The Iodine/Iodide/Starch Supramolecular Complex
Abstract
:1. Introduction
2. General Considerations on the Reaction of Starch with Iodine
3. Dependence on the Nature of the Organic (Bio)Polymer
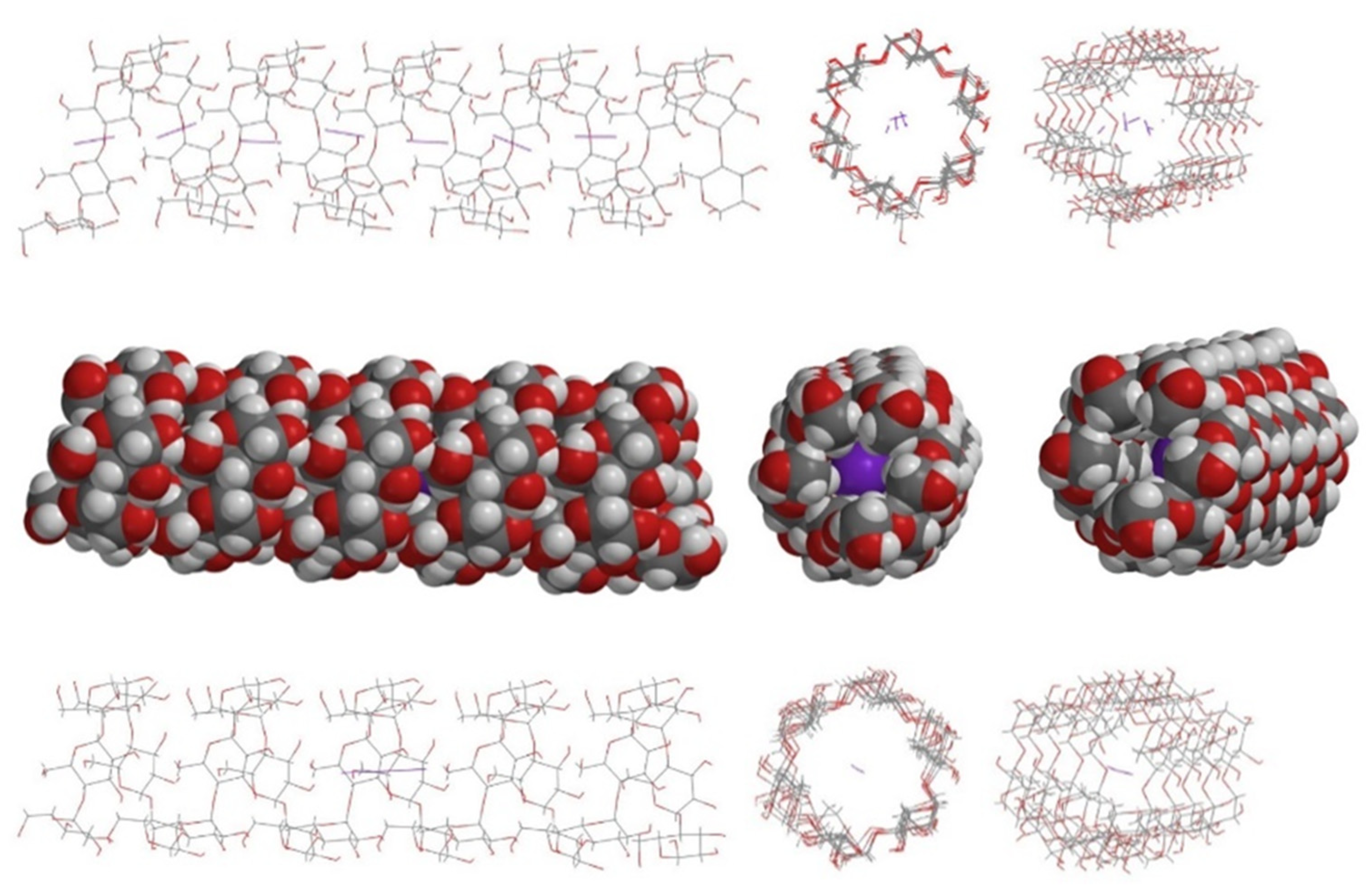
4. Geometrical Data
5. The I2-Only Hypothesis
6. Poly-Iodine Anions as Candidates
7. Structural Role of the Solvent
8. The I5−-I2 Hypothesis
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nelson, D.L.; Cox, M.M. Principles of Biochemistry. In Principles of Biochemistry, 5th ed.; W.H. Freeman & Company: New York, NY, USA, 2008. [Google Scholar]
- Wang, K.; Vilaplana, F.; Wu, A.; Hasjim, J.; Gilbert, R.G. The Size Dependence of the Average Number of Branches in Amylose. Carbohydr. Polym. 2019, 223, 115134. [Google Scholar] [CrossRef]
- Green, M.M.; Blankenhorn, G.; Hart, H. Which Starch Fraction Is Water-Soluble, Amylose or Amylopectin? J. Chem. Educ. 1975, 52, 729. [Google Scholar] [CrossRef]
- Nouri, A.; Khoee, S. Preparation of Amylose-Poly(Methyl Methacrylate) Inclusion Complex as a Smart Nanocarrier with Switchable Surface Hydrophilicity. Carbohydr. Polym. 2020, 246, 116662. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Guan, L.; Wang, S.; Zhang, J.; Tan, L.; Kong, L.; Zhang, H. Lipophilization and Amylose Inclusion Complexation Enhance the Stability and Release of Catechin. Carbohydr. Polym. 2021, 269, 118251. [Google Scholar] [CrossRef]
- Prasher, P.; Fatima, R.; Sharma, M. Therapeutic Delivery with V-Amylose. Drug Dev. Res. 2021, 82, 727–729. [Google Scholar] [CrossRef]
- Katz, J.R. Abhandlungen Zur Physikalischen Chemie Der Stärke Und Der Brotbereitung. Z. Phys. Chem. 1930, 150A, 37–59. [Google Scholar] [CrossRef]
- