Radnai, B.; Tucsek, Z.; Gallyas, F.; Sumegi, B.; Veres, B. TRAF6 Is Functional in Inhibition of TLR4-Mediated NF-κB Activation by Resveratrol. J. Nutr. Biochem. 2013, 24, 819–823. [Google Scholar] [CrossRef]
- Kawabata, T.; Tokuda, H.; Fujita, K.; Kainuma, S.; Sakai, G.; Matsushima-Nishiwaki, R.; Kozawa, O.; Otsuka, T. Resveratrol Inhibits the Epidermal Growth Factor-Induced Migration of Osteoblasts: The Suppression of SAPK/JNK and Akt. Cell. Physiol. Biochem. 2017, 43, 1025–1036. [Google Scholar] [CrossRef]
- Yi, C.-O.; Jeon, B.T.; Shin, H.J.; Jeong, E.A.; Chang, K.C.; Lee, J.E.; Lee, D.H.; Kim, H.J.; Kang, S.S.; Cho, G.J.; et al. Resveratrol Activates AMPK and Suppresses LPS-Induced NF-κB-Dependent COX-2 Activation in RAW 264.7 Macrophage Cells. Anat. Cell Biol. 2011, 44, 194. [Google Scholar] [CrossRef] [PubMed]
- Salt, I.P.; Palmer, T.M. Exploiting the Anti-Inflammatory Effects of AMP-Activated Protein Kinase Activation. Expert Opin. Investig. Drugs 2012, 21, 1155–1167. [Google Scholar] [CrossRef]
- Vaez, H.; Soraya, H.; Garjani, A.; Gholikhani, T. Toll-Like Receptor 4 (TLR4) and AMPK Relevance in Cardiovascular Disease. Adv. Pharm. Bull. 2023, 13, 36–47. [Google Scholar] [CrossRef]
- Kikuchi, H.; Mimuro, H.; Kuribayashi, F. Resveratrol Strongly Enhances the Retinoic Acid-Induced Superoxide Generating Activity via up-Regulation of Gp91-Phox Gene Expression in U937 Cells. Biochem. Biophys. Res. Commun. 2018, 495, 1195–1200. [Google Scholar] [CrossRef]
- Hsieh, M.-J.; Chin, M.-C.; Lin, C.-C.; His, Y.-T.; Lo, Y.-S.; Chuang, Y.-C.; Chen, M.-K. Pinostilbene Hydrate Suppresses Human Oral Cancer Cell Metastasis by Downregulation of Matrix Metalloproteinase-2 Through the Mitogen-Activated Protein Kinase Signaling Pathway. Cell. Physiol. Biochem. 2018, 50, 911–923. [Google Scholar] [CrossRef]
- Pinheiro, D.M.L.; De Oliveira, A.H.S.; Coutinho, L.G.; Fontes, F.L.; De Medeiros Oliveira, R.K.; Oliveira, T.T.; Faustino, A.L.F.; Lira Da Silva, V.; De Melo Campos, J.T.A.; Lajus, T.B.P.; et al. Resveratrol Decreases the Expression of Genes Involved in Inflammation through Transcriptional Regulation. Free Radic. Biol. Med. 2019, 130, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Balestrieri, C.; Felice, F.; Piacente, S.; Pizza, C.; Montoro, P.; Oleszek, W.; Visciano, V.; Balestrieri, M.L. Relative Effects of Phenolic Constituents from Yucca Schidigera Roezl. Bark on Kaposi’s Sarcoma Cell Proliferation, Migration, and PAF Synthesis. Biochem. Pharmacol. 