Development of an Electrochemical Paper-Based Device Modified with Functionalized Biochar for the Screening of Paracetamol in Substandard Medicines
Abstract
1. Introduction
2. Results
2.1. Morphological Characterization
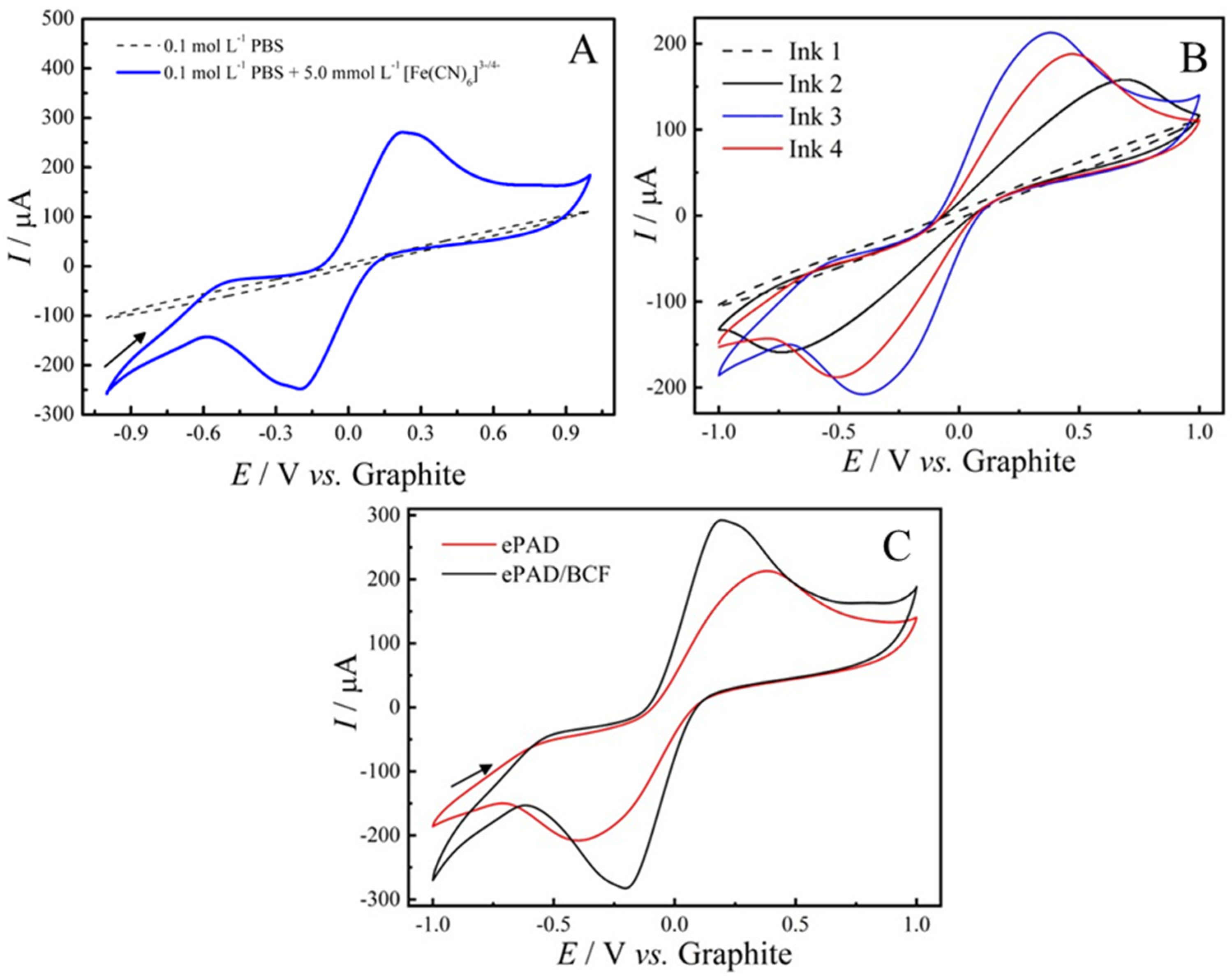
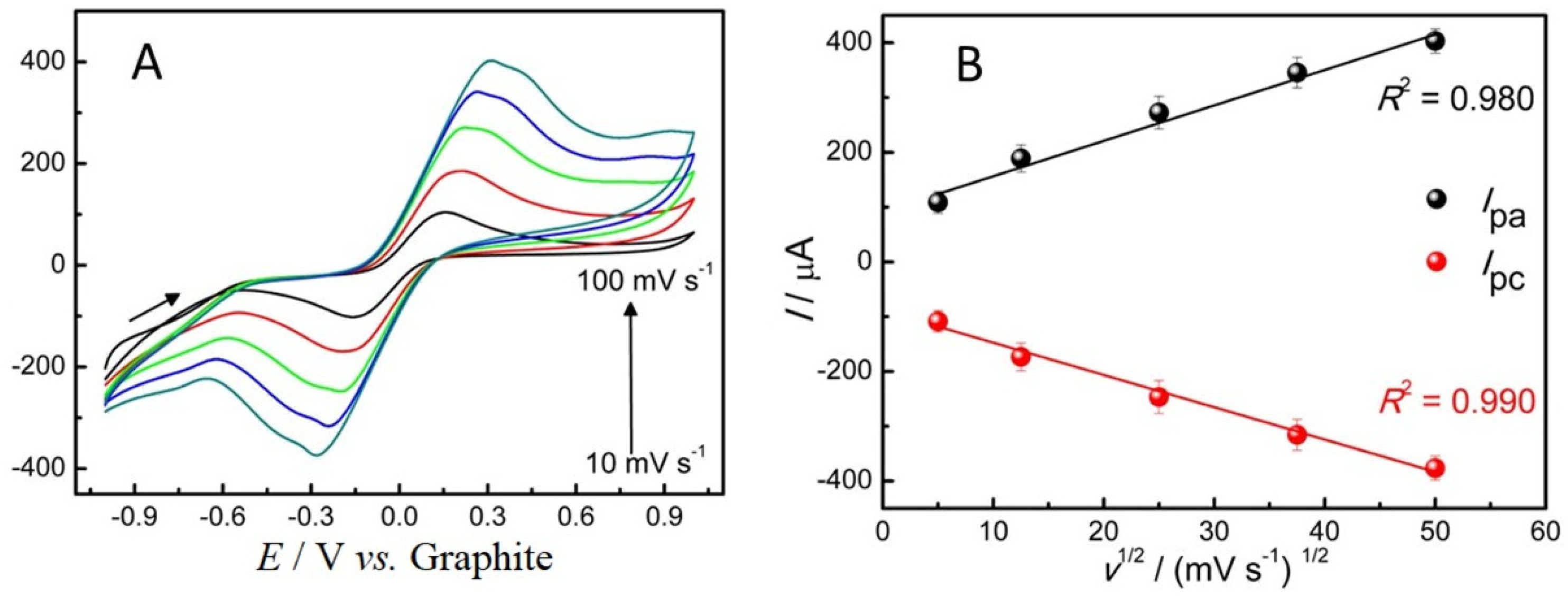
2.2. Electrochemical Characterization of ePADs
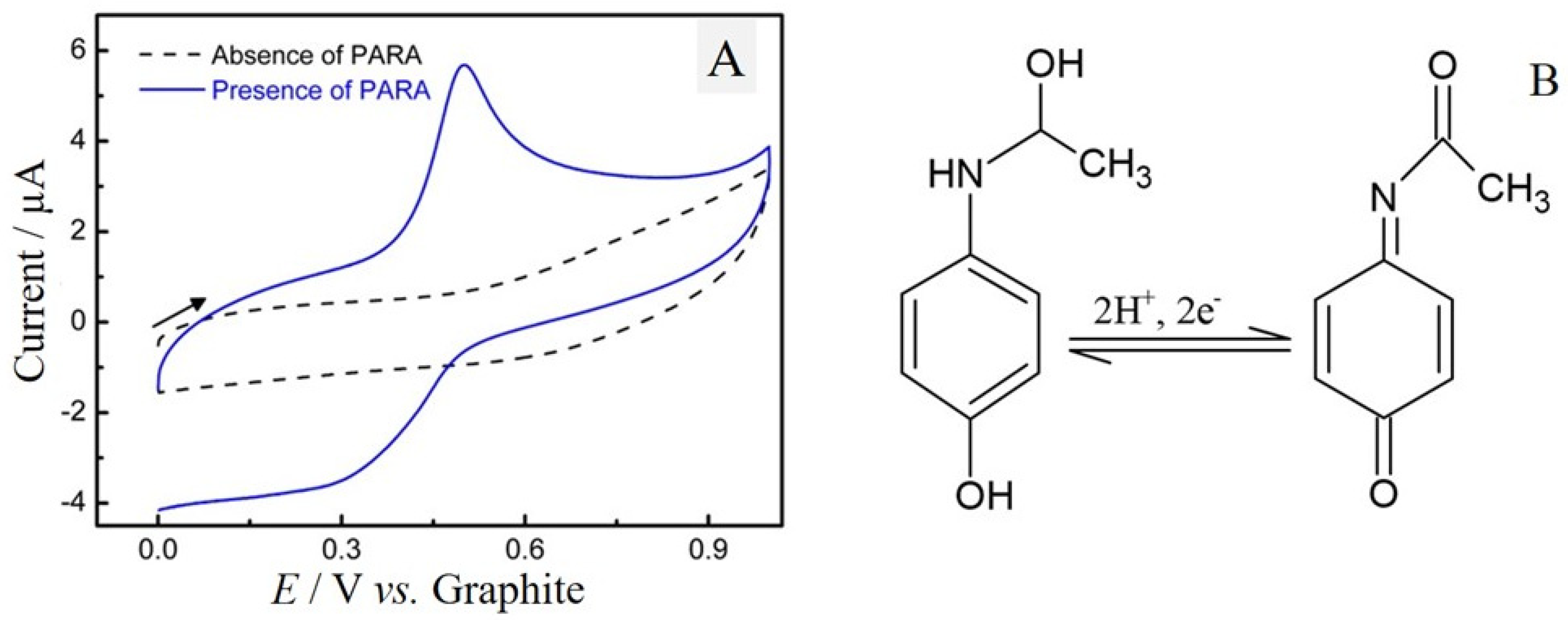
2.3. Electrochemical Behavior of Paracetamol
2.4. Calibration Curve
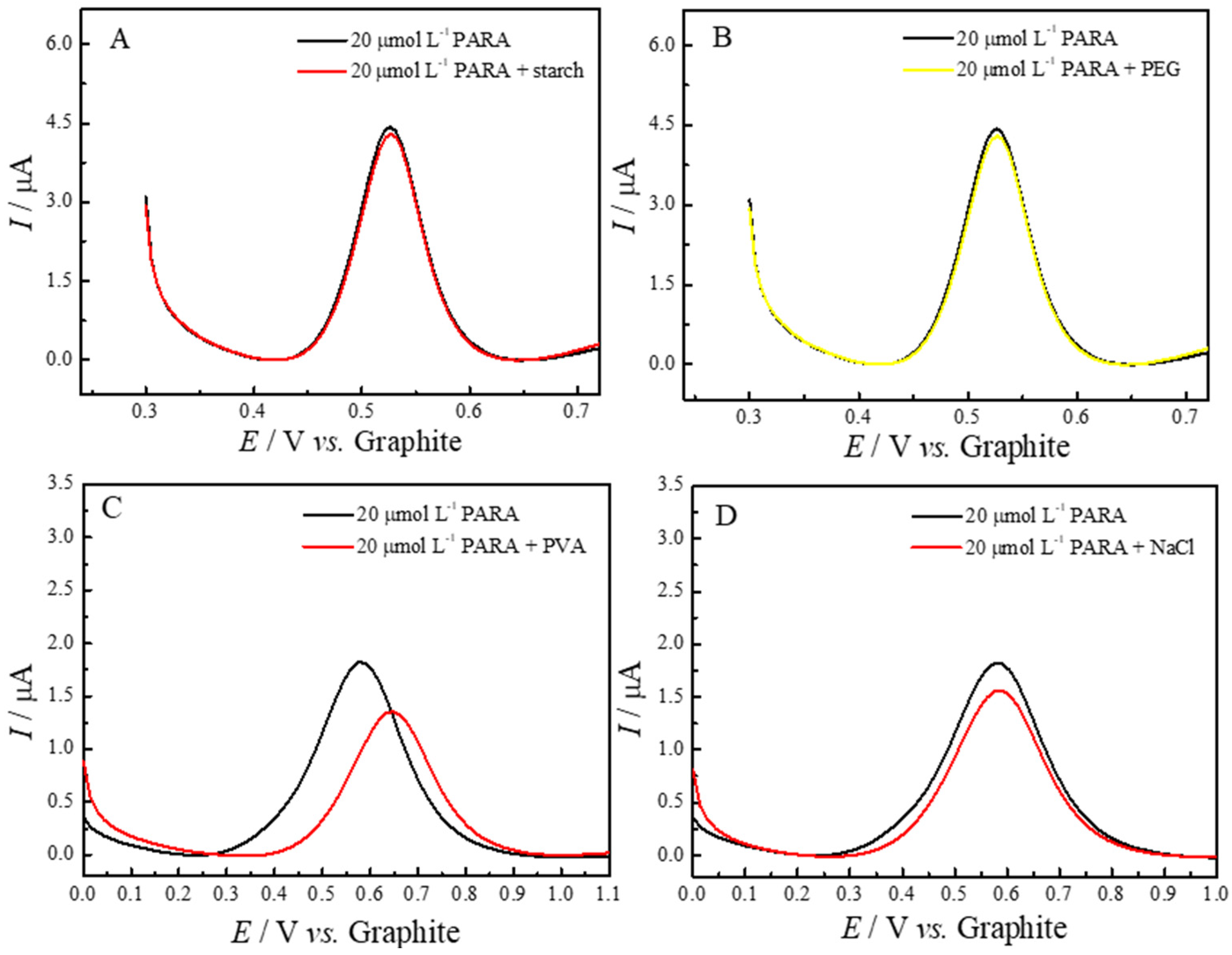
2.5. Application of the ePAD/BCF Sensor in the Quantification of Paracetamol
3. Materials and Methods
3.1. Material and Reagents
3.2. Instruments
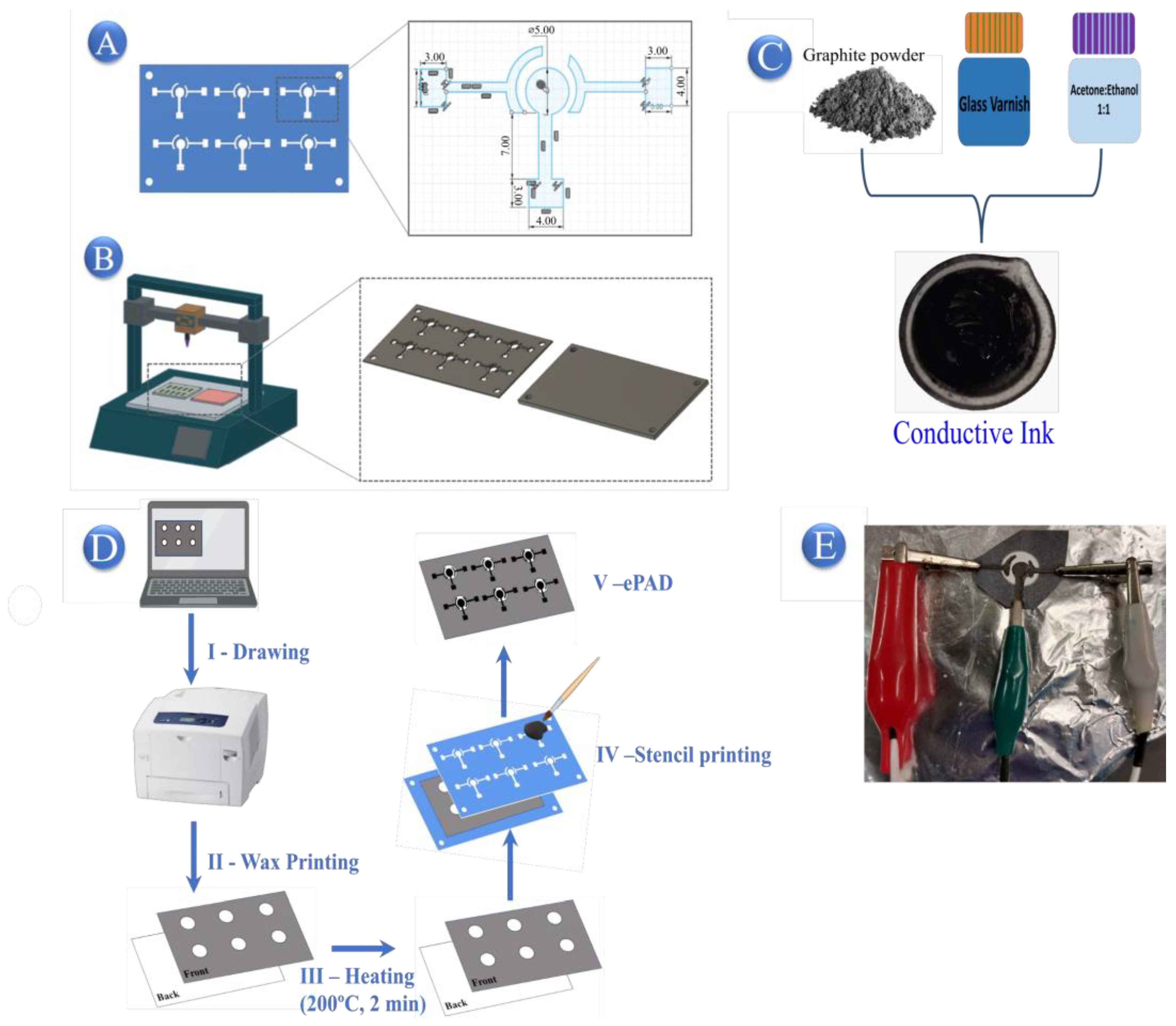
3.3. Fabrication and Modification of ePADs
3.4. Sample Preparation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roncone, A.; Kelly, S.D.; Giannioti, Z.; Hauk, C.; Caillet, C.; Newton, P.N.; Perez-Mon, C.; Bontempo, L. Stable Isotope Ratio Analysis: An Emerging Tool to Trace the Origin of Falsified Medicines. TrAC Trends Anal. Chem. 2024, 174, 117666. [Google Scholar] [CrossRef]
- Sandeep, D.H.; Krushna, B.R.R.; Sharma, S.C.; Ravindran, P.; Sivayogana, R.; Ramesha, H.; Hemalatha, N.; Rashmi, H.; Devaraju, K.S.; Krithika, C.; et al. Eco-Friendly Synthesis of CQDs from Pistachio Shells: Versatile Applications in Anti-Counterfeiting, Flexible Films, Latent Fingerprints and Potential Anti-Cancer Activity. J. Alloys Compd. 2024, 991, 174311. [Google Scholar] [CrossRef]
- Pal, T.; Mathai, T.; Mukherji, S. Colorimetric Chemosensor for Rapid Detection of Fluoroquinolone Load in Environmental Water Bodies, Urine, and Counterfeit Drug Testing. Biosens. Bioelectron. X 2023, 14, 100384. [Google Scholar] [CrossRef]
- Mazurków, J.M.; Montiel, N.F.; Van Echelpoel, R.; Kusior, A.; De Wael, K. The Potential of Electrochemical Sensors to Unveil Counterfeits: Xanax as a Case Study. Electrochim. Acta 2024, 494, 144458. [Google Scholar] [CrossRef]
