Light-Responsive and Dual-Targeting Liposomes: From Mechanisms to Targeting Strategies
Abstract
:1. Introduction
1.1. Liposomes as Drug Nanocarriers
1.2. Targeting Mechanisms of Liposomes
1.2.1. Passive Targeting of Liposomes
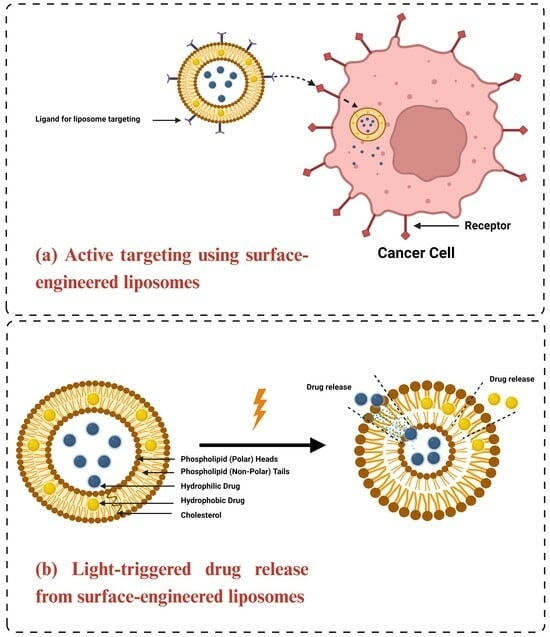
1.2.2. Active Targeting of Liposomes
1.3. Functionalized Liposomes
1.3.1. Long-Circulating PEGylated Liposomes
1.3.2. Ligand-Functionalized Liposomes
1.3.3. Stimuli-Responsive Liposomes
2. Mechanisms of Light-Triggered Drug Release from Liposomes
2.1. Photoisomerization
2.2. Photocleavage (Photo-Oxidation)
2.3. Surface Plasmon Resonance Absorption (Photothermal Activation)
2.4. Photochemical Hydrophobicity Change (Photochemical Activation)
2.5. Photo-Crosslinking and De-Crosslinking
3. Mechanisms of NIR Light-Triggered Drug Release
3.1. Photothermal Effect
3.2. Two-Photon Absorption (TPA)
3.3. Up-Converting Nanoparticles (UCNPs)
4. Strategies for Light-Targeting Drug Delivery
4.1. Light-Targeting through the Activation of Targeting Ligands
4.2. Light-Targeting through Particle Size Reduction
4.3. Light-Targeting through Blood Vessel Disruption
5. Light-Responsive Liposomes for Drug Delivery
5.1. Formulation, Design, and Optimization
5.1.1. Liposomal Size
5.1.2. Surface Charge
5.2. Light Source Selection
5.2.1. Light Penetration Depth
5.2.2. Photodamage
6. Dual-Targeting Stimuli-Triggered Liposomes
6.1. Light/pH Dual-Responsive Liposomes
6.2. Light/Temperature Dual-Responsive Liposomes
7. Challenges in Light-Triggered Drug Release from Liposomes
8. Emerging Trends and Future Prospects
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| ABC | Accelerated blood clearance |
| ALA | 5-aminolevulinic acid |
| Azo SM | N-[(E)-4-(4-((4-butylphenyl)diazenyl)phenyl)butanoyl]-D-erythro-sphingosylphosphorylcholine |
| BHA | 4-butylazobenzene-4-hexyloxy-trimethyl-ammoniumtrifluoro-acetate |
| BHA-cur-lipo | BHA-curcumin-liposomes |
| Bhc | 6-bromo-7-hydroxy-4-hydroxycoumarin |
| BTSL | Bubble-generating thermosensitive liposomes |
| CHEMS | Cholesteryl hemisuccinate |
| CJM-Chol | CJM126 mixed with cholesterol derivative |
| CMA | 7-(2-methacryloyloxyethoxy)-4-methylcoumarin |
| CMC value | Critical micelle concertation value |
| P(MEOMA-co-CMA) | Coumarin-containing poly(2-(2-methoxyethoxy)ethyl methacrylate) |
| CPPs | Cell-penetrating peptides |
| CPT | Chemophototherapy |
| CTX | Cabazitaxel |
| CTX-PoP-Lip | Cabazitaxel-loaded porphyrin-phospholipid liposomes |
| CuAAC | Copper-catalyzed azide alkyne cyclo-addition |
| DNQ | 2-diazo-1,2-naphthoquinone |
| DMPC | 1,2-dimyristoyl-sn-glycero-3-phosphocholine |
| DMPG-Na | 1,2-Dimyristoyl-sn-glycero-3-phosphoglycerol, sodium salt |
| DNA | Deoxyribonucleic acid |
| DOPC | 1,2-Dioleoyl-sn-glycero-3-phosphocholine |
| DOPE | Dioleoyl-phosphatidylethanolamine |
| DOTMA | 1,2-di-O-octadecenyl-3-trimethylammonium propane (chloride salt) |
| DOTAP | 1,2-dioleoyl-3-trimethylanmmonium-propane (chloride salt) |
| DPPC | 1,2-dipalmitoyl-sn-glycero-3-phosphocholine |
| DPPG-Na | 1,2-Dipalmitoyl-sn-glycero-3-phosphoglycerol, sodium salt |
| DSPC | 1,2-distearoyl-sn-glycero-3-phosphocholine |
| DSPE | 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine |
| DSPG-Na | 1,2-Distearoyl-sn-glycero-3-phosphatidylglycerol, sodium salt |
| EE% | Percent encapsulation efficiency |
| EGFR | Epidermal growth factor receptor |
| EM | Electromagnetic |
| EMA | European Medicines Agency |
| EMC | Electronic Medicines Compendium |
| EPR | Enhanced permeability and retention |
| Fab | Antigen-binding fragment |
| FDA | U.S. Food and Drug Administration |
| FOE | Fiber optic endoscopy |
| FR | Folate receptor |
| GNOLs | Gold nanoshell-coated oleanolic acid liposomes |
| HLB | Hydrophilic-lipophilic balance |
| HSPC | L-α-phosphatidylcholine, hydrogenated (soy) |
| ICG | Indocyanine green |
| IgM | Immunoglobulin M |
| IR | Infrared |
| LC% | Percentage of loading capacity |
| LEDs | Light-emitting diodes |
| LUVs | Large unilamellar vesicles |
| MAA | Methacrylic acid |
| mAbs | Monoclonal antibodies |
| MAL | Methyl aminolevulinate |
| MC | Merocyanine |
| MEO2MA | 2-(2-methoxyethoxy)ethyl methacrylate |
| MLVs | Multilamellar large vesicles |
| MPEG-2000-DPPE-Na | n-(methoxypolyethylene glycol 2000 carbamoyl)-1,2-dipalmitoyl-sn-glycero-3-phosphatidylethanolamine, monosodium salt |
| MPEG-5000-DPPE-Na | n-(methoxypolyethylene glycol 5000 carbamoyl)-1,2-dipalmitoyl-sn-glycero-3-phosphatidylethanolamine, monosodium salt |
| MPS | Mononuclear phagocyte systems |
| m-THPC | Meta-tetrahydroxy phenyl cholorin |
| NIR | Near-infrared |
| ONB | o-nitrobenzyl |
| PC | Phosphatidylcholine |
| PDT | Photodynamic therapy |
| PEG | Polyethylene glycol |
| PEG-PLEA | Poly(ethylene glycol)-poly(lactic acid-ethanolic acid) |
| PEG-PMI-DTE | PEGylated perylenemonoimide-dithienylethene |
| PEO | Polyethylene oxide |
| PET | Photo-induced electron transfer |
| PIT | Photoimmunotherapy |
| Pheo-a | Pheophorbide-a |
| PNB | Poly (4,5-dimethoxy-2-nitrobenzyl methacrylate |
| POPC | 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine |
| PTT | Photothermal therapy |
| Pyro-a | Pyropheophorbide-a |
| RES | Reticuloendothelial system |
| RNA | Ribonucleic acid |
| ROS | Reactive oxygen species |
| SC-CO2 | Supercritical carbon dioxide |
| scFv | Single-chain variable fragment |
| SP | Spiropyran |
| SPR | Surface plasmon resonance |
| SP-to-MC | Spiropyran-to-merocyanine |
| SPTPC | Spiropyran-containing triazole-phosphatidylcholine |
| SUVs | Small unilamellar vesicles |
| TFR | Transferrin receptor |
| TPA | Two-photon absorption |
| TPC | Triazole-phosphatidylcholine |
| TTA | Triplet–triplet annihilation |
| UCNPs | Up-Converting nanoparticles |
| uPA | Urokinase-plasminogen activator |
| UV | Ultraviolet |
| VPTT | Volume-phase transition temperature |
| 1O2 | Singlet oxygen |
| 3O2 | Molecular oxygen |
| 18:0-Azo PC | 1-stearoyl-2-[(E)-4-(4-((4-butylphenyl)diazenyl)phenyl)butanoyl]-sn-glycero-3-phosphocholine (CAS No.: 2098674-45-2) |
| 18:0-PhoDAG | 1-stearoyl-2-[(E)-4-(4-((4-butylphenyl)diazenyl)phenyl)butanoyl]-sn-glycerol |
References
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238-IN27. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Chiang, C.L.; Cheng, M.H.; Lin, C.H. From nanoparticles to cancer nanomedicine: Old problems with new solutions. Nanomater 2021, 11, 1727. [Google Scholar] [CrossRef]
- Langer, R. Polymers for the sustained release of proteins and other molecules. Nature 1976, 262, 1954–1962. [Google Scholar]
