A Fine Analysis of Zn Species Structure and Distribution in Zn/ZSM-5 Catalysts by Linear Combination Fitting Analysis of XANES Spectra
Abstract
:1. Introduction
2. Results and Discussion
2.1. Textural and Physical Properties of Zeolite Catalysts
2.2. Acid Properties
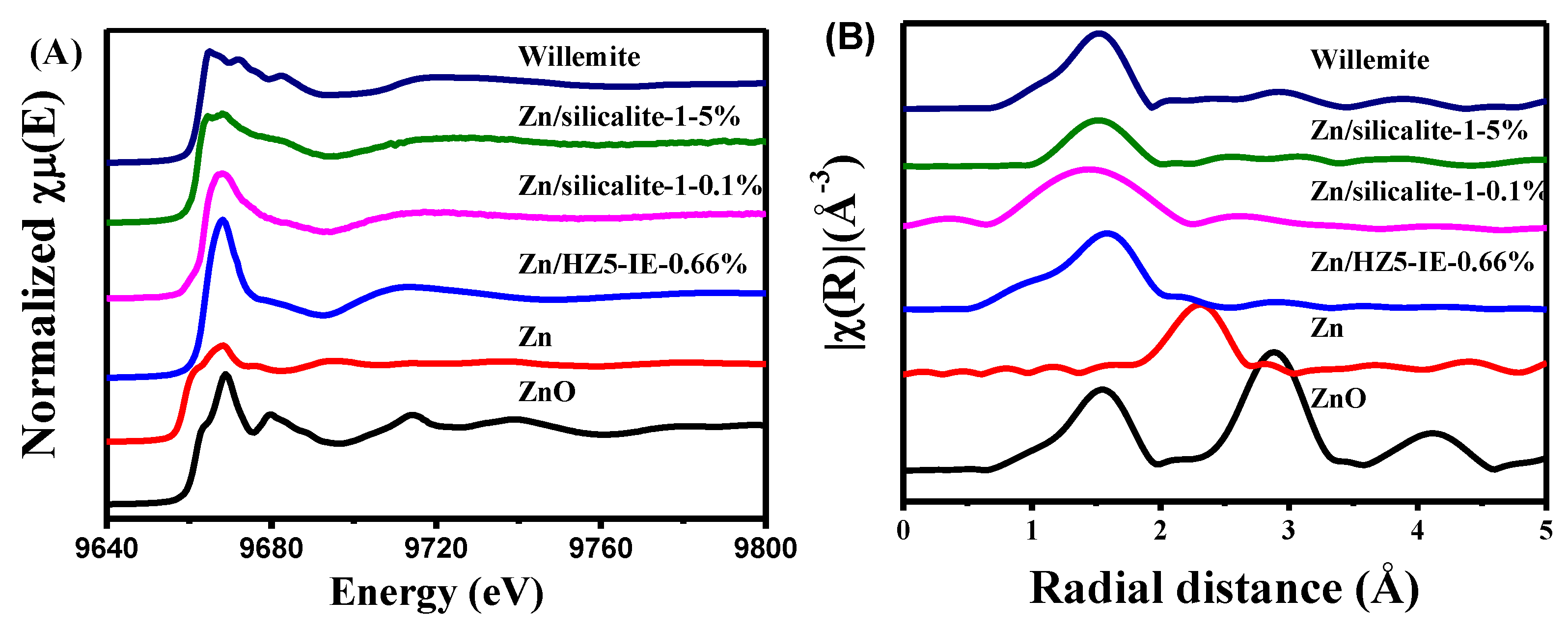
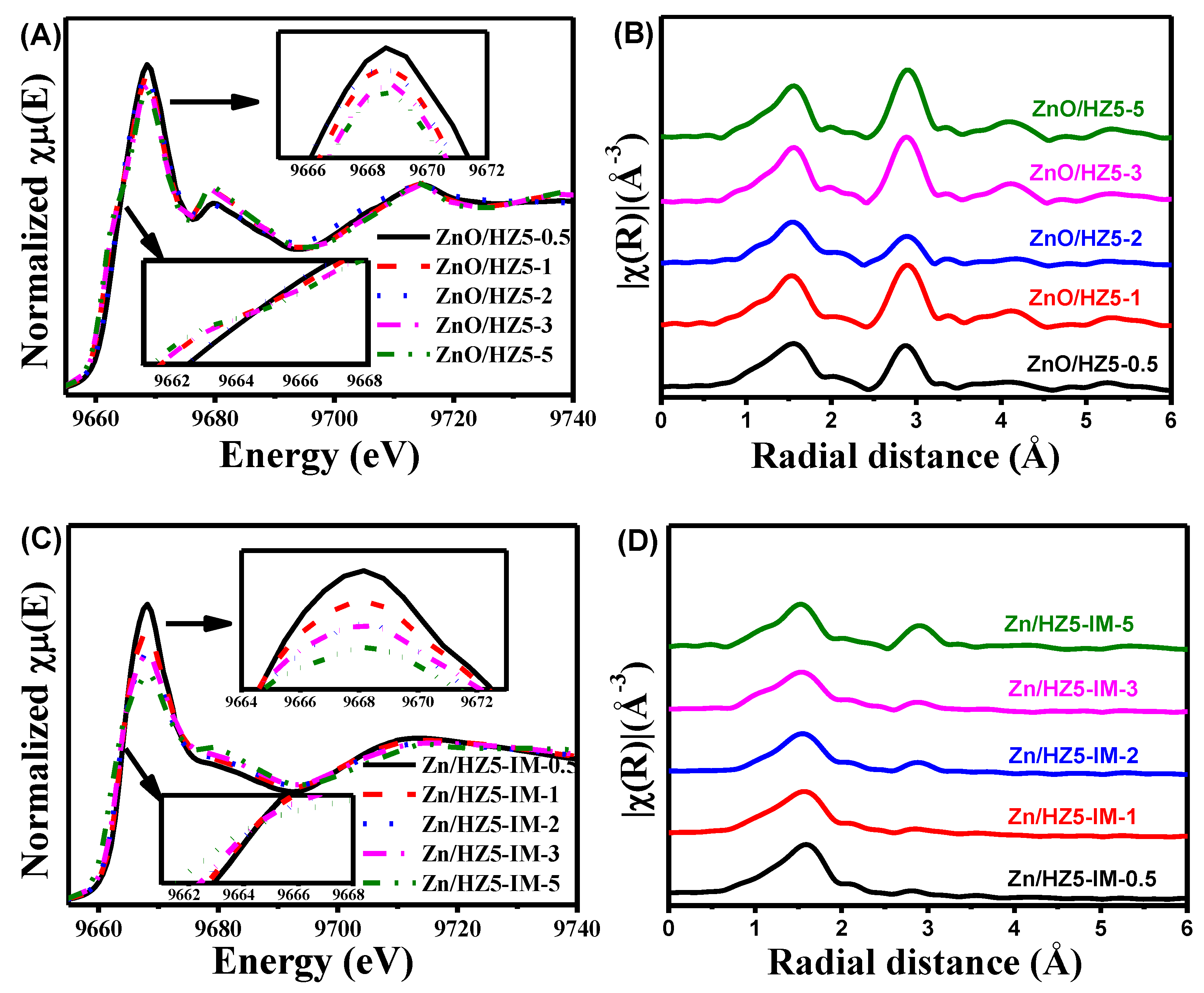
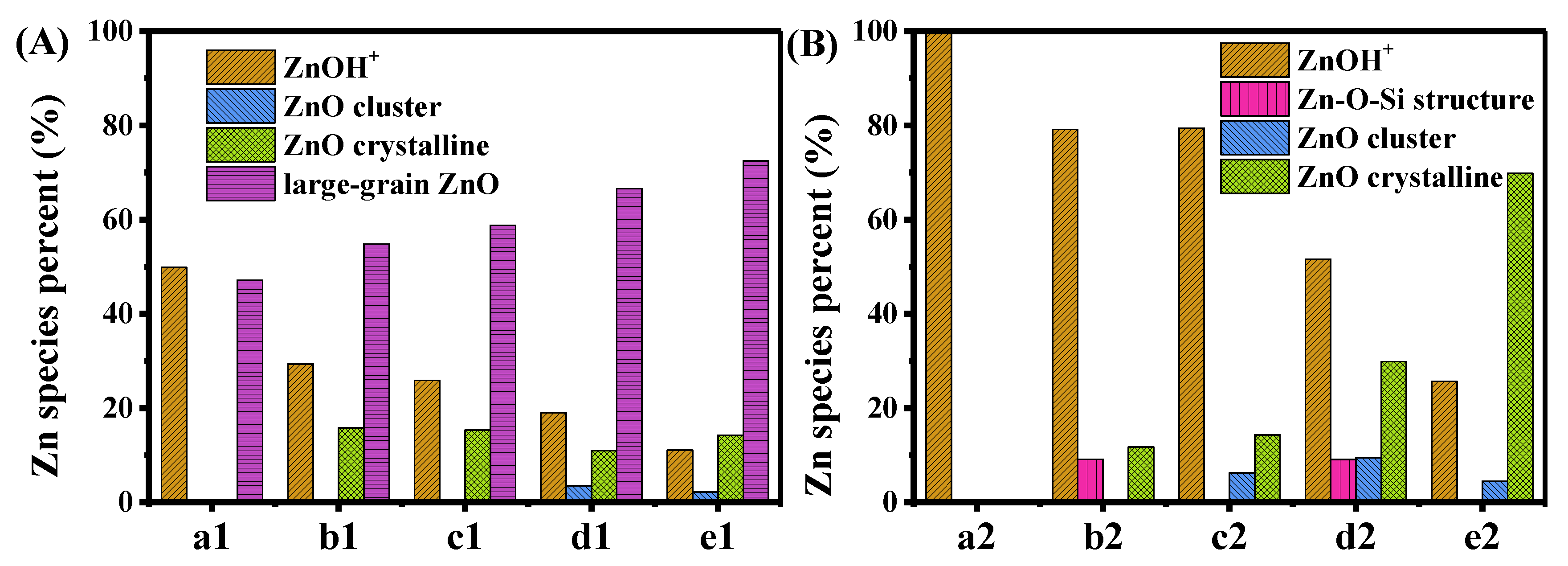
2.3. Analyses of Zn Distribution by EXAFS and LCF of XANES Spectra
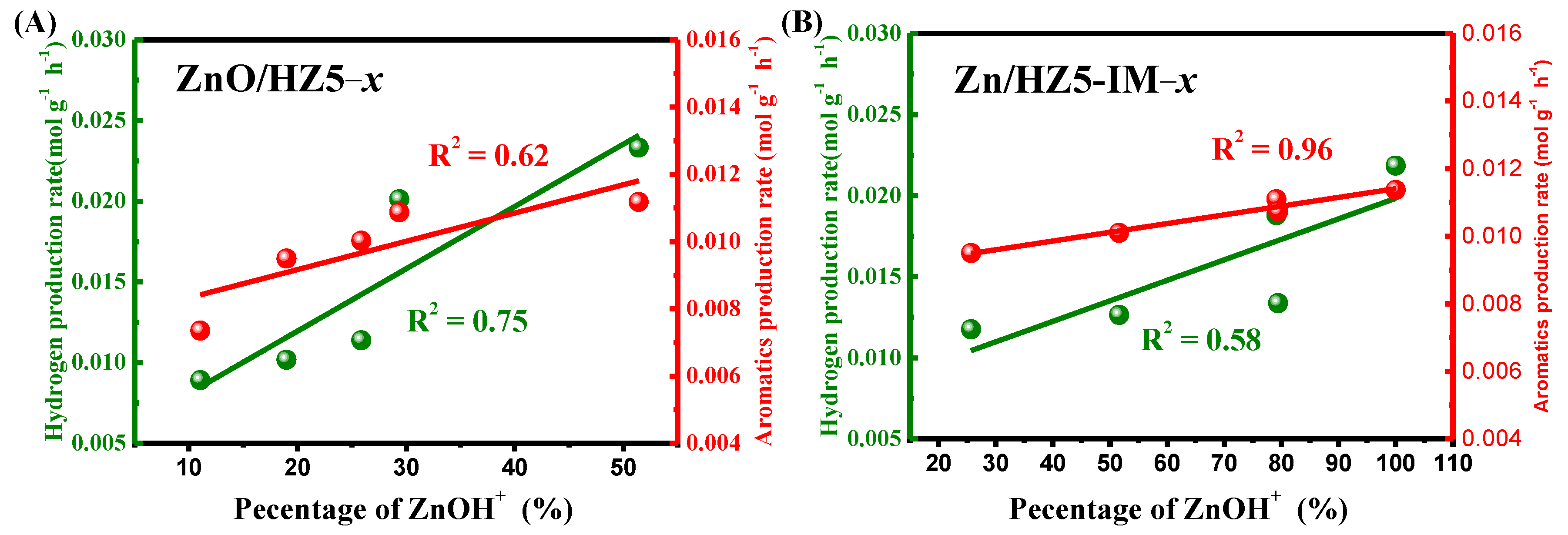
2.4. Catalytic Performance of Various Catalysts
3. Experimental
3.1. Sample Preparation
3.2. Characteristics of the Catalyst
3.3. Catalyst Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lalik, E.; Liu, X.S.; Klinowski, J. Role of Gallium in the Catalytic Activity of Zeolite [Si,Ga]-ZSM-5 for Methanol Conversion. J. Phys. Chem. 1992, 96, 805–809. [Google Scholar] [CrossRef]
- Ono, Y.; Kanae, K. Transformation of Butanes over ZSM-5 Zeolites. Part 2.—Formation of Aromatic Hydrocarbons over Zn-ZSM-5 and Ga-ZSM-5. J. Chem. Soc. Faraday Trans. 1991, 87, 669–675. [Google Scholar] [CrossRef]
- Ono, Y.; Adachi, H.; Senoda, Y. Selective Conversion of Methanol into Aromatic Hydrocarbons over Zinc-Exchanged ZSM-5 Zeolites. J. Chem. Soc. Faraday Trans. I 1988, 84, 1091–1099. [Google Scholar] [CrossRef]
- Guisnet, M.; Gnep, N.S.; Alario, F. Aromatization of Short Chain Alkanes on Zeolite Catalysts. Appl. Catal. A Gen. 1992, 89, 1–30. [Google Scholar] [CrossRef]
- Ono, Y. Transformation of Lower Alkanes into Aromatic Hydrocarbons over ZSM-5 Zeolites. Catal. Rev. Sci. Eng. 1992, 34, 179–226. [Google Scholar] [CrossRef]
- Mores, D.; Kornatowski, J.; Olsbye, U.; Weckhuysen, B.M. Coke Formation during the Methanol-to-Olefin Conversion: In Situ Microspectroscopy on Individual H-ZSM-5 Crystals with Different Bronsted Acidity. Chem. Eur. J. 2011, 17, 2874–2884. [Google Scholar] [CrossRef]
- Liang, T.Y.; Chen, J.L.; Qin, Z.F.; Li, J.F.; Wang, P.F.; Wang, S.; Wang, G.F.; Dong, M.; Fan, W.B.; Wang, J.G. Conversion of Methanol to Olefins over H-ZSM-5 Zeolite: Reaction Pathway Is Related to the Framework Aluminum Siting. ACS Catal. 2016, 6, 7311–7325. [Google Scholar] [CrossRef]
