Preparation, Characterization, and Antioxidant Activities of Extracts from Amygdalus persica L. Flowers
Abstract
1. Introduction
2. Result and Discussion
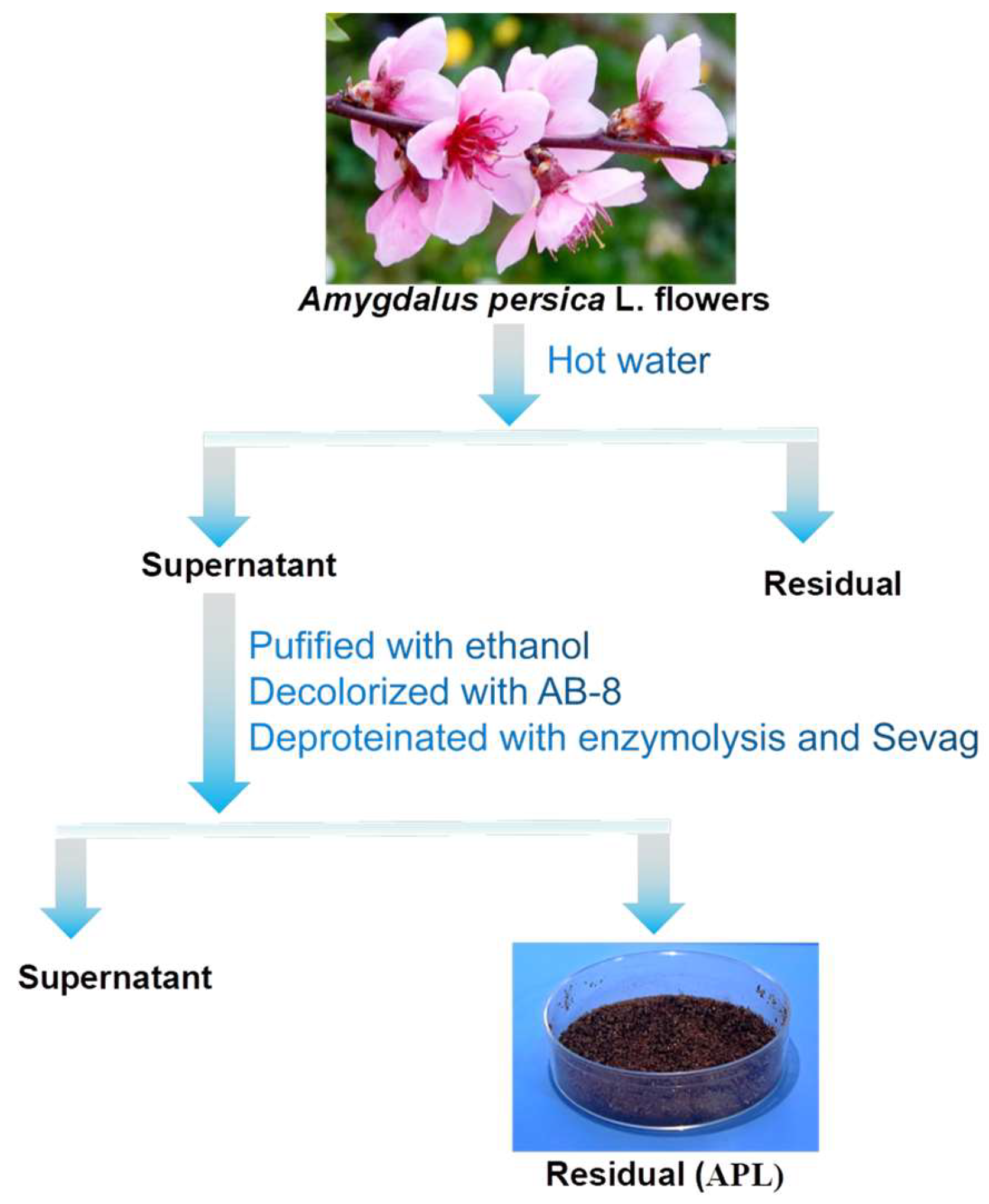
2.1. Isolation and Purification of Polysaccharide
2.2. Characterization of APL
2.3. In Vitro Antioxidant Activity Analysis
2.3.1. Scavenging Activity of DPPH Radicals
2.3.2. Scavenging Activity of ABTS Radicals
2.3.3. Hydroxyl Radical Scavenging Activity
2.3.4. Superoxide Radical Scavenging Activity
2.3.5. Reducing Power
2.4. Validation of Antioxidant Activity In Vivo
2.4.1. Effect of APL on Fluorescence Intensity of ROS in Zebrafish
2.4.2. Effect of APL on Tyrosinase Activity and Melanin Distribution
3. Materials and Methods
3.1. Materials
3.2. Exaction and Purification of Polysaccharides
3.3. Analysis of Monosaccharide Components
3.4. Determination of Molecular Weight Distribution
3.5. FT-IR Spectroscopic Analysis
3.6. In Vitro Assays of Antioxidant Activity
3.6.1. DPPH Radical Scavenging Activity
3.6.2. ABTS Radical Scavenging Activity
3.6.3. Hydroxyl Radical Scavenging Activity
3.6.4. Superoxide Radical Scavenging Activity
3.6.5. Reducing Power
3.7. In Vivo Assays of Antioxidant Activity
3.7.1. Zebrafish Culture
3.7.2. Assay of Antioxidant Effect in Zebrafish
3.7.3. Assay of Depigmentation Effect and Tyrosinase Activity in Zebrafish
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Griendling, K.K.; Camargo, L.L.; Rios, F.J.; Alves-Lopes, R.; Montezano, A.C.; Touyz, R.M. Oxidative Stress and Hypertension. Circ. Res. 2021, 128, 993–1020. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Vatner, S.F.; Zhang, J.; Oydanich, M.; Berkman, T.; Naftalovich, R.; Vatner, D.E. Healthful aging mediated by inhibition of oxidative stress. Ageing Res. Rev. 2020, 64, 101194. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fan, W.; Li, X.; Wang, W.-X.; Liu, S. Enhanced Removal of Free Radicals by Aqueous Hydrogen Nanobubbles and Their Role in Oxidative Stress. Environ. Sci. Technol. 2022, 56, 15096–15107. [Google Scholar] [CrossRef]
- Ienco, E.C.; LoGerfo, A.; Carlesi, C.; Orsucci, D.; Ricci, G.; Mancuso, M.; Siciliano, G. Oxidative stress treatment for clinical trials in neurodegenerative diseases. J. Alzheimer’s Dis. 2011, 24, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Cross, C.E.; Halliwell, B.; Borish, E.T.; Pryor, W.A.; Ames, B.N.; Saul, R.L.; Mccord, J.M.; Harman, D. Oxygen radicals and human disease. Ann. Intern. Med. 1987, 107, 526–545. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Bosco, D.A.; Fowler, D.M.; Zhang, Q.; Nieva, J.; Powers, E.T.; Wentworth, P.; Lerner, R.A.; Kelly, J.W. Elevated levels of oxidized cholesterol metabolites in Lewy body disease brains accelerate α-synuclein fibrilization. Nat. Chem. Biol. 2006, 2, 249–253. [Google Scholar] [CrossRef]