2006, 71, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Tarui, M.; Shindou, H.; Kumagai, K.; Morimoto, R.; Harayama, T.; Hashidate, T.; Kojima, H.; Okabe, T.; Nagano, T.; Nagase, T.; et al. Selective Inhibitors of a PAF Biosynthetic Enzyme Lysophosphatidylcholine Acyltransferase 2. J. Lipid Res. 2014, 55, 1386–1396. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wu, X.; Cai, X.; Song, M.; Zheng, J.; Pan, C.; Qiu, P.; Zhang, L.; Zhou, S.; Tang, Z.; et al. Identification of Pinostilbene as a Major Colonic Metabolite of Pterostilbene and Its Inhibitory Effects on Colon Cancer Cells. Mol. Nutr. Food Res. 2016, 60, 1924–1932. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Huang, I.-T.; Shih, H.-J.; Chang, Y.-Y.; Kao, M.-C.; Shih, P.-C.; Huang, C.-J. Cluster of Differentiation 14 and Toll-like Receptor 4 Are Involved in the Anti-Inflammatory Effects of Tyrosol. J. Funct. Foods 2019, 53, 93–104. [Google Scholar] [CrossRef]
- Serreli, G.; Melis, M.P.; Corona, G.; Deiana, M. Modulation of LPS-Induced Nitric Oxide Production in Intestinal Cells by Hydroxytyrosol and Tyrosol Metabolites: Insight into the Mechanism of Action. Food Chem. Toxicol. 2019, 125, 520–527. [Google Scholar] [CrossRef]
- Kim, Y.-Y.; Lee, S.; Kim, M.-J.; Kang, B.-C.; Dhakal, H.; Choi, Y.-A.; Park, P.-H.; Choi, H.; Shin, T.-Y.; Choi, H.G.; et al. Tyrosol Attenuates Lipopolysaccharide-Induced Acute Lung Injury by Inhibiting the Inflammatory Response and Maintaining the Alveolar Capillary Barrier. Food Chem. Toxicol. 2017, 109, 526–533. [Google Scholar] [CrossRef]
- Muriana, F.J.G.; Montserrat-de La Paz, S.; Lucas, R.; Bermudez, B.; Jaramillo, S.; Morales, J.C.; Abia, R.; Lopez, S. Tyrosol and Its Metabolites as Antioxidative and Anti-Inflammatory Molecules in Human Endothelial Cells. Food Funct. 2017, 8, 2905–2914. [Google Scholar] [CrossRef] [PubMed]
- Serra, G.; Deiana, M.; Spencer, J.P.E.; Corona, G. Olive Oil Phenolics Prevent Oxysterol-Induced Proinflammatory Cytokine Secretion and Reactive Oxygen Species Production in Human Peripheral Blood Mononuclear Cells, Through Modulation of P38 and JNK Pathways. Mol. Nutr. Food Res. 2017, 61, 1700283. [Google Scholar] [CrossRef]
