Exploring the Therapeutic Efficacy of Parsley (Petroselinum crispum Mill.) as a Functional Food: Implications in Immunological Tolerability, Reduction of Muscle Cramps, and Treatment of Dermatitis
Abstract
1. Introduction
2. Results
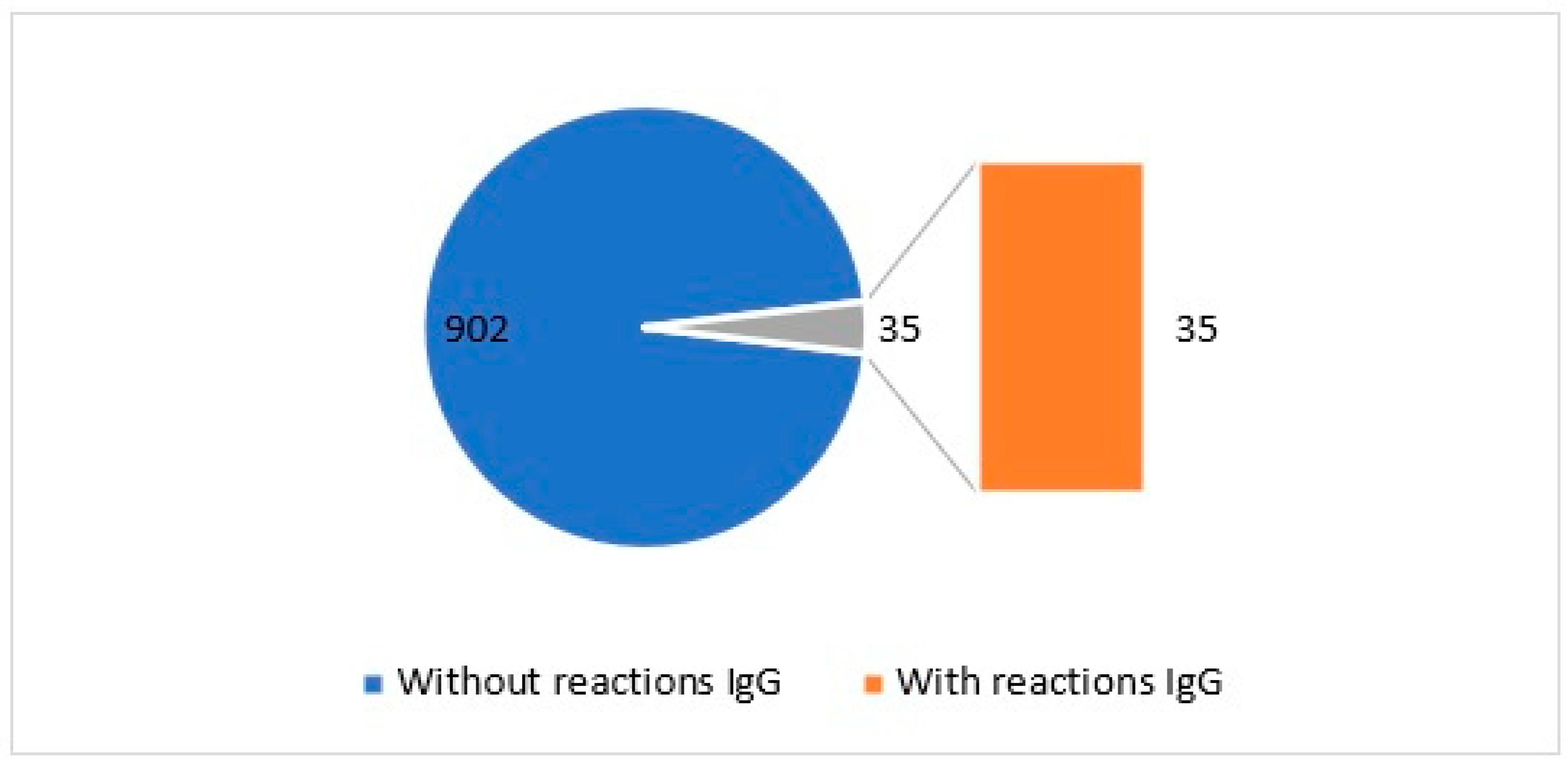