- Nakabeppu, Y.; Tsuchimoto, D.; Yamaguchi, H.; Sakumi, K. Oxidative damage in nucleic acids and Parkinson’s disease. J. Neurosci. Res. 2007, 85, 919–934. [Google Scholar] [CrossRef]
- Figtree, G.A.; Karimi, G.K.; Liu, C.-C.; Rasmussen, H.H. Oxidative regulation of the Na+–K+ pump in the cardiovascular system. Free Radic. Biol. Med. 2012, 53, 2263–2268. [Google Scholar] [CrossRef]
- Rasmussen, H.H.; Hamilton, E.J.; Liu, C.-C.; Figtree, G.A. Reversible oxidative modification: Implications for cardiovascular physiology and pathophysiology. Trends Cardiovasc. Med. 2010, 20, 85–90. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Colombo, G.; Gagliano, N.; Colombo, R.; Giustarini, D.; Rossi, R.; Milzani, A. S-Glutathiolation in life and death decisions of the cell. Free Radic. Res. 2011, 45, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Weisbrod, R.M.; Pimentel, D.R.; Ying, J.; Sharov, V.S.; Schöneich, C.; Cohen, R.A. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat. Med. 2004, 10, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Yu, X.; Maninder, M.; Xu, B. Total phenolics and antioxidants profiles of commonly consumed edible flowers in China. Int. J. Food Prop. 2018, 21, 1524–1540. [Google Scholar] [CrossRef]
- Liu, H.-M.; Tang, W.; Lei, S.-N.; Zhang, Y.; Cheng, M.-Y.; Liu, Q.-L.; Wang, W. Extraction Optimization, Characterization and Biological Activities of Polysaccharide Extracts from Nymphaea hybrid. Int. J. Mol. Sci. 2023, 24, 8974. [Google Scholar] [CrossRef] [PubMed]
- Ślusarczyk, S.; Hajnos, M.; Skalicka-Woźniak, K.; Matkowski, A. Antioxidant activity of polyphenols from Lycopus lucidus Turcz. Food Chem. 2009, 113, 134–138. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, X.; Yan, X.-H.; Zhang, J.-L.; Wang, L.-Y.; Xue, H.; Jiang, G.-C.; Ma, X.-T.; Liu, X.-J. Characterization, hypolipidemic and antioxidant activities of degraded polysaccharides from Ganoderma lucidum. Int. J. Biol. Macromol. 2019, 135, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Jiao, R.; Liu, Y.; Gao, H.; Xiao, J.; So, K.F. The Anti-Oxidant and Antitumor Properties of Plant Polysaccharides. Am. J. Chin. Med. 2016, 44, 463–488. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E. Edible flowers: A review of the nutritional, antioxidant, antimicrobial properties and effects on human health. J. Food Compos. Anal. 2017, 60, 38–50. [Google Scholar] [CrossRef]
- Prabawati, N.B.; Oktavirina, V.; Palma, M.; Setyaningsih, W. Edible Flowers: Antioxidant Compounds and Their Functional Properties. Horticulturae 2021, 7, 66. [Google Scholar] [CrossRef]
- Chen, G.-L.; Chen, S.-G.; Xiao, Y.; Fu, N.-L. Antioxidant capacities and total phenolic contents of 30 flowers. Ind. Crop. Prod. 2018, 111, 430–445. [Google Scholar] [CrossRef]
