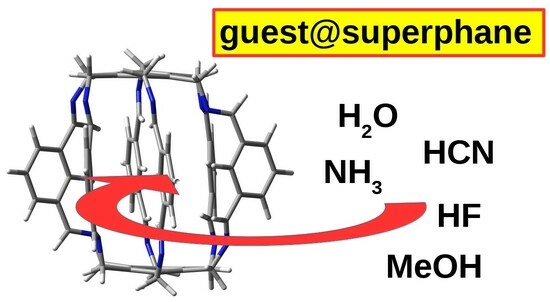
Characteristics of Intermolecular Interactions between Encapsulated Molecules and the Lantern-Like Carcerand Superphanes
Abstract
:1. Introduction
2. Results and Discussion
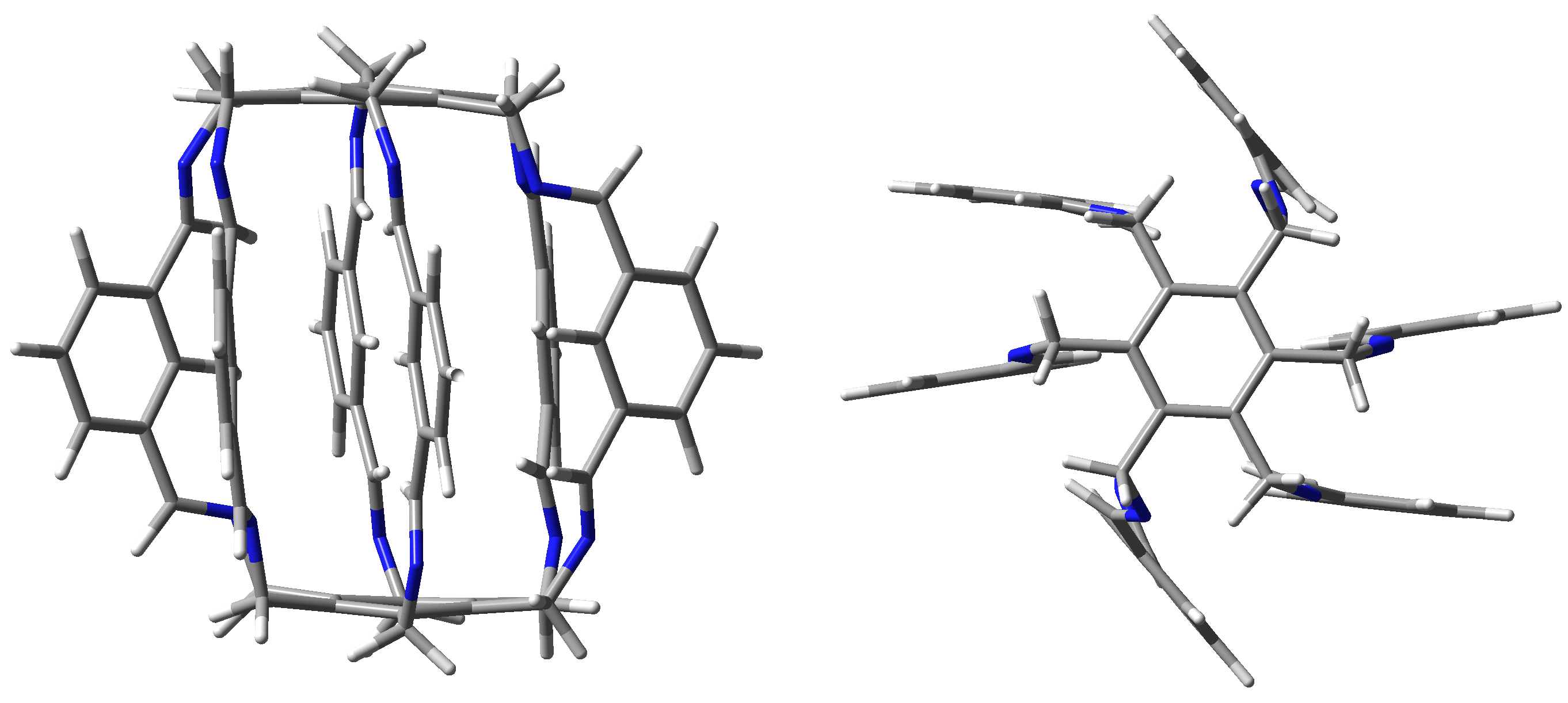
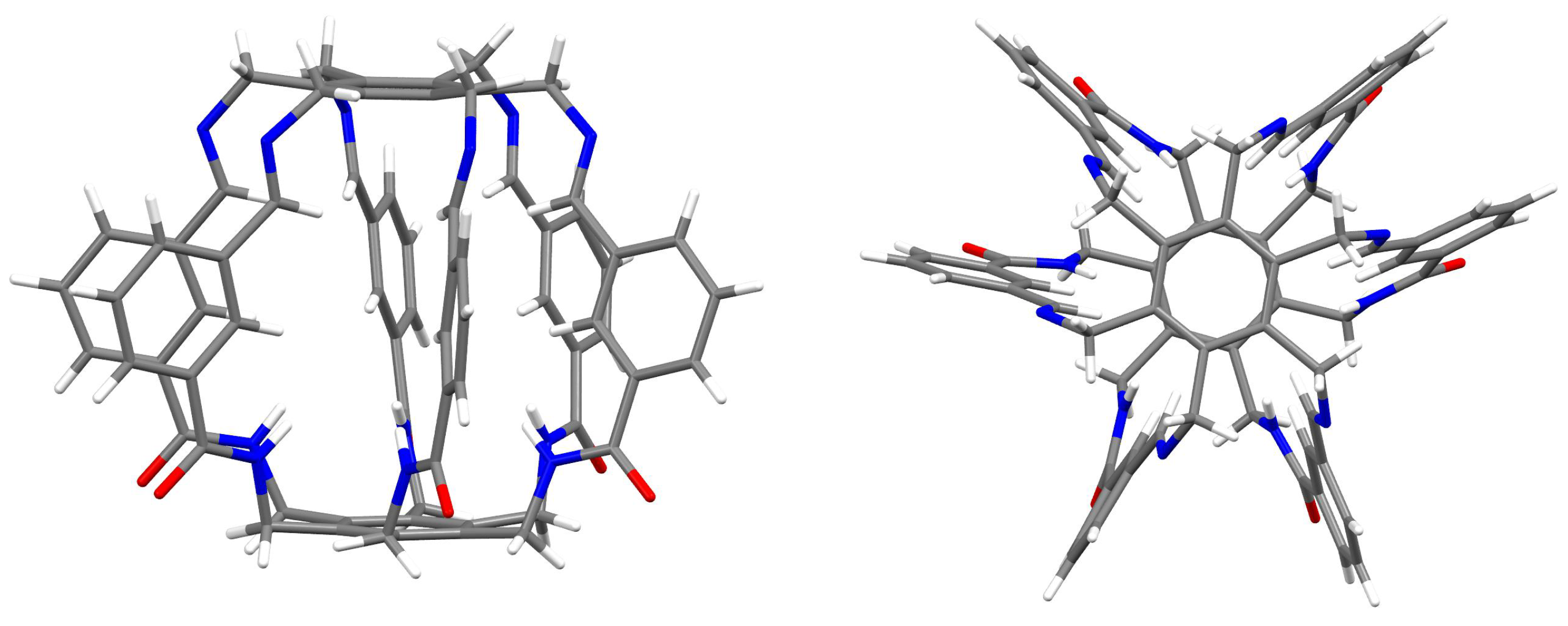
2.1. Characteristics of Intermolecular Interactions in Carceplexes Guest@1
2.1.1. Intermolecular Interactions in O@1
2.1.2. Intermolecular Interactions in @1
2.1.3. Intermolecular Interactions in HF@1
2.1.4. Intermolecular Interactions in HCN@1
2.1.5. Intermolecular Interactions in MeOH@1
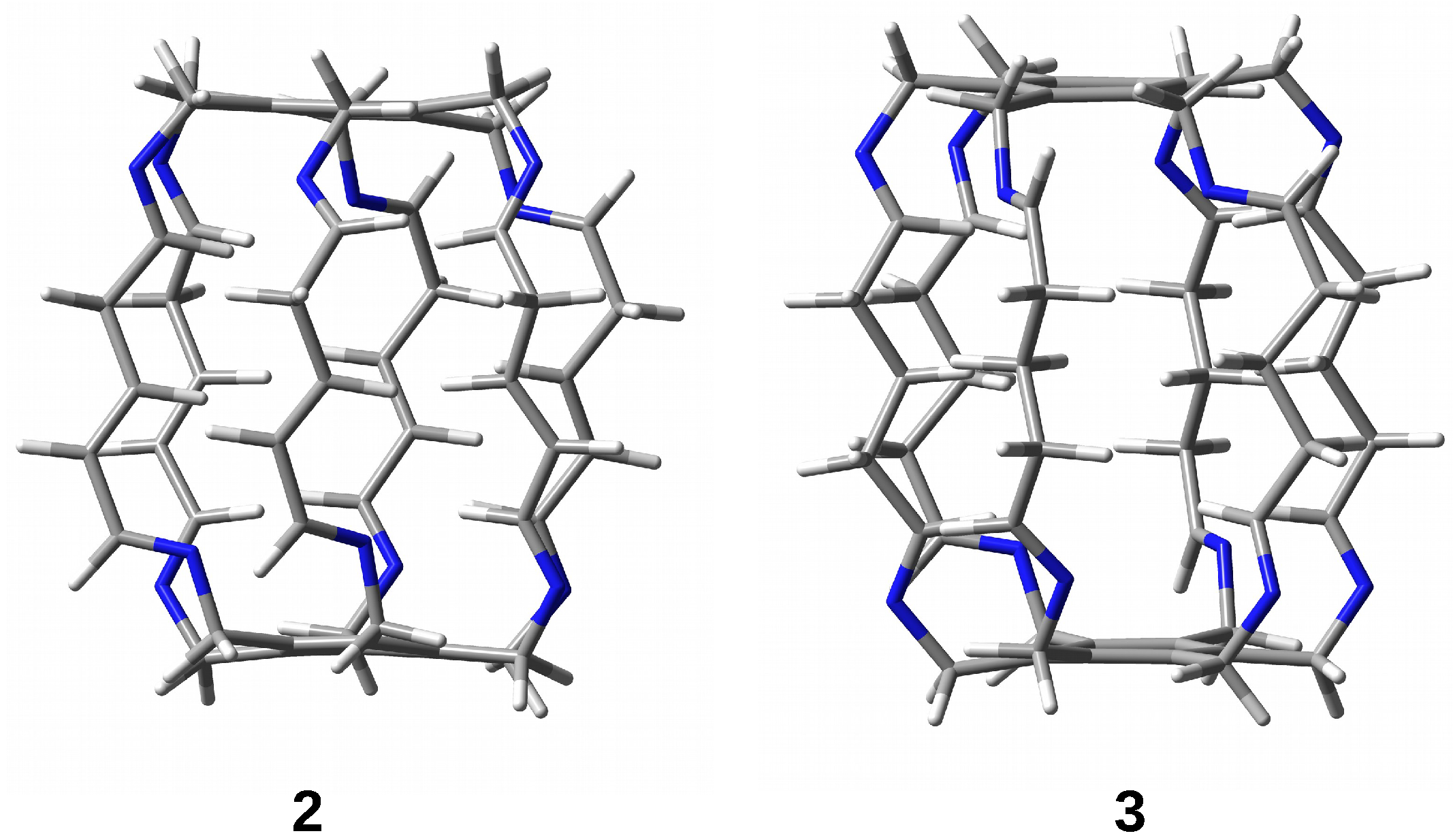
2.2. Influence of the Type of Side Chain
2.2.1. Influence of the Type of Side Chain on the Height of the Cage
2.2.2. Influence of the Type of Side Chain on the Encapsulation Energy
3. Methodology
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gleiter, R.; Hopf, H. (Eds.) Modern Cyclophane Chemistry; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Ghasemabadi, P.G.; Yao, T.; Bodwell, G.J. Cyclophanes containing large polycyclic aromatic hydrocarbons. Chem. Soc. Rev. 2015, 44, 6494–6518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-X.; Li, J.; Niu, Y.-Y. A Review of Crystalline Multibridged Cyclophane Cages: Synthesis, Their Conformational Behavior, and Properties. Molecules 2022, 27, 7083. [Google Scholar] [CrossRef]
- Gleiter, R.; Kratz, D. “Super” Phanes. Acc. Chem. Res. 1993, 26, 311–318. [Google Scholar] [CrossRef]
- Sekine, Y.; Brown, M.; Boekelheide, V. [2.2.2.2.2.2](l,2,3,4,5,6)Cyclophane: Superphane. J. Am. Chem. Soc. 1979, 101, 3126–3127. [Google Scholar] [CrossRef]
- Iwamura, H.; Katoh, M.; Kihara, H. How Strained Is the “Flat” Benzene Ring in Superphane ? Tetrahedron Lett. 1980, 21, 1757–1760. [Google Scholar] [CrossRef]