- Mohammad, M.A.A.; Elkady, E.F.; Fouad, M.A.; Salem, W.A. Analysis of Aspirin, Prasugrel and Clopidogrel in Counterfeit Pharmaceutical and Herbal Products: Plackett-Burman Screening and Box-Behnken Optimization. J. Chromatogr. Sci. 2021, 59, 730–747. [Google Scholar] [CrossRef] [PubMed]
- Schwartzman, G.H.; Dekker, P.K.; Silverstein, A.S.; Fontecilla, N.M.; Norton, S.A. Dermatologic Consequences of Substandard, Spurious, Falsely Labeled, Falsified, and Counterfeit Medications. Dermatol. Clin. 2022, 40, 227–236. [Google Scholar] [CrossRef]
- Vandy, A.; Conteh, E.; Lahai, M.; Kolipha-Kamara, M.; Marah, M.; Marah, F.; Suma, K.M.; Mattia, S.C.; Tucker, K.D.S.; Wray, V.S.E.; et al. Physicochemical Quality Assessment of Various Brands of Paracetamol Tablets Sold in Freetown Municipality. Heliyon 2024, 10, e25502. [Google Scholar] [CrossRef]
- Achache, M.; Elouilali Idrissi, G.; Chraka, A.; Ben Seddik, N.; Draoui, K.; Bouchta, D.; Mohamed, C. Detection of Paracetamol by a Montmorillonite-Modified Carbon Paste Sensor: A Study Combining MC Simulation, DFT Computation and Electrochemical Investigations. Talanta 2024, 274, 126027. [Google Scholar] [CrossRef]
- Theyagarajan, K.; Lakshmi, B.A.; Kim, Y.J. Electrochemical Sensing of Acetaminophen in Biofluids, Pharmaceutical and Environmental Samples Using Cobalt Hexacyanoferrate Decorated Iron Terephthalate Metal Organic Framework. Electrochim. Acta 2024, 488, 144229. [Google Scholar] [CrossRef]
- Omar, J.; Boix, A.; Ulberth, F. Raman Spectroscopy for Quality Control and Detection of Substandard Painkillers. Vib. Spectrosc. 2020, 111, 103147. [Google Scholar] [CrossRef]
- de Sá, B.S.; Stefano, J.S.; Luiz, L.R.; Perfecto, T.M.; Mazon, T.; Volanti, D.P.; Janegitz, B.C.; Ribeiro, C. Methane-Derived Electrochemical Sensor for Determination of Paracetamol and Diquat. Mater. Chem. Phys. 2024, 315, 129025. [Google Scholar] [CrossRef]