- Le Saux, S.; Aubert-Pouëssel, A.; Ouchait, L.; Mohamed, K.E.; Martineau, P.; Guglielmi, L.; Devoisselle, J.M.; Legrand, P.; Chopineau, J.; Morille, M. Nanotechnologies for intracellular protein delivery: Recent progress in inorganic and organic nanocarriers. Adv. Ther. 2021, 4, 2100009. [Google Scholar] [CrossRef]
- Saleh, T.A. Nanomaterials: Classification, properties, and environmental toxicities. Environ. Technol. Innov. 2020, 20, 101067. [Google Scholar] [CrossRef]
- Bangham, A.D.; Horne, R.W. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. JMB 1964, 8, 660-IN10. [Google Scholar] [CrossRef]
- Bangham, A.D. Lipid bilayers and biomembranes. Annu. Rev. Biochem. 1972, 41, 753–776. [Google Scholar] [CrossRef]
- Laouini, A.; Jaafar-Maalej, C.; Limayem-Blouza, I.; Sfar, S.; Charcosset, C.; Fessi, H. Preparation, characterization and applications of liposomes: State of the art. J. Colloid Sci. Biotechnol. 2012, 1, 147–168. [Google Scholar] [CrossRef]
- Agiba, A.M.; Nasr, M.; Abdel-Hamid, S.; Eldin, A.B.; Geneidi, A.S. Enhancing the intestinal permeation of the chondroprotective nutraceuticals glucosamine sulphate and chondroitin sulphate using conventional and modified liposomes. Curr. Drug Deliv. 2018, 15, 907–916. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Alinaghi, A.; Rouini, M.R.; Daha, F.J.; Moghimi, H.R. The influence of lipid composition and surface charge on biodistribution of intact liposomes releasing from hydrogel-embedded vesicles. Int. J. Pharm. 2014, 459, 30–39. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Szebeni, J. Stealth liposomes and long circulating nanoparticles: Critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog. Lipid Res. 2003, 42, 463–478. [Google Scholar] [CrossRef]
- Marzban, E.; Alavizadeh, S.H.; Ghiadi, M.; Khoshangosht, M.; Khashayarmanesh, Z.; Abbasi, A.; Jaafari, M.R. Optimizing the therapeutic efficacy of cisplatin PEGylated liposomes via incorporation of different DPPG ratios: In vitro and in vivo studies. Colloids Surf. B 2015, 136, 885–891. [Google Scholar] [CrossRef]
- Nicolosi, D.; Cupri, S.; Genovese, C.; Tempera, G.; Mattina, R.; Pignatello, R. Nanotechnology approaches for antibacterial drug delivery: Preparation and microbiological evaluation of fusogenic liposomes carrying fusidic acid. Int. J. Antimicrob. Agents 2015, 45, 622–626. [Google Scholar] [CrossRef]
- Nakhaei, P.; Margiana, R.; Bokov, D.O.; Abdelbasset, W.K.; Jadidi Kouhbanani, M.A.; Varma, R.S.; Marofi, F.; Jarahian, M.; Beheshtkhoo, N. Liposomes: Structure, biomedical applications, and stability parameters with emphasis on cholesterol. Front. Bioeng. Biotechnol. 2021, 9, 705886. [Google Scholar] [CrossRef]
- Chaves, M.A.; Baldino, L.; Pinho, S.C.; Reverchon, E. Supercritical CO2 assisted process for the production of mixed phospholipid nanoliposomes: Unloaded and vitamin D3-loaded vesicles. J. Food Eng. 2022, 316, 110851. [Google Scholar] [CrossRef]
- Campardelli, R.; Baldino, L.; Reverchon, E. Supercritical fluids applications in nanomedicine. J. Supercrit. Fluids 2015, 101, 193–214. [Google Scholar] [CrossRef]
- Trucillo, P.; Campardelli, R.; Reverchon, E. Supercritical CO2 assisted liposomes formation: Optimization of the lipidic layer for an efficient hydrophilic drug loading. J. CO2 Util. 2017, 18, 181–188. [Google Scholar] [CrossRef]
- US FDA. Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/approved-drug-products-therapeutic-equivalence-evaluations-orange-book (accessed on 3 January 2024).
- EMC. Electronic Medicines Compendium: Approved and Regulated Prescribing and Patient Information for Licensed Medicines. Available online: https://www.medicines.org.uk/emc#gref (accessed on 3 January 2024).
- Danhier, F.; Feron, O.; Préat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef]
- Iyer, A.K.; Khaled, G.; Fang, J.; Maeda, H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov. Today 2006, 11, 812–818. [Google Scholar] [CrossRef]
- Alavi, M.; Hamidi, M. Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles. Drug Metab. Pers. Ther. 2019, 34, 20180032. [Google Scholar] [CrossRef]
- Bae, Y.H.; Park, K. Targeted drug delivery to tumors: Myths, reality and possibility. J. Control. Release 2011, 153, 198. [Google Scholar] [CrossRef]
- Dumont, N.; Merrigan, S.; Turpin, J.; Lavoie, C.; Papavasiliou, V.; Geretti, E.; Espelin, C.W.; Luus, L.; Kamoun, W.S.; Ghasemi, O.; et al. Nanoliposome targeting in breast cancer is influenced by the tumor microenvironment. Nanomed. Nanotech. Biol. Med. 2019, 17, 71–81. [Google Scholar] [CrossRef]
- Wöll, S.; Dickgiesser, S.; Rasche, N.; Schiller, S.; Scherließ, R. Sortagged anti-EGFR immunoliposomes exhibit increased cytotoxicity on target cells. Eur. J. Pharm. Biopharm. 2019, 136, 203–212. [Google Scholar] [CrossRef]
- Nassir, A.M.; Ibrahim, I.A.A.; Md, S.; Waris, M.; Ain, M.R.; Ahmad, I.; Shahzad, N. Surface functionalized folate targeted oleuropein nano-liposomes for prostate tumor targeting: In vitro and in vivo activity. Life Sci. 2019, 220, 136–146. [Google Scholar] [CrossRef]
- Yoon, H.Y.; Chang, I.H.; Goo, Y.T.; Kim, C.H.; Kang, T.H.; Kim, S.Y.; Lee, S.J.; Song, S.H.; Whang, Y.M.; Choi, Y.W. Intravesical delivery of rapamycin via folate-modified liposomes dispersed in thermo-reversible hydrogel. Int. J. Nanomed. 2019, 14, 6249. [Google Scholar] [CrossRef]
- Akhtar, A.; Ghali, L.; Wang, S.X.; Bell, C.; Li, D.; Wen, X. Optimisation of folate-mediated liposomal encapsulated arsenic trioxide for treating HPV-positive cervical cancer cells in vitro. Int. J. Mol. Sci. 2019, 20, 2156. [Google Scholar] [CrossRef]
- Sakpakdeejaroen, I.; Somani, S.; Laskar, P.; Mullin, M.; Dufès, C. Transferrin-bearing liposomes entrapping plumbagin for targeted cancer therapy. J. Interdiscip. Nanomed. 2019, 4, 54–71. [Google Scholar] [CrossRef]
- Jhaveri, A.; Deshpande, P.; Pattni, B.; Torchilin, V. Transferrin-targeted, resveratrol-loaded liposomes for the treatment of glioblastoma. J. Control. Release 2018, 277, 89–101. [Google Scholar] [CrossRef]
- Ye, J.; Yang, Y.; Jin, J.; Ji, M.; Gao, Y.; Feng, Y.; Wang, H.; Chen, X.; Liu, Y. Targeted delivery of chlorogenic acid by mannosylated liposomes to effectively promote the polarization of TAMs for the treatment of glioblastoma. Bioact. Mater. 2020, 5, 694–708. [Google Scholar] [CrossRef]