- Fakhri, S.; Tomas, M.; Capanoglu, E.; Hussain, Y.; Abbaszadeh, F.; Lu, B.; Hu, X.; Wu, J.; Zou, L.; Smeriglio, A.; et al. Antioxidant and anticancer potentials of edible flowers: Where do we stand? Crit. Rev. Food Sci. Nutr. 2022, 62, 8589–8645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yin, Z.; Chen, L.; Yang, B.; Zhang, W.; Kang, W. Chemical Constituents and Coagulation Activity of Amygdalus persica L. Flowers. J. Food Qual. 2021, 2021, 9914508. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.; Yin, Z.; Guo, Q.; Yang, B.; Feng, M.; Li, X.; Chen, L.; Zhang, W.; Kang, W. The Content Variation of Four Active Components in Amygdalus persica L. during Different Harvesting Periods. J. Food Qual. 2022, 2022, 1017674. [Google Scholar] [CrossRef]
- Huang, G.; Chen, F.; Yang, W.; Huang, H. Preparation, deproteinization and comparison of bioactive polysaccharides. Trends Food Sci. Technol. 2021, 109, 564–568. [Google Scholar] [CrossRef]
- Cheng, H.; Huang, G. Extraction, characterisation and antioxidant activity of Allium sativum polysaccharide. Int. J. Biol. Macromol. 2018, 114, 415–419. [Google Scholar] [CrossRef]
- Chen, F.; Huang, G.; Yang, Z.; Hou, Y. Antioxidant activity of Momordica charantia polysaccharide and its derivatives. Int. J. Biol. Macromol. 2019, 138, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Shi, L. Bioactivities, isolation and purification methods of polysaccharides from natural products: A review. Int. J. Biol. Macromol. 2016, 92, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Coelho, E.; Rocha, M.A.M.; Saraiva, J.A.; Coimbra, M.A. Microwave superheated water and dilute alkali extraction of brewers’ spent grain arabinoxylans and arabinoxylo-oligosaccharides. Carbohydr. Polym. 2014, 99, 415–422. [Google Scholar] [CrossRef]
- Song, Y.-R.; Han, A.-R.; Park, S.-G.; Cho, C.-W.; Rhee, Y.-K.; Hong, H.-D. Effect of enzyme-assisted extraction on the physicochemical properties and bioactive potential of lotus leaf polysaccharides. Int. J. Biol. Macromol. 2020, 153, 169–179. [Google Scholar] [CrossRef]
- Hashemifesharaki, R.; Xanthakis, E.; Altintas, Z.; Guo, Y.; Gharibzahedi, S.M.T. Microwave-assisted extraction of polysaccharides from the marshmallow roots: Optimization, purification, structure, and bioactivity. Carbohydr. Polym. 2020, 240, 116301. [Google Scholar] [CrossRef] [PubMed]
- Sorourian, R.; Khajehrahimi, A.E.; Tadayoni, M.; Azizi, M.H.; Hojjati, M. Ultrasound-assisted extraction of polysaccharides from Typha domingensis: Structural characterization and functional properties. Int. J. Biol. Macromol. 2020, 160, 758–768. [Google Scholar] [CrossRef]
- Liew, S.Q.; Teoh, W.H.; Tan, C.K.; Yusoff, R.; Ngoh, G.C. Subcritical water extraction of low methoxyl pectin from pomelo (Citrus grandis (L.) Osbeck) peels. Int. J. Biol. Macromol. 2018, 116, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Liu, D.; Yin, J.-Y.; Nie, S.-P. Consecutive and progressive purification of food-derived natural polysaccharide: Based on material, extraction process and crude polysaccharide. Trends Food Sci. Technol. 2020, 99, 76–87. [Google Scholar] [CrossRef]
