Photochemical Hydroxyl Group Abstraction from N-Hydroxypyridine-2(1H)-thione Isolated in a Solid Hydrogen Matrix: Photogeneration of 2-Mercaptopyridine
Abstract
:1. Introduction
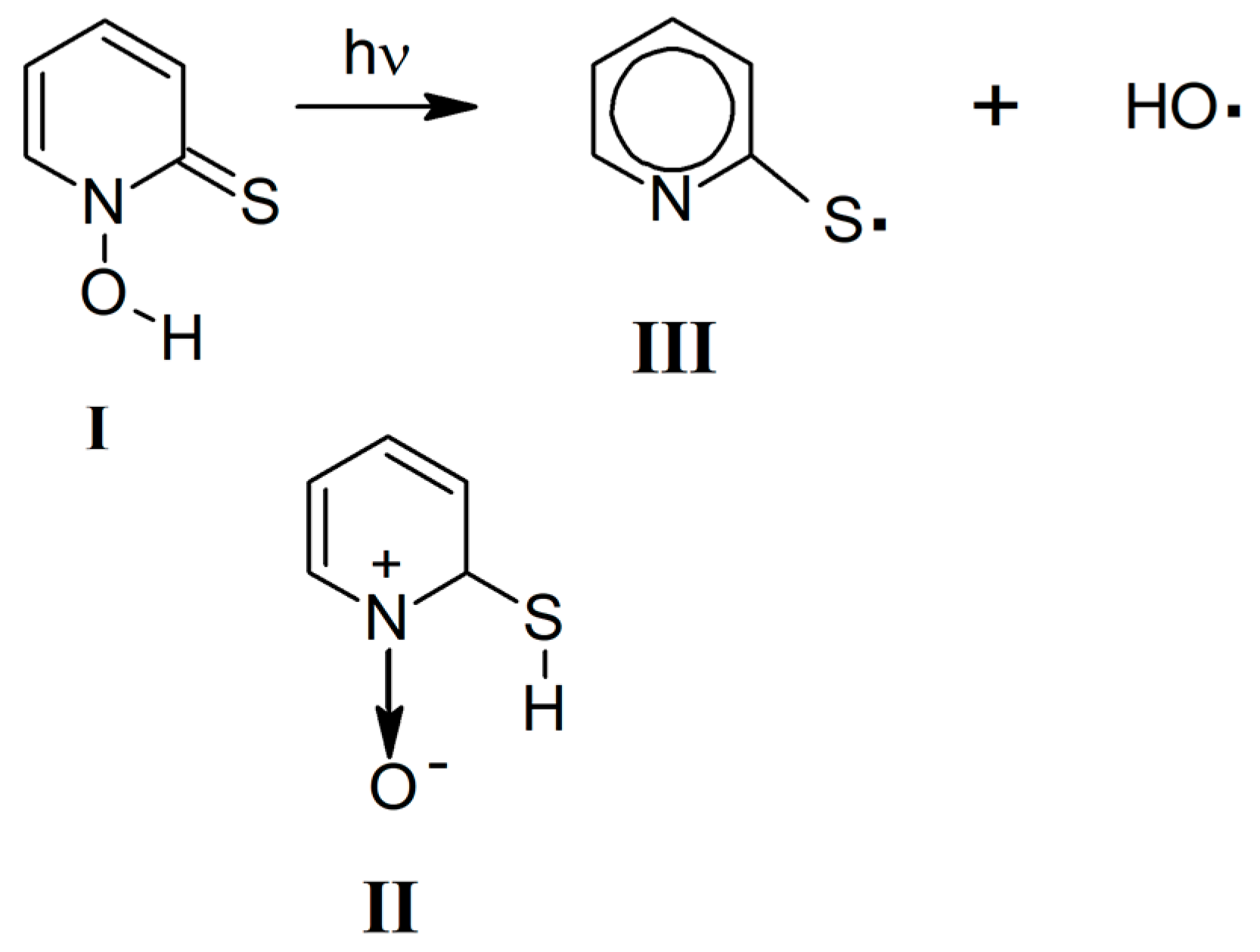
2. Results
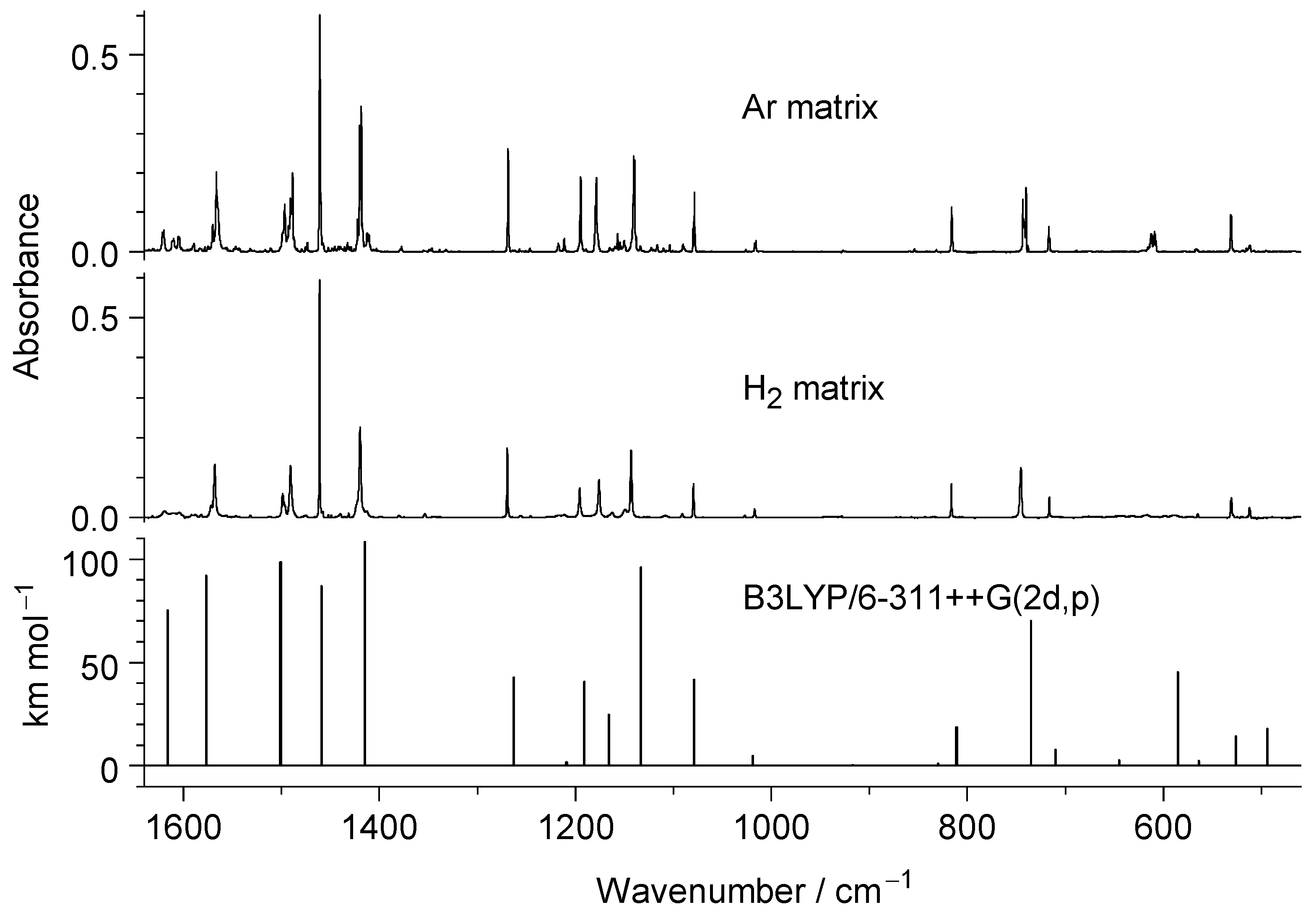
2.1. Structure and Infrared Spectrum of N-Hydroxypyridine-2(1H)-thione Monomers Isolated in Low-Temperature n-H2 Matrix
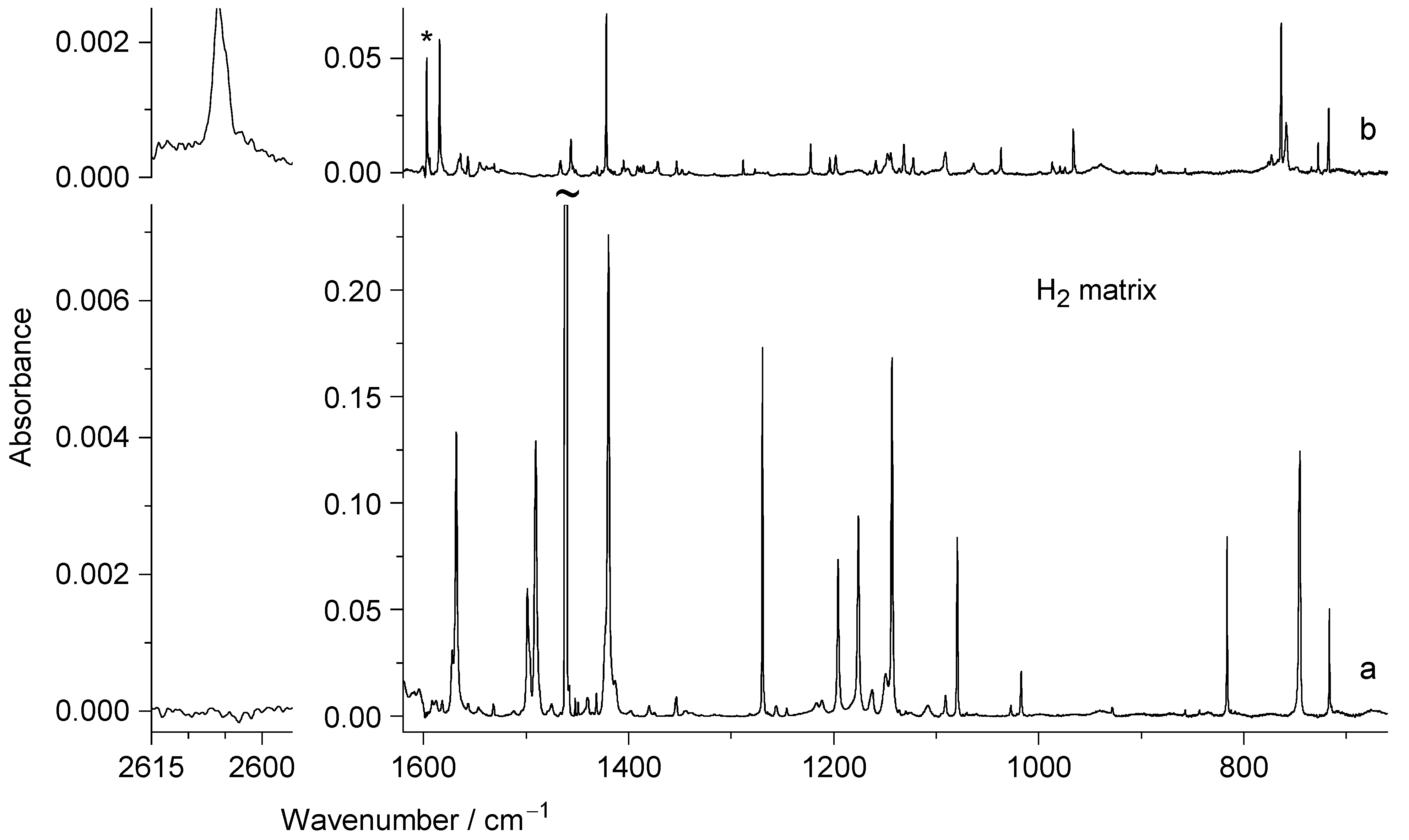
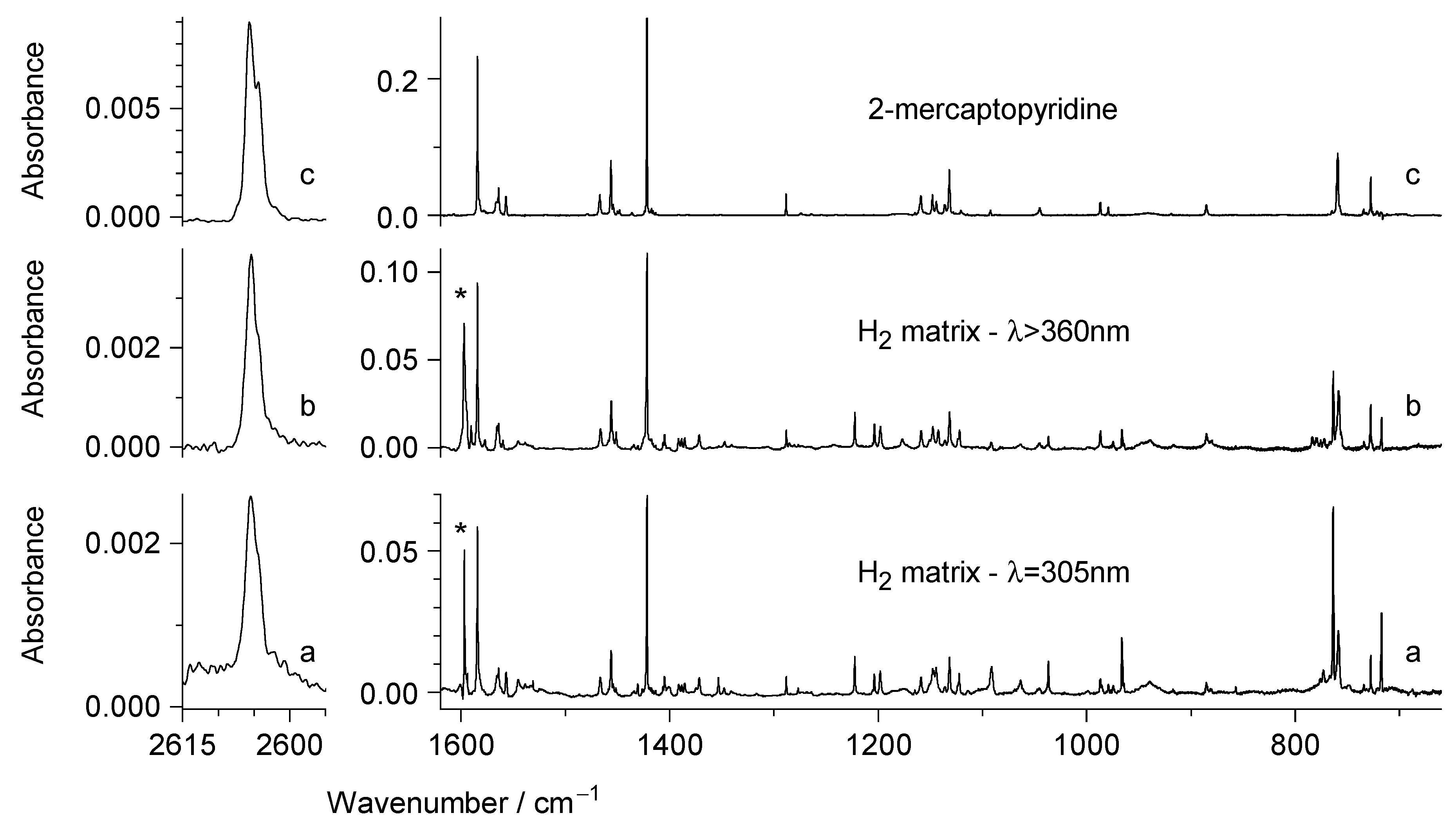
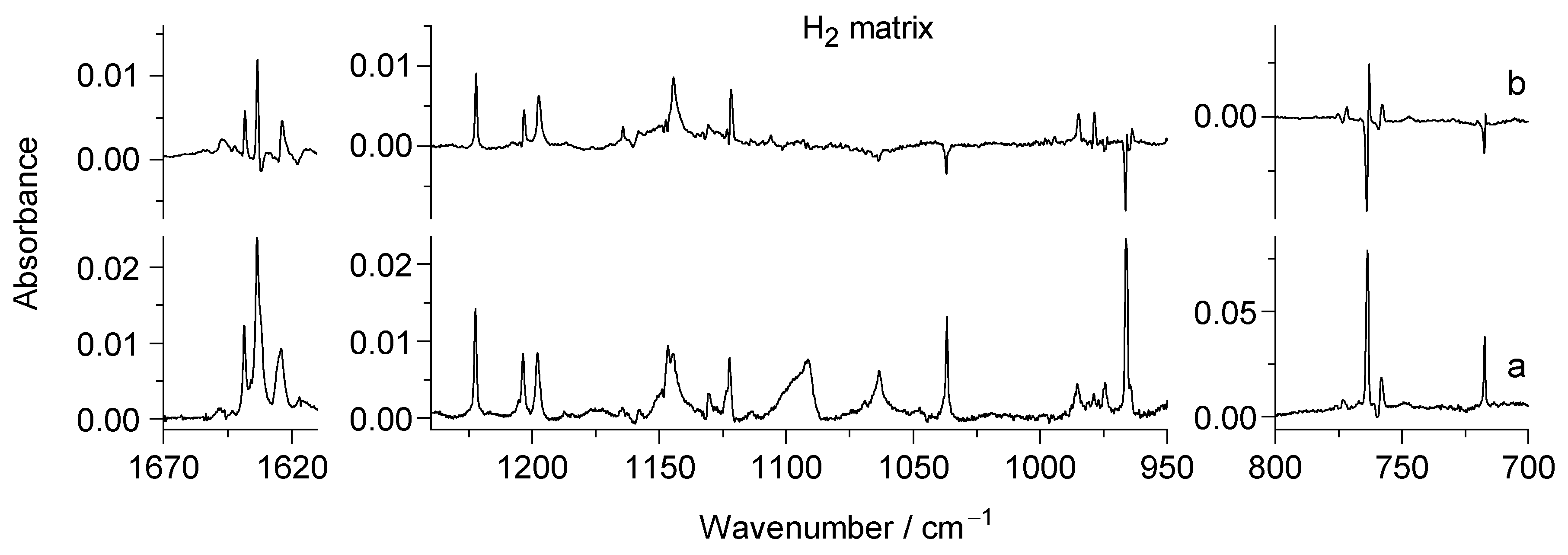
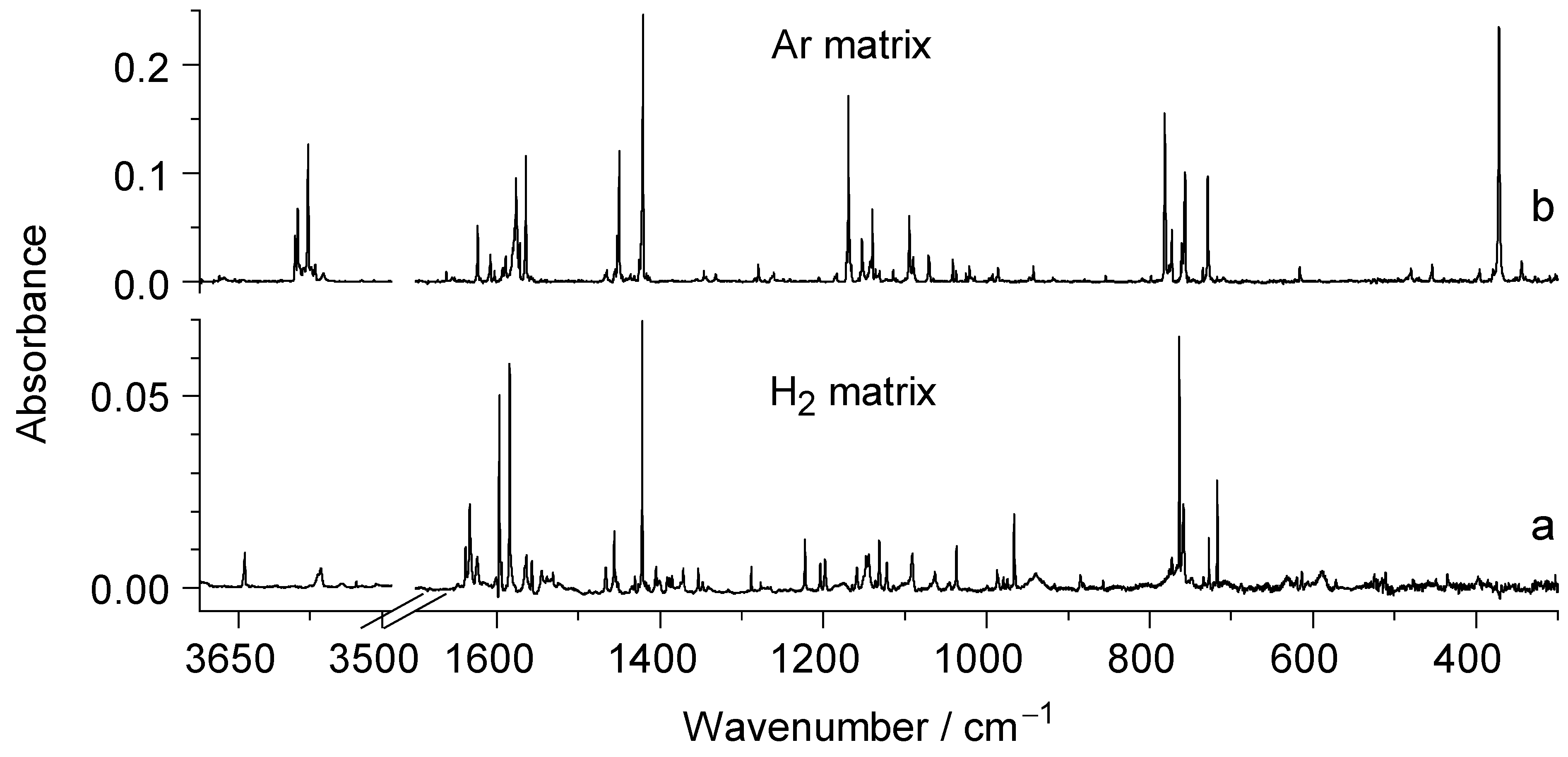
2.2. UV Irradiation of N-Hydroxypyridine-2(1H)-thione Isolated in a Low-Temperature n-H2 Matrix
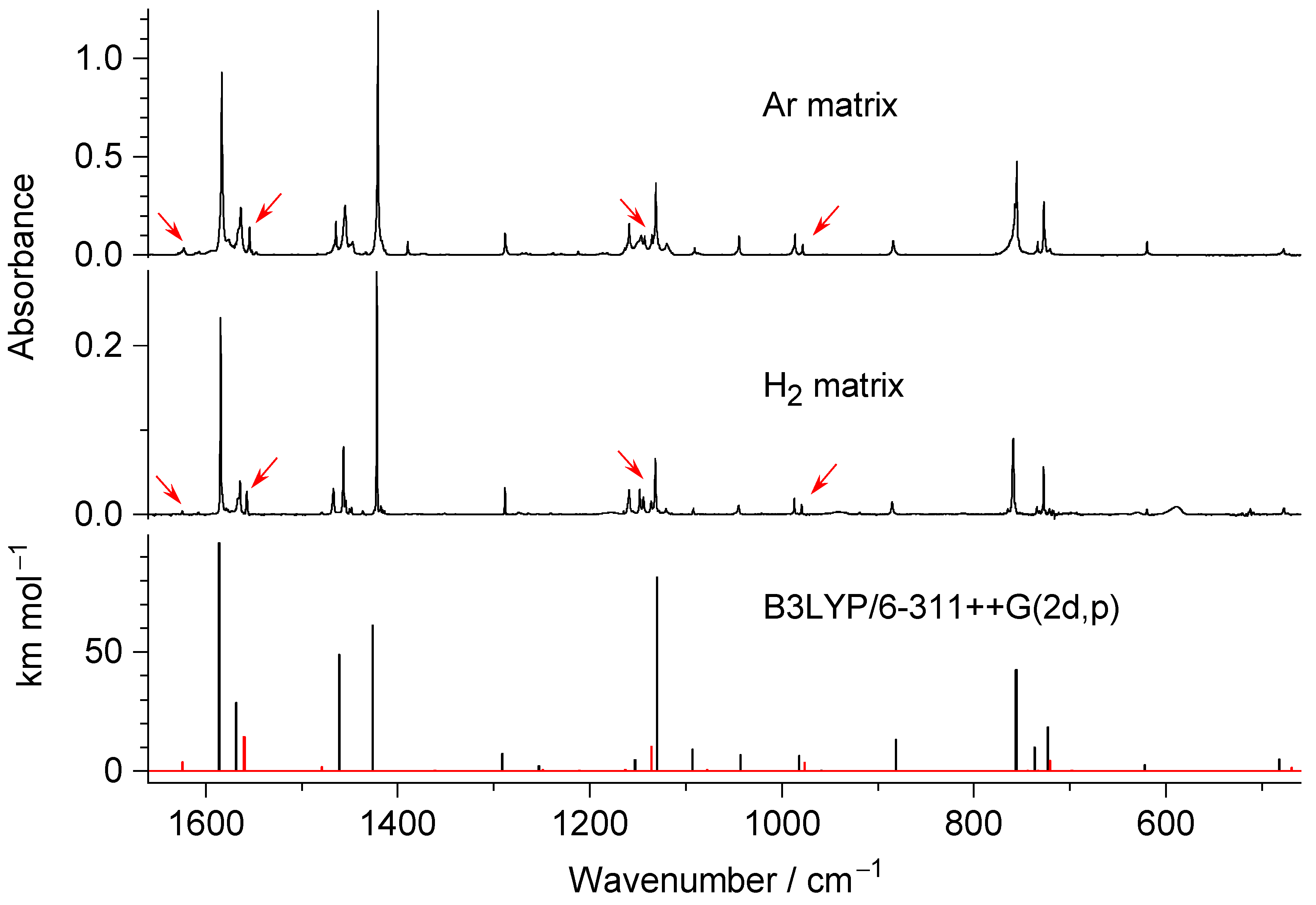
2.3. Comparison of Photoproducts Generated upon UV Irradiation of N-Hydroxypyridine-2(1H)-thione Isolated in n-H2 Matrices with Those Photoproduced from the Compound Isolated in Ar or N2 Matrices
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jones, B.A.; Katritzky, A.R. N-Oxides and Related Compounds. Part XVII. The Tautomerism of Mercapto- and Acylamino-pyridine 1-Oxides. J. Chem. Soc. 1960, 2937–2942. [Google Scholar] [CrossRef]
- Fukuda, R.; Ehara, M. Electronic Excitation and Ionization Behavior of N-Hydroxypyridine-2(1H)-thione and its Deprotonated Anion in a Polarizable Medium Studied Using Quantum Chemical Computations. Theor. Chem. Acc. 2016, 135, 105. [Google Scholar] [CrossRef]