- Keizers, P.H.J.; Bakker, F.; Ferreira, J.; Wackers, P.F.K.; van Kollenburg, D.; van der Aa, E.; van Beers, A. Benchtop NMR Spectroscopy in the Analysis of Substandard and Falsified Medicines as Well as Illegal Drugs. J. Pharm. Biomed. Anal. 2020, 178, 112939. [Google Scholar] [CrossRef] [PubMed]
- Sakuda, M.; Yoshida, N.; Koide, T.; Keila, T.; Kimura, K.; Tsuboi, H. Clarification of the Internal Structure and Factors of Poor Dissolution of Substandard Roxithromycin Tablets by Near-Infrared Chemical Imaging. Int. J. Pharm. 2021, 596, 120232. [Google Scholar] [CrossRef]
- Melaré, A.G.; Barreto, F.C.; Silva, M.K.L.; Simões, R.P.; Cesarino, I. Determination of Fluoxetine in Weight Loss Herbal Medicine Using an Electrochemical Sensor Based on RGO-CuNPs. Molecules 2023, 28, 6361. [Google Scholar] [CrossRef] [PubMed]
- Vazan, M.; Tashkhourian, J.; Haghighi, B. A Novel Electrochemical Sensor Based on MoO3 Nanobelt-Graphene Oxide Composite for the Simultaneous Determination of Paracetamol and 4-Aminophenol. Diam. Relat. Mater. 2023, 140, 110549. [Google Scholar] [CrossRef]
- Silva, L.R.G.; Stefano, J.S.; Crapnell, R.D.; Banks, C.E.; Janegitz, B.C. Additive Manufactured Microfluidic Device for Electrochemical Detection of Carbendazim in Honey Samples. Talanta Open 2023, 7, 100213. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, Q.; Zhang, X.; Yuan, W.; Wu, J. A Laser-Induced Graphene-Based Sensor Modified with CeO2 for Determination of Organophosphorus Pesticides with Improved Performance. Sensors 2023, 23, 9605. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, A. Exploring the Potential of Ionic Liquid-Based Electrochemical Biosensors for Real-Time Biomolecule Monitoring in Pharmaceutical Applications: From Lab to Life. Results Eng. 2023, 20, 101533. [Google Scholar] [CrossRef]