- Kadja, G.T.M.; Suprianti, T.R.; Ilmi, M.M.; Khalil, M.; Mukti, R.R.; Subagjo. Sequential Mechanochemical and Recrystallization Methods for Synthesizing Hierarchically Porous ZSM-5 Zeolites. Microporous Mesoporous Mat. 2020, 308, 110550. [Google Scholar] [CrossRef]
- Kadja, G.T.M.; Azhari, N.J.; Mardiana, S.; Khalil, M.; Subagjo; Mahyuddin, M.H. Accelerated, Mesoporogen-Free Synthesis of Hierarchical Nanorod ZSM-48 Assisted by Hydroxyl Radicals. Ind. Eng. Chem. Res. 2021, 60, 17786–17791. [Google Scholar] [CrossRef]
- Kadja, G.T.M.; Azhari, N.J.; Mukti, R.R.; Khalil, M. A Mechanistic Investigation of Sustainable Solvent-Free, Seed-Directed Synthesis of ZSM-5 Zeolites in the Absence of an Organic Structure-Directing Agent. ACS Omega 2021, 6, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Pashkova, V.; Klein, P.; Dedecek, J.; Tokarová, V.; Wichterlová, B. Incorporation of Al at ZSM-5 Hydrothermal Synthesis. Tuning of Al Pairs in the Framework. Microporous Mesoporous Mat. 2015, 202, 138–146. [Google Scholar] [CrossRef]
- Dedecek, J.; Balgová, V.; Pashkova, V.; Klein, P.; Wichterlová, B. Synthesis of ZSM-5 Zeolites with Defined Distribution of Al Atoms in the Framework and Multinuclear MAS NMR Analysis of the Control of Al Distribution. Chem. Mater. 2012, 24, 3231–3239. [Google Scholar] [CrossRef]
- Bayat, A.; Sadrameli, S.M. Production of Renewable Aromatic Hydrocarbons via Conversion of Canola Oil Methyl Ester (CME) over Zinc Promoted HZSM-5 Catalysts. Renew. Energy 2017, 106, 62–67. [Google Scholar] [CrossRef]
- Ramos, R.; García, A.; Botas, J.A.; Serrano, D.P. Enhanced Production of Aromatic Hydrocarbons by Rapeseed Oil Conversion over Ga and Zn Modified ZSM-5 Catalysts. Ind. Eng. Chem. Res. 2016, 55, 12723–12732. [Google Scholar] [CrossRef]
- Dufresne, L.A.; Levanmao, R. Hydrogen Back-Spillover Effects in the Aromatization of Ethylene on Hybrid ZSM-5 Catalysts. Catal. Lett. 1994, 25, 371–383. [Google Scholar] [CrossRef]
- Pidko, E.A.; Hensen, E.J.M.; van Santen, R.A. Anionic Oligomerization of Ethylene over Ga/ZSM-5 Zeolite: A Theoretical Study. J. Phys. Chem. C 2008, 112, 19604–19611. [Google Scholar] [CrossRef]
- Shi, D.Z.; Wang, S.; Wang, H.; Wang, P.F.; Zhang, L.; Qin, Z.F.; Wang, J.G.; Zhu, H.Q.; Fan, W.B. Synthesis of HZSM-5 Rich in Paired Al and Its Catalytic Performance for Propane Aromatization. Catalysts 2020, 10, 622. [Google Scholar] [CrossRef]