- Prasad, A.; Rossi, C.; Manoharan, R.R.; Sedlářová, M.; Cangeloni, L.; Rathi, D.; Tamasi, G.; Pospíšil, P.; Consumi, M. Bioactive Compounds and Their Impact on Protein Modification in Human Cells. Int. J. Mol. Sci. 2022, 23, 7424. [Google Scholar] [CrossRef]
- Francescangeli, E.; Boila, A.; Goracci, G. Properties and Regulation of Microsomal PAF-Synthesizing Enzymes in Rat Brain Cortex. Neurochem. Res. 2000, 25, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Sundström, C.; Nilsson, K. Establishment and Characterization of a Human Histiocytic Lymphoma Cell Line (U-937). Int. J. Cancer 1976, 17, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Gavriil, L.; Detopoulou, M.; Petsini, F.; Antonopoulou, S.; Fragopoulou, E. Consumption of Plant Extract Supplement Reduces Platelet Activating Factor-Induced Platelet Aggregation and Increases Platelet Activating Factor Catabolism: A Randomised, Double-Blind and Placebo-Controlled Trial. Br. J. Nutr. 2019, 121, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]

| Short-Time Effect | Long-Time Effect | |||
|---|---|---|---|---|
| Compound | Concentration (μM) | % of Baseline | Concentration (μM) | % of Baseline |
| R | 100 | 95.9 ± 9.8 | 100 | 83.8 ± 1.6 |
| RMeth2 | 100 | 88.2 ± 3.4 | 20 | 84.2 ± 1.7 |
| RAc3 | 100 | 98.2 ± 8.3 | 75 | 97.2 ± 2.2 |
| T | N.A. | 100 | 86.1 ± 5.7 | |
| TAc2 | N.A. | 5 | 88.4 ± 3.1 | |
| TAc3 | N.A. | 5 | 86.2 ± 2.2 | |
| U-73122 | 1.5 | 86.7 ± 1.5 | N.A. | |
| PD 98059 | 25 | 96.7 ± 1.3 | N.A. | |
| SB 203580 | 20 | 108.7 ± 2.0 | N.A. | |
| RO-31-8425 | 2.5 | 93.6 ± 1.7 | N.A. | |
| SP 600125 | 5 | 98.3 ± 2.5 | N.A. | |
| PAF-CPT, IL-1β and Inhibitors, 30 min | |||||
|---|---|---|---|---|---|
| IL-1β 3.3 ng/mL | U-73122 1.5 μM | PD 98059 25 μM | SB 203580 20 μM | RO-31-8425 2.5 μM | |
| p-Value (vs. baseline) * | <0.0001 | 0.0165 | 0.006 | 0.0082 | 0.3836 |
| %Change (vs. baseline) | 43.6 ± 23.5 | 21.8 ± 17.7 | 31.5 ± 22.9 | 32.2 ± 27.0 | −11.3 ± 29.6 |
| p-Value (vs. IL-1β) * | 0.0155 | 0.2303 | 0.2912 | 0.0017 | |
| Lyso-PAF-ATC, IL-1β and Inhibitors, 3 h | |||||
|---|---|---|---|---|---|
| IL-1β 3.3 ng/mL | U-73122 1.5 μM | PD 98059 25 μM | SB 203580 20 μM | SP 600125 5 μM | |
| p-Value (vs. baseline) * | <0.0001 | 0.5704 | <0.0001 | 0.1741 | 0.8005 |
| %Change (vs. baseline) | 23.8 ± 16.4 | −2.6 ± 10.0 | 46.7 ± 13.8 | −8.3 ± 13.7 | 1.3 ± 10.4 |
| p-Value (vs. IL-1β) * | 0.0006 | <0.0001 | <0.0001 | 0.0006 | |
| PAF-CPT | LysoPAF-ATC | ||||