- Hu, J.; Dai, J.; Huang, C.; Zeng, X.; Wei, W.; Wang, Z.; Lin, P. Organic Electrochemical Transistor with MoS2 Nanosheets Modified Gate Electrode for Sensitive Glucose Sensing. Sensors 2023, 23, 7449. [Google Scholar] [CrossRef]
- Eldeeb, M.A.; Dhamu, V.N.; Paul, A.; Muthukumar, S.; Prasad, S. Espial: Electrochemical Soil PH Sensor for In Situ Real-Time Monitoring. Micromachines 2023, 14, 2188. [Google Scholar] [CrossRef]
- Wang, Z.; Shi, Y.; Xu, Z.; Sun, M.; Shen, X.; Wu, K.; Yu, M.; Zhang, L.; Yu, G. Development of a C3N4-Based Electrochemical Sensing Platform for the Detection of Circulating Tumor Cells in Blood. Alex. Eng. J. 2024, 98, 170–176. [Google Scholar] [CrossRef]
- Barreto, F.C.; dos Santos, G.T.V.; Leao, A.L.; Goonetilleke, A.; Cesarino, I. Determination and Electro-Remediation of Sulfamethazine Using Carbon Nanotubes and Silver Nanoparticles as Electrode Modifiers. J. Solid State Electrochem. 2024, 1–10. [Google Scholar] [CrossRef]
- Cancelliere, R.; Cianciaruso, M.; Carbone, K.; Micheli, L. Biochar: A Sustainable Alternative in the Development of Electrochemical Printed Platforms. Chemosensors 2022, 10, 344. [Google Scholar] [CrossRef]
- Barreto, F.C.; Ito, E.Y.; Mounienguet, N.K.; Dal’ Evedove Soares, L.; Yang, J.; He, Q.; Cesarino, I. Electrochemical Sensor Based on Spent Coffee Grounds Hydrochar and Metal Nanoparticles for Simultaneous Detection of Emerging Contaminants in Natural Water. Chemosensors 2023, 11, 562. [Google Scholar] [CrossRef]
- Hassan, Q.; Meng, Z.; Noroozifar, M.; Kerman, K. Methylene Blue-Modified Biochar from Sugarcane for the Simultaneous Electrochemical Detection of Four DNA Bases. Chemosensors 2023, 11, 169. [Google Scholar] [CrossRef]
- Gomes, G.C.; da Silva, M.K.L.; Barreto, F.C.; Cesarino, I. Electrochemical Sensing Platform Based on Renewable Carbon Modified with Antimony Nanoparticles for Methylparaben Detection in Personal Care Products. Chemosensors 2023, 11, 141. [Google Scholar] [CrossRef]