- Lapinski, L.; Gerega, A.; Sobolewski, A.L.; Nowak, M.J. Thioperoxy Derivative Generated by UV-Induced Transformation of N-Hydroxypyridine-2(1H)-thione Isolated in Low-Temperature Matrixes. J. Phys. Chem. A 2008, 112, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Bond, A.; Jones, W. 1-Hydroxy-2(1H)-pyridinethione. Acta Crystallogr. C. 1999, 55, 1536–1538. [Google Scholar] [CrossRef]
- Aveline, B.M.; Kochevar, I.E.; Redmond, R.W. Photochemistry of the Nonspecific Hydroxyl Radical Generator, N-Hydroxypyridine-2(1H)-thione. J. Am. Chem. Soc. 1996, 118, 10113–10123. [Google Scholar] [CrossRef]
- Poole, J.S. Chapter 5. Recent Advances in the Photochemistry of Heterocyclic N-Oxides and Their Derivatives. In Heterocyclic N-Oxides; Larionov, O.V., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 111–151. [Google Scholar] [CrossRef]
- Epe, B.; Ballmaier, D.; Adam, W.; Grimm, G.N.; Saha-Möller, C.R. Photolysis of N-Hydroxypyridinethiones: A New Source of Hydroxyl Radicals for the Direct Damage of Cell-Free and Cellular DNA. Nucleic Acids Res. 1996, 24, 1625–1631. [Google Scholar] [CrossRef]
- Möller, M.; Adam, W.; Saha-Möller, C.R.; Stopper, H. Studies on Cytotoxic and Genotoxic Effects of N-Hydroxypyridine-2-thione (Omadine) in L5178Y Mouse Lymphoma Cells. Toxicol. Lett. 2002, 136, 77–84. [Google Scholar] [CrossRef]