- Vakhshiteh, F.; Khabazian, E.; Atyabi, F.; Ostad, S.N.; Madjd, Z.; Dinarvand, R. Peptide-conjugated liposomes for targeted miR-34a delivery to suppress breast cancer and cancer stem-like population. J. Drug Deliv. Sci. Technol. 2020, 57, 101687. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Dong, S.; Lee, R.J.; Yang, D.; Zhang, H.; Teng, L. Cell-penetrating peptide and transferrin co-modified liposomes for targeted therapy of glioma. Molecules 2019, 24, 3540. [Google Scholar] [CrossRef]
- Lin, Y.L.; Tsai, N.M.; Chen, C.H.; Liu, Y.K.; Lee, C.J.; Chan, Y.L.; Wang, Y.S.; Chang, Y.C.; Lin, C.H.; Huang, T.H.; et al. Specific drug delivery efficiently induced human breast tumor regression using a lipoplex by non-covalent association with anti-tumor antibodies. J. Nanobiotechnol. 2019, 17, 25. [Google Scholar] [CrossRef]
- Yu, S.; Bi, X.; Yang, L.; Wu, S.; Yu, Y.; Jiang, B.; Zhang, A.; Lan, K.; Duan, S. Co-delivery of paclitaxel and PLK1-targeted siRNA using aptamer-functionalized cationic liposome for synergistic anti-breast cancer effects in vivo. J. Biomed. Nanotechnol. 2019, 15, 1135–1148. [Google Scholar] [CrossRef]
- Riaz, M.K.; Riaz, M.A.; Zhang, X.; Lin, C.; Wong, K.H.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Surface functionalization and targeting strategies of liposomes in solid tumor therapy: A review. Int. J. Mol. Sci. 2018, 19, 195. [Google Scholar] [CrossRef]
- Nazeer, N.; Panicker, J.T.; Rajalekshmi, S.M.; Shaiju, S.D.A. A Review on Surface Modified Sterically Stabilized Liposomes. Int. J. Innov. Sci. Res. Technol. 2019, 4, 795–801. [Google Scholar]
- Rowe, R.C. Handbook of Pharmaceutical Excipients; Libros Digitales-Pharmaceutical Press: London, UK, 2020. [Google Scholar]
- Allen, C.D.S.N.; Dos Santos, N.; Gallagher, R.; Chiu, G.N.C.; Shu, Y.; Li, W.M.; Johnstone, S.A.; Janoff, A.S.; Mayer, L.D.; Webb, M.S.; et al. Controlling the physical behavior and biological performance of liposome formulations through use of surface grafted poly (ethylene glycol). Biosci. Rep. 2002, 22, 225–250. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Akita, H.; Harashima, H. The polyethyleneglycol dilemma: Advantage and disadvantage of PEGylation of liposomes for systemic genes and nucleic acids delivery to tumors. Biol. Pharm. Bull. 2013, 36, 892–899. [Google Scholar] [CrossRef]
- AlSawaftah, N.; Pitt, W.G.; Husseini, G.A. Dual-targeting and stimuli-triggered liposomal drug delivery in cancer treatment. ACS Pharmacol. Transl. Sci. 2021, 4, 1028–1049. [Google Scholar] [CrossRef]
- Jain, A.; Jain, S.K. Advances in tumor targeted liposomes. Curr. Mol. Med. 2018, 18, 44–57. [Google Scholar] [CrossRef]
- Zhou, J.; Rossi, J.J. Cell-specific aptamer-mediated targeted drug delivery. Oligonucleotides 2011, 21, 1–10. [Google Scholar] [CrossRef]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef]
- Derycke, A.S.; De Witte, P.A. Transferrin-mediated targeting of hypericin embedded in sterically stabilized PEG-liposomes. Int. J. Oncol. 2002, 20, 181–187. [Google Scholar] [CrossRef]
- Derycke, A.S.; Kamuhabwa, A.; Gijsens, A.; Roskams, T.; De Vos, D.; Kasran, A.; Huwyler, J.; Missiaen, L.; de Witte, P.A. Transferrin- conjugated liposome targeting of photosensitizer AlPcS4 to rat bladder carcinoma cells. J. Natl. Cancer Inst. 2004, 96, 1620–1630. [Google Scholar] [CrossRef]
- Hilgenbrink, A.R.; Low, P.S. Folate receptor-mediated drug targeting: From therapeutics to diagnostics. J. Pharm. Sci. 2005, 94, 2135–2146. [Google Scholar] [CrossRef]
- Paulos, C.M.; Reddy, J.A.; Leamon, C.P.; Turk, M.J.; Low, P.S. Ligand binding and kinetics of folate receptor recycling in vivo: Impact on receptor-mediated drug delivery. Mol. Pharmacol. 2004, 66, 1406–1414. [Google Scholar] [CrossRef]
- Sofou, S.; Sgouros, G. Antibody-targeted liposomes in cancer therapy and imaging. Expert Opin Drug Deliv. 2008, 5, 189–204. [Google Scholar] [CrossRef]
- Ichikawa, K.; Hikita, T.; Maeda, N.; Yonezawa, S.; Takeuchi, Y.; Asai, T.; Namba, Y.; Oku, N. Antiangiogenic photodynamic therapy (PDT) by using long-circulating liposomes modified with peptide specific to angiogenic vessels. Biochim. Biophys. Acta Biomembr. 2005, 1669, 69–74. [Google Scholar] [CrossRef]
- Meng, S.; Su, B.; Li, W.; Ding, Y.; Tang, L.; Zhou, W.; Song, Y.; Li, H.; Zhou, C. Enhanced antitumor effect of novel dual targeted paclitaxel liposomes. Nanotechnology 2010, 21, 415103. [Google Scholar] [CrossRef]
- Saul, J.M.; Annapragada, A.V.; Bellamkonda, R.V. A dual-ligand approach for enhancing targeting selectivity of therapeutic nanocarriers. J. Control. Release 2006, 114, 277–287. [Google Scholar] [CrossRef]
- Zhu, Y.; Feijen, J.; Zhong, Z. Dual-targeted nanomedicines for enhanced tumor treatment. Nano Today 2018, 18, 65–85. [Google Scholar] [CrossRef]
- Mei, L.; Zhang, Q.; Yang, Y.; He, Q.; Gao, H. Angiopep-2 and activatable cell penetrating peptide dual modified nanoparticles for enhanced tumor targeting and penetrating. Int. J. Pharm. 2014, 474, 95–102. [Google Scholar] [CrossRef]
- Li, H.; Tsui, T.Y.; Ma, W. Intracellular delivery of molecular cargo using cell-penetrating peptides and the combination strategies. Int. J. Mol. Sci. 2015, 16, 19518–19536. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, Y.; Wang, H.; Wang, J.; Shin, M.C.; Byun, Y.; He, H.; Liang, Y.; Yang, V.C. Curb challenges of the “Trojan Horse” approach: Smart strategies in achieving effective yet safe cell-penetrating peptide-based drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 1299–1315. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, Q.; Yu, Z.; He, Q. Cell-penetrating peptide-based intelligent liposomal systems for enhanced drug delivery. Curr. Pharm. Biotechnol. 2014, 15, 210–219. [Google Scholar] [CrossRef]
- Desale, K.; Kuche, K.; Jain, S. Cell-penetrating peptides (CPPs): An overview of applications for improving the potential of nanotherapeutics. Biomater. Sci. 2021, 9, 1153–1188. [Google Scholar] [CrossRef]
- Zangabad, P.S.; Mirkiani, S.; Shahsavari, S.; Masoudi, B.; Masroor, M.; Hamed, H.; Jafari, Z.; Taghipour, Y.D.; Hashemi, H.; Karimi, M.; et al. Stimulus-Responsive Liposomes as Smart Nanoplatforms for Drug Delivery Applications. Nanotechnol. Rev. 2018, 7, 95–122. [Google Scholar] [CrossRef]
- Lee, Y.; Thompson, D.H. Stimuli-Responsive Liposomes for Drug Delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1450. [Google Scholar] [CrossRef]
- Tao, Y.; Chan, H.F.; Shi, B.; Li, M.; Leong, K.W. Light: A magical tool for controlled drug delivery. Adv. Funct. Mater. 2020, 30, 2005029. [Google Scholar] [CrossRef] [PubMed]