- Baharfar, M.; Rahbar, M.; Tajik, M.; Liu, G. Engineering Strategies for Enhancing the Performance of Electrochemical Paper-Based Analytical Devices. Biosens. Bioelectron. 2020, 167, 112506. [Google Scholar] [CrossRef]
- Silva, M.K.L.; Sousa, G.S.; Simoes, R.P.; Cesarino, I. Fabrication of Paper-Based Analytical Devices Using a PLA 3D-Printed Stencil for Electrochemical Determination of Chloroquine and Escitalopram. J. Solid State Electrochem. 2022, 26, 581–586. [Google Scholar] [CrossRef]
- Mustafa, F.; Finny, A.S.; Kirk, K.A.; Andreescu, S. Printed Paper-Based (Bio)Sensors: Design, Fabrication and Applications. Compr. Anal. Chem. 2020, 89, 63–89. [Google Scholar] [CrossRef]
- Squissato, A.L.; Almeida, E.S.; Silva, S.G.; Richter, E.M.; Batista, A.D.; Munoz, R.A.A. Screen-Printed Electrodes for Quality Control of Liquid (Bio)Fuels. TrAC Trends Anal. Chem. 2018, 108, 210–220. [Google Scholar] [CrossRef]
- Nunes, E.W.; Silva, M.K.L.; Rascón, J.; Leiva-Tafur, D.; Lapa, R.M.L.; Cesarino, I. Acetylcholinesterase Biosensor Based on Functionalized Renewable Carbon Platform for Detection of Carbaryl in Food. Biosensors 2022, 12, 486. [Google Scholar] [CrossRef] [PubMed]
- Kalinke, C.; Oliveira, P.R.; Oliveira, G.A.; Mangrich, A.S.; Marcolino-Junior, L.H.; Bergamini, M.F. Activated Biochar: Preparation, Characterization and Electroanalytical Application in an Alternative Strategy of Nickel Determination. Anal. Chim. Acta 2017, 983, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Pradela-Filho, L.A.; Andreotti, I.A.A.; Carvalho, J.H.S.; Araújo, D.A.G.; Orzari, L.O.; Gatti, A.; Takeuchi, R.M.; Santos, A.L.; Janegitz, B.C. Glass Varnish-Based Carbon Conductive Ink: A New Way to Produce Disposable Electrochemical Sensors. Sensors Actuators B Chem. 2020, 305, 127433. [Google Scholar] [CrossRef]