- Li, D.D.; Han, R.M.; Liang, R.; Chen, C.H.; Lai, W.; Zhang, J.P.; Skibsted, L.H. Hydroxyl Radical Reaction with trans-Resveratrol: Initial Carbon Radical Adduct Formation Followed by Rearrangement to Phenoxyl Radical. J. Phys. Chem. B 2012, 116, 7154–7161. [Google Scholar] [CrossRef]
- Chen, C.H.; Han, R.M.; Liang, R.; Fu, L.M.; Wang, P.; Ai, X.C.; Zhang, J.P.; Skibsted, L.H. Direct Observation of the β-Carotene Reaction with Hydroxyl Radical. J. Phys. Chem. B 2011, 115, 2082–2089. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford Academic: Oxford, UK, 2015. [Google Scholar] [CrossRef]
- Halliwell, B.; Adhikary, A.; Dingfelder, M.; Dizdaroglu, M. Hydroxyl Radical is a Significant Player in Oxidative DNA Damage in vivo. Chem. Soc. Rev. 2021, 50, 8355–8360. [Google Scholar] [CrossRef]
- Demidchik, V.; Cuin, T.A.; Svistunenko, D.; Smith, S.J.; Miller, A.J.; Shabala, S.; Sokolik, A.; Yurin, V. Arabidopsis Root K+-Efflux Conductance Activated by Hydroxyl Radicals: Single-Channel Properties, Genetic Basis and Involvement in Stress-Induced Cell Death. J. Cell Sci. 2010, 123, 1468–1479. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Shabala, S.N.; Coutts, K.B.; Mark, A.; Tester, M.A.; Davies, J.M. Free Oxygen Radicals Regulate Plasma Membrane Ca2+ and K+ Permeable Channels in Plant Root Cells. J. Cell Sci. 2003, 116, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Gorini, F.; Scala, G.; Cooke, M.S.; Majello, B.; Amente, S. Towards