2.1. IgG Test: Parsley Tolerability Assessment
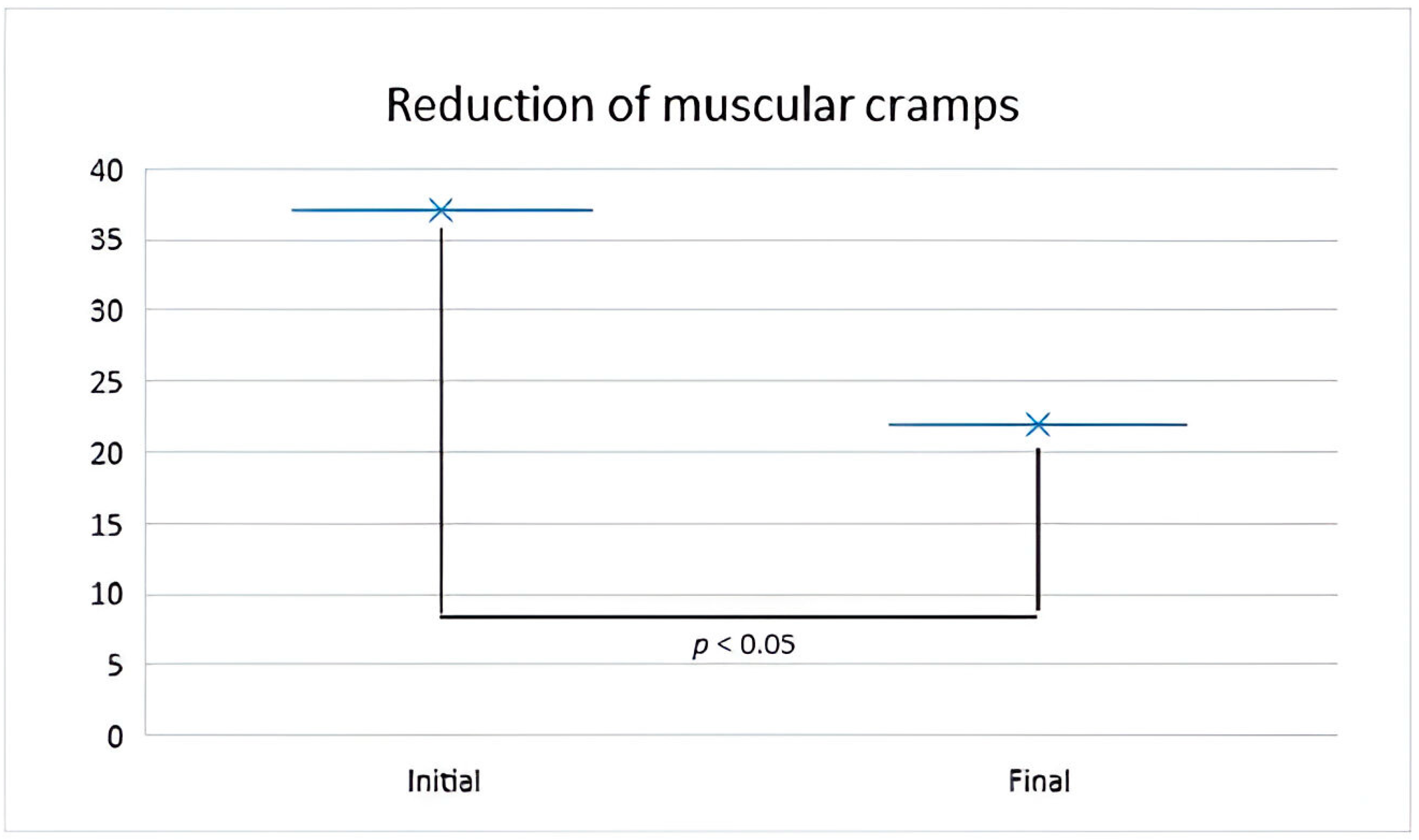
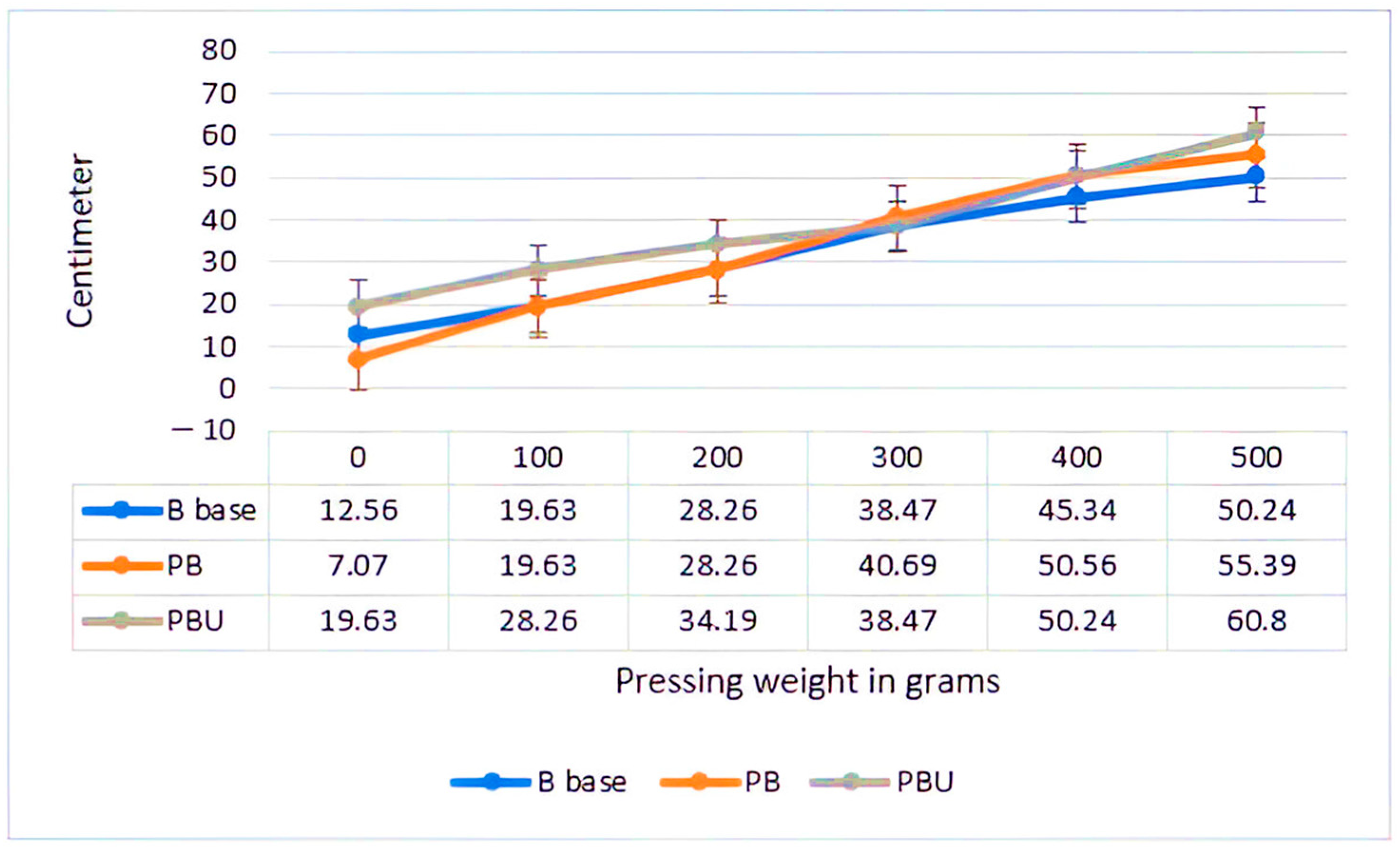
2.2. Mineral Water for Muscle Cramps
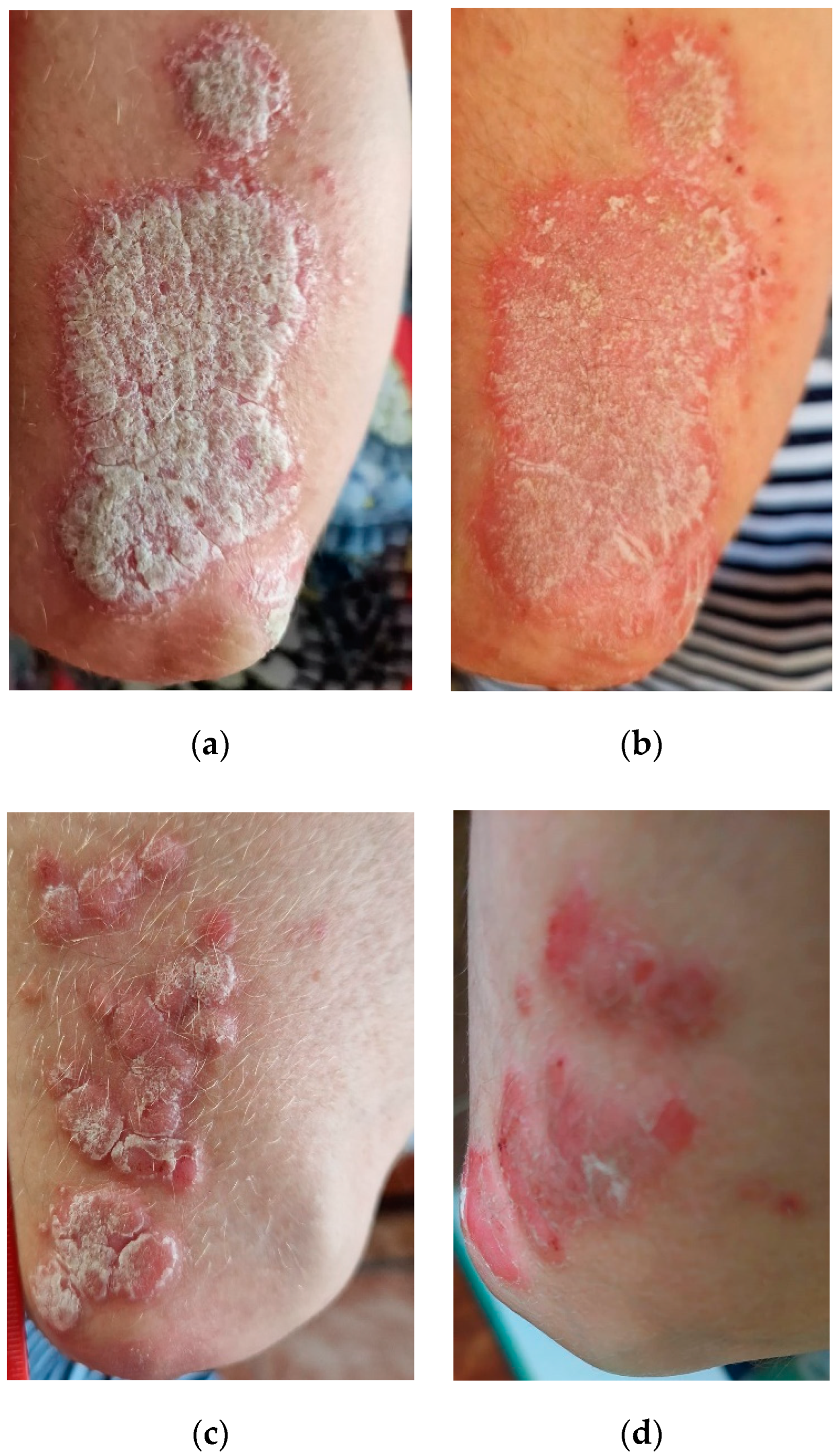
2.3. Dermatological Applications
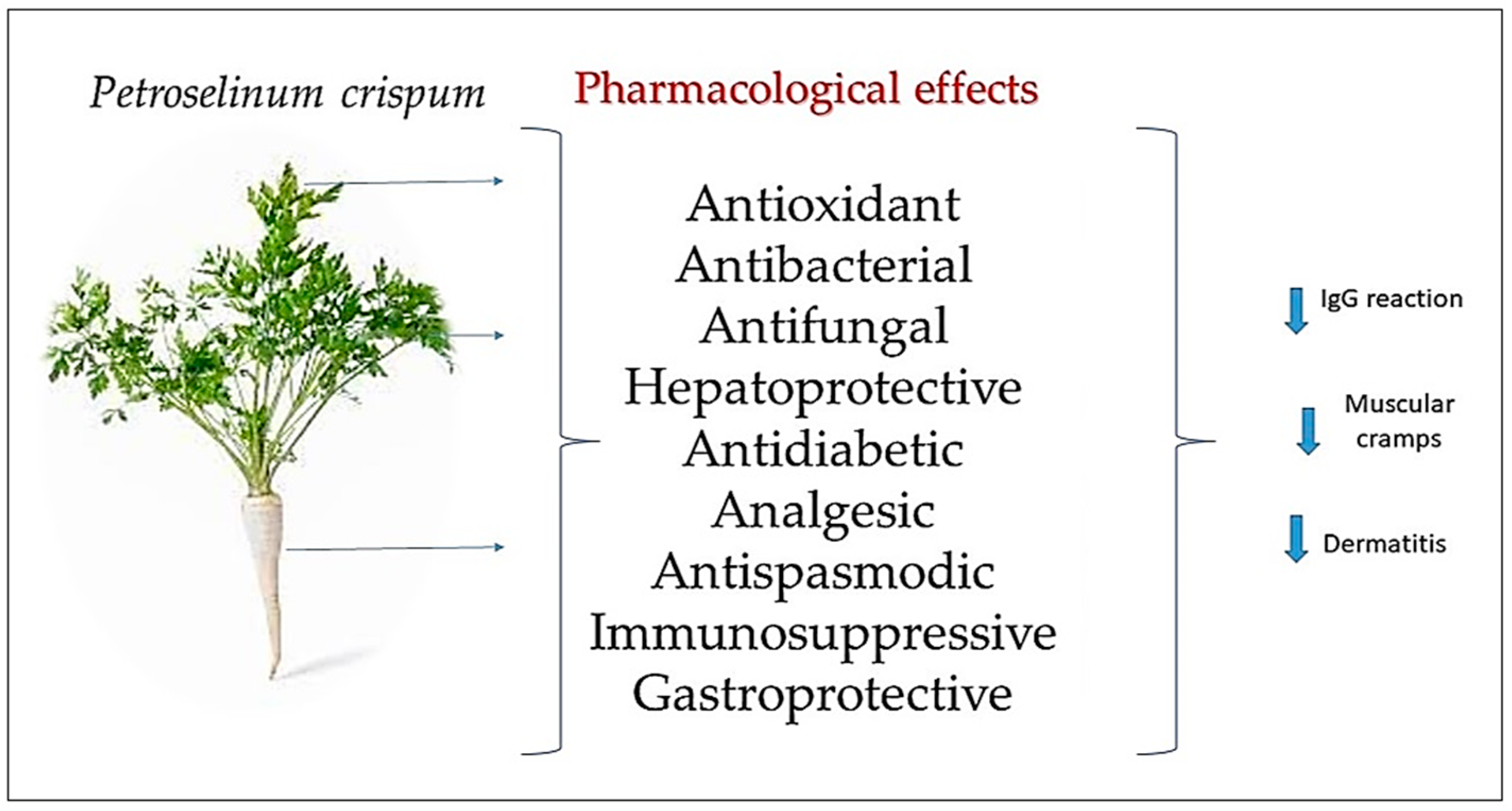
3. Discussion
4. Materials and Methods
4.1. Structure of the Study
4.2. Chemicals, Reagents, and Plant Material
4.3. Evaluation of Lyophilized Product with Gas Chromatograph (GC-MS)
- RI = retention index of “i”;
- i = constituent of essential oil subjected to analysis;
- n = carbon number of the alkane eluting before “i”;
- m = number of carbons of the alkane eluting after “i”;
- tri = retention time of “i”;
- trn = retention time of the alkane eluting before “i”;
- trm = retention time of the alkane eluting after “i”.