- Sekine, Y.; Boekelheide, V. A Study of the Synthesis and Properties of [26](1,2,3,4,5,6)Cyclophane (Superphane). J. Am. Chem. Soc. 1981, 103, 1777–1785. [Google Scholar] [CrossRef]
- Dodziuk, H.; Vetokhina, V.; Hopf, H.; Luboradzki, R.; Gaweł, P.; Waluk, J. Electronic states of cyclophanes with small bridges. J. Chem. Soc. 2012, 136, 074201. [Google Scholar] [CrossRef]
- Jabłoński, M. Does the Presence of a Bond Path Really Mean Interatomic Stabilization? The Case of the Ng@Superphane (Ng = He, Ne, Ar, and Kr) Endohedral Complexes. Symmetry 2021, 13, 2241. [Google Scholar] [CrossRef]
- Jabłoński, M. Determining Repulsion in Cyclophane Cages. Molecules 2022, 27, 3969. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Miyoshi, N.; Shinmyozu, T. Synthesis of a “Molecular Pinwheel”: [3.3.3.3.3.3](1,2,3,4,5,6)Cyclophane. Angew. Chem. Int. Ed. Engl. 1996, 35, 549–550. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Miyoshi, N.; Hirakida, M.; Kusumoto, S.; Kawase, H.; Rudzinski, J.M.; Shinmyozu, T. Syntheses, Structures, and Transannular π–π Interactions of Multibridged [3n]Cyclophanes. J. Am. Chem. Soc. 1996, 118, 12267–12275. [Google Scholar] [CrossRef]
- Hori, K.; Sentou, W.; Shinmyozu, T. Ab Initio Molecular Orbital Study on Inversion Mechanism of Trimethylene Bridges of [33](1,3,5)- and [36](1,2,3,4,5,6) Cyclophanes. Tetrahedron Lett. 1997, 38, 8955–8958. [Google Scholar] [CrossRef]
- Bettinger, H.F.; Schleyer, P.v.R.; Schaefer III, H.F. [36](1,2,3,4,5,6)Cyclophane–A Molecular Pinwheel and Its Correlated Inversion: NMR and Energetic Considerations. J. Am. Chem. Soc. 1998, 120, 1074–1075. [Google Scholar] [CrossRef]
- Yasutake, M.; Sakamoto, Y.; Onaka, S.; Sako, K.; Tatemitsu, H.; Shinmyozu, T. Crystal structural properties of a pinwheel compound: [36](1,2,3,4,5,6)cyclophane. Tetrahedron Lett. 2000, 41, 7933–7938. [Google Scholar] [CrossRef]
- Yasutake, M.; Koga, T.; Sakamoto, Y.; Komatsu, S.; Zhou, M.; Sako, K.; Tatemitsu, H.; Onaka, S.; Aso, Y.; Inoue, S.; et al. An Alternative Synthetic Route of [35](1,2,3,4,5)Cyclophane, and Structural Properties of Multibridged [3n]Cyclophanes and Their Charge-Transfer Complexes in the Solid State. J. Am. Chem. Soc. 2002, 124, 10136–10145. [Google Scholar] [CrossRef]
- Nogita, R.; Matohara, K.; Yamaji, M.; Oda, T.; Sakamoto, Y.; Kumagai, T.; Lim, C.; Yasutake, M.; Shimo, T.; Jefford, C.W.; et al. Photochemical Study of [33](1,3,5)Cyclophane and Emission Spectral Properties of [3n]Cyclophanes (n = 2–6). J. Am. Chem. Soc. 2004, 126, 13732–13741. [Google Scholar] [CrossRef] [PubMed]
- Fujitsuka, M.; Tojo, S.; Shinmyozu, T.; Majima, T. Intramolecular dimer radical anions of [3n] cyclophanes: Transannular distance dependent stabilization energy. Chem. Commun. 2009, 1553–1555. [Google Scholar] [CrossRef] [PubMed]
- Jabłoński, M. Bader’s Topological Bond Path Does Not Necessarily Indicate Stabilizing Interaction–Proof Studies Based on the Ng@[3n]cyclophane Endohedral Complexes. Molecules 2023, 28, 6353. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Xiong, S.; Zhou, W.; Zhai, H.; Liu, Y.; He, Q. Superphane: A new lantern-like receptor for encapsulation of a water dimer. Chem. Commun. 2021, 57, 4496–4499. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Liu, Y.; Zhou, W.; Jiang, Y.; He, Q. Superphanes: Facile and efficient preparation, functionalization and unique properties. Tetrahedron Chem. 2022, 1, 100006. [Google Scholar] [CrossRef]
- Zhou, W.; Li, A.; Gale, P.A.; He, Q. A highly selective superphane for recognition and extraction. Cell Rep. Phys. Sci. 2022, 3, 100875. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, F.; Li, A.; Bai, S.; Feng, X.; He, Q. A Superphane-based carcerand for arsenic detoxification via imprisoning arsenate. Cell Rep. Phys. Sci. 2023, 4, 101295. [Google Scholar] [CrossRef]
- Zhou, W.; Li, A.; Zhou, M.; Xu, Y.; Zhang, Y.; He, Q. Nonporous amorphous superadsorbents for highly effective and selective adsorption of iodine in water. Nat. Commun. 2023, 14, 5388. [Google Scholar] [CrossRef]
- Xie, H.; Finnegan, T.J.; Gunawardana, V.W.L.; Pavlović, R.Z.; Moore, C.E.; Badjić, J.D. A Hexapodal Capsule for the Recognition of Anions. J. Am. Chem. Soc. 2021, 143, 3874–3880. [Google Scholar] [CrossRef]
- Xie, H.; Gunawardana, V.W.L.; Finnegan, T.J.; Xie, W.; Badjić, J.D. Picking on Carbonate: Kinetic Selectivity in the Encapsulation of Anions. Angew. Chem. Int. Ed. 2022, 61, e202116518. [Google Scholar] [CrossRef]
- Oh, J.H.; Kim, J.H.; Kim, D.S.; Han, H.J.; Lynch, V.M.; Sessler, J.L.; Kim, S.K. Synthesis and Anion Recognition Features of a Molecular Cage Containing Both Hydrogen Bond Donors and Acceptors. Org. Lett. 2019, 21, 4336–4339. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xiong, S.; Zhang, J.; P, J.; Ding, W.; Chen, X.; He, Q.; Zhang, Z. A hexapyrrolic molecular cage and the anion-binding studies in chloroform. J. Mol. Struct. 2023, 1293, 136232. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: New York, NY, USA, 1990. [Google Scholar]
- Popelier, P.L.A. Atoms in Molecules. An Introduction; Longman: Singapore, 2000. [Google Scholar]
- Matta, C.F.; Boyd, R.J. The Quantum Theory of Atoms in Molecules; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Jabłoński, M. On the Coexistence of the Carbene⋯H-D Hydrogen Bond and Other Accompanying Interactions in Forty Dimers of N-Heterocyclic-Carbenes (I, IMe2, IiPr2, ItBu2, IMes2, IDipp2, IAd2; I = imidazol-2-ylidene) and Some Fundamental Proton Donors (HF, HCN, H2O, MeOH, NH3). Molecules 2022, 27, 5712. [Google Scholar]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density-Functional Theory of Atoms and Molecules; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Jensen, F. Introduction to Computational Chemistry; John Wiley & Sons Ltd.: Chichester, UK, 2007. [Google Scholar]
- Pritchard, B.P.; Altarawy, D.; Didier, B.; Gibson, T.D.; Windus, T.L. New Basis Set Exchange: An Open, Up-to-Date Resource for the Molecular Sciences Community. J. Chem. Inf. Model. 2019, 59, 4814–4820. [Google Scholar] [CrossRef] [PubMed]
- Mardirossian, N.; Head-Gordon, M. Thirty years of density functional theory in computational chemistry: An overview and extensive assessment of 200 density functionals. Mol. Phys. 2017, 115, 2315–2372. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
- Dennington, R.; Keith, T.; Millam, J. (Eds.) GaussView, Version 6; Semichem Inc.: Shawnee Mission, KS, USA, 2019. [Google Scholar]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen bond strength revealed by topological analyses of experimentally observed electron densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Jabłoński, M.; Monaco, G. Different Zeroes of Interaction Energies As the Cause of Opposite Results on the Stabilizing Nature of C–H⋯O Intramolecular Interactions. J. Chem. Inf. Model. 2013, 53, 1661–1675. [Google Scholar] [CrossRef]
- Jabłoński, M. A Critical Overview of Current Theoretical Methods of Estimating the Energy of Intramolecular Interactions. Molecules 2020, 25, 5512. [Google Scholar] [CrossRef]