- Allende, S.; Liu, Y.; Jacob, M.V. Electrochemical Sensing of Paracetamol Based on Sugarcane Bagasse-Activated Biochar. Ind. Crops Prod. 2024, 211, 118241. [Google Scholar] [CrossRef]
- Zeghioud, H.; Fryda, L.; Djelal, H.; Assadi, A.; Kane, A. A Comprehensive Review of Biochar in Removal of Organic Pollutants from Wastewater: Characterization, Toxicity, Activation/Functionalization and Influencing Treatment Factors. J. Water Process Eng. 2022, 47, 102801. [Google Scholar] [CrossRef]
- Araújo, D.A.G.; Camargo, J.R.; Pradela-Filho, L.A.; Lima, A.P.; Muñoz, R.A.A.; Takeuchi, R.M.; Janegitz, B.C.; Santos, A.L. A Lab-Made Screen-Printed Electrode as a Platform to Study the Effect of the Size and Functionalization of Carbon Nanotubes on the Voltammetric Determination of Caffeic Acid. Microchem. J. 2020, 158, 105297. [Google Scholar] [CrossRef]
- Pradela-Filho, L.A.; Araújo, D.A.G.; Takeuchi, R.M.; Santos, A.L. Nail Polish and Carbon Powder: An Attractive Mixture to Prepare Paper-Based Electrodes. Electrochim. Acta 2017, 258, 786–792. [Google Scholar] [CrossRef]
- Crapnell, R.D.; Banks, C.E. Perspective: What Constitutes a Quality Paper in Electroanalysis? Talanta Open 2021, 4, 100065. [Google Scholar] [CrossRef]
- Ferrari, A.G.M.; Foster, C.W.; Kelly, P.J.; Brownson, D.A.C.; Banks, C.E. Determination of the Electrochemical Area of Screen-Printed Electrochemical Sensing Platforms. Biosensors 2018, 8, 53. [Google Scholar] [CrossRef]
- Camargo, J.R.; Andreotti, I.A.A.; Kalinke, C.; Henrique, J.M.; Bonacin, J.A.; Janegitz, B.C. Waterproof Paper as a New Substrate to Construct a Disposable Sensor for the Electrochemical Determination of Paracetamol and Melatonin. Talanta 2020, 208, 120458. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: Hoboken, NJ, USA, 2001; ISBN 0471043729. [Google Scholar]