- Mikhailov, M.N.; Mishin, I.V.; Kustov, L.M.; Lapidus, A.L. Structure and Reactivity of Pt/GaZSM-5 Aromatization Catalyst. Microporous Mesoporous Mat. 2007, 104, 145–150. [Google Scholar] [CrossRef]
- Samanta, A.; Bai, X.W.; Robinson, B.; Chen, H.; Hu, J.L. Conversion of Light Alkane to Value-Added Chemicals over ZSM-5/Metal Promoted Catalysts. Ind. Eng. Chem. Res. 2017, 56, 11006–11012. [Google Scholar] [CrossRef]
- Mehdad, A.; Lobo, R.F. Ethane and Ethylene Aromatization on Zinc-Containing Zeolites. Catal. Sci. Technol. 2017, 7, 3562–3572. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, W.Y.; Xiao, G.M. Atomic Layer Deposition of Zinc Oxide on HZSM-5 Template and Its Methanol Aromatization Performance. Catal. Lett. 2015, 145, 860–867. [Google Scholar] [CrossRef]
- Biscardi, J.A.; Iglesia, E. Structure and Function of Metal Cations in Light Alkane Reactions Catalyzed by Modified H-ZSM5. Catal. Today 1996, 31, 207–231. [Google Scholar] [CrossRef]
- She, L.Q.; Wang, D.C.; Li, X.W.; Liu, X.Y.; Han, M. The States of Zinc in ZnZSM-5 Zeolites and Their Catalysis. Acta Phys.-Chim. Sin. 1994, 10, 246. [Google Scholar]
- Wei, C.L.; Gao, J.; Wang, K.; Dong, M.; Fan, W.B.; Qin, Z.F.; Wang, J.G. Effect of Hydrogen Pre-Treatment on the Catalytic Properties of Zn/HZSM-5 Zeolite for Ethylene Aromatization Reaction. Acta Phys.-Chim. Sin. 2017, 33, 1483–1491. [Google Scholar] [CrossRef]
- Kolyagin, Y.; Ordomsky, V.; Khimyak, Y.; Rebrov, A.; Fajula, F.; Ivanova, I. Initial Stages of Propane Activation over Zn/MFI Catalyst Studied by in Situ NMR and Ir Spectroscopic Techniques. J. Catal. 2006, 238, 122–133. [Google Scholar] [CrossRef]
- Berndt, H.; Lietz, G.; Lucke, B.; Volter, J. Zinc Promoted H-ZSM-5 Catalysts for Conversion of Propane to Aromatics I. Acidity and Activity. Appl. Catal. A Gen. 1996, 146, 351–363, Erratum in Appl. Catal. A Gen. 1997, 155, 121–123. [Google Scholar] [CrossRef]
- Berndt, H.; Lietz, G.; Volter, J. Zinc Promoted H-ZSM-5 Catalysts for Conversion of Propane to Aromatics II. Nature of the Active Sites and Their Activation. Appl. Catal. A Gen. 1996, 146, 365–379. [Google Scholar] [CrossRef]