a Comprehensive View of 8-Oxo-7,8-dihydro-2′-deoxyguanosine: Highlighting the Intertwined Roles of DNA Damage and Epigenetics in Genomic Instability. DNA Repair 2021, 97, 103027. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Sage, E.; Douki, T. Ultraviolet Radiation-Mediated Damage to Cellular DNA. Mutat. Res. 2005, 571, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Douki, T.; Gasparutto, D.; Ravanat, J.-L. Oxidative Damage to DNA: Formation, Measurement and Biochemical Features. Mutat. Res. 2003, 531, 5–23. [Google Scholar] [CrossRef]
- Chaulk, S.G.; Pezacki, J.P.; MacMillan, A.M. Studies of RNA Cleavage by Photolysis of N-Hydroxypyridine-2(1H)-thione. A New Photochemical Footprinting Method. Biochemistry 2000, 39, 10448–10453. [Google Scholar] [CrossRef]
- Aveline, B.M.; Kochevar, I.E.; Redmond, R.W. Photochemistry of N-Hydroxypyridine-2-thione Derivatives: Involvement of the 2-Pyridylthiyl Radical in the Radical Chain Reaction Mechanism. J. Am. Chem. Soc. 1995, 117, 9699–9708. [Google Scholar] [CrossRef]
- Aveline, B.M.; Kochevar, I.E.; Redmond, R.W. N-Hydroxypyridine-2(1H)-thione: Not a Selective Generator of Hydroxyl Radicals in Aqueous Solution. J. Am. Chem. Soc. 1996, 118, 289–290. [Google Scholar] [CrossRef]
