A Turn-On Fluorescent Probe for Highly Selective Detection and Visualization of Hydrogen Sulfide in Fungi
Abstract
:1. Introduction
2. Results and Discussion
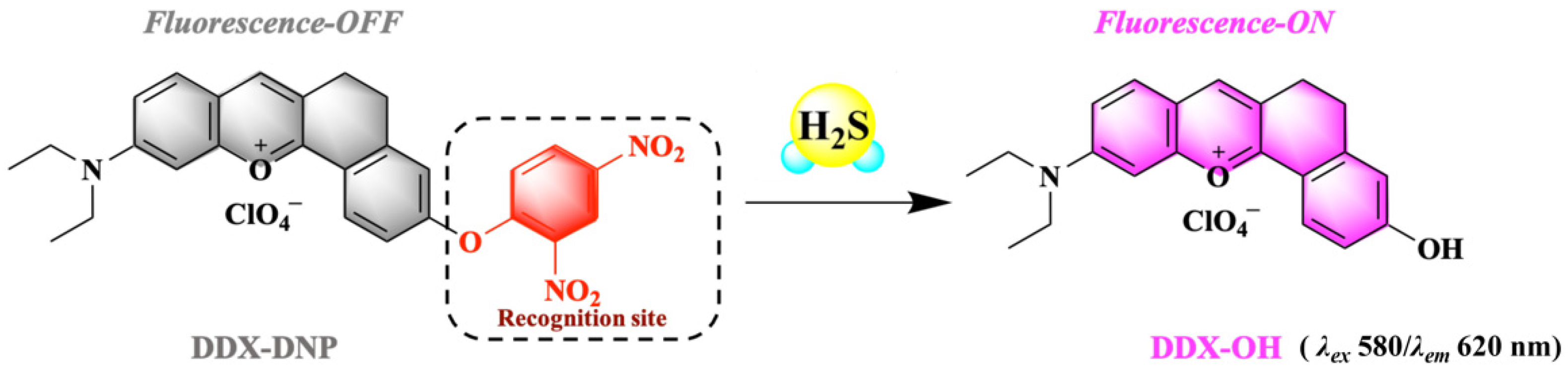
2.1. Design of DDX-DNP and H2S Response
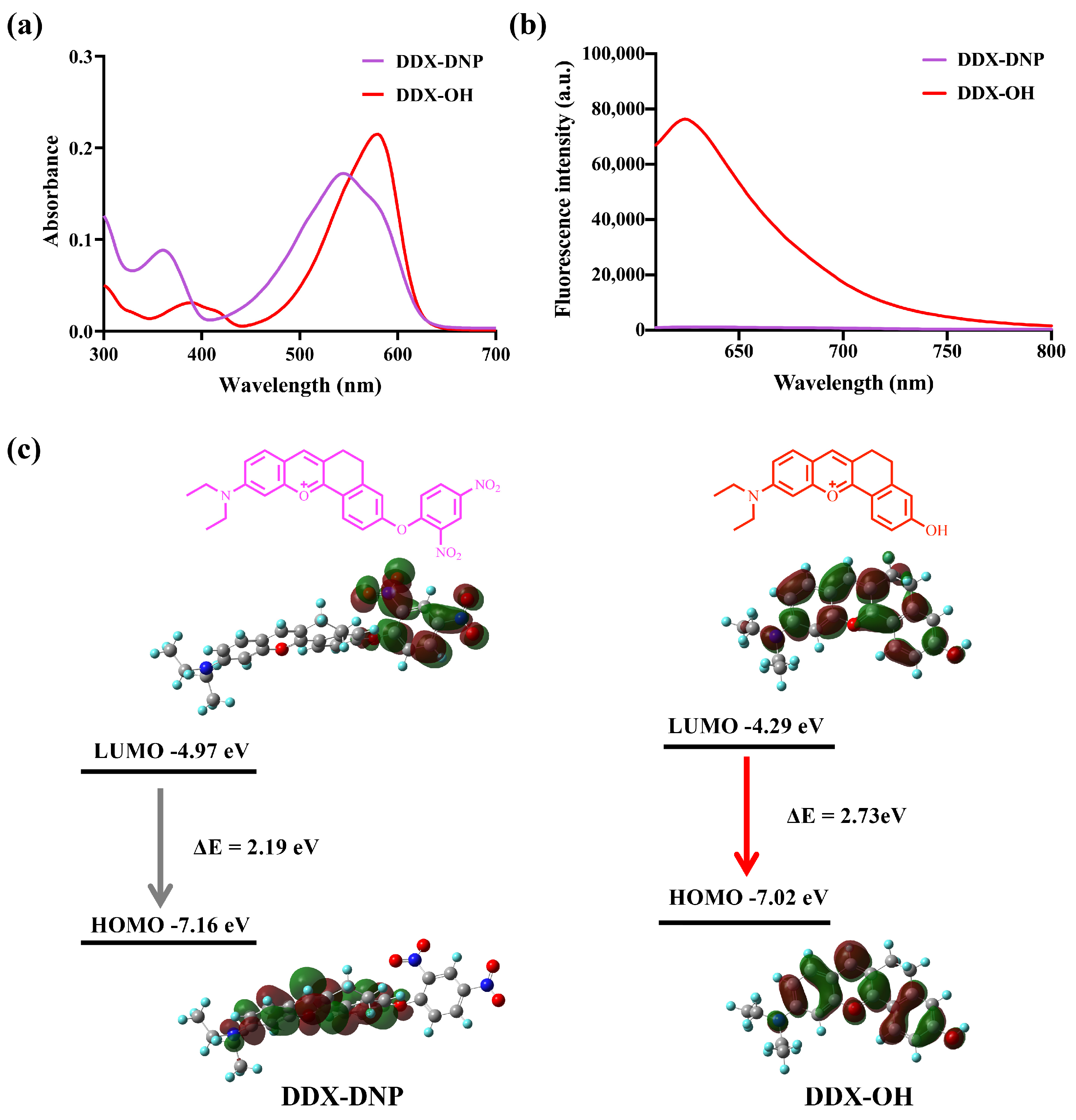
2.2. Spectral Characteristics and DFT Calculations of DDX-DNP and DDX-OH
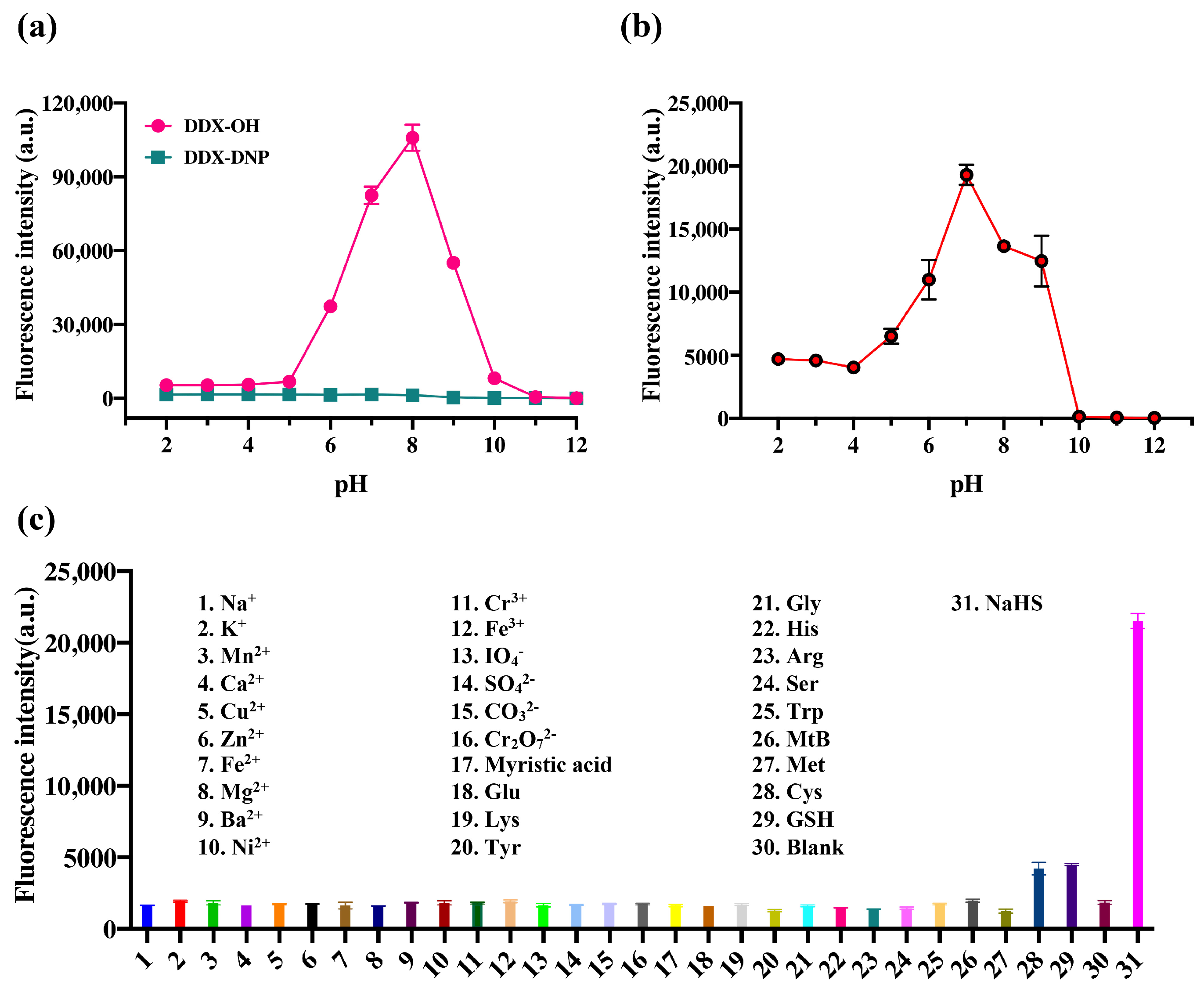
2.3. Selectivity of DDX-DNP for the Detection of H2S
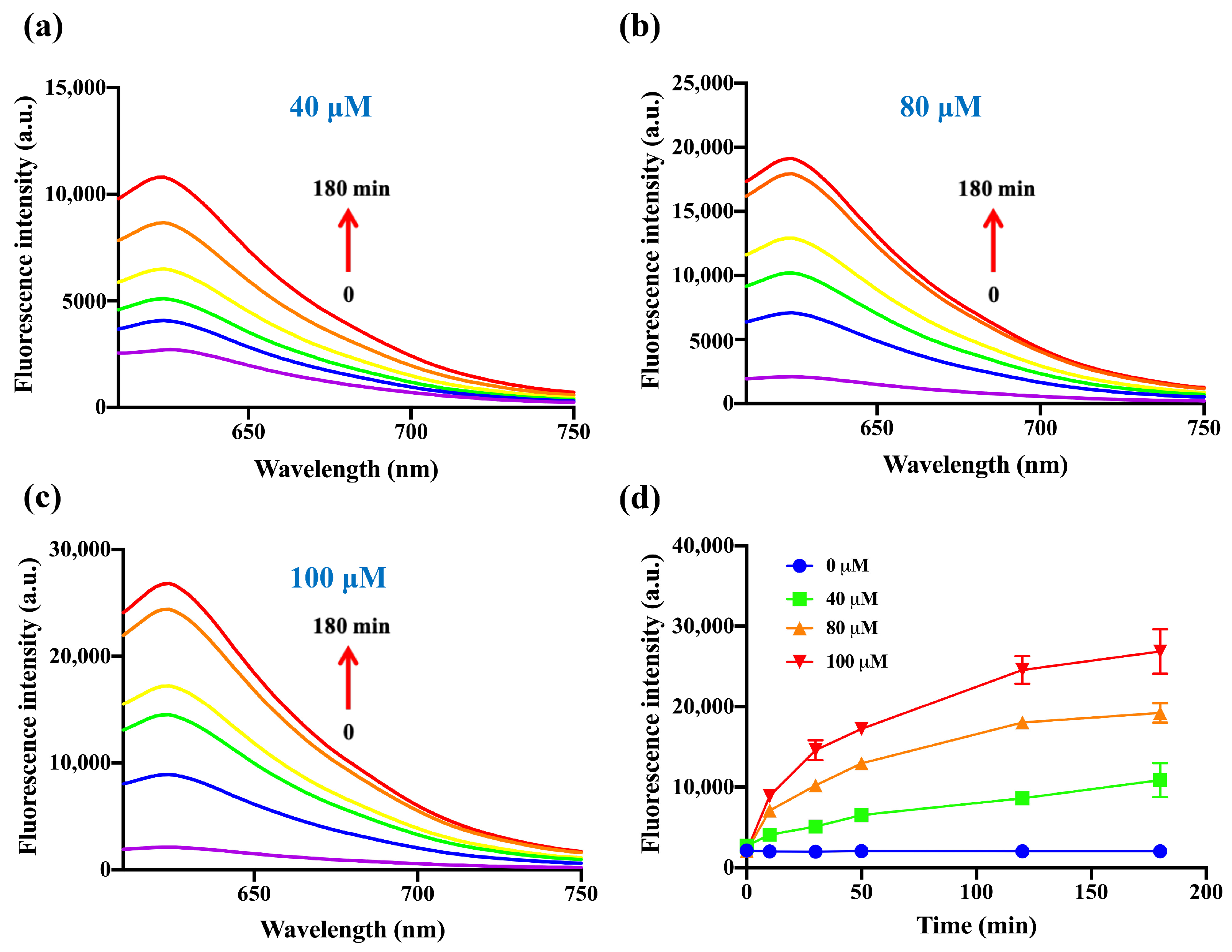
2.4. Confirmation about the Reaction between DDX-DNP and H2S
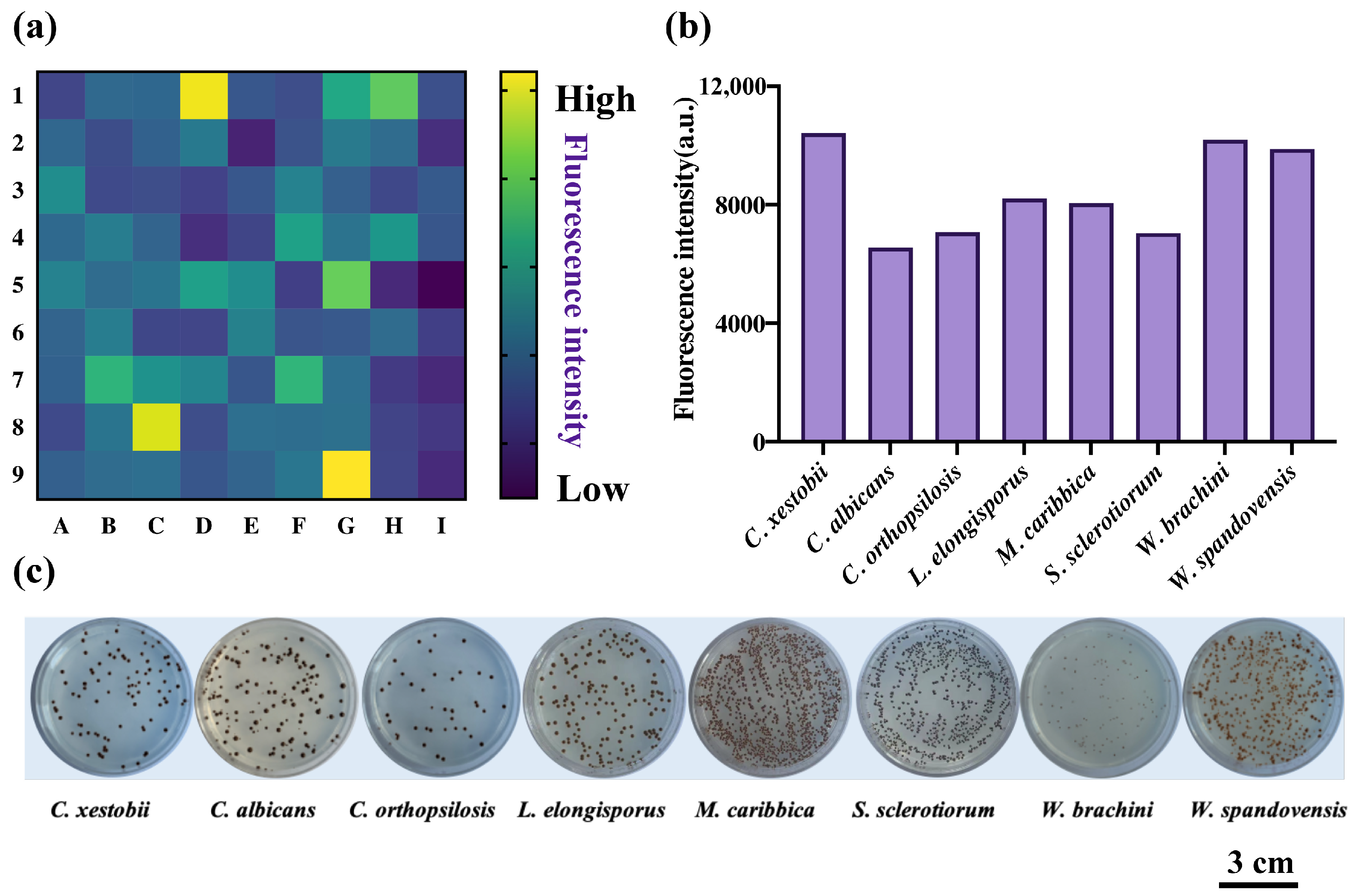
2.5. High-Throughput Screening for Fungi-Produced H2S from Tongue-Coating Fungi Strains Based on DDX-DNP
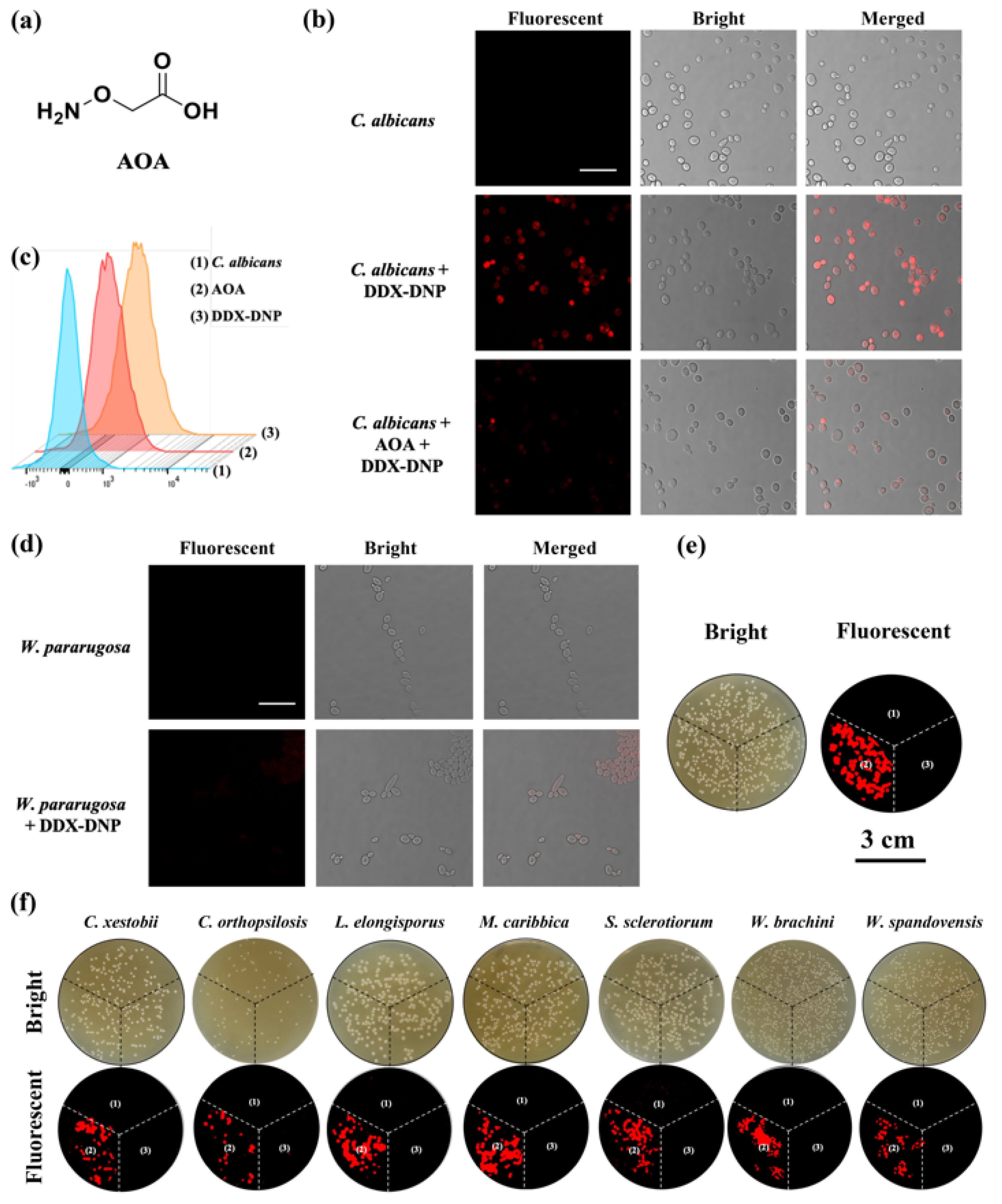
2.6. Fluorescence Imaging of Fungi via H2S Monitoring
3. Materials and Methods
3.1. Materials and Instruments
3.2. Synthetic Procedure of Probe DDX-DNP
3.2.1. Synthesis of DDX-OH
3.2.2. Synthesis of DDX-DNP
3.3. Density Functional Theory (DFT) Calculations
3.4. High-Performance Liquid Chromatography–Mass Spectrometry of DDX-DNP and DDX-OH
3.5. Spectroscopic Measurements
3.6. Kinetic Studies [38]
3.7. Selectivity Evaluation
3.8. Fungi Strains and Cultivation
3.9. DDX-DNP High-Throughput Screening for H2S Production by Tongue-Coating Fungi
3.10. Fluorescence Imaging of Fungi via the Detection of H2S