- Martins, T.S.; Bott-Neto, J.L.; Oliveira, O.N.; Machado, S.A.S. Paper-Based Electrochemical Sensors with Reduced Graphene Nanoribbons for Simultaneous Detection of Sulfamethoxazole and Trimethoprim in Water Samples. J. Electroanal. Chem. 2021, 882, 114985. [Google Scholar] [CrossRef]
- Deroco, P.B.; Fatibello-Filho, O.; Arduini, F.; Moscone, D. Electrochemical Determination of Capsaicin in Pepper Samples Using Sustainable Paper-Based Screen-Printed Bulk Modified with Carbon Black. Electrochim. Acta 2020, 354, 136628. [Google Scholar] [CrossRef]
- Jemmeli, D.; Marcoccio, E.; Moscone, D.; Dridi, C.; Arduini, F. Highly Sensitive Paper-Based Electrochemical Sensor for Reagent Free Detection of Bisphenol A. Talanta 2020, 216, 120924. [Google Scholar] [CrossRef] [PubMed]




| Scheme | GR-GV Ratio | Ipa (µA) | Ipc (µA) | ΔEp (mV) |
|---|---|---|---|---|
| Ink 01—80:20 (m/m) | N/A | N/A | N/A | |
| ePAD | Ink 02—70:30 (m/m) | 158 | −159 | 1420 |
| Ink 03—60:40 (m/m) | 213 | −208 | 772 | |
| Ink 04—50:50 (m/m) | 187 | −190 | 980 | |
| ePAD/BCF | 294 | −284 | 387 |
| Manufacturing Method | Conductive Ink | Analyte | LOD (µmol L−1) | Ref. |
|---|---|---|---|---|
| Spread with brush | Graphite/nail polish 80:20% (m/m) | Dopamine | 5.2 | [37] |
| Adhesive mask | Graphite/glass varnish 80:20% (m:m) | Dopamine | 4.1 | [33] |
| Catechol | 9.0 | |||
| Hydroquinone | 5.3 | |||
| Estriol | 0.08 | |||
| Graphite/nail polish 52:48% (m/m) + 20 µL of MWCNT suspension | Caffeic acid | 0.2 | [36] | |
| Screen-printed | Graphite-based ink and Ag/AgCl ink | Bisphenol-A | 0.03 | [44] |
| Carbon powder (10% m/m) with 9.0 g of graphite ink | Capsaicin | 0.085 | [43] | |
| Carbon ink and rGNRs | Sulfamethoxazole | 0.09 | [42] | |
| Trimethoprim | 0.04 | |||
| 3D-printed stencil | GR-GV 60:40% (m/m) + 400 μL acetone/ethanol (1:1) | PARA | 3.50 | * |
| Interferents | Interference (%) |
|---|---|
| Starch | −3.20 |
| PEG | −5.00 |
| PVA | −9.20 |
| Sodium Chloride | −8.50 |