4.4. Chemical Composition of the Dry Matter
4.5. Formulation and Evaluation of Bioadhesive Preparation with Parsley
Preparation of Bioadhesive Products
4.6. Quality Control of Bioadhesive Preparations
4.7. Immunological Tests
4.8. Muscle Cramp Verification Test
4.9. Dermatological Evaluation
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gnintoungbe, G.S.; Medehouenou, T.C.M.; Adounkpe, F.; Akpovi, C.; Loko, F. Phytochemical Screening, Antioxidant Activity and Safety of Petroselinum crispum (Mill.) AW Hill Apiaceae Leaves Grown in Benin. Open J. Appl. Sci. 2023, 13, 36–50. [Google Scholar] [CrossRef]
- Nouioura, G.; Kettani, T.; Tourabi, M.; Elousrouti, L.T.; Al kamaly, O.; Alshawwa, S.Z.; Shahat, A.A.; Alhalmi, A.; Lyoussi, B.; Derwich, E. The Protective Potential of Petroselinum crispum (Mill.) Fuss. on Paracetamol-Induced Hepatio-Renal Toxicity and Antiproteinuric Effect: A Biochemical, Hematological, and Histopathological Study. Medicina 2023, 59, 1814. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, R.; Handog, E.B.; Das, A.; Bansal, A.; Macarayo, M.J.; Keshavmurthy, V.; Narayan, V.; Jagadeesan, S.; Pipo III, E.; Ibaviosa, G.M. Topical and Systemic Therapies in Melasma: A Systematic Review. Indian Dermatol. Online J. 2023, 14, 769–781. [Google Scholar] [CrossRef]
- Vikou, C.; Semassa, J.; Diaconeasa, Z.; Roko, G.; Tohoyessou, M.; Dah-Nouvlessounon, D.; Sina, H.; Stanilă, A.; Baba-Moussa, L. Diversity, Chemical Compositions and Beneficial Effects of Some Spices and Aromatic Leaves Consumed in Benin and in the World: Critical Review. Am. J. Plant Sci. 2023, 14, 569–598. [Google Scholar] [CrossRef]
- Herrera-Calderon, O.; Saleh, A.M.; Mahmood, A.A.R.; Khalaf, M.A.; Calva, J.; Loyola-Gonzales, E.; Tataje-Napuri, F.E.; Chávez, H.; Almeida-Galindo, J.S.; Chavez-Espinoza, J.H.; et al. The Essential Oil of Petroselinum crispum (Mill) Fuss Seeds from Peru: Phytotoxic Activity and In Silico Evaluation on the Target Enzyme of the Glyphosate Herbicide. Plants 2023, 12, 2288. [Google Scholar] [CrossRef]
- Bampidis, V.; Azimonti, G.; Bastos, M.; Christensen, H.; Durjava, M.; Kouba, M.; López-Alonso, M.; López, S.; Marcon, F.; Mayo, B.; et al. Safety and efficacy of a feed additive consisting of a tincture derived from the fruit of Petroselinum crispum (Mill.) Fuss (parsley tincture) for use in all animal species (FEFANA asbl). EFSA J. 2023, 21, e07694. [Google Scholar] [CrossRef]
- Choi, J.-E.; Moon, J.-S.; Choi, J.-E.; Moon, J.-S. Physiological activities of parsley extracts as an ingredient of functional cosmetics. Asian J. Beauty Cosmetol. 2017, 15, 501–511. [Google Scholar] [CrossRef]
- Yousofi, A.; Daneshmandi, S.; Soleimani, N.; Bagheri, K.; Karimi, M.H. Immunomodulatory effect of Parsley (Petroselinum crispum) essential oil on immune cells: Mitogen-activated splenocytes and peritoneal macrophages. Immunopharmacol. Immunotoxicol. 2012, 34, 303–308. [Google Scholar] [CrossRef]
- Mara de Menezes Epifanio, N.; Rykiel Iglesias Cavalcanti, L.; Falcão dos Santos, K.; Soares Coutinho Duarte, P.; Kachlicki, P.; Ożarowski, M.; Jorge Riger, C.; Siqueira de Almeida Chaves, D. Chemical characterization and in vivo antioxidant activity of parsley (Petroselinum crispum) aqueous extract. Food Funct. 2020, 11, 5346–5356. [Google Scholar] [CrossRef]
- Abd-Alla, H.I. The potential use of some members belong to Apiaceae and Asteraceae plant family as immune boosters in livestock production. Egypt. J. Anim. Prod. 2022, 59, 45–55. [Google Scholar] [CrossRef]
- Ajebli, M.; Eddouks, M. Antihypertensive activity of Petroselinum crispum through inhibition of vascular calcium channels in rats. J. Ethnopharmacol. 2019, 242, 112039. [Google Scholar] [CrossRef] [PubMed]
- Khosravan, S.; Alami, A.; Mohammadzadeh-Moghadam, H.; Ramezani, V.; Sheikhnavesi, M. The efficacy of topical use of Petroselinum crispum (parsley) versus hydroquinone cream for reduction of epidermal melasma: A randomized clinical trial. Avicenna J. Phytomed. 2015, 5, 126. [Google Scholar]
- Papp, N.; Czégényi, D.; Tóth, M.; Dénes, T.; Bartha, S.G.; Csepregi, R.; Gyergyák, K.; Bukovics, P.; Stranczinger, S.; Varga, E. Ethnomedicine survey on folk dermatology in Transylvania, Romania. Clin. Dermatol. 2022, 40, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Hasanuddin, S.; Gozali, D.; Arba, M.; Ramadhan, D.S.F.; Mustarichie, R. In silico prediction of metabolite in petroselinum crispum in inhibiting androgen receptor as treatment for alopecia. Res. J. Pharm. Technol. 2022, 15, 1211–1218. [Google Scholar] [CrossRef]
- Şener, G.; Karakadıoglu, G.; Ozbeyli, D.; Ede, S.; Yanardag, R.; Sacan, O.; Aykac, A. Petroselinum crispum extract ameliorates scopolamine-induced cognitive dysfunction: Role on apoptosis, inflammation and oxidative stress. Food Sci. Hum. Wellness 2022, 11, 1290–1298. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, F.; Wang, X.; Yao, H.-Y. Evaluation of antioxidant activity of parsley (Petroselinum crispum) essential oil and identification of its antioxidant constituents. Food Res. Int. 2006, 39, 833–839. [Google Scholar] [CrossRef]
- Papuc, C.; Predescu, C.; Nicorescu, V.; Stefan, G.; Nicorescu, I. Antioxidant properties of a parsley (Petroselinum crispum) juice rich in polyphenols and nitrites. Curr. Res. Nutr. Food Sci. J. 2016, 4, 114–118. [Google Scholar] [CrossRef]
- Leahu, A.; Damian, C.; Oroian, M.; Miclescu, V.; Ropciuc, S. Variation in content of antioxidant and free radical scavenging activity of basil (Ocimum basilicum), dill (Anethum graveolens) and parsley (Petroselinum sativum). Food Environ. Saf. J. 2016, 12, 347–353. [Google Scholar]
- Agyare, C.; Appiah, T.; Boakye, Y.D.; Apenteng, J.A. Petroselinum crispum: A review. In Medicinal Spices and Vegetables from Africa; Kuete, V., Ed.; Elsevier Inc.: Cambridge, MA, USA, 2017; pp. 527–547. [Google Scholar]
- Slighoua, M.; Mahdi, I.; Amrati, F.E.-Z.; Di Cristo, F.; Amaghnouje, A.; Grafov, A.; Boucetta, N.; Bari, A.; Bousta, D. Assessment of in vivo estrogenic and anti-inflammatory activities of the hydro-ethanolic extract and polyphenolic fraction of parsley (Petroselinum sativum Hoffm.). J. Ethnopharmacol. 2021, 265, 113290. [Google Scholar] [CrossRef]
- Ede, S.; Özbeyli, D.; Erdoğan, Ö.; Çevik, Ö.; Kanpalta, F.; Ercan, F.; Yanardağ, R.; Saçan, Ö.; Ertik, O.; Yüksel, M. Hepatoprotective effects of parsley (Petroselinum crispum) extract in rats with bile duct ligation. Arab J. Gastroenterol. 2023, 24, 45–51. [Google Scholar] [CrossRef]
- Shcherazade, O.-S.F.; Pétronille, A.-Z.; Zana, C.; John, A.; Bertin, Y.K. Study of the anti-anemic activity of the aqueous extract of the leaves of Petroselinum crispum (Apiaceae) in the white mouse (Mus musculus) of SWISS strain. J. Blood Disord. Ther. 2022, 2, 101. [Google Scholar]
- Akpaso, M.I.; Enefe, K.; Igiri, A.O. Improved Fertility Potential of Ethanolic Leaf Extracts of Petroselinum crispum. Trop. J. Med. Res. 2023, 22, 157–163. [Google Scholar]
- Thangavelu, S.; Balasubramanian, B.; Palanisamy, S.; Shanmugam, V.; Natchiappan, S.; Kalibulla, S.I.; Rathinasamy, B.; Arumugam, V.A. Characterization and phytoconstituents of Petroselinum crispum (Mill) and Coriandrum sativum (Linn) and their impacts on inflammation—An in vitro analysis against human adenocarcinoma cells with molecular docking. S. Afr. J. Bot. 2022, 146, 776–788. [Google Scholar] [CrossRef]
- Piras, A.; Porcedda, S.; Falconieri, D.; Fais, A.; Era, B.; Carta, G.; Rosa, A. Supercritical extraction of volatile and fixed oils from Petroselinum crispum L. seeds: Chemical composition and biological activity. Nat. Prod. Res. 2022, 36, 1883–1888. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.u.; Abbas, S.Q.; Ali, F.; Ishaq, M.; Bano, I.; Hassan, M.; Jin, H.-Z.; Bungau, S.G. A Comprehensive In Silico Exploration of Pharmacological Properties, Bioactivities, Molecular Docking, and Anticancer Potential of Vieloplain F from Xylopia vielana Targeting B-Raf Kinase. Molecules 2022, 27, 917. [Google Scholar] [CrossRef] [PubMed]
- Galkin, A.; Arkhypova, M.; Starosyla, D.; Deriabin, O.; Vasylchenko, O.; Rybalko, S.; Golembiovska, O. Antiviral Activity of New Flavonoids-Containing Phytopreparation against Human Alphaherpesvirus 2, Hepatitis C virus, and Coronavirus. [Preprint.]. 27 June 2022. Available online: https://doi.org/10.21203/rs.3.rs-1773246/v1 (accessed on 16 December 2023).
- Zare-Bidaki, M.; Ghasempour, A.; Mohammadparast-Tabas, P.; Ghoreishi, S.M.; Alamzadeh, E.; Javanshir, R.; Le, B.N.; Barakchi, M.; Fattahi, M.; Mortazavi-Derazkola, S. Enhanced in vivo wound healing efficacy and excellent antibacterial, antifungal, antioxidant and anticancer activities via AgNPs@ PCS. Arab. J. Chem. 2023, 16, 105194. [Google Scholar] [CrossRef]
- Mirmohammadmakki, F.; Gharachorloo, M.; Ghavami, M.; Abdossi, V.; Azizinezhad, R. Quantitative Changes in Ascorbic Acid and Chlorophyll Contents of Parsley (Petroselinum crispum) and Dill (Anethum graveolens) Harvested in Three Consecutive Months of Spring. J. Food Biosci. Technol. 2023, 13, 1–12. [Google Scholar]
- Byers, H.L.; McHenry, L.J.; Grundl, T.J. XRF techniques to quantify heavy metals in vegetables at low detection limits. Food Chem. X 2019, 1, 100001. [Google Scholar] [CrossRef]
- Muresan, M.; Micle, O.; Antal, L.; Dobjanschi, L.; Antonescu, A.; Vicas, L.; Bodog, F.; Dorofteiu, M. Correlation between reaktiveoxygene species and homocysteine levels in normal pregnancy. Farmacia 2011, 59, 179–190. [Google Scholar]

| Parameters | Groups | Total | p | ||||
|---|---|---|---|---|---|---|---|
| Control Group | Study Group | ||||||
| Count | % | Count | % | ||||
| Redness initial | 0% | 0 | 0.0 | 0 | 0.0 | 36 | 0.002 ** |
| <10% | 3 | 9.4 | 5 | 15.6 | |||
| 10–29% | 8 | 25.0 | 7 | 21.9 | |||
| 30–49% | 5 | 15.6 | 4 | 12.5 | |||
| p | 0.477 | ||||||
| Redness final | 0% | 0 | 0.0 | 0 | 0.0 | ||
| <10% | 4 | 12.5 | 10 | 31.2 | |||
| 10–29% | 7 | 21.9 | 5 | 15.6 | |||
| 30–49% | 5 | 15.6 | 1 | 3.1 | |||
| p | 0.020 * | ||||||
| Thickness initial | 0% | 0 | 0.0 | 0 | 0.0 | 0.001 ** | |
| <10% | 6 | 18.8 | 6 | 18.8 | |||
| 10–29% | 7 | 21.9 | 8 | 25.0 | |||
| 30–49% | 3 | 9.4 | 2 | 6.2 | |||
| p | 0.837 | ||||||
| Thickness final | 0% | 0 | 0.0 | 1 | 3.1 | ||
| <10% | 6 | 18.8 | 12 | 37.5 | |||
| 10–29% | 8 | 25.0 | 3 | 9.4 | |||
| 30–49% | 2 | 6.2 | 0 | 0.0 | |||
| p | 0.008 ** | ||||||
| Scaling initial | 0% | 0 | 0.0 | 0 | 0.0 | 0.001 ** | |
| <10% | 5 | 15.6 | 8 | 25.0 | |||
| 10–29% | 7 | 21.9 | 5 | 15.6 | |||
| 30–49% | 4 | 12.5 | 3 | 9.4 | |||
| p | 0.343 | ||||||
| Scaling final | 0% | 1 | 3.1 | 4 | 12.5 | ||
| <10% | 5 | 15.6 | 10 | 31.2 | |||
| 10–29% | 6 | 18.8 | 2 | 6.2 | |||
| 30–49% | 4 | 12.5 | 0 | 0.0 | |||
| p | 0.003 ** | ||||||
| Product | Substance | Retention Time (min) | Peak Number | Kovats Index |
|---|---|---|---|---|
| Green parsley powder | Acetic acid | 2.88 | 9 | Reference |
| Thiourea | 3.40 | 10 | 18.05 | |
| Triethylfluorosilane | 4.06 | 11 | 40.97 | |
| 1,2,3,4-diepoxybutane | 4.35 | 12 | 51.04 | |
| Hydrazoic acid | 4.55 | 13 | 57.98 | |
| N-Methoxyformamide | 5.16 | 14 | 79.16 | |
| Glyceraldehyde | 5.46 | 15 | 89.58 | |
| Pyruvic acid, methyl ester | 6.59 | 16 | 129.81 | |
| 2-Methylcyclopentanol | 6.72 | 17 | 133.33 | |
| Beta-Myrcene | 9.15 | 19 | 217.70 | |
| 1-Isopropyl-4-methylenebicyclo(3,1,0)-hexane | 10.10 | 20 | 250.69 | |
| Maltol | 11.20 | 21 | 288.88 | |
| p-Cymenene | 11.59 | 22 | 302.43 | |
| p-Mentha-1,3,8-triene | 12.16 | 23 | 322.22 | |
| 1-Vinyl-cyclohexanol | 12.39 | 24 | 330.20 | |
| Glyceraldehyde | 12.73 | 25 | 342.01 | |
| 3,5-Dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one | 12.88 | 26 | 347.22 | |
| 3-Methylacetophenone | 13.93 | 27 | 383.68 | |
| 5-Hydroxymethylfurfural | 14.87 | 28 | 416.31 | |
| 1-Acetate-1,2,3-propanetriol | 15.18 | 29 | 427.08 | |
| 8a-Chlorooctahydro-1(H)-naphthalenone | 18.81 | 31 | 553.12 | |