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lefer, D. A new gaseous signaling molecule emerges: Cardioprotective role of hydrogen sulfide. Proc. Natl. Acad. Sci. USA 2007, 104, 17907–17908. [Google Scholar] [CrossRef] [PubMed]
- Wang, R. Hydrogen sulfide: The third gasotransmitter in biology and medicine. Antioxid. Redox Signal. 2010, 12, 1061–1064. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Rose, P.; Moore, P. Hydrogen sulfide and cell signaling. Annu. Rev. Pharmacol. 2011, 51, 169–187. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Zhang, M.; Jin, X.; Zhang, H.; Zheng, H.; Zheng, S.; Qiao, Y.; Yu, H.; Sun, B.; Hou, X.; et al. Inhibition of fungal pathogenicity by targeting the H2S-synthesizing enzyme cystathionine β-synthase. Sci. Adv. 2022, 8, eadd5366. [Google Scholar] [CrossRef] [PubMed]
- Shatalin, K.; Shatalina, E.; Mironov, A.; Nudler, E. H2S: A universal defense against antibiotics in bacteria. Science 2011, 334, 986–990. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, M.; Jiang, D.; Xue, X.; Zhang, J.; Zhang, G.; Wang, Y.; Zhao, H. Ultrafast response fluorescent probe with red-emission for monitoring hydrogen sulfide in vivo and in vitro. J. Photochem. Photobiol. A 2019, 382, 111974. [Google Scholar] [CrossRef]
- Yang, G.; Wu, L.; Jiang, B.; Yang, W.; Qi, J.; Cao, K.; Meng, Q.; Mustafa, A.; Mu, W.; Zhang, S.; et al. H2S as a Physiologic Vasorelaxant: Hypertension in Mice with Deletion of Cystathionine γ-Lyase. Science 2008, 322, 587. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H. Hydrogen sulfide: Its production, release and functions. Amino Acids 2011, 41, 113–121. [Google Scholar] [CrossRef]