- Chen, X.C.; Dong, M.; Niu, X.J.; Wang, K.; Chen, G.; Fan, W.B.; Wang, J.G.; Qin, Z.F. Influence of Zn Species in HZSM-5 on Ethylene Aromatization. Chin. J. Catal. 2015, 36, 880–888. [Google Scholar] [CrossRef]
- Gong, T.; Qin, L.J.; Lu, J.; Feng, H. ZnO Modified ZSM-5 and Y Zeolites Fabricated by Atomic Layer Deposition for Propane Conversion. PCCP Phys. Chem. Chem. Phys. 2016, 18, 601–614. [Google Scholar] [CrossRef]
- Scurrell, M.S. Factors Affecting the Selectivity of the Aromatization of Light Alkanes on Modified ZSM-5 Catalysts. Appl. Catal. 1988, 41, 89–98. [Google Scholar] [CrossRef]
- Biscardi, J.A.; Meitzner, G.D.; Iglesia, E. Structure and Density of Active Zn Species in Zn/H-ZSM5 Propane Aromatization Catalysts. J. Catal. 1998, 179, 192–202. [Google Scholar] [CrossRef]
- Gao, D.; Zhi, Y.B.; Cao, L.Y.; Zhao, L.; Gao, J.; Xu, C.M.; Ma, M.Z.; Hao, P.F. Influence of Zinc State on the Catalyst Properties of Zn/HZSM-5 Zeolite in 1-Hexene Aromatization and Cyclohexane Dehydrogenation. Chin. J. Chem. Eng. 2022, 43, 124–134. [Google Scholar] [CrossRef]
- Niu, X.J.; Gao, J.; Miao, Q.; Dong, M.; Wang, G.F.; Fan, W.B.; Qin, Z.F.; Wang, J.G. Influence of Preparation Method on the Performance of Zn-Containing HZSM-5 Catalysts in Methanol-to-Aromatics. Microporous Mesoporous Mat. 2014, 197, 252–261. [Google Scholar] [CrossRef]
- Li, Y.N.; Liu, S.L.; Xie, S.J.; Xu, L.Y. Promoted Metal Utilization Capacity of Alkali-Treated Zeolite: Preparation of Zn/ZSM-5 and Its Application in 1-Hexene Aromatization. Appl. Catal. A Gen. 2009, 360, 8–16. [Google Scholar] [CrossRef]
- Smiesková, A.; Rojasová, E.; Hudec, P.; Sabo, L. Study of the Role of Zn in Aromatization of Light Alkanes with Probe Molecules. React. Kinet. Catal. Lett. 2004, 82, 227–234. [Google Scholar] [CrossRef]
- Gao, J.; Wei, C.L.; Dong, M.; Wang, G.F.; Li, Z.K.; Qin, Z.F.; Wang, J.G.; Fan, W.B. Evolution of Zn Species on Zn/HZSM-5 Catalyst under H2 Pretreated and its Effect on Ethylene Aromatization. ChemCatChem 2019, 11, 3892–3902. [Google Scholar] [CrossRef]
- Boiko, M.E.; Sharkov, M.D.; Boiko, A.M.; Konnikov, S.G.; Bobyl, A.V.; Budkina, N.S. Investigation of the Atomic, Crystal, and Domain Structures of Materials Based on X-ray Diffraction and Absorption Data: A Review. Tech. Phys. 2015, 60, 1575–1600. [Google Scholar] [CrossRef]
- Geng, R.; Liu, Y.; Gao, J.; Guo, Y.; Dong, M.; Wang, S.; Fan, W.; Wang, J.; Qin, Z. The Migration of Zn Species on Zn/ZSM-5 Catalyst during the Process of Ethylene Aromatization. Catal. Sci. Technol. 2022, 12, 4201–4210. [Google Scholar] [CrossRef]
- Geng, R.; Liu, Y.C.; Guo, Y.X.; Wang, P.F.; Dong, M.; Wang, S.; Wang, J.G.; Qin, Z.F.; Fan, W.B. Structure Evolution of Zn Species on Fresh, Deactivated, and Regenerated Zn/ZSM-5 Catalysts in Ethylene Aromatization. ACS Catal. 2022, 12, 14735–14747. [Google Scholar] [CrossRef]
- Bordiga, S.; Lamberti, C.; Ricchiardi, G.; Regli, L.; Bonino, F.; Damin, A.; Lillerud, K.P.; Bjorgen, M.; Zecchina, A. Electronic and Vibrational Properties of a MOF-5 Metal-Organic Framework: ZnO Quantum Dot Behaviour. Chem. Commun. 2004, 2300–2301. [Google Scholar] [CrossRef]
- Shen, X.Q.; Kang, J.C.; Niu, W.; Wang, M.H.; Zhang, Q.H.; Wang, Y. Impact of Hierarchical Pore Structure on the Catalytic Performances of MFI Zeolites Modified by ZnO for the Conversion of Methanol to Aromatics. Catal. Sci. Technol. 2017, 7, 3598–3612. [Google Scholar] [CrossRef]
- Wark, M.; Kessler, H.; SchulzEkloff, G. Growth and Reactivity of Zinc and Cadmium Oxide Nano-Particles in Zeolites. Microporous Mater. 1997, 8, 241–253. [Google Scholar] [CrossRef]
- Pan, T.; Wu, Z.J.; Zhou, K.Y. In Situ Incorporation of Zn into Hierarchical ZSM-5 Zeolites for Olefin Hydroisomerization. Ind. Eng. Chem. Res. 2020, 59, 12371–12380. [Google Scholar] [CrossRef]
- Shang, X.; Zhuo, H.Y.; Han, Q.; Yang, X.F.; Hou, G.J.; Liu, G.D.; Su, X.; Huang, Y.Q.; Zhang, T. Xylene Synthesis through Tandem CO2 Hydrogenation and Toluene Methylation over a Composite ZnZrO Zeolite Catalyst. Angew. Chem. Int. Edit. 2023, 62, e202309377. [Google Scholar] [CrossRef] [PubMed]
- Rittner, F.; Seidel, A.; Boddenberg, B. Zeolites as Matrices for the Stabilization of Unusual Cationic Zinc Species. Microporous Mesoporous Mat. 1998, 24, 127–131. [Google Scholar] [CrossRef]
- Li, L.; Li, G.D.; Yan, C.; Mu, X.Y.; Pan, X.L.; Zou, X.X.; Wang, K.X.; Chen, J.S. Efficient Sunlight-Driven Dehydrogenative Coupling of Methane to Ethane over a Zn+-Modified Zeolite. Angew. Chem. Int. Edit. 2011, 50, 8299–8303. [Google Scholar] [CrossRef]