- Gatti, C.; May, E.; Destro, R.; Cargnoni, F. Fundamental Properties and Nature of CH··O Interactions in Crystals on the Basis of Experimental and Theoretical Charge Densities. The Case of 3,4-Bis(dimethylamino)-3-cyclobutene-1,2-dione (DMACB) Crystal. J. Phys. Chem. A 2002, 106, 2707–2720. [Google Scholar] [CrossRef]
- Afonin, A.V.; Vashchenko, A.V.; Sigalov, M.V. Estimating the energy of intramolecular hydrogen bonds from 1H NMR and QTAIM calculations. Org. Biomol. Chem. 2016, 14, 11199–11211. [Google Scholar] [CrossRef]
- Emamian, S.; Lu, T.; Kruse, H.; Emamian, H. Exploring Nature and Predicting Strength of Hydrogen Bonds: A Correlation Analysis Between Atoms-in-Molecules Descriptors, Binding Energies, and Energy Components of Symmetry-Adapted Perturbation Theory. J. Comput. Chem. 2019, 40, 2868–2881. [Google Scholar] [CrossRef]
- Keith, T.A. AIMAll (Version 15.05.18); TK Gristmill Software: Overland Park, KS, USA, 2015. [Google Scholar]


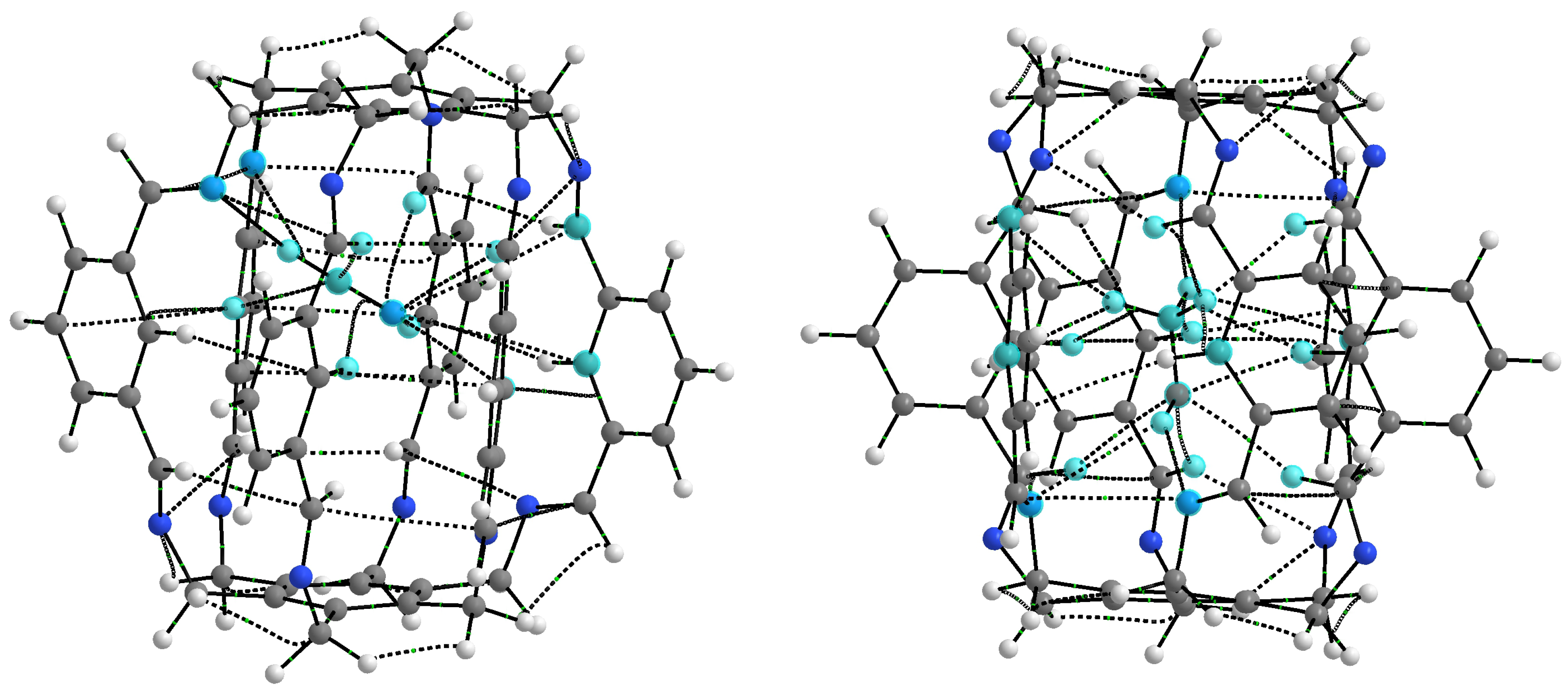
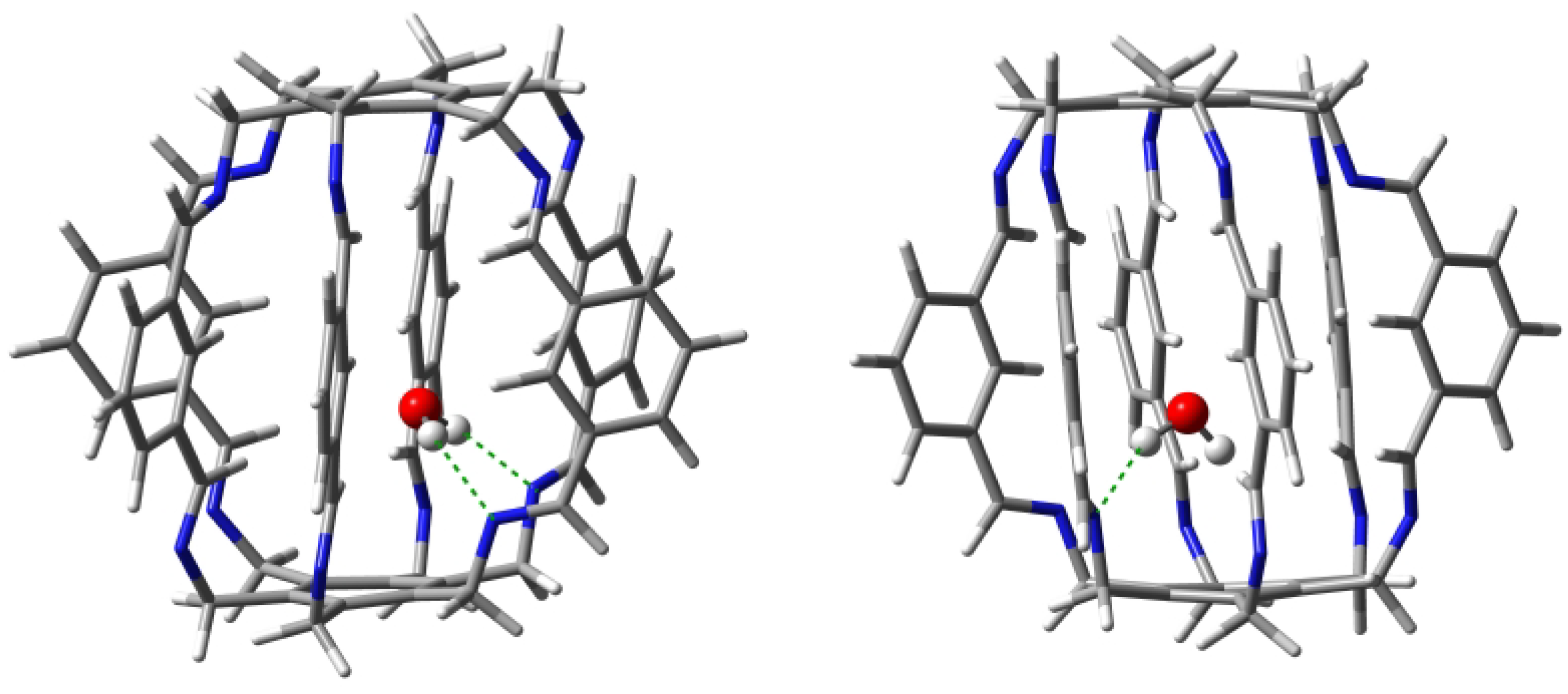
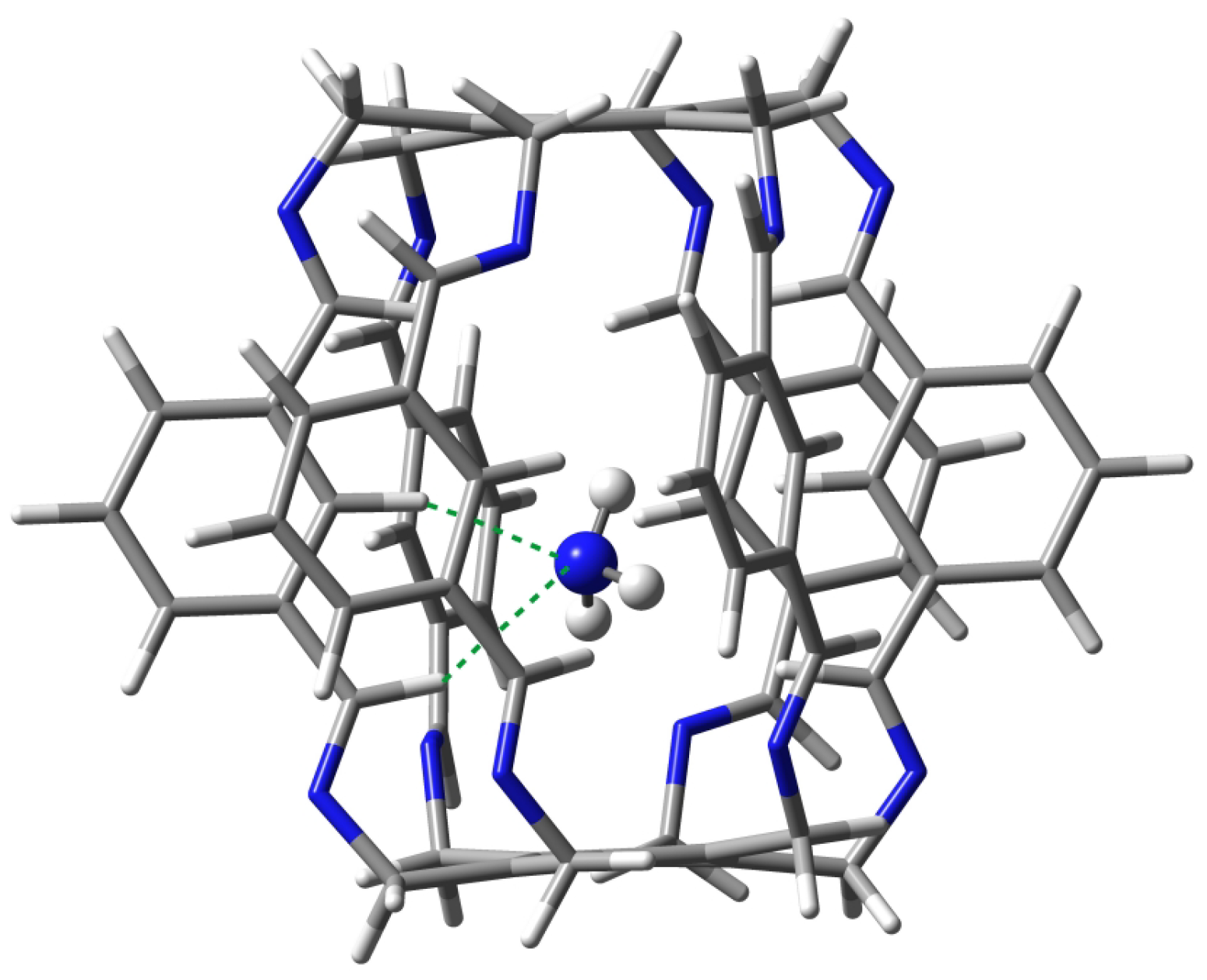
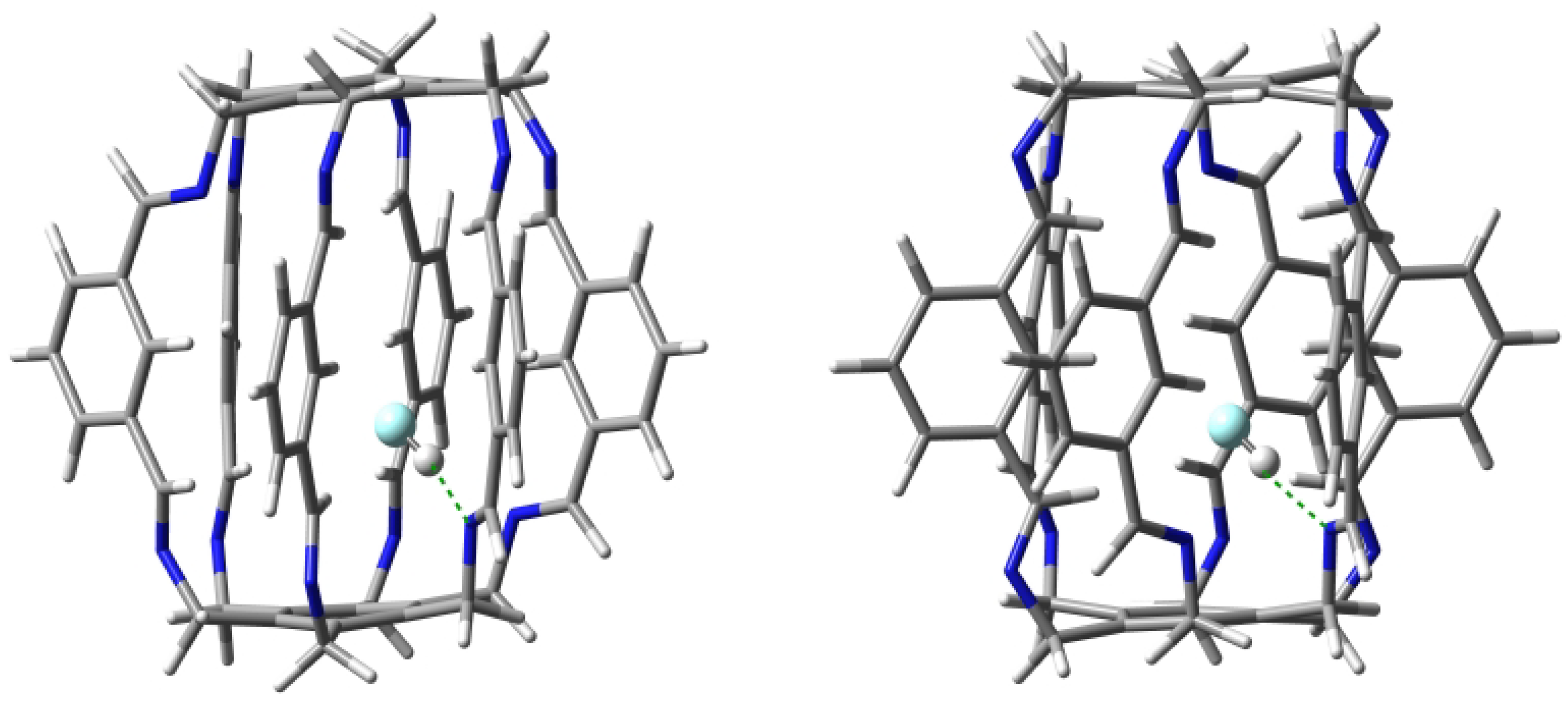
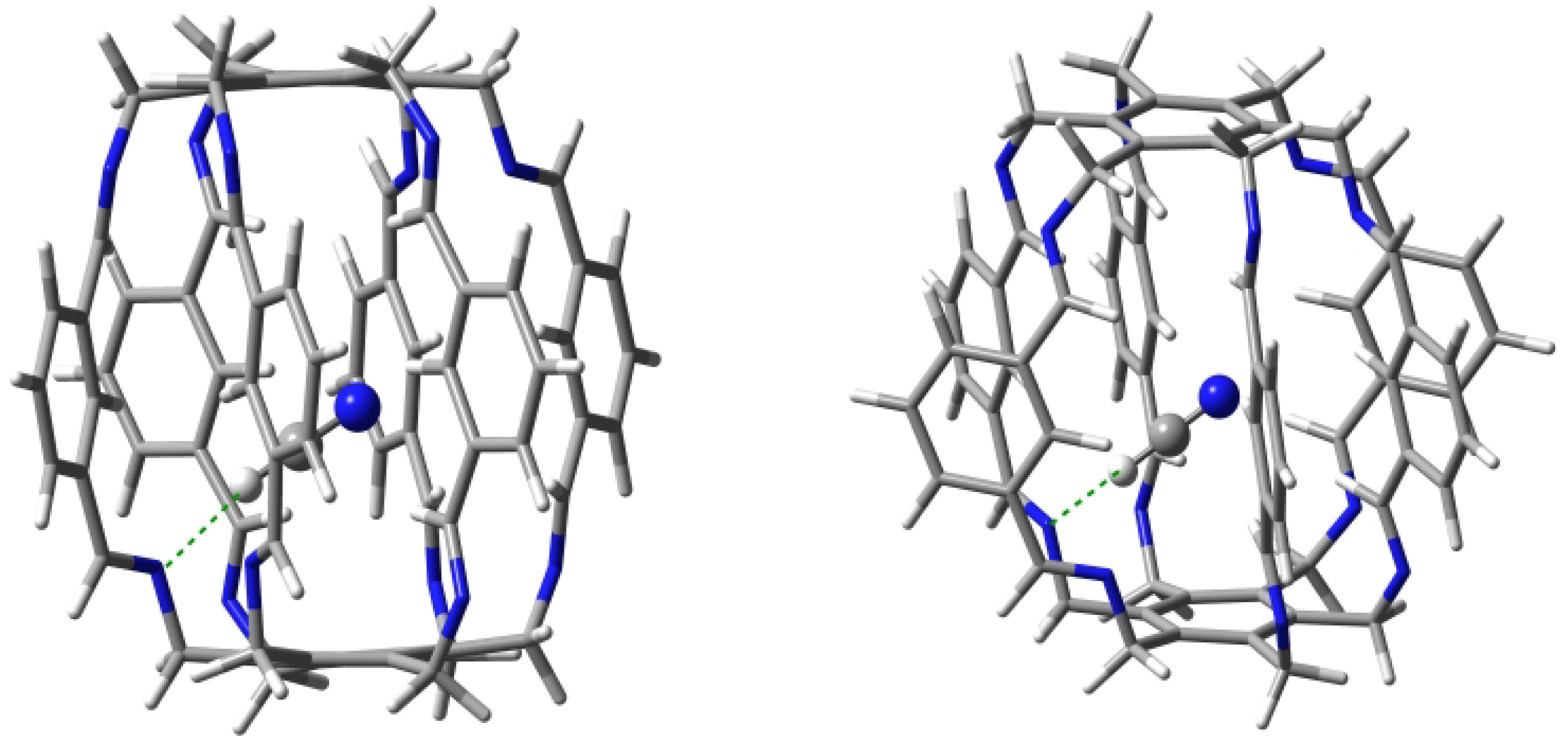
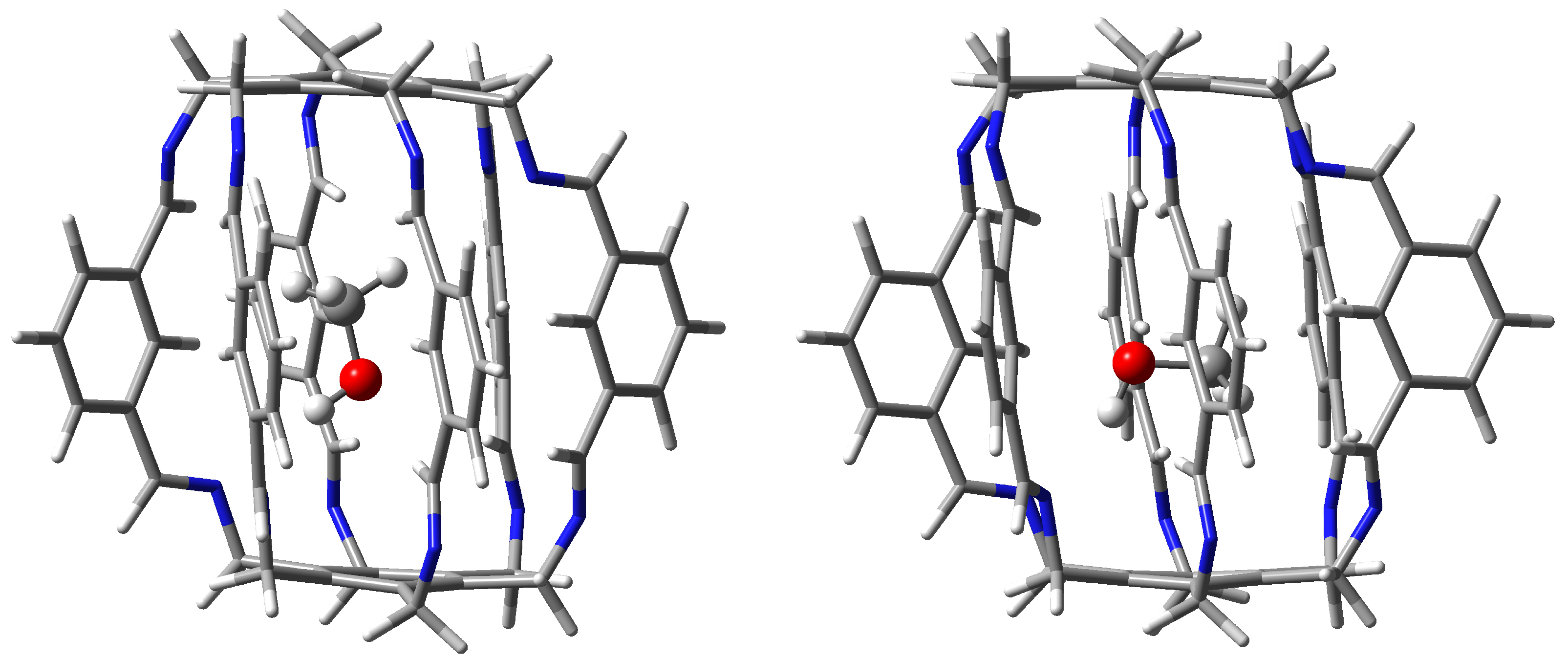
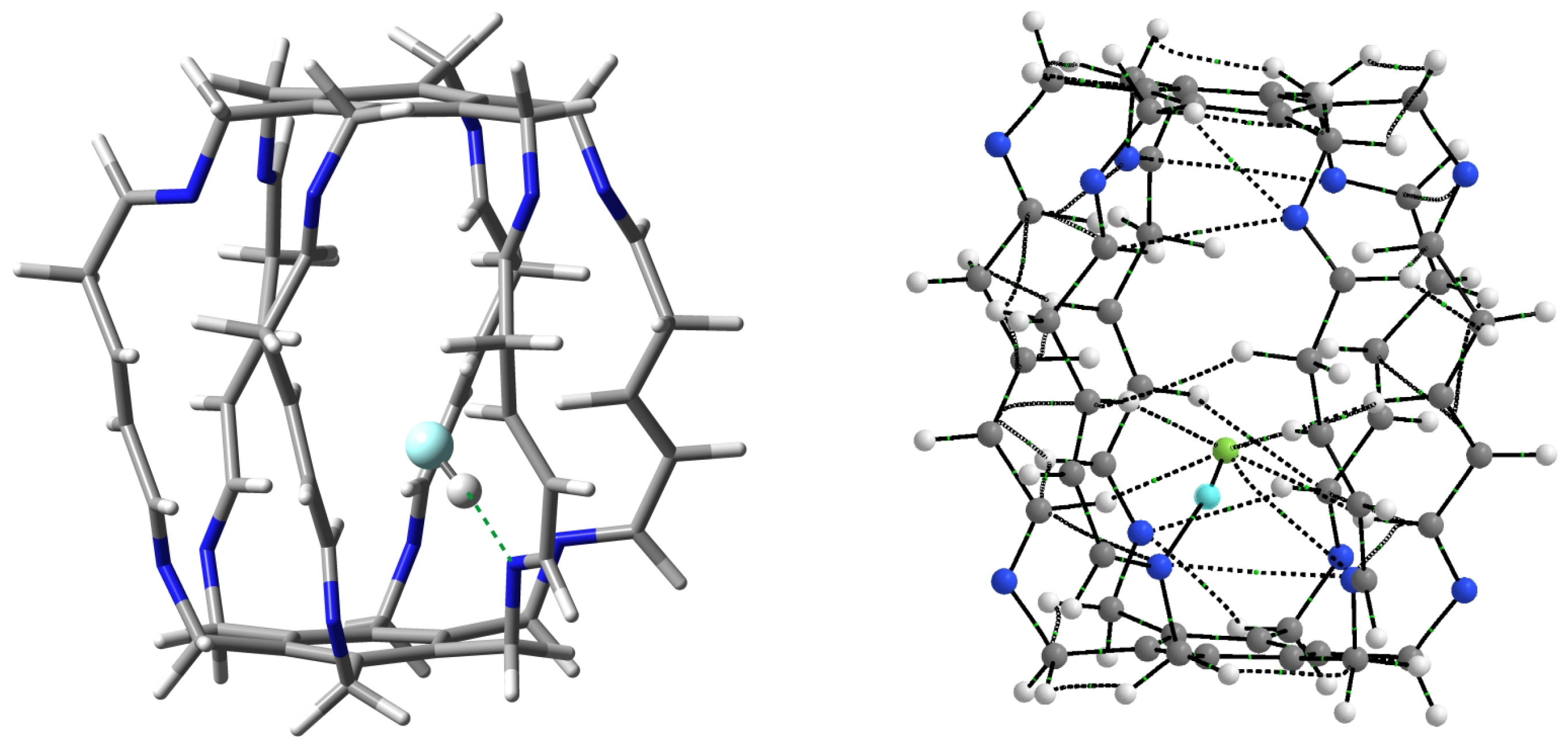
| Carceplex | SP | Guest | a | ∑BE b | Intermolecular Interaction | |||
|---|---|---|---|---|---|---|---|---|
| n c | Type d | e | BE f | |||||
| 11a | 1 | O | −18.8 | −14.2 | 2 | N⋯H-O | 0.024 and 0.030 | −4.7 and −5.9 |
| 1 | C-O | 0.006 | −0.7 | |||||
| 3 | C-O | 0.005–0.010 | −0.4 to −1.4 | |||||
| 11b | 1 | O | −16.8 | −11.6 | 2 | N⋯H-O | 0.006 and 0.022 | −0.6 and −4.1 |
| 2 | C-O | 0.010 and 0.013 | −1.5 and −2.2 | |||||
| 2 | C-H-O | 0.008 and 0.010 | −1.0 and −1.6 | |||||
| 1 | C()⋯O | 0.006 | −0.6 | |||||
| 1 | C-H-O | 0.004 | 0.0 | |||||
| 12 | 1 | N | −12.9 | −9.9 | 1 | C-N | 0.012 | −1.8 |
| 5 | C-Hc⋯N | 0.002–0.019 | 0.3 to −3.4 | |||||
| 2 | C-H-N | 0.004 and 0.008 | −0.2 and −1.1 | |||||