- Li, L.; Bhatia, M.; Zhu, Y.; Zhu, Y.; Ramnath, R.; Wang, Z.; Whiteman, M.; Salto-Tellez, M.; Moore, P. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J. 2005, 19, 1196–1198. [Google Scholar] [CrossRef]
- Kamoun, P.; Belardinelli, M.; Chabli, A.; Lallouchi, K.; Chadefaux-Vekemans, B. Endogenous Hydrogen Sulfide Overproduction in Down Syndrome. Am. J. Med. Genet. A 2003, 116, 310–311. [Google Scholar] [CrossRef]
- Eto, K.; Asada, T.; Arima, K.; Makifuchi, T.; Kimura, H. Brain hydrogen sulfide is severely decreased in Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2002, 293, 1485–1488. [Google Scholar] [CrossRef]
- Wang, R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol. Rev. 2012, 92, 791–896. [Google Scholar] [CrossRef] [PubMed]
- Vadovics, M.; Igaz, N.; Alfldi, R. Candida albicans enhances the progression of oral squamous cell cancrinoma in vitro and in vivo. mBio 2021, 13, e03144-21. [Google Scholar] [PubMed]
- Ma, Z.; Bi, Q.; Wang, Y. Hydrogen sulfide accelerates cell cycle progression in oral squamous cell carcinoma cell lines. Oral Dis. 2015, 21, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Yoneda, M.; Hanioka, T.; Takeshita, T.; Hirofuji, T. Induction and inhibition of oral malodor. Mol. Oral Microbiol. 