- Municoy, S.; Álvarez Echazú, M.I.; Antezana, P.E.; Galdopórpora, J.M.; Olivetti, C.; Mebert, A.M.; Foglia, M.L.; Tuttolomondo, M.V.; Alvarez, G.S.; Hardy, J.G.; et al. Stimuli-responsive materials for tissue engineering and drug delivery. Int. J. Mol. Sci. 2020, 21, 4724. [Google Scholar] [CrossRef] [PubMed]
- Linsley, C.S.; Wu, B.M. Recent advances in light-responsive on-demand drug-delivery systems. Ther. Deliv. 2017, 8, 89–107. [Google Scholar] [CrossRef] [PubMed]
- Rapp, T.L.; DeForest, C.A. Targeting drug delivery with light: A highly focused approach. Adv. Drug Deliv. Rev. 2021, 171, 94–107. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, Y.; Wang, Q.; Liu, T.; Sun, J.; Zhang, R. Remote light-responsive nanocarriers for controlled drug delivery: Advances and perspectives. Small 2019, 15, 1903060. [Google Scholar] [CrossRef]
- Seynhaeve, A.L.B.; Amin, M.; Haemmerich, D.; Van Rhoon, G.C.; Ten Hagen, T.L.M. Hyperthermia and smart drug delivery systems for solid tumor therapy. Adv. Drug Deliv. Rev. 2020, 163, 125–144. [Google Scholar] [CrossRef]
- Otto, D.P.; de Villiers, M.M. What is the future of heated transdermal delivery systems? Ther. Deliv. 2014, 5, 961–964. [Google Scholar] [CrossRef]
- Banerjee, R. Trigger-responsive nanoparticles: Control switches for cancer therapy. Nanomedicine 2011, 6, 1657–1660. [Google Scholar] [CrossRef]
- Schmaljohann, D. Thermo-and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Wahajuddin, S.A. Superparamagnetic iron oxide nanoparticles: Magnetic nanoplatforms as drug carriers. Int. J. Nanomed. 2012, 7, 3445. [Google Scholar] [CrossRef]
- Liu, J.F.; Jang, B.; Issadore, D.; Tsourkas, A. Use of magnetic fields and nanoparticles to trigger drug release and improve tumor targeting. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1571. [Google Scholar] [CrossRef]
- Anderson, S.D.; Gwenin, V.V.; Gwenin, C.D. Magnetic functionalized nanoparticles for biomedical, drug delivery and imaging applications. Nanoscale Res. Lett. 2019, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Jiang, Y.; Lin, M.; Zhang, J.; Guo, H.; Yang, F.; Leung, W.; Xu, C. Ultrasound-responsive materials for drug/gene delivery. Front. Pharmacol. 2020, 10, 1650. [Google Scholar] [CrossRef] [PubMed]
- Mehier-Humbert, S.; Bettinger, T.; Yan, F.; Guy, R.H. Plasma membrane poration induced by ultrasound exposure: Implication for drug delivery. J. Control. Release 2005, 104, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Leung, S.J.; Romanowski, M. Light-activated content release from liposomes. Theranostics 2012, 2, 1020. [Google Scholar] [CrossRef]
- Goulet-Hanssens, A.; Eisenreich, F.; Hecht, S. Enlightening materials with photoswitches. Adv. Mater. 2020, 32, 1905966. [Google Scholar] [CrossRef]
- Russew, M.M.; Hecht, S. Photoswitches: From molecules to materials. Adv. Mater. 2010, 22, 3348–3360. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, X.; Xia, F.; Dai, Y. Azobenzene-based photoswitchable catalysts: State of the art and perspectives. J. Catal. 2022, 409, 33–40. [Google Scholar] [CrossRef]
- Sponza, A.D.; Liu, D.; Chen, E.P.; Shaw, A.; Diawara, L.; Chiu, M. Synthesis strategies for non-symmetric, photochromic diarylethenes. Org. Biomol. Chem. 2020, 18, 7238–7252. [Google Scholar] [CrossRef]
- Kortekaas, L.; Browne, W.R. The evolution of spiropyran: Fundamentals and progress of an extraordinarily versatile photochrome. Chem Soc. Rev. 2019, 48, 3406–3424. [Google Scholar] [CrossRef]
- Liu, Y.; An, X. Preparation, microstructure and function of liposome with light responsive switch. Colloids Surf. B 2019, 178, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Shah, P.K.; Culver, H.R.; David, S.N.; Stansbury, J.W.; Yin, X.; Bowman, C.N. Photo-responsive liposomes composed of spiropyran-containing triazole-phosphatidylcholine: Investigation of merocyanine-stacking effects on liposome–fiber assembly-transition. Soft Matter 2019, 15, 3740–3750. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.X.; Xin, B.; Li, C.; Xie, N.H.; Gong, W.L.; Huang, Z.L.; Zhu, M.Q. PEGylated perylenemonoimide-dithienylethene for super-resolution imaging of liposomes. ACS Appl. Mater. Interfaces 2017, 9, 10338–10343. [Google Scholar] [CrossRef] [PubMed]
- Dariva, C.G.; Coelho, J.F.; Serra, A.C. Near infrared light-triggered nanoparticles using singlet oxygen photocleavage for drug delivery systems. J. Control. Release 2019, 294, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.S.; Gomes, E.R.; Roque, M.C.; Oliveira, M.C. Triggered drug release from liposomes: Exploiting the outer and inner tumor environment. Front. Oncol. 2021, 11, 623760. [Google Scholar] [CrossRef]
- Hrycay, E.G.; Bandiera, S.M. Involvement of cytochrome P450 in reactive oxygen species formation and cancer. Adv. Pharmacol. 2015, 74, 35–84. [Google Scholar]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic therapy review: Principles, photosensitizers, applications, and future directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Plaetzer, K.; Krammer, B.; Berlanda, J.; Berr, F.; Kiesslich, T. Photophysics and photochemistry of photodynamic therapy: Fundamental aspects. Lasers Med. Sci. 2009, 24, 259–268. [Google Scholar] [CrossRef]
- Kudinova, N.V.; Berezov, T.T. Photodynamic therapy of cancer: Search for ideal photosensitizer. Biochemistry 2010, 4, 95–103. [Google Scholar] [CrossRef]
- O’Connor, A.E.; Gallagher, W.M.; Byrne, A.T. Porphyrin and nonporphyrin photosensitizers in oncology: Preclinical and clinical advances in photodynamic therapy. Photochem. Photobiol. 2009, 85, 1053–1074. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Ghosh, S.; He, X.; Huang, W.C.; Quinn, B.; Tian, M.; Jahagirdar, D.; Mabrouk, M.T.; Ortega, J.; Zhang, Y.; et al. Anti-cancer liposomal chemophototherapy using bilayer-localized photosensitizer and cabazitaxel. Nano Res. 2022, 15, 4302–4309. [Google Scholar] [CrossRef]
- Lovell, J.F.; Jin, C.S.; Huynh, E.; Jin, H.; Kim, C.; Rubinstein, J.L.; Chan, W.C.W.; Cao, W.; Wang, L.V.; Zheng, G. Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nat. Mater. 2011, 10, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Carter, K.A.; Shao, S.; Hoopes, M.I.; Luo, D.; Ahsan, B.; Grigoryants, V.M.; Song, W.; Huang, H.; Zhang, G.; Pandey, R.K.; et al. Porphyrin-phospholipid liposomes permeabilized by near-infrared light. Nat. Commun. 2014, 5, 3546. [Google Scholar] [CrossRef] [PubMed]