| 2 | N⋯H-N | 0.006 and 0.010 | −0.6 and −1.4 | |||||
| 13a | 1 | HF | −19.7 | −16.4 | 1 | N⋯H-F | 0.052 | −10.8 |
| 2 | C-F | 0.006 and 0.010 | −0.5 and −1.5 | |||||
| 3 | C-F | 0.004–0.010 | −0.2 to −1.6 | |||||
| 1 | C()⋯F | 0.003 | 0.2 | |||||
| 1 | N⋯F | 0.006 | −0.7 | |||||
| 13b | 1 | HF | −18.5 | −14.7 | 1 | N⋯H-F | 0.046 | −9.5 |
| 2 | C-F | 0.004 and 0.012 | −0.2 and −2.0 | |||||
| 3 | C-F | 0.003–0.011 | 0.1 to −1.7 | |||||
| 1 | N⋯F | 0.007 | −0.8 | |||||
| 14a | 1 | HCN | −16.2 | −13.9 | 1 | N⋯H-C | 0.027 | −5.3 |
| 1 | N⋯(H-C) | 0.009 | −1.2 | |||||
| 1 | C-C | 0.005 | −0.4 | |||||
| 1 | C-C | 0.010 | −1.5 | |||||
| 3 | C-N | 0.004–0.015 | −0.2 to −2.6 | |||||
| 2 | C-N | 0.006 and 0.008 | −0.5 and −1.2 | |||||
| 1 | C()⋯N | 0.003 | 0.1 | |||||
| 1 | N | 0.002 | 0.2 | |||||
| 14b | 1 | HCN | −16.2 | −14.6 | 2 | N⋯H-C | 0.008 and 0.029 | −1.1 and −5.8 |
| 1 | C-C | 0.007 | −0.8 | |||||
| 1 | C-C | 0.009 | −1.2 | |||||
| 1 | C()⋯C | 0.004 | 0.0 | |||||
| 3 | C-N | 0.005–0.014 | −0.4 to −2.3 | |||||
| 2 | C-N | 0.006–0.008 | −0.6 and −0.9 | |||||
| 1 | C()⋯N | 0.002 | 0.3 | |||||
| 15a | 1 | MeOH | −19.2 | −13.0 | 2 | N⋯H-O | 0.004 and 0.012 | −0.2 and −1.9 |
| 1 | C-Hc⋯O | 0.011 | −1.7 | |||||
| 3 | C-O | 0.003–0.008 | 0.2 to −1.1 | |||||
| 3 | C()⋯H-C | 0.001–0.009 | 0.5 to −1.2 | |||||
| 2 | C-Hc⋯H-C | 0.002 and 0.011 | 0.3 and −1.8 | |||||
| 3 | C-H-C | 0.007–0.009 | −0.8 to −1.2 | |||||
| 1 | C-Hc⋯(H-C) | 0.010 | −1.6 | |||||
| 1 | H-C | 0.005 | −0.5 | |||||
| 1 | N⋯H-C | 0.003 | 0.1 | |||||
| 15b | 1 | MeOH | −18.3 | −13.3 | 2 | N⋯H-O | 0.006 and 0.010 | −0.6 and −1.5 |
| 3 | C-Hc⋯O | 0.010–0.013 | −1.4 to −2.1 | |||||
| 1 | C()⋯O | 0.004 | −0.1 | |||||
| 2 | C-H-C | 0.010 and 0.010 | −1.5 and −1.5 | |||||