2019, 34, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Zhong, K.; Deng, L.; Zhao, J.; Yan, X.; Sun, T.; Li, J.; Tang, L. A novel near-infrared fluorescent probe for highly selective recognition of hydrogen sulfide and imaging in living cells. RSC Adv. 2018, 8, 23924–23929. [Google Scholar] [CrossRef] [PubMed]
- Jimnez, D.; Martinez-Manez, R.; Sancenon, F.; Ros-Lis, J.V.; Benito, A.; Soto, J. A new chromo-chemodosimeter selective for sulfide anion. J. Am. Chem. Soc. 2003, 125, 9000–9001. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; He, M.; Yu, H.; Li, D. An H2S Sensor Based on Electrochemistry for Chicken Coops. Sensors 2016, 16, 1398. [Google Scholar] [CrossRef] [PubMed]
- Vitvitsky, V.; Banerjee, R. H2S analysis in biological samples using gas chromatography with sulfur chemiluminescence detection. Methods Enzymol. 2015, 554, 111–123. [Google Scholar]
- Feng, L.; Yan, Q.; Zhang, B.; Tian, X.; Wang, C.; Yu, Z.; Cui, J.; Guo, D.; Ma, X.; James, T. Ratiometric fluorescent probe for sensing Streptococcus mutans glucosyltransferase, a key factor in the formation of dental caries. Chem. Commun. 2019, 55, 3548–3551. [Google Scholar] [CrossRef]