- Massiot, J.; Rosilio, V.; Makky, A. Photo-triggerable liposomal drug delivery systems: From simple porphyrin insertion in the lipid bilayer towards supramolecular assemblies of lipid–porphyrin conjugates. J. Mater. Chem. B 2019, 7, 1805–1823. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Lindstrom, A.; Li, Y. Porphyrin-based nanomedicines for cancer treatment. Bioconjug. Chem. 2019, 30, 1585–1603. [Google Scholar] [CrossRef]
- Cressey, P.; Bronstein, L.G.; Benmahmoudi, R.; Rosilio, V.; Regeard, C.; Makky, A. Novel liposome-like assemblies composed of phospholipid-porphyrin conjugates with photothermal and photodynamic activities against bacterial biofilms. Int. J. Pharm. 2022, 623, 121915. [Google Scholar] [CrossRef]
- Massiot, J.; Abuillan, W.; Konovalov, O.; Makky, A. Photo-triggerable liposomes based on lipid-porphyrin conjugate and cholesterol combination: Formulation and mechanistic study on monolayers and bilayers. Biochim. Biophys. Acta Biomembr. 2022, 1864, 183812. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef]
- Salkho, N.M.; Awad, N.S.; Pitt, W.G.; Husseini, G.A. Photo-induced drug release from polymeric micelles and liposomes: Phototriggering mechanisms in drug delivery systems. Polymers 2022, 14, 1286. [Google Scholar] [CrossRef]
- Rubio-Camacho, M.; Martínez-Tomé, M.J.; Cuestas-Ayllón, C.; de la Fuente, J.M.; Esquembre, R.; Mateo, C.R. Tailoring the plasmonic properties of gold-liposome nanohybrids as a potential powerful tool for light-mediated therapies. Colloids Interface Sci. Commun. 2023, 52, 100690. [Google Scholar] [CrossRef]
- Liu, Y.; He, M.; Niu, M.; Zhao, Y.; Zhu, Y.; Li, Z.; Feng, N. Delivery of vincristine sulfate-conjugated gold nanoparticles using liposomes: A light-responsive nanocarrier with enhanced antitumor efficiency. Int. J. Nanomed. 2015, 10, 3081–3095. [Google Scholar]
- Fomina, N.; Sankaranarayanan, J.; Almutairi, A. Photochemical mechanisms of light-triggered release from nanocarriers. Adv. Drug Deliv. Rev. 2012, 64, 1005–1020. [Google Scholar] [CrossRef]
- Zhao, X.; Fang, X.; Yang, S.; Zhang, S.; Yu, G.; Liu, Y.; Zhou, Y.; Feng, Y.; Li, J. Light-tuning amphiphility of host-guest Alginate-based supramolecular assemblies for photo-responsive Pickering emulsions. Carbohydr. Polym. 2021, 251, 117072. [Google Scholar] [CrossRef]
- Rideau, E.; Dimova, R.; Schwille, P.; Wurm, F.R.; Landfester, K. Liposomes and polymersomes: A comparative review towards cell mimicking. Chem. Soc. Rev. 2018, 47, 8572–8610. [Google Scholar] [CrossRef] [PubMed]
- Leong, J.; Teo, J.Y.; Aakalu, V.K.; Yang, Y.Y.; Kong, H. Engineering Polymersomes for Diagnostics and Therapy. Adv. Healthc. Mater. 2018, 7, 1701276. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Yamada, T.; Kubo, G.; Sakurai, K.; Yamaguchi, K.; Nakanishi, J. Preparation of a series of photoresponsive polymersomes bearing photocleavable a 2-nitrobenzyl group at the hydrophobic/hydrophilic interfaces and their payload releasing behaviors. Polymers 2019, 11, 1254. [Google Scholar] [CrossRef] [PubMed]
- Regen, S.L.; Singh, A.; Oehme, G.; Singh, M. Polymerized phosphatidylcholine vesicles. Stabilized and controllable time-release carriers. Biochem. Biophys. Res. Commun. 1981, 101, 131–136. [Google Scholar] [CrossRef]
- Nakamura, S.; Uehara, H.; Hasegawa, T.; Fujimoto, K. Phototriggered Sequence-specific DNA Transportation into Liposomes Using Ultrafast DNA Photocrosslinking. Chem. Lett. 2017, 46, 1839–1841. [Google Scholar] [CrossRef]
- He, J.; Tong, X.; Zhao, Y. Photoresponsive nanogels based on photocontrollable cross-links. Macromolecules 2009, 42, 4845–4852. [Google Scholar] [CrossRef]
- Lu, D.; Zhu, M.; Wu, S.; Wang, W.; Lian, Q.; Saunders, B.R. Triply responsive coumarin-based microgels with remarkably large photo-switchable swelling. Polym. Chem. 2019, 10, 2516–2526. [Google Scholar] [CrossRef]
- Heidarli, E.; Dadashzadeh, S.; Haeri, A. State of the art of stimuli-responsive liposomes for cancer therapy. Iran. J. Pharm. Res. 2017, 16, 1273–1304. [Google Scholar] [PubMed]
- Raza, A.; Rasheed, T.; Nabeel, F.; Hayat, U.; Bilal, M.; Iqbal, H.M.N. Endogenous and exogenous stimuli-responsive drug delivery systems for programmed site-specific release. Molecules 2019, 24, 1117. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, X.; Zhou, Z.; Wang, K.; Li, C.; Qiao, H.; Oupicky, D.; Sun, M. Near-Infrared light-triggered drug release from a multiple lipid carrier complex using an all-in one strategy. J. Controll. Release 2017, 261, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Refaat, A.; Del Rosal, B.; Palasubramaniam, J.; Pietersz, G.; Wang, X.; Moulton, S.E.; Peter, K. Near-infrared light-responsive liposomes for protein delivery: Towards bleeding-free photothermally-assisted thrombolysis. J. Control. Release 2021, 337, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Liu, J.; Wu, Y.; Feng, L.; Liu, Z. Near-Infrared-Light responsive nanoscale drug delivery systems for cancer treatment. Coord. Chem. Rev. 2016, 320–321, 100–117. [Google Scholar] [CrossRef]
- Zeng, X.L.; Zhou, X.C.; Wu, S. Red and near-infrared light-cleavable polymers. Macromol. Rapid Commun. 2018, 39, 1800034. [Google Scholar] [CrossRef]
- Zhu, X.J.; Su, Q.Q.; Feng, W.; Li, F.Y. Anti-Stokes shift luminescent materials for bio-applications. Chem. Soc. Rev. 2017, 46, 1025–1039. [Google Scholar] [CrossRef]
- Sun, Y.; Ji, Y.; Yu, H.; Wang, D.; Cao, M.; Wang, J. Near-infrared light-sensitive liposomes for controlled release. RSC Adv. 2016, 6, 81245–81249. [Google Scholar] [CrossRef]
- Gwon, K.; Jo, E.-J.; Sahu, A.; Lee, J.Y.; Kim, M.-G.; Tae, G. Improved near infrared-mediated hydrogel formation using diacrylated Pluronic F127-Coated upconversion nanoparticles. Mater. Sci. Eng. C 2018, 90, 77–84. [Google Scholar] [CrossRef]
- Wu, S.; Butt, H.J. Near-infrared-sensitive materials based on upconverting nanoparticles. Adv. Mater. 2016, 28, 1208–1226. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.H.; Zhou, J.J.; Zheng, K.Z.; Bednarkiewicz, A.; Liu, X.G.; Jin, D.Y. Advances in highly doped upconversion nanoparticles. Nat. Commun. 2018, 9, 2415. [Google Scholar] [CrossRef]