- Luo, Y.J.; Miao, C.X.; Yue, Y.H.; Hua, W.M.; Gao, Z. ZnO Supported on Silicalite-1 as an Efficient Catalyst for Isobutane Dehydrogenation to Isobutene Assisted by CO2. Microporous Mesoporous Mat. 2020, 294, 109864. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, J.; Yang, L.Q.; Wang, Q.F. The Dehydration of Hemimorphite. Acta Petrol. Sin. 2005, 21, 993–998. [Google Scholar]
- Pinilla-Herrero, I.; Borfecchia, E.; Holzinger, J.; Mentzel, U.V.; Joensen, F.; Lomachenko, K.A.; Bordiga, S.; Lamberti, C.; Berlier, G.; Olsbye, U.; et al. High Zn/Al Ratios Enhance Dehydrogenation vs Hydrogen Transfer Reactions of Zn-ZSM-5 Catalytic Systems in Methanol Conversion to Aromatics. J. Catal. 2018, 362, 146–163. [Google Scholar] [CrossRef]
- Yin, D.H.; Yin, D.L. Study on the Solid-State Reaction of ZnCl2 and Y Zeolites under Microwave Irradiation. Acta Phys. Chim. Sin. 1998, 14, 448–452. [Google Scholar] [CrossRef]
- Xie, Y.C.; Wang, C.B.; Tang, Y.Q. Solid States Ion-Exchange between Halides and Zeolites. Acta Chim. Sin. 1993, 51, 774–779. [Google Scholar]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data Analysis for X-ray Absorption Spectroscopy Using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Lengke, M.F.; Ravel, B.; Fleet, M.E.; Wanger, G.; Gordon, R.A.; Southam, G. Mechanisms of Gold Bioaccumulation by Filamentous Cyanobacteria from gold(III)—Chloride Complex. Environ. Sci. Technol. 2006, 40, 6304–6309. [Google Scholar] [CrossRef]
- Ressler, T.; Wong, J.; Roos, J.; Smith, I.L. Quantitative Speciation of Mn-Bearing Particulates Emitted from Autos Burning (Methylcyclopentadienyl)Manganese Tricarbonyl-Added Gasolines Using XANES Spectroscopy. Environ. Sci. Technol. 2000, 34, 950–958. [Google Scholar] [CrossRef]
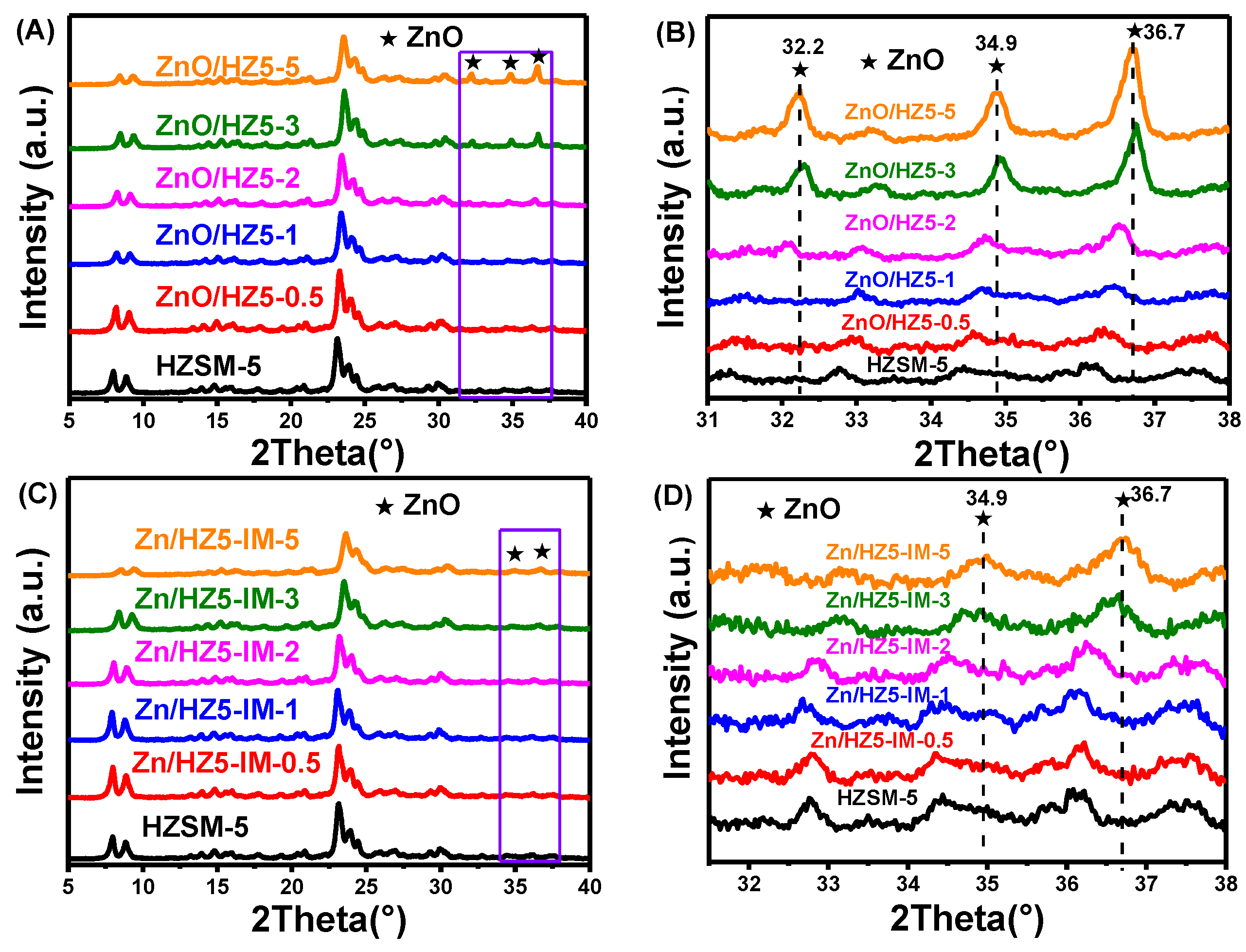
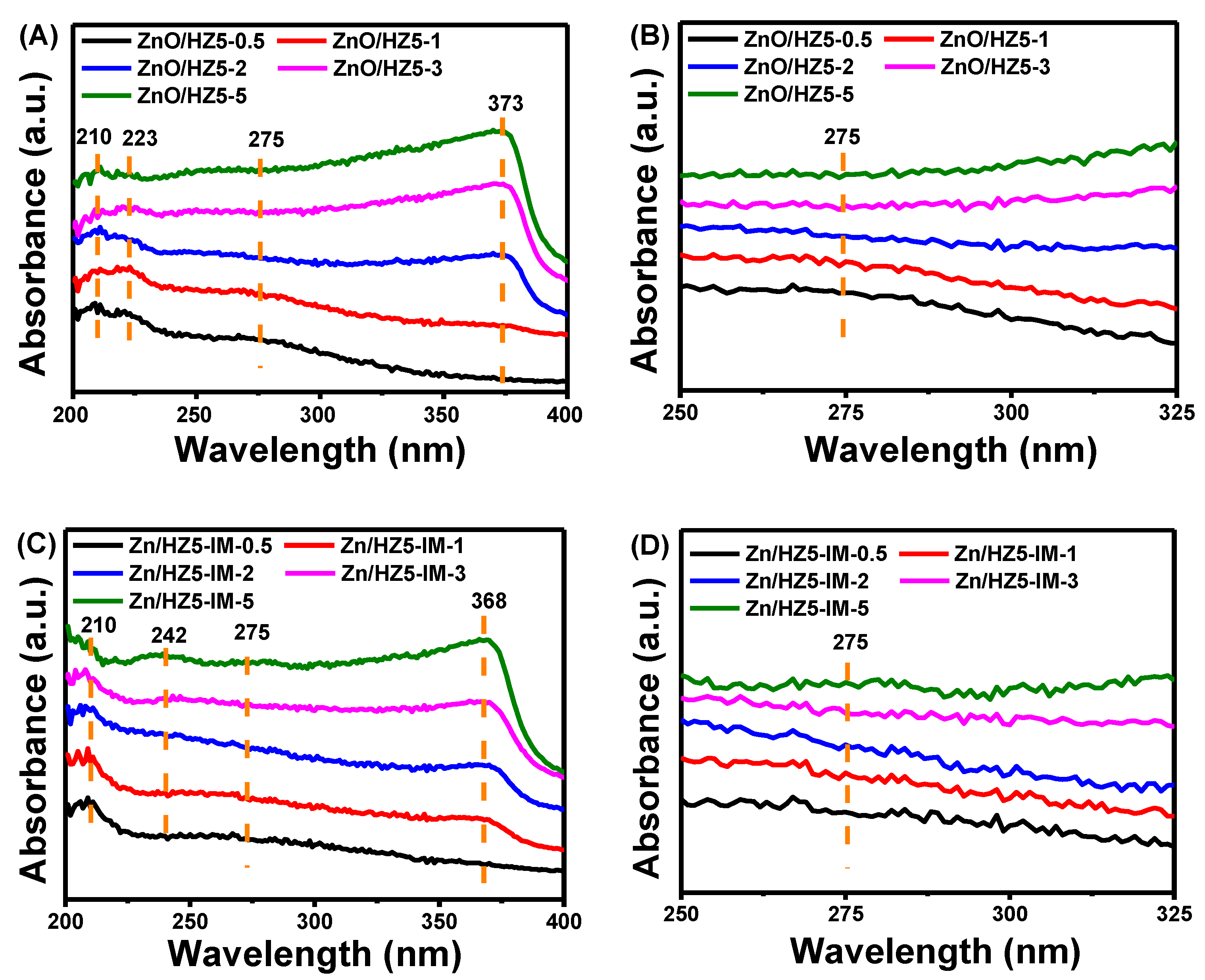
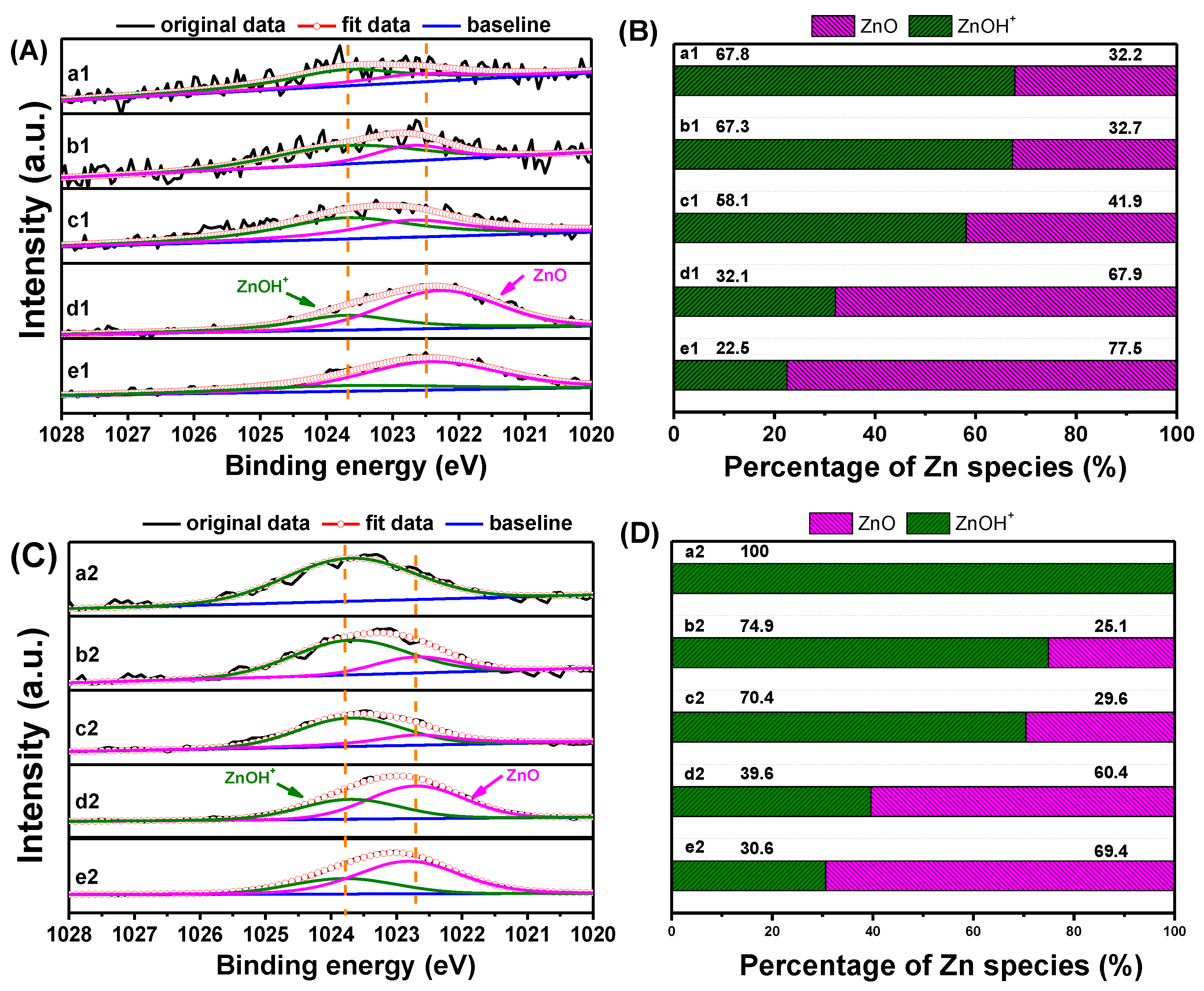
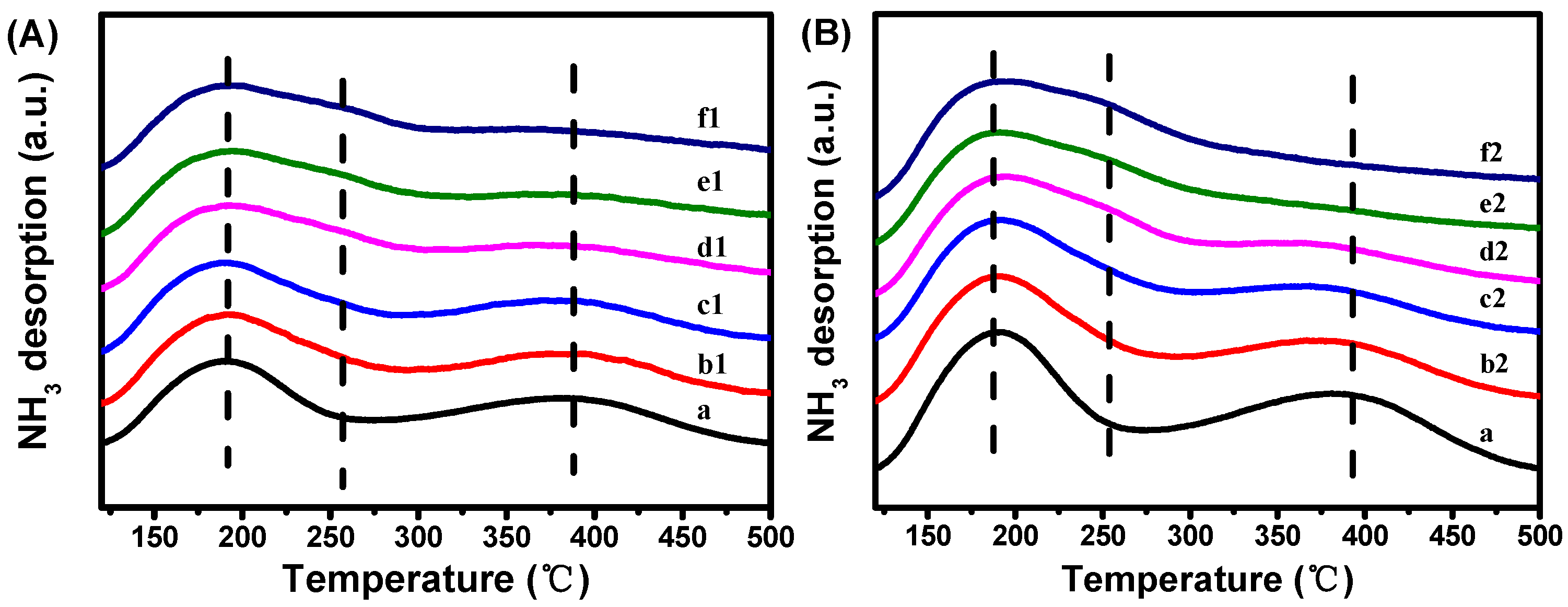
| Samples | Crystallinity a (%) | Zn Content b/ (wt.%) | Asurface/(m2·g−1) | Pore Volume/(cm3·g−1) | |||
|---|---|---|---|---|---|---|---|
| Total | External | Total | Micro | Meso | |||
| HZ5 | 100 | - | 373 | 113 | 0.36 | 0.10 | 0.26 |
| ZnO/HZ5−0.5 | 97.97 | 0.52 | 333 | 116 | 0.36 | 0.11 | 0.25 |
| ZnO/HZ5−1 | 96.78 | 0.81 | 319 | 109 | 0.33 | 0.10 | 0.23 |
| ZnO/HZ5−2 | 97.06 | 2.07 | 308 | 99 | 0.33 | 0.10 | 0.23 |
| ZnO/HZ5−3 | 89.64 | 3.77 | 304 | 101 | 0.32 | 0.09 | 0.23 |
| ZnO/HZ5−5 | 80.08 | 5.49 | 297 | 100 | 0.33 | 0.09 | 0.24 |
| Zn/HZ5-IM−0.5 | 98.23 | 0.56 | 350 | 108 | 0.33 | 0.10 | 0.23 |
| Zn/HZ5-IM−1 | 94.98 | 1.04 | 344 | 105 | 0.33 | 0.10 | 0.23 |
| Zn/HZ5-IM−2 | 95.39 | 1.86 | 338 | 100 | 0.34 | 0.10 | 0.24 |
| Zn/HZ5-IM−3 | 89.01 | 3.10 | 341 | 100 | 0.32 | 0.10 | 0.22 |
| Zn/HZ5-IM−5 | 84.61 | 4.99 | 306 | 90 | 0.30 | 0.09 | 0.21 |
| Samples | Acidity Density (mmol·g−1) | |||
|---|---|---|---|---|
| Weak | Medium | Strong | Total | |
| HZ5 | 0.39 | - | 0.38 | 0.77 |
| ZnO/HZ5−0.5 | 0.41 | - | 0.21 | 0.62 |
| ZnO/HZ5−1 | 0.44 | - | 0.20 | 0.64 |
| ZnO/HZ5−2 | 0.33 | 0.08 | 0.12 | 0.59 |
| ZnO/HZ5−3 | 0.24 | 0.22 | 0.11 | 0.57 |
| ZnO/HZ5−5 | 0.19 | 0.26 | 0.12 | 0.57 |
| Zn/HZ5-IM−0.5 | 0.44 | - | 0.24 | 0.68 |
| Zn/HZ5-IM−1 | 0.43 | - | 0.21 | 0.64 |
| Zn/HZ5-IM−2 | 0.19 | 0.25 | 0.20 | 0.64 |
| Zn/HZ5-IM−3 | 0.18 | 0.24 | 0.16 | 0.58 |
| Zn/HZ5-IM−5 | 0.14 | 0.20 | 0.13 | 0.47 |
| Samples | Description | E0 (eV) | Zn K-Edge EXAFS Fit Parameters a | |||||
|---|---|---|---|---|---|---|---|---|
| Contribution | CN | R (Å) | S02 | σ2 | R-Factor | |||
| ZnO | Large-grain ZnO | 9661.7 | Zn−O | 4 | 1.97 | 0.91 | 0.005 | 0.001 |
| Zn/HZ5-IE−0.66% | ZnOH+ | 9664.1 | Zn−O | 5.74 | 2.07 | 0.91 | 0.010 | 0.005 |
| Zn/silicalite-1−0.1% | ZnO cluster | 9663.7 | Zn−O | 3.77 | 2.02 | 0.91 | 0.007 | 0.010 |
| Zn/silicalite-1−5% | ZnO crystalline | 9662.1 | Zn−O | 3.90 | 1.97 | 0.91 | 0.007 | 0.005 |
| Willemite | Zn-O-Si structure | 9662.5 | Zn−O | 3.52 | 1.95 | 0.91 | 0.004 | 0.020 |
| Samples | E0 (eV) | Zn K-Edge EXAFS Fit Parameters a | |||||
|---|---|---|---|---|---|---|---|
| Contribution | CN | R (Å) | S02 | σ2 | R-Factor | ||
| ZnO | 9661.7 | Zn−O | 4.0 | 1.97 | 0.91 | 0.005 | 0.001 |
| ZnO/HZ5−0.5 | 9662.6 | Zn−O | 4.9 | 2.02 | 0.91 | 0.008 | 0.011 |
| ZnO/HZ5−1.0 | 9661.9 | Zn−O | 4.3 | 1.98 | 0.91 | 0.006 | 0.013 |
| ZnO/HZ5−2.0 | 9661.7 | Zn−O | 4.1 | 2.01 | 0.91 | 0.007 | 0.016 |
| ZnO/HZ5−3.0 | 9661.4 | Zn−O | 3.8 | 1.98 | 0.91 | 0.004 | 0.015 |
| ZnO/HZ5−5.0 | 9661.2 | Zn−O | 3.8 | 1.97 | 0.91 | 0.004 | 0.012 |
| Zn/HZ5-IM−0.5 | 9664.0 | Zn−O | 6.2 | 2.08 | 0.91 | 0.009 | 0.005 |