- Tian, Z.; Yan, Q.; Feng, L.; Deng, S.; Wang, C.; Cui, J.; Wang, C.; Zhang, Z.; James, T.; Ma, X. A far-red fluorescent probe for sensing laccase in fungi and its application in developing an effective biocatalyst for the biosynthesis of antituberculous dicoumarin. Chem. Commun. 2019, 55, 3951–3954. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Zhao, X.; Li, R.; Han, X.; Yan, Q.; Feng, L.; Xin, X.; Cui, J.; Ma, X. High-throughput fluorescent screening of thioredoxin reductase inhibitors to inhibit Mycobacterium tuberculosis. Chin. Chem. Lett. 2024, 35, 108504. [Google Scholar] [CrossRef]
- Ji, G.; Zheng, T.; Gao, X.; Liu, Z. A highly selective turn-on luminescent logic gates probe based on post-synthetic MOF for aspartic acid detection. Sens. Actuators B 2019, 284, 91–95. [Google Scholar] [CrossRef]
- Liu, C.; Pan, J.; Li, S.; Zhao, Y.; Berkman, C.; Whorton, A.; Xian, M. Capture and visualization of Hydrogen Sulfide by a fluorescent probe. Angew. Chem. Int. Ed. 2011, 50, 10327–10329. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Huo, F.; Zhang, Y.; Wen, Y.; Yin, C. HS− induced thiolysis reaction to regulate UV- and fluorescence spectra change and their bioimaging in living cells. Spectrochim. Acta A 2019, 215, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Hammers, M.; Taormina, M.; Cerda, M.; Montoya, L.; Seidenkranz, D.; Parthasarathy, R.; Pluth, M. A bright fluorescent probe for H2S enables analyte-responsive, 3D imaging in live Zebrafish using light sheet fluorescence microscopy. J. Am. Chem. Soc. 2015, 137, 10216–10223. [Google Scholar] [CrossRef]