- Xiang, J.; Tong, X.; Shi, F.; Yan, Q.; Yu, B.; Zhao, Y. Near-Infrared light-triggered drug release from UV-responsive di-block copolymer-coated upconversion nanoparticles with high monodispersity. J. Mater. Chem. B 2018, 6, 3531–3540. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Yu, Z.; Ren, Q.; Liu, X.; Wang, Y.; Sun, X.; Yin, S.; Pan, J.; Huang, X. Nanoscale ZnO-Based photosensitizers for photodynamic therapy. Photodiagnosis Photodyn. Ther. 2020, 30, 101694. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, W.; Di, H.; Luo, L.; Zhu, C.; Yang, J.; Yin, X.; Yin, H.; Gao, J.; Du, Y.; et al. A photosensitive liposome with NIR light triggered doxorubicin release as a combined photodynamic-chemo therapy system. J. Control. Release 2018, 277, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Wang, W. Phototriggered targeting of nanocarriers for drug delivery. Nano Res. 2018, 11, 5424–5438. [Google Scholar] [CrossRef]
- Xie, X.; Yang, Y.; Yang, Y.; Mei, X. Photolabile-caged peptide-conjugated liposomes for siRNA delivery. J. Drug Target. 2015, 23, 789–799. [Google Scholar] [CrossRef]
- Xie, X.; Yang, Y.; Yang, Y.; Zhang, H.; Li, Y.; Mei, X. A photo-responsive peptide-and asparagine–glycine–arginine (NGR) peptide-mediated liposomal delivery system. Drug Deliv. 2016, 23, 2445–2456. [Google Scholar] [CrossRef]
- Hansen, M.B.; Van Gaal, E.; Minten, I.; Storm, G.; Van Hest, J.C.; Löwik, D.W. Constrained and UV-activatable cell-penetrating peptides for intracellular delivery of liposomes. J. Control. Release 2012, 164, 87–94. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Y.; Xie, X.; Wang, Z.; Gong, W.; Zhang, H.; Li, Y.; Yu, F.; Li, Z.; Mei, X. Dual-modified liposomes with a two-photon-sensitive cell penetrating peptide and NGR ligand for siRNA targeting delivery. Biomaterials 2015, 48, 84–96. [Google Scholar] [CrossRef]
- Mizukami, S.; Hosoda, M.; Satake, T.; Okada, S.; Hori, Y.; Furuta, T.; Kikuchi, K. Photocontrolled compound release system using caged antimicrobial peptide. J. Am. Chem. Soc. 2010, 132, 9524–9525. [Google Scholar] [CrossRef] [PubMed]
- Fan, N.C.; Cheng, F.Y.; Ho, J.A.A.; Yeh, C.S. Photocontrolled targeted drug delivery: Photocaged biologically active folic acid as a light-responsive tumor-targeting molecule. Angew. Chem. 2012, 124, 8936–8940. [Google Scholar] [CrossRef]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef] [PubMed]
- Tong, R.; Chiang, H.H.; Kohane, D.S. Photoswitchable nanoparticles for in vivo cancer chemotherapy. Proc. Natl. Acad. Sci. USA 2013, 110, 19048–19053. [Google Scholar] [CrossRef] [PubMed]
- Cabral, H.; Matsumoto, Y.; Mizuno, K.; Chen, Q.; Murakami, M.; Kimura, M.; Terada, Y.; Kano, M.R.; Miyazono, K.; Uesaka, M.J.N.N.; et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 2011, 6, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Chen, T.; Öçsoy, I.; Yasun, E.; Wu, C.; Zhu, G.; You, M.; Han, D.; Jiang, J.; Yu, R.; et al. A cell-targeted, size-photocontrollable, nuclear-uptake nanodrug delivery system for drug-resistant cancer therapy. Nano Lett. 2015, 15, 457–463. [Google Scholar] [CrossRef]
- Ojha, T.; Pathak, V.; Shi, Y.; Hennink, W.E.; Moonen, C.T.; Storm, G.; Kiessling, F.; Lammers, T. Pharmacological and physical vessel modulation strategies to improve EPR-mediated drug targeting to tumors. Adv. Drug Deliv. Rev. 2017, 119, 44–60. [Google Scholar] [CrossRef]
- Tour, O.; Meijer, R.M.; Zacharias, D.A.; Adams, S.R.; Tsien, R.Y. Genetically targeted chromophore-assisted light inactivation. Nat. Biotechnol. 2003, 21, 1505–1508. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Mitsunaga, M.; Ogawa, M.; Kosaka, N.; Rosenblum, L.T.; Choyke, P.L.; Kobayashi, H. Cancer cell–selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat. Med. 2011, 17, 1685–1691. [Google Scholar] [CrossRef]
- Sano, K.; Nakajima, T.; Choyke, P.L.; Kobayashi, H. Markedly enhanced permeability and retention effects induced by photo-immunotherapy of tumors. ACS Nano 2013, 7, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Puri, A. Phototriggerable liposomes: Current research and future perspectives. Pharmaceutics 2014, 6, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Miranda, D.; Lovell, J.F. Mechanisms of light-induced liposome permeabilization. Bioeng. Transl. Med. 2016, 1, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Mathiyazhakan, M.; Wiraja, C.; Xu, C. A concise review of gold nanoparticles-based photo-responsive liposomes for controlled drug delivery. Nanomicro Lett. 2018, 10, 10. [Google Scholar] [CrossRef]
- Paasonen, L.; Sipilä, T.; Subrizi, A.; Laurinmäki, P.; Butcher, S.J.; Rappolt, M.; Yaghmur, A.; Urtti, A.; Yliperttula, M. Gold-embedded photosensitive liposomes for drug delivery: Triggering mechanism and intracellular release. J. Control. Release 2010, 147, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Wei, W.; Yue, Z.; Lv, P.; Wang, L.; Ma, G.; Su, Z. Particle size affects the cellular response in macrophages. Eur. J. Pharm. Sci. 2010, 41, 650–657. [Google Scholar] [CrossRef]
- Jasinski, D.L.; Li, H.; Guo, P. The effect of size and shape of RNA nanoparticles on biodistribution. Mol. Ther. 2018, 26, 784–792. [Google Scholar] [CrossRef]
- Hardonk, M.J.; Harms, G.; Koudstaal, J. Zonal heterogeneity of rat hepatocytes in the in vivo uptake of 17nm colloidal gold granules. Histochemistry 1985, 83, 473–477. [Google Scholar] [CrossRef]
- Di, J.; Gao, X.; Du, Y.; Zhang, H.; Gao, J.; Zheng, A. Size, shape, charge and “stealthy” surface: Carrier properties affect the drug circulation time in vivo. Asian J. Pharm. Sci. 2021, 16, 444–458. [Google Scholar] [CrossRef]
- Woodle, M.C.; Papahadjopoulos, D. Liposome preparation and size characterization. Methods Enzymol. 1989, 171, 193–217. [Google Scholar]
- Kulkarni, S.B.; Betageri, G.V.; Singh, M. Factors affecting microencapsulation of drugs in liposomes. J. Microencapsul. 1995, 12, 229–246. [Google Scholar] [CrossRef]
- Perkins, W.R.; Minchey, S.R.; Ahl, P.L.; Janoff, A.S. The determination of liposome captured volume. Chem. Phys. Lipids. 1993, 64, 197–217. [Google Scholar] [CrossRef] [PubMed]
- Filipczak, N.; Pan, J.; Yalamarty, S.S.K.; Torchilin, V.P. Recent advancements in liposome technology. Adv. Drug Deliv. Rev. 2020, 156, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Myint, K.T.; Sahoo, S.; Thein, A.W.; Moe, S.; Ni, H. Laser therapy for retinopathy in sickle cell disease. Cochrane Database Syst. Rev. 2022, 12, CD010790. [Google Scholar] [PubMed]
- Jelínková, H. Lasers for Medical Applications: Diagnostics, Therapy and Surgery; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Bastos, J.; Lizarelli, R.; Parizotto, N. Comparative study of laser and LED systems of low intensity applied to tendon healing. Laser Phys. 2009, 19, 1925–1931. [Google Scholar] [CrossRef]