|---|---|---|---|---|---|
| Phenolic Compound | %Change | p-Value * | %Change | p-Value * | |
| R | 100 μM | −11.7 ± 11.4 | 0.160 | −17.8 ± 7.2 | 0.1313 |
| R | 50 μM | −2.2 ± 14.4 | 0.774 | 14.5 ± 19.5 | 0.0424 |
| R | 10 μM | 8.4 ± 18.0 | 0.257 | 35.0± 16.4 | <0.0001 |
| RMeth2 | 100 μM | 3.0 ± 8.8 | 0.682 | 44.8 ± 12.7 | <0.0001 |
| RMeth2 | 50 μM | −0.1 ± 15.8 | 0.992 | 28.7 ± 24.3 | 0.0001 |
| RMeth2 | 10 μM | 7.9 ± 7.8 | 0.289 | 13.0 ± 22.1 | 0.0682 |
| RAc3 | 100 μM | 3.9 ± 14.7 | 0.600 | 28.0 ± 11.7 | 0.0058 |
| RAc3 | 50 μM | −8.2 ± 16.0 | 0.268 | 10.1 ± 17.3 | 0.1679 |
| RAc3 | 10 μM | 0.9 ± 10.6 | 0.902 | −3.4 ± 37.4 | 0.6409 |
| Compared to | Baseline | Phenolic Compound | ||
|---|---|---|---|---|
| %Change | p-Value * | %Change | p-Value * | |
| R 50 μΜ | 14.5 ± 19.5 | 0.0413 | ||
| R 50 μM + PD 98059 | 12.4 ± 17.4 | 0.2454 | −1.8 ± 15.2 | 0.8503 |
| R 10 μM | 35.0 ± 16.4 | <0.0001 | ||
| R 10 μM + PD 98059 | 1.5 ± 15.0 | 0.8887 | −24.8 ± 11.1 | 0.0037 |
| RMeth2 50 μM | 28.7 ± 24.3 | <0.0001 | ||
| RMeth2 50 μM +PD 98059 | 40.8 ± 27.1 | <0.0001 | 9.4 ± 21.0 | 0.2375 |
| RMeth2 10 μM | 13.0 ± 22.1 | 0.0669 | ||
| RMeth2 10 μM + PD 98059 | 49.3 ± 10.7 | <0.0001 | 32.1 ± 9.5 | 0.0011 |
| RAc3 50 μM | 10.1 ± 17.3 | 0.1660 | ||
| RAc3 50 μM + PD 98059 | 1.7 ± 14.0 | 0.8657 | −7.7 ± 12.7 | 0.4128 |
| RAc3 10 μM | −3.4 ± 37.4 | 0.6395 | ||
| RAc3 10 μM + PD 98059 | 31.0 ± 7.9 | 0.0022 | 35.6 ± 8.2 | 0.0011 |
| Compared to | Baseline | Phenolic Compound | ||
|---|---|---|---|---|
| %Change | p-Value * | %Change | p-Value * | |
| R 50 μΜ | 14.5 ± 19.5 | 0.0402 | ||
| R 50 μM + U-73122 | 39.9 ± 20.5 | <0.0001 | 22.2 ± 17.9 | 0.0127 |
| R 10 μM | 35.0 ± 16.4 | <0.0001 | ||
| R 10 μM + U-73122 | 44.0 ± 24.1 | 0.0002 | 6.7 ± 17.9 | 0.4646 |
| RMeth2 50 μM | 28.7 ± 24.3 | <0.0001 | ||
| RMeth2 50 μM + U-73122 | 32.1 ± 16.2 | 0.0014 | 2.6 ± 12.6 | 0.7398 |
| RMeth2 10 μM | 13.0 ± 22.1 | 0.0654 | ||
| RMeth2 10 μM + U-73122 | 21.9 ± 14.8 | 0.0402 | 7.9 ± 13.1 | 0.4074 |
| RAc3 50 μM | 10.1 ± 17.3 | 0.1636 | ||
| RAc3 50 μM + U-73122 | 24.2 ± 14.4 | 0.0151 | 12.8 ± 13.1 | 0.1696 |
| RAc3 10 μM | −3.4 ± 37.4 | 0.6376 | ||
| RAc3 10 μM + U-73122 | −3.0 ± 8.5 | 0.7816 | 0.5 ± 8.8 | 0.9656 |
| Compared to | Baseline | Phenolic Compound | ||
|---|---|---|---|---|
| %Change | p-Value * | %Change | p-Value * | |
| R 50 μΜ | 14.5 ± 19.5 | 0.0404 | ||