- Tao, L.; Zhang, J.; Lan, W.; Yu, L.; Bi, Y.; Song, S.; Xiong, B.; Wang, H. Polysaccharide decolorization: Methods, principles of action, structural and functional characterization, and limitations of current research. Trends Food Sci. Technol. 2023, 138, 284–296. [Google Scholar] [CrossRef]
- Zheng, M.; Ma, M.; Yang, Y.; Liu, Z.; Liu, S.; Hong, T.; Ni, H.; Jiang, Z. Structural characterization and antioxidant activity of polysaccharides extracted from Porphyra haitanensis by different methods. Int. J. Biol. Macromol. 2023, 242, 125003. [Google Scholar] [CrossRef]
- Mei, X.; Yang, W.; Huang, G.; Huang, H. The antioxidant activities of balsam pear polysaccharide. Int. J. Biol. Macromol. 2020, 142, 232–236. [Google Scholar] [CrossRef]
- Xu, D.; Wang, C.; Zhuo, Z.; Ye, M.; Pu, B. Extraction, purification and antioxidant activity of polysaccharide from cold pressed oil cake of ‘Tengjiao’ seed. Int. J. Biol. Macromol. 2020, 163, 508–518. [Google Scholar] [CrossRef]
- Ma, J.-S.; Liu, H.; Han, C.-R.; Zeng, S.-J.; Xu, X.-J.; Lu, D.-J.; He, H.-J. Extraction, characterization and antioxidant activity of polysaccharide from Pouteria campechiana seed. Carbohydr. Polym. 2020, 229, 115409. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Pu, Q.; Qiu, H.; Di, D. Polysaccharides isolated from Lycium barbarum L. by integrated tandem hybrid membrane technology exert antioxidant activities in mitochondria. Ind. Crop. Prod. 2021, 168, 113547. [Google Scholar] [CrossRef]
- Wang, X.; Gao, A.; Jiao, Y.; Zhao, Y.; Yang, X. Antitumor effect and molecular mechanism of antioxidant polysaccharides from Salvia miltiorrhiza Bunge in human colorectal carcinoma LoVo cells. Int. J. Biol. Macromol. 2018, 108, 625–634. [Google Scholar] [CrossRef] [PubMed]
- El-Kenawi, A.; Ruffell, B. Inflammation, ROS, and Mutagenesis. Cancer Cell 2017, 32, 727–729. [Google Scholar] [CrossRef]
- Noworyta-Sokołowska, K.; Górska, A.; Gołembiowska, K. LPS-induced oxidative stress and inflammatory reaction in the rat striatum. Pharmacol. Rep. 2013, 65, 863–869. [Google Scholar] [CrossRef]
- Casey, M.J.; Stewart, R.A. Pediatric Cancer Models in Zebrafish. Trends Cancer 2020, 6, 407–418. [Google Scholar] [CrossRef]
- Fan, M.; Zhang, G.; Hu, X.; Xu, X.; Gong, D. Quercetin as a tyrosinase inhibitor: Inhibitory activity, conformational change and mechanism. Food Res. Int. 2017, 100, 226–233. [Google Scholar] [CrossRef]