- van Kranendonk, J. Chapter 5. Lattice Vibrations and Elastic Properties. In Solid Hydrogen: Theory of the Properties of Solid H2, HD and D2, 1st ed.; van Kranendonk, J., Ed.; Springer: New York, NY, USA, 1983; pp. 131–172. Available online: https://link.springer.com/chapter/10.1007/978-1-4684-4301-1_5 (accessed on 20 September 2024).
- Lapinski, L.; Nowak, M.J.; Rostkowska, H. Solid H2 versus Solid Noble-gas Environment: Influence on Photoinduced Hydrogen-Atom Transfer in Matrix-Isolated 4(3H)-Pyrimidinone. J. Chem. Phys. 2017, 146, 094306. [Google Scholar] [CrossRef]
- Rostkowska, H.; Luchowska, A.; Lapinski, L.; Nowak, M.J. Effect of a Solid hydrogen Environment on UV-Induced Hydrogen-Atom Transfer in Matrix-Isolated Heterocyclic Thione Compounds. J. Phys. Chem. A 2021, 125, 7437–7448. [Google Scholar] [CrossRef]
- Nowak, M.J.; Reva, I.; Rostkowska, H.; Lapinski, L. UV-induced Hydrogen-Atom Transfer and Hydrogen-Atom Detachment in Monomeric 7-Azaindole Isolated in Ar and n-H2 Matrices. Phys. Chem. Chem. Phys. 2017, 19, 11447–11454. [Google Scholar] [CrossRef]
- Góbi, S.; Keresztes, B.; Schneiker, A.; Tarczay, G. Hydrogen-Atom-Assisted Processes on Thioacetamide in para-H2 matrix—Formation of Thiol Tautomers. Phys. Chem. Chem. Phys. 2024, 26, 21589–21597. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.M.; Mitchell, E.G.; Sanchez, D.A.; Block, E.; Kukolich, S.G. Microwave Spectra and Gas Phase Structural Parameters for N-Hydroxypyridine-2(1H)-thione. J. Phys. Chem. A 2011, 115, 14526–14530. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.J.; Lapinski, L.; Rostkowska, H.; Leś, A.; Adamowicz, L. Theoretical and Matrix-Isolation Experimental Study on 2(1H)-Pyridinethione/2-Pyridinethiol. J. Phys. Chem. 1990, 94, 7406–7414. [Google Scholar] [CrossRef]
- Rostkowska, H.; Lapinski, L.; Reva, I.; Almeida, B.J.A.N.; Nowak, M.J.; Fausto, R. UV-Induced Hydrogen-Atom Transfer in 3,6-Dithiopyridazine and in Model Compounds 2-Thiopyridine and 3-Thiopyridazine. J. Phys. Chem. A 2011, 115, 12142–12149. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.M.; Viegas, L.P.; Wood, S.A.; Roque, J.P.L.; McMahon, R.J.; Fausto, R. Heavy-Atom Tunneling Through Crossing Potential Energy Surfaces: Cyclization of a Triplet 2-Formylarylnitrene to a Singlet 2,1-Benzisoxazole. Angew. Chem. Int. Ed. 2020, 59, 17622–17627. [Google Scholar] [CrossRef]
- Nunes, C.M.; Pinto, S.M.V.; Reva, I.; Rosado, M.T.S.; Fausto, R. Photochemistry of Matrix-Isolated 3-Chloro-1,2-benzisoxazole: Generation and Characterization of 2-Cyanophenoxyl Radical and Other Reactive Intermediates. J. Molec. Struct. 2018, 1172, 34–41. [Google Scholar] [CrossRef]
- Haupa, K.A.; Tielens, A.G.G.M.; Lee, Y.-P. Reaction of H + HONO in Solid para-Hydrogen: Infrared Spectrum of •ONH(OH). Phys. Chem. Chem. Phys. 2017, 19, 16169–16177. [Google Scholar] [CrossRef]