| R 50 μM + SB 203580 | −25.8 ± 9.6 | 0.0165 | −35.2 ± 8.4 | 0.0003 |
| R 10 μM | 35.0 ± 16.4 | <0.0001 | ||
| R 10 μM + SB 203580 | −49.1 ± 8.3 | <0.0001 | −62.3 ± 6.2 | <0.0001 |
| RMeth2 50 μM | 28.7 ± 24.3 | <0.0001 | ||
| RMeth2 50 μM + SB 203580 | −21.5 ± 16.7 | 0.0444 | −39.0 ± 13.0 | <0.0001 |
| RMeth2 10 μM | 13.0 ± 22.1 | 0.0656 | ||
| RMeth2 10 μM + SB 203580 | −7.3 ± 22.4 | 0.4619 | −18.0 ± 19.9 | 0.0461 |
| RAc3 50 μM | 10.1 ± 17.3 | 0.1639 | ||
| RAc3 50 μM + SB 203580 | −20.1 ± 18.1 | 0.0604 | −27.5 ± 16.5 | 0.0066 |
| RAc3 10 μM | −3.4 ± 37.4 | 0.6378 | ||
| RAc3 10 μM + SB 203580 | −23.7 ± 4.1 | 0.0435 | −21.0 ± 4.3 | 0.0918 |
| Phenolic Compound | PAF-CPT | LysoPAF-ATC | LysoPAF-ATE | ||||
|---|---|---|---|---|---|---|---|
| %Change | p-Value * | %Change | p-Value * | %Change | p-Value * | ||
| R | 100 μM | −14.8 (−31.3, −7.6) | 0.0004 | −43.4 (−47.7, −33.9) | <0.0001 | −41.6 (−48.3, −28.2) | <0.0001 |
| R | 50 μM | −26.7 (−44.9, −26.7) | 0.0005 | −24.0 (−38.6, −18.5) | <0.0001 | −32.6 (−39.3, −25.8) | <0.0001 |
| R | 10 μM | −12.7 (−26.7, 7.2) | 0.0105 | −5.4 (−21.3, 23.5) | 0.5838 | 0.6 (−28.6, 11.0) | 0.3062 |
| R | 5 μM | −8.5 (−13.8, −3.0) | 0.1289 | 17.6 (4.4, 26.1) | 0.0624 | 7.1 (−4.5, 7.6) | 0.5607 |
| R | 1 μM | 3.4 (−2.4, 8.9) | 0.6101 | 1.6 (−6.9, 14.0) | 0.6932 | 17.9 (−7.9, 26.2) | 0.2139 |
| R | 0.1 μM | −10.1 (−18.1, −1.4) | 0.0702 | 4.6 (−3.6, 17.4) | 0.418 | 14.1 (−12.2, 26.2) | 0.3424 |
| RMeth2 | 20 μM | −39.3 (−47.2, −33.7) | <0.0001 | 12.7 (−3.7, 14.2) | 0.8145 | −21.0 (−23.7, −18.5) | 0.0035 |
| RMeth2 | 10 μM | −20.2 (−40.9, −6.9) | 0.0005 | −36.3 (−51.3, −26.1) | <0.0001 | −13.5 (−21.9, −3.8) | 0.1534 |
| RMeth | 5 μM | −15.0 (−17.9, −5.4) | 0.0269 | −11.1 (−24.0, −4.5) | 0.0366 | 1.1 (−5.3, 13.4) | 0.5612 |
| RAc3 | 75 μM | −14.7 (−64.1, −10.0) | <0.0001 | −23.0 (−32.6, −14.7) | 0.0028 | −10.8 (−23.0, −9.1) | 0.0936 |
| RAc3 | 50 μM | −26.7 (−37.1, −21.5) | <0.0001 | 3.2 (−4.7, 11.9) | 0.7164 | −1.3 (−9.6, 8.3) | 0.7816 |
| RAc3 | 10 μM | −24.2 (−33.6, −3.9) | 0.0005 | 4.1 (−10.1, 22.5) | 0.701 | 7.1 (−15.5, 25.0) | 0.4637 |
| Phenolic Compound | PAF-CPT | LysoPAF-ATC | LysoPAF-ATE | ||||
|---|---|---|---|---|---|---|---|
| %Change | p-Value * | %Change | p-Value * | %Change | p-Value * | ||
| T | 100 μM | 8.7 (−2.5, 18.4) | 0.2891 | −11.8 (−20.9, 3.9) | 0.0193 | 8.3 (−17.5, 17.9) | 0.5867 |