| Zn/HZ5-IM−1.0 | 9663.5 | Zn−O | 5.8 | 2.06 | 0.91 | 0.010 | 0.004 |
| Zn/HZ5-IM−2.0 | 9663.2 | Zn−O | 4.4 | 2.05 | 0.91 | 0.009 | 0.010 |
| Zn/HZ5-IM−3.0 | 9662.9 | Zn−O | 4.6 | 2.03 | 0.91 | 0.010 | 0.008 |
| Zn/HZ5-IM−5.0 | 9661.1 | Zn−O | 3.9 | 1.98 | 0.91 | 0.005 | 0.005 |
| Sample | C2H4 Conv. (%) | Product Selectivity (C Molar %) | H2 Selectivity (Molar %) | ||||
|---|---|---|---|---|---|---|---|
| CH4 | C20-C40 | C3=-C4= | C5 and C5+ | Aromatics | |||
| ZnO/HZ5−0.5 | 97.7 | 5.5 | 25.3 | 3.3 | 4.0 | 61.9 | 41.6 |
| ZnO/HZ5−1 | 98.3 | 6.3 | 23.0 | 2.8 | 4.1 | 63.8 | 39.0 |
| ZnO/HZ5−2 | 99.6 | 6.0 | 28.7 | 1.3 | 3.5 | 60.6 | 25.5 |
| ZnO/HZ5−3 | 98.4 | 5.9 | 29.6 | 3.3 | 2.5 | 58.7 | 23.2 |
| ZnO/HZ5−5 | 98.0 | 7.4 | 33.6 | 0.8 | 1.0 | 57.2 | 21.5 |
| Zn/HZ5-IM−0.5 | 98.1 | 5.0 | 23.9 | 3.7 | 2.1 | 65.4 | 42.2 |
| Zn/HZ5-IM−1 | 98.7 | 6.5 | 26.0 | 2.3 | 1.8 | 63.4 | 35.2 |
| Zn/HZ5-IM−2 | 98.2 | 5.7 | 25.5 | 3.5 | 3.1 | 62.2 | 28.6 |
| Zn/HZ5-IM−3 | 98.4 | 5.0 | 30.6 | 3.3 | 1.8 | 59.3 | 26.8 |
| Zn/HZ5-IM−5 | 98.8 | 4.8 | 32.3 | 2.8 | 1.8 | 58.3 | 24.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Gao, J.; Shao, J.; Geng, R.; Qin, Z.; Wang, J.; Fan, W.; Dong, M. A Fine Analysis of Zn Species Structure and Distribution in Zn/ZSM-5 Catalysts by Linear Combination Fitting Analysis of XANES Spectra. Molecules 2024, 29, 631. https://doi.org/10.3390/molecules29030631
Li B, Gao J, Shao J, Geng R, Qin Z, Wang J, Fan W, Dong M. A Fine Analysis of Zn Species Structure and Distribution in Zn/ZSM-5 Catalysts by Linear Combination Fitting Analysis of XANES Spectra. Molecules. 2024; 29(3):631. https://doi.org/10.3390/molecules29030631
Chicago/Turabian StyleLi, Baichao, Jie Gao, Jiabei Shao, Rui Geng, Zhangfeng Qin, Jianguo Wang, Weibin Fan, and Mei Dong. 2024. "A Fine Analysis of Zn Species Structure and Distribution in Zn/ZSM-5 Catalysts by Linear Combination Fitting Analysis of XANES Spectra" Molecules 29, no. 3: 631. https://doi.org/10.3390/molecules29030631
APA StyleLi, B., Gao, J., Shao, J., Geng, R., Qin, Z., Wang, J., Fan, W., & Dong, M. (2024). A Fine Analysis of Zn Species Structure and Distribution in Zn/ZSM-5 Catalysts by Linear Combination Fitting Analysis of XANES Spectra. Molecules, 29(3), 631. https://doi.org/10.3390/molecules29030631