| m-Cymen-8-ol | 18.92 | 32 | 556.94 | |
| 3-Isopropenyl-2,5-dimethyl-3,4-hexadien-2-ol | 20.98 | 34 | 628.47 | |
| 5-allyl-1-methoxy-2,3-(methylenedioxy)-benzene | 21.42 | 35 | 643.75 | |
| Sesquisabinenes isomer | 21.47 | 36 | 645.48 | |
| 5,6,7,7a-tetrahydro-4,4,7A-trimethyl-2(4H)-benzofuranone | 21.67 | 37 | 652.43 | |
| 3-Deoxy-d-mannoic-lactones | 22.46 | - | 679.86 | |
| 1,2,3,5-Cyclohexanetetrol | 22.88 | 38 | 694.44 | |
| Heptose | 23.04 | 39 | 700.00 | |
| Apiol | 24.51 | 40 | 751.04 | |
| 6-Hydroxy-4,4,7a-trimethyl-5,6,7,7a-tetrahydrobenzofuran-2(4H)-one | 26.06 | 41 | 804.86 | |
| Ficusin | 27.12 | 42 | 841.66 | |
| Neophytadians | 27.21 | 43 | 844.79 | |
| 3,7,11,15-Tetramethyl-2-hexanedecen-1-ol | 27.63 | 44 | 859.37 | |
| Hexanoic acid-2-phenylethyl ester | 27.94 | 45 | 870.13 | |
| Palmitic acid | 29.20 | 47 | 913.88 | |
| Methoxsalen | 30.59 | 49 | 962.15 | |
| Bergapten | 30.92 | 50 | 973.61 | |
| Pythol | 31.62 | 53 | 998.26 | |
| Linoleic acid | 31.88 | 54 | 1006.94 | |
| Linolenic acid | 31.99 | 55 | 1010.76 | |
| 1-Methyl-8-(1-methylethyl)-tricyclo[4,4,0,0(2,7)]dec-3-ene-3-methanol | 32.10 | 56 | 1014.58 | |
| 1-Methyl-3[(1-methylethyldiene)-cyclopropyl]-benzene | 32.19 | 57 | 1017.70 | |
| Stearic acid | 32.30 | 58 | 1021.52 | |
| Octanoic acid 2-dimethylaminoethyl ester | 34.10 | 59 | 1084.02 | |
| 8-13-epoxy-1,15,16-trinor-8-xi-labdan-6beta-ol | 34.35 | 60 | 1092.70 | |
| 3-Cyclopentylpropionic acid 2-dimethylaminoethyl ester | 36.43 | 61 | 1164.93 | |
| Oxypeucedarin | 36.83 | 63 | 1178.81 | |
| 2-Monopalmitin | 37.13 | 64 | 1189.23 |
| Metal | λ (nm) | Lamp Current (mA) | Slit Width | WHO Standard |
|---|---|---|---|---|
| Ni | 232 | 4 | 0.2 | Not specified |
| Cr | 357.9 | 7 | 0.2 | Not specified |
| Cu | 324.8 | 4 | 0.5 | Not specified |
| Cd | 228.8 | 4 | 0.5 | 0.005 ppm |
| Mn | 279.5 | 5 | 0.2 | 0.1 ppm |
| Zn | 213 | 5 | 1 | Not specified |
| Fe | 248.3 | 5 | 0.2 | 0.3 ppm |
| Pb | 283.3 | 10 | 1.2 | 0.05 ppm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganea, M.; Vicaș, L.G.; Gligor, O.; Sarac, I.; Onisan, E.; Nagy, C.; Moisa, C.; Ghitea, T.C. Exploring the Therapeutic Efficacy of Parsley (Petroselinum crispum Mill.) as a Functional Food: Implications in Immunological Tolerability, Reduction of Muscle Cramps, and Treatment of Dermatitis. Molecules 2024, 29, 608. https://doi.org/10.3390/molecules29030608
Ganea M, Vicaș LG, Gligor O, Sarac I, Onisan E, Nagy C, Moisa C, Ghitea TC. Exploring the Therapeutic Efficacy of Parsley (Petroselinum crispum Mill.) as a Functional Food: Implications in Immunological Tolerability, Reduction of Muscle Cramps, and Treatment of Dermatitis. Molecules. 2024; 29(3):608. https://doi.org/10.3390/molecules29030608
Chicago/Turabian StyleGanea, Mariana, Laura Grațiela Vicaș, Octavia Gligor, Ioan Sarac, Emilian Onisan, Csaba Nagy, Corina Moisa, and Timea Claudia Ghitea. 2024. "Exploring the Therapeutic Efficacy of Parsley (Petroselinum crispum Mill.) as a Functional Food: Implications in Immunological Tolerability, Reduction of Muscle Cramps, and Treatment of Dermatitis" Molecules 29, no. 3: 608. https://doi.org/10.3390/molecules29030608
APA StyleGanea, M., Vicaș, L. G., Gligor, O., Sarac, I., Onisan, E., Nagy, C., Moisa, C., & Ghitea, T. C. (2024). Exploring the Therapeutic Efficacy of Parsley (Petroselinum crispum Mill.) as a Functional Food: Implications in Immunological Tolerability, Reduction of Muscle Cramps, and Treatment of Dermatitis. Molecules, 29(3), 608. https://doi.org/10.3390/molecules29030608