| 2 | H-C | 0.007 and 0.008 | −0.8 and −1.0 | |||||
| 2 | C()⋯H-C | 0.005 and 0.006 | −0.3 and −0.7 | |||||
| Superphane | H2O | NH3 | HF | HCN | MeOH | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | c | a | b | a | b | a | b | a | b | ||
| 1 | 9.15 | 9.18 | 9.16 | n/a | 9.11 | n/a | 9.21 | 9.18 | 9.10 | 9.10 | 9.08 | 9.05 |
| 2 | 8.96 | 8.97 | 9.01 | n/a | 8.91 | 8.93 | 9.03 | n/a | 8.94 | n/a | 8.81 | n/a |
| 3 | 9.05 | 9.09 | 9.07 | 9.08 | 9.02 | 9.04 | 9.12 | 9.10 | 9.02 | n/a | 8.99 | n/a |
| Superphane | H2O | NH3 | HF | HCN | MeOH | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | c | a | b | a | b | a | b | a | b | |
| 1 | −18.8 | −16.8 | n/a | −12.9 | n/a | −19.7 | −18.5 | −16.2 | −16.2 | −19.2 | −18.3 |
| 2 | −19.6 | −16.5 | n/a | −13.1 | −13.0 | −23.1 | n/a | −18.4 | n/a | −17.5 | n/a |
| 3 | −16.6 | −15.6 | −14.2 | −12.9 | −12.5 | −18.3 | −16.4 | −15.8 | n/a | −18.0 | n/a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabłoński, M. Characteristics of Intermolecular Interactions between Encapsulated Molecules and the Lantern-Like Carcerand Superphanes. Molecules 2024, 29, 601. https://doi.org/10.3390/molecules29030601
Jabłoński M. Characteristics of Intermolecular Interactions between Encapsulated Molecules and the Lantern-Like Carcerand Superphanes. Molecules. 2024; 29(3):601. https://doi.org/10.3390/molecules29030601
Chicago/Turabian StyleJabłoński, Mirosław. 2024. "Characteristics of Intermolecular Interactions between Encapsulated Molecules and the Lantern-Like Carcerand Superphanes" Molecules 29, no. 3: 601. https://doi.org/10.3390/molecules29030601
APA StyleJabłoński, M. (2024). Characteristics of Intermolecular Interactions between Encapsulated Molecules and the Lantern-Like Carcerand Superphanes. Molecules, 29(3), 601. https://doi.org/10.3390/molecules29030601