- Lin, V.; Lippert, A.; Chang, C. Azide-based fluorescent probes: Imaging hydrogen sulfide in living systems. Methods Enzymol. 2015, 554, 63–80. [Google Scholar]
- Men, J.; Yang, X.; Zhang, H.; Zhou, J. A near-infrared fluorescent probe based on nucleophilic substitution−cyclization for selective detection of hydrogen sulfide and bioimaging. Dye. Pigment. 2018, 153, 206–212. [Google Scholar] [CrossRef]
- Kaushik, R.; Ghosh, A.; Jose, D. Recent progress in hydrogen sulphide (H2S) sensors by metal displacement approach. Coord. Chem. Rev. 2017, 347, 141–157. [Google Scholar] [CrossRef]
- Sasakura, K.; Hanaoka, K.; Shibuya, N.; Mikami, Y.; Kimura, Y.; Komatsu, T.; Ueno, T.; Terai, T.; Kimura, H.; Nagano, T. Development of a highly selective fluorescence probe for hydrogen sulfide. J. Am. Chem. Soc. 2011, 133, 1803–1805. [Google Scholar] [CrossRef]
- Jose, D.; Sakla, R.; Sharma, N.; Gadiyaram, S.; Kaushik, R.; Ghosh, A. Sensing and Bioimaging of the Gaseous Signaling Molecule Hydrogen Sulfide by Near-Infrared Fluorescent Probes. ACS Sens. 2020, 5, 3365–3391. [Google Scholar] [CrossRef]
- Wan, D.; Pan, T.; Ou, P.; Zhou, R.; Ouyang, Z.; Luo, L.; Xiao, Z.; Peng, Y. Construct a lysosome-targeting and highly selective fluorescent probe for imaging of hydrogen sulfide in living cells and inflamed tissues. Spectrochim. Acta A 2021, 249, 119311. [Google Scholar] [CrossRef]