| Samples Primary Ingredients | Labeled (mg) | Added (µmol L−1) | Quantified (µmol L−1) | Error (µmol L−1) | Error (%) | Recovery (%) | Standard Error of Recovery (%) |
|---|---|---|---|---|---|---|---|
| Paracetamol Tablet | 500 mg/tablet | 20.00 | 18.70 ± 0.03 | 0.03 | −6.50% | 93.50 | 0.15 |
| Flu medicine capsule | Paracetamol 400 mg Chlorpheniramine Maleate 4 mg Phenylephrine Hydrochloride 4 mg | 20.00 | 18.50 ± 0.05 | 0.05 | −7.50% | 92.50 | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.K.L.d.; Barreto, F.C.; Sousa, G.d.S.; Simões, R.P.; Ahuja, G.; Dutta, S.; Mulchandani, A.; Cesarino, I. Development of an Electrochemical Paper-Based Device Modified with Functionalized Biochar for the Screening of Paracetamol in Substandard Medicines. Molecules 2024, 29, 5468. https://doi.org/10.3390/molecules29225468
Silva MKLd, Barreto FC, Sousa GdS, Simões RP, Ahuja G, Dutta S, Mulchandani A, Cesarino I. Development of an Electrochemical Paper-Based Device Modified with Functionalized Biochar for the Screening of Paracetamol in Substandard Medicines. Molecules. 2024; 29(22):5468. https://doi.org/10.3390/molecules29225468
Chicago/Turabian StyleSilva, Martin Kassio Leme da, Francisco Contini Barreto, Guilherme dos Santos Sousa, Rafael Plana Simões, Gaurav Ahuja, Samriddha Dutta, Ashok Mulchandani, and Ivana Cesarino. 2024. "Development of an Electrochemical Paper-Based Device Modified with Functionalized Biochar for the Screening of Paracetamol in Substandard Medicines" Molecules 29, no. 22: 5468. https://doi.org/10.3390/molecules29225468
APA StyleSilva, M. K. L. d., Barreto, F. C., Sousa, G. d. S., Simões, R. P., Ahuja, G., Dutta, S., Mulchandani, A., & Cesarino, I. (2024). Development of an Electrochemical Paper-Based Device Modified with Functionalized Biochar for the Screening of Paracetamol in Substandard Medicines. Molecules, 29(22), 5468. https://doi.org/10.3390/molecules29225468