- Tong, R.; Kohane, D.S. Shedding light on nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012, 4, 638–662. [Google Scholar] [CrossRef]
- Rwei, A.Y.; Wang, W.; Kohane, D.S. Photoresponsive nanoparticles for drug delivery. Nano Today 2015, 10, 451–467. [Google Scholar] [CrossRef]
- Barat, K. Laser Safety: Tools and Training; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Ash, C.; Dubec, M.; Donne, K.; Bashford, T. Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Lasers Med Sci. 2017, 32, 1909–1918. [Google Scholar] [CrossRef]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic therapy for the treatment and diagnosis of cancer—A review of the current clinical status. Front. Chem. 2021, 9, 686303. [Google Scholar] [CrossRef]
- Gonzaga, E.R. Role of UV light in photodamage, skin aging, and skin cancer: Importance of photoprotection. Am. J. Clin. Dermatol. 2009, 10, 19–24. [Google Scholar] [CrossRef]
- Organisciak, D.T.; Vaughan, D.K. Retinal light damage: Mechanisms and protection. Prog. Retin. Eye Res. 2010, 29, 113–134. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Jones, C.; Baek, Y.; Park, G.K.; Kashiwagi, S.; Choi, H.S. Near-infrared fluorescence imaging in immunotherapy. Adv. Drug Deliv. Rev. 2020, 167, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Mi, P. Stimuli-responsive nanocarriers for drug delivery, tumor imaging, therapy and theranostics. Theranostics 2020, 10, 4557. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Zhang, H.; Zhang, X.; Liu, D.; Chen, D.; Zhang, W.; Zhang, L.; Santos, H.A.; Hai, M. Biodegradable photothermal and pH responsive calcium carbonate@ phospholipid@ acetalated dextran hybrid platform for advancing biomedical applications. Adv. Funct. Mater. 2016, 26, 6158–6169. [Google Scholar] [CrossRef]
- Chen, M.M.; Song, F.F.; Feng, M.; Liu, Y.; Liu, Y.Y.; Tian, J.; Lv, F.; Zhang, Q.Q. pH-sensitive charge-conversional and NIR responsive bubble-generating liposomal system for synergetic thermo-chemotherapy. Colloids Surf. B 2018, 167, 104–114. [Google Scholar] [CrossRef]
- You, C.; Wang, M.; Wu, H.; An, P.; Pan, M.; Luo, Y.; Sun, B. Near infrared radiated stimulus-responsive liposomes based on photothermal conversion as drug carriers for co-delivery of CJM126 and cisplatin. Mater. Sci. Eng. C 2017, 80, 362–370. [Google Scholar] [CrossRef]
- Luo, L.; Bian, Y.; Liu, Y.; Zhang, X.; Wang, M.; Xing, S.; Li, L.; Gao, D. Combined near infrared photothermal therapy and chemotherapy using gold nanoshells coated liposomes to enhance antitumor effect. Small 2016, 12, 4103–4112. [Google Scholar] [CrossRef]
- Lin, H.C.; Li, W.T.; Madanayake, T.W.; Tao, C.; Niu, Q.; Yan, S.Q.; Gao, B.A.; Ping, Z. Aptamer-guided upconversion nanoplatform for targeted drug delivery and near-infrared light-triggered photodynamic therapy. J. Biomater. Appl. 2020, 34, 875–888. [Google Scholar] [CrossRef]
- Tian, M.; Xin, X.; Wu, R.; Guan, W.; Zhou, W. Advances in intelligent-responsive nanocarriers for cancer therapy. Pharmacol. Res. 2022, 178, 106184. [Google Scholar] [CrossRef]
- Chamundeeswari, M.; Jeslin, J.; Verma, M.L. Nanocarriers for drug delivery applications. Environ. Chem. Lett. 2019, 17, 849–865. [Google Scholar] [CrossRef]
- Fournier, L.; Gauron, C.; Xu, L.; Aujard, I.; Le Saux, T.; Gagey-Eilstein, N.; Maurin, S.; Dubruille, S.; Baudin, J.B.; Bensimon, D.; et al. A blue-absorbing photolabile protecting group for in vivo chromatically orthogonal photoactivation. ACS Chem. Biol. 2013, 8, 1528–1536. [Google Scholar] [CrossRef]
- Olson, J.P.; Kwon, H.B.; Takasaki, K.T.; Chiu, C.Q.; Higley, M.J.; Sabatini, B.L.; Ellis-Davies, G.C. Optically selective two-photon uncaging of glutamate at 900 nm. J. Am. Chem. Soc. 2013, 135, 5954–5957. [Google Scholar] [CrossRef] [PubMed]
- Fournier, L.; Aujard, I.; Le Saux, T.; Maurin, S.; Beaupierre, S.; Baudin, J.B.; Jullien, L. Coumarinylmethyl caging groups with redshifted absorption. Chem. Eur. J. 2013, 19, 17494–17507. [Google Scholar] [CrossRef] [PubMed]
- Gandioso, A.; Cano, M.; Massaguer, A.; Marchán, V. A green light-triggerable RGD peptide for photocontrolled targeted drug delivery: Synthesis and photolysis studies. J. Org. Chem. 2016, 81, 11556–11564. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhao, Y.; Zhang, H.; Huang, K.; Yang, J.; Han, G. Expanding anti-Stokes shifting in triplet–triplet annihilation upconversion for in vivo anticancer prodrug activation. Angew. Chem. 2017, 129, 14592–14596. [Google Scholar] [CrossRef]
- Li, D.; Ma, Y.; Du, J.; Tao, W.; Du, X.; Yang, X.; Wang, J. Tumor acidity/NIR controlled interaction of transformable nanoparticle with biological systems for cancer therapy. Nano Lett. 2017, 17, 2871–2878. [Google Scholar] [CrossRef]
- Lin, Q.; Bao, C.; Yang, Y.; Liang, Q.; Zhang, D.; Cheng, S.; Zhu, L. Highly discriminating photorelease of anticancer drugs based on hypoxia activatable phototrigger conjugated chitosan nanoparticles. Adv. Mater. 2013, 25, 1981–1986. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Kumar, E.M.; Chavali, M.S. Updates on Responsive Drug Delivery Based on Liposome Vehicles for Cancer Treatment. Pharmaceutics 2022, 14, 2195. [Google Scholar] [CrossRef]
- Nsairat, H.; AlShaer, W.; Odeh, F.; Essawi, E.; Khater, D.; Al Bawab, A.; El-Tanani, M.; Awidi, A.; Mubarak, M.S. Recent Advances in Using Liposomes for Delivery of Nucleic Acid-Based Therapeutics. OpenNano 2023, 11, 100132. [Google Scholar] [CrossRef]
- Magar, K.T.; Boafo, G.F.; Li, X.; Chen, Z.; He, W. Liposome-based delivery of biological drugs. Chin. Chem. Lett. 2022, 33, 587–596. [Google Scholar] [CrossRef]













| Lipid Name and CAS No. | Synonym | Molecular Formula | Chemical Structure |
|---|---|---|---|
| Neutral | |||
| Cholesterol (CAS No.: 57-88-5) | --- | C27H46O |  |
| Anionic | |||
| 1,2-Dimyristoyl-sn-glycero-3-phosphoglycerol, sodium salt (CAS No.: 200880-40-6) | DMPG-Na | C34H66NaO10PNa |  |
| 1,2-Dipalmitoyl-sn-glycero-3-phosphoglycerol, sodium salt (CAS No.: 200880-41-7) | DPPG-Na | C38H74NaO10PNa |  |
| 1,2-Distearoyl-sn-glycero-3-phosphatidylglycerol, sodium salt (CAS No.: 200880-42-8) | DSPG-Na | C42H82NaO10PNa |  |
| N-(Methoxypolyethylene glycol 5000 carbamoyl)-1,2-dipalmitoyl-sn-glycero-3-phosphatidylethanolamine, monosodium salt (CAS No.: 205494-72-0) | MPEG-5000-DPPE-Na | (C2H4O)nC39H76NO10PNa |  |
| N-(Methoxypolyethylene glycol 2000 carbamoyl)-1,2-dipalmitoyl-sn-glycero-3-phosphatidylethanolamine, monosodium salt (CAS No.: 384835-61-4) | MPEG-2000-DPPE-Na | (C2H4O)nC39H76NO10PNa |  |