- Chang, T.-S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Li, L.; Hu, S.-Q. Molecular inhibitory mechanism of tricin on tyrosinase. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 107, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Jee, J.-G. Repositioning of Thiourea-Containing Drugs as Tyrosinase Inhibitors. Int. J. Mol. Sci. 2015, 16, 28534–28548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-H.; Wu, J.; Weng, L.; Zhang, H.; Zhang, J.; Wu, A. An improved phenol-sulfuric acid method for the determination of carbohydrates in the presence of persulfate. Carbohydr. Polym. 2020, 227, 115332. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, J.; Li, X.; Zhu, X.; Zhang, Z.; Li, D. Study on the structure, antioxidant activity and degradation pattern of polysaccharides isolated from lotus seedpod. Carbohydr. Polym. 2023, 316, 121065. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Zhang, H.; Yao, H.; Zhou, J.; Duan, Y.; Ma, H. Comparison of characterization, antioxidant and immunological activities of three polysaccharides from Sagittaria sagittifolia L. Carbohydr. Polym. 2020, 235, 115939. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Castiblanco, T.; Mejía-Giraldo, J.C.; Puertas-Mejía, M. Lentinula edodes, a Novel Source of Polysaccharides with Antioxidant Power. Antioxidants 2022, 11, 1770. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Ma, X.; Liu, X.; Yang, M.; Fan, W.; Ren, H.; Efehi, N.; Wang, X.; Zhu, X. Extraction, purification, characterization and antioxidant activities of polysaccharides from Zizyphus jujuba cv. Linzexiaozao. Int. J. Biol. Macromol. 2018, 118, 2138–2148. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, J.; Wu, L.; Ma, T. Effects of 4-epianhydrotetracycline on oxidative stress in zebrafish (Danio rerio) embryos. Sci. Total Environ. 2021, 796, 149047. [Google Scholar] [CrossRef] [PubMed]





| Items | Amount |
|---|---|
| Rhamnose (molar ratio) | 0.17 |
| Arabinose (molar ratio) | 0.034 |
| Mannose (molar ratio) | 1.0 |
| Glucose (molar ratio) | 0.17 |
| Weight-average molecular weight (Mw, kDa) | 208.53 (56.2%) |
| Weight-average molecular weight (Mw, kDa) | 15.19 (33.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Q.; Li, W.; Liang, M.; Li, G.; Wu, Z.; Long, J.; Yuan, C.; Mei, W.; Xia, X. Preparation, Characterization, and Antioxidant Activities of Extracts from Amygdalus persica L. Flowers. Molecules 2024, 29, 633. https://doi.org/10.3390/molecules29030633
Yu Q, Li W, Liang M, Li G, Wu Z, Long J, Yuan C, Mei W, Xia X. Preparation, Characterization, and Antioxidant Activities of Extracts from Amygdalus persica L. Flowers. Molecules. 2024; 29(3):633. https://doi.org/10.3390/molecules29030633
Chicago/Turabian StyleYu, Qingtao, Wenzhi Li, Ming Liang, Guohu Li, Zhuoyan Wu, Jieyi Long, Chanling Yuan, Wenjie Mei, and Xiaole Xia. 2024. "Preparation, Characterization, and Antioxidant Activities of Extracts from Amygdalus persica L. Flowers" Molecules 29, no. 3: 633. https://doi.org/10.3390/molecules29030633
APA StyleYu, Q., Li, W., Liang, M., Li, G., Wu, Z., Long, J., Yuan, C., Mei, W., & Xia, X. (2024). Preparation, Characterization, and Antioxidant Activities of Extracts from Amygdalus persica L. Flowers. Molecules, 29(3), 633. https://doi.org/10.3390/molecules29030633