| T | 50 μM | −44.7 (−48.2, −23.2) | 0.0005 | 44.1 (35.3, 52.5) | 0.002 | 32.4 (16.2, 39.2) | 0.003 |
| T | 10 μM | −9.0 (−19.8, 6.6) | 0.1597 | −1.1 (−7.4, 12.8) | 0.4879 | 0.0 (−17.9, 28.2) | 0.56 |
| T | 5 μM | −16.3 (−36.6, 7.1) | 0.0238 | 6.8 (0.8, 13.1) | 0.1201 | 3.3 (−14.9, 8.9) | 0.9267 |
| T | 1 μM | −16.1 (−29.4, −3.0) | 0.0015 | 3.3 (−7.9, 14.7) | 0.3912 | 4.1 (−6.3, 13.0) | 0.3321 |
| T | 0.1 μM | −23.7 (−30.7, −10.1) | 0.0001 | 4.4 (−11.7, 20.1) | 0.3465 | 10.8 (−8.9, 27.0) | 0.1117 |
| TAc2 | 5 μM | 3.5 (−14.8, 17.3) | 0.7534 | −13.6 (−18.6, −4.2) | 0.0179 | −6.6 (−14.4, 5.9) | 0.2404 |
| TAc2 | 1 μM | 2.3 (−18.2, 30.2) | 0.9294 | 3.8 (−3.6, 20.1) | 0.228 | 5.4 (−8.9, 14.6) | 0.3435 |
| TAc2 | 0.1 μM | 13.1 (−18.1, 26.7) | 0.7818 | −2.1 (−8.3, 2.2) | 0.8988 | 5.8 (−11.3, 11.4) | 0.5235 |
| TAc3 | 5 μM | −2.9 (19.8, 16.2) | 0.3879 | 1.9 (−15.7, 12.7) | 0.77 | −9.0 (−13.8, 3.2) | 0.3635 |
| TAc3 | 1 μM | −11.3 (−26.3, 7.7) | 0.0806 | −8.5 (−17.0, 7.9) | 0.14 | −21.8 (−29.3, −1.3) | 0.0027 |
| TAc3 | 0.1 μM | −13.0 −34.5, 8.2) | 0.0491 | −9.8 (−19.9, 4.5) | 0.1397 | −0.2 (−6.5, 6.5) | 0.8604 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petsini, F.; Detopoulou, M.; Choleva, M.; Kostakis, I.K.; Fragopoulou, E.; Antonopoulou, S. Exploring the Effect of Resveratrol, Tyrosol, and Their Derivatives on Platelet-Activating Factor Biosynthesis in U937 Cells. Molecules 2024, 29, 5419. https://doi.org/10.3390/molecules29225419
Petsini F, Detopoulou M, Choleva M, Kostakis IK, Fragopoulou E, Antonopoulou S. Exploring the Effect of Resveratrol, Tyrosol, and Their Derivatives on Platelet-Activating Factor Biosynthesis in U937 Cells. Molecules. 2024; 29(22):5419. https://doi.org/10.3390/molecules29225419
Chicago/Turabian StylePetsini, Filio, Maria Detopoulou, Maria Choleva, Ioannis K. Kostakis, Elizabeth Fragopoulou, and Smaragdi Antonopoulou. 2024. "Exploring the Effect of Resveratrol, Tyrosol, and Their Derivatives on Platelet-Activating Factor Biosynthesis in U937 Cells" Molecules 29, no. 22: 5419. https://doi.org/10.3390/molecules29225419
APA StylePetsini, F., Detopoulou, M., Choleva, M., Kostakis, I. K., Fragopoulou, E., & Antonopoulou, S. (2024). Exploring the Effect of Resveratrol, Tyrosol, and Their Derivatives on Platelet-Activating Factor Biosynthesis in U937 Cells. Molecules, 29(22), 5419. https://doi.org/10.3390/molecules29225419