- Apkarian, V.A.; Schwentner, N. Molecular Photodynamics in Rare Gas Solids. Chem. Rev. 1999, 99, 1481–1514. [Google Scholar] [CrossRef]
- Bahou, M.; Das, P.; Lee, Y.-F.; Wu, Y.-J.; Lee, Y.-P. Infrared Spectra of Free Radicals and Protonated Species Produced in para-Hydrogen Matrices. Phys. Chem. Chem. Phys. 2014, 16, 2200–2210. [Google Scholar] [CrossRef]
- Silvera, I.F. The Solid Molecular Hydrogens in the Condensed Phase: Fundamentals and Static Properties. Rev. Mod. Phys. 1980, 52, 393–452. [Google Scholar] [CrossRef]
- Hoshina, H.; Fushitani, M.; Momose, T.; Shida, T. Tunneling Chemical Reactions in Solid Parahydrogen: Direct Measurement of the Rate Constants of R+H2→RH+H (R=CD3, CD2H, CDH2, CH3) at 5 K. J. Chem. Phys. 2004, 120, 3706–3715. [Google Scholar] [CrossRef] [PubMed]
- Momose, T.; Hoshina, H.; Sogoshi, N.; Katsuki, H.; Wakabayashi, T.; Shida, T. Tunneling Chemical Reactions in Solid Parahydrogen: A Case of CD3+H2→CD3H+H at 5K. J. Chem. Phys. 1998, 108, 7334–7338. [Google Scholar] [CrossRef]
- Ruzi, M.; Anderson, D.T. Fourier Transform Infrared Studies of Ammonia Photochemistry in Solid Parahydrogen. J. Phys. Chem. A 2013, 117, 13832–13842. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Exchange-Energy Approximation with Correct Asymptotic Behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Lee, C.T.; Yang, W.T.