| Cationic | |||
| 1,2-dioleoyl-3-trimethylanmmonium-propane (chloride salt) (CAS No.: 132172-61-3) | DOTAP | C42H80NO4Cl |  |
| 1,2-di-O-octadecenyl-3-trimethylammonium propane (chloride salt) (CAS No.: 104872-42-6) | DOTMA | C42H84ClNO2 |  |
| Zwitterion | |||
| 1,2-dimyristoyl-sn-glycero-3-phosphocholine (CAS No.: 18194-24-6) | DMPC | C36H72NO8P |  |
| 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine (CAS No.: 63-89-8) | DPPC | C40H80NO8P |  |
| 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine (CAS No.: 1069-79-0) | DSPE | C41H82NO8P |  |
| L-α-phosphatidylcholine, hydrogenated (soy) (CAS No.: 97281-48-6) | HSPC | C42H84NO8P |  |
| 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (CAS No.: 26853-31-6) | POPC | C42H82NO8P |  |
| 1,2-Dioleoyl-sn-Glycero-3-phosphocholine (CAS No.: 4235-95-4) | DOPC | C44H84NO8P |  |
| 1,2-Distearoyl-sn-glycero-3-phosphocholine (CAS No.: 816-94-4) | DSPC | C44H88NO8P |  |
| Photoswitchable Lipids | |||
| 1-stearoyl-2-[(E)-4-(4-((4-butylphenyl)diazenyl)phenyl)butanoyl]-sn-glycerol (CAS No.: 1985595-31-0) | 18:0-PhoDAG | C41H64N2O5 |  |
| N-[(E)-4-(4-((4-butylphenyl)diazenyl)phenyl)butanoyl]-D-erythro-sphingosylphosphorylcholine (CAS No.: 2260670-56-0) | Azo SM | C43H71N4O6P |  |
| 1-stearoyl-2-[(E)-4-(4-((4-butylphenyl)diazenyl)phenyl)butanoyl]-sn-glycero-3-phosphocholine (CAS No.: 2098674-45-2) | 18:0-azo PC | C46H76N3O8P |  |
| Product Name | Approval Date | Product Description | Liposome Composition | Indication and Usage | Manufacturer |
|---|---|---|---|---|---|
| Doxil® | FDA: 1995 EMA: 1996 | Doxorubicin encapsulated in stealth liposomes. | MPEG-DSPE, HSPC, cholesterol. | Ovarian cancer, AIDS-related Kaposi’s sarcoma. | Janssen Pharmaceuticals (Beerse, Belgium) |
| Abelcet® | FDA: 2005 | Amphotericin B lipid complex injection. | DMPC, DMPG. | Invasive fungal infections. | Leadiant Biosciences, Inc. (Gaithersburg, MD, USA) |
| DaunoXome® | FDA: 1996 EMA: 2004 | Daunorubicin encapsulated in liposomes. | DSPC, cholesterol. | Advanced HIV-associated Kaposi’s sarcoma. | Galen Ltd. (Craigavon, UK) |
| AmBisome® | FDA: 1997 EMA: 2006 | Amphotericin B liposome for injection. | HSPC, cholesterol, DSPG, alpha tocopherol. | Cryptococcal meningitis in HIV-infected patients. | Gilead Sciences, Inc. (Foster City, CA, USA) |
| DepoCyt® | FDA: 1999 EMA: 2001 | Cytarabine liposome injection. | Cholesterol, triolein, DOPC, DPPG. | Lymphomatous meningitis. | Pacira Pharmaceuticals, Inc. (Parsippany, NJ, USA) |
| Myocet® | FDA: 2000 EMA: 2000 | Non-PEGylated liposomal doxorubicin. | Phosphatidylcholine, cholesterol. | Metastatic breast cancer in adult women. | Teva Pharmaceuticals (Tel Aviv, Israel) |
| Mepact® | FDA: 2001 EMA: 2009 | A liposomal suspension of mifamurtide. | POPC, OOPS. | High-grade resectable non-metastatic osteosarcoma. | Takeda Pharmaceutical Company (Tokyo, Japan) |
| Exparel® | FDA: 2011 EMA: 2021 | Bupivacaine liposome injectable suspension. | Cholesterol, DPPG, DEPC. | Postsurgical regional analgesia. | Pacira Pharmaceuticals, Inc. (Parsippany, NJ, USA) |
| Onivyde® | EMA: 2016 | Irinotecan sucrosofate in PEGylated liposomes. | DSPC, cholesterol, MPEG-2000-DSPE. | Metastatic adenocarcinoma of the pancreas. | Laboratoires Servier (Servier) (Suresnes, France) |
| Vyxeos® | FDA: 2017 | Cytarabine and daunorubicin liposome injection. | DSPC, DSPG, cholesterol. | Acute myeloid leukemia. | Jazz Pharmaceuticals plc (Dublin, Ireland) |
| Arikayce® | FDA: 2018 EMA: 2020 | Amikacin liposome inhalation suspension. | Cholesterol, DPPC. | Non-tuberculous mycobacterial (NTM) lung infections. | Almac Pharma Services Ltd. (Athlone, Ireland) |
| Zolsketil® | EMA: 2022 | Doxorubicin in PEGylated liposomes. | MPEG 2000-DSPE, HSPC, cholesterol. | Ovarian neoplasms, sarcoma, Kaposi, multiple myeloma. | Accord Healthcare S.L.U. (Barcelona, Spain) |
| Active Targeting Ligand | Encapsulated Drug | Preparation Method | Reference |
|---|---|---|---|
| PEGylated Liposomes | |||
| mAbs (MM-302) | Doxorubicin | Thin-film hydration | [26] |
| mAbs (Sortagged anti-EGFR) | Doxorubicin | Ethanol injection | [27] |
| Folate | Oleuropein | Thin-film hydration | [28] |
| Folate | Rapamycin | Thin-film hydration | [29] |
| Folate | Arsenic trioxide | Thin-film hydration | [30] |
| Transferrin | Plumbagin | Thin-film hydration | [31] |
| Transferrin | Resveratrol | Thin-film hydration | [32] |
| Mannose | Chlorogenic acid | Thin-film hydration | [33] |
| cRGD | microRNA | Thin-film hydration | [34] |
| Cationic Liposomes | |||
| Transferrin | Doxorubicin | Ethanol injection | [35] |
| mAbs (Herceptin) | Curcumin | Thin-film hydration | [36] |
| Aptamer (AS1411) | Paclitaxel and siRNA | Thin-film hydration | [37] |
| Stimuli | Advantages | Limitations | References |
|---|---|---|---|
| Light |
|
| [64,65,66,67,68] |
| Heat |
|
| [69,70] |
| pH |
|
| [71,72] |
| Electrical fields |
|
| [73,74] |
| Magnetic fields |
|
| [75,76] |
| Ultrasound waves |
|
| [77,78] |
| Encapsulating Drug | Ligand Type | Caging/Shielding Group | Irradiation Source | Reference |
|---|---|---|---|---|
| siRNA | CPP/PCP | PEG | NIR | [130] |
| Vinorelbine bitartrate | PSP/NGR | PEG | NIR | [131] |
| ----- | TAT | PEG | UV | [132] |
| siRNA | pcCPP/NGR | PEG | NIR | [133] |
| 5(6)-carboxyfluorescein | AMP (BTL) | ε-amino group of the Lys in TL | UV | [134] |
| Paclitaxel | Folate | o-nitrobenzylamine | UV | [135] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agiba, A.M.; Arreola-Ramírez, J.L.; Carbajal, V.; Segura-Medina, P. Light-Responsive and Dual-Targeting Liposomes: From Mechanisms to Targeting Strategies. Molecules 2024, 29, 636. https://doi.org/10.3390/molecules29030636
Agiba AM, Arreola-Ramírez JL, Carbajal V, Segura-Medina P. Light-Responsive and Dual-Targeting Liposomes: From Mechanisms to Targeting Strategies. Molecules. 2024; 29(3):636. https://doi.org/10.3390/molecules29030636
Chicago/Turabian StyleAgiba, Ahmed M., José Luis Arreola-Ramírez, Verónica Carbajal, and Patricia Segura-Medina. 2024. "Light-Responsive and Dual-Targeting Liposomes: From Mechanisms to Targeting Strategies" Molecules 29, no. 3: 636. https://doi.org/10.3390/molecules29030636
APA StyleAgiba, A. M., Arreola-Ramírez, J. L., Carbajal, V., & Segura-Medina, P. (2024). Light-Responsive and Dual-Targeting Liposomes: From Mechanisms to Targeting Strategies. Molecules, 29(3), 636. https://doi.org/10.3390/molecules29030636