- Montoya, L.; Pluth, M. Selective turn-on fluorescent probes for imaging hydrogen sulfide in living cells. Chem. Commun. 2012, 48, 4767–4769. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Shen, Y.; Gong, X.; Zhang, X.; Yuan, L. Highly Selective Fluorescent Probe Design for Visualizing Hepatic Hydrogen Sulfide in the Pathological Progression of Nonalcoholic Fatty Liver. Anal. Chem. 2021, 93, 16673–16682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L. A novel “turn-on” fluorescent probe based on hydroxy functionalized naphthalimide as a logic platform for visual recognition of H2S in environment and living cells. Spectrochim. Acta A 2020, 235, 11833. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Zhang, L.; Pu, Z.; Xia, J.; Chen, L.; Xia, Y.; Cui, H.; Wang, J.; Peng, X. A NIR fluorescent probe for the detection and visualization of hydrogen sulfide using the aldehyde group assisted thiolysis of dinitrophenyl ether strategy. J. Mater. Chem. B 2018, 6, 7916–7925. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, Y.; Li, Z.; Dong, G.; Guo, Y.; Ma, S.; Li, J.; Du, L.; Li, M. Bright chemiluminescent dioxetane probes for the detection of gaseous transmitter H2S. Bioorganic Med. Chem. Lett. 2021, 46, 128148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, J.; Liu, J.; Ning, L.; Zhu, X.; Yu, B.; Liu, X.; Yao, X.; Zhang, H. Near-Infrared and Naked-Eye Fluorescence Probe for Direct and Highly Selective Detection of Cysteine and Its Application in Living Cells. Anal. Chem. 2015, 87, 4856–4863. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.; Ru, J.; Yao, X.; Yang, Y.; Li, X.; Tang, X.; Zhang, G.; Liu, W. A naked-eye visible and turn-on fluorescence probe for Fe3+ and its bioimaging application in living cells. Sens. Actuators B 2016, 237, 501–508. [Google Scholar] [CrossRef]
- Jiang, W.; Chen, H.; Pan, Y.; Lin, W. A long-wavelength fluorescent turn-on probe for video detection of biological thiols in living cells. Anal. Methods 2015, 7, 4168–4172. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Q.; He, S.; Feng, L.; Zhang, M.; Han, C.; Wu, Y.; Wang, C.; Ma, X.; Ma, T. A Turn-On Fluorescent Probe for Highly Selective Detection and Visualization of Hydrogen Sulfide in Fungi. Molecules 2024, 29, 577. https://doi.org/10.3390/molecules29030577
Yan Q, He S, Feng L, Zhang M, Han C, Wu Y, Wang C, Ma X, Ma T. A Turn-On Fluorescent Probe for Highly Selective Detection and Visualization of Hydrogen Sulfide in Fungi. Molecules. 2024; 29(3):577. https://doi.org/10.3390/molecules29030577
Chicago/Turabian StyleYan, Qingsong, Shengui He, Lei Feng, Ming Zhang, Chaoyan Han, Yuzhuo Wu, Chao Wang, Xiaochi Ma, and Tonghui Ma. 2024. "A Turn-On Fluorescent Probe for Highly Selective Detection and Visualization of Hydrogen Sulfide in Fungi" Molecules 29, no. 3: 577. https://doi.org/10.3390/molecules29030577