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron-Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate Spin-Dependent Electron Liquid Correlation Energies for Local Spin Density Calculations: A Critical Analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rostkowska, H.; Nowak, M.J.; Reva, I.; Lapinski, L. Photochemical Hydroxyl Group Abstraction from N-Hydroxypyridine-2(1H)-thione Isolated in a Solid Hydrogen Matrix: Photogeneration of 2-Mercaptopyridine. Molecules 2024, 29, 5472. https://doi.org/10.3390/molecules29225472
Rostkowska H, Nowak MJ, Reva I, Lapinski L. Photochemical Hydroxyl Group Abstraction from N-Hydroxypyridine-2(1H)-thione Isolated in a Solid Hydrogen Matrix: Photogeneration of 2-Mercaptopyridine. Molecules. 2024; 29(22):5472. https://doi.org/10.3390/molecules29225472
Chicago/Turabian StyleRostkowska, Hanna, Maciej J. Nowak, Igor Reva, and Leszek Lapinski. 2024. "Photochemical Hydroxyl Group Abstraction from N-Hydroxypyridine-2(1H)-thione Isolated in a Solid Hydrogen Matrix: Photogeneration of 2-Mercaptopyridine" Molecules 29, no. 22: 5472. https://doi.org/10.3390/molecules29225472
APA StyleRostkowska, H., Nowak, M. J., Reva, I., & Lapinski, L. (2024). Photochemical Hydroxyl Group Abstraction from N-Hydroxypyridine-2(1H)-thione Isolated in a Solid Hydrogen Matrix: Photogeneration of 2-Mercaptopyridine. Molecules, 29(22), 5472. https://doi.org/10.3390/molecules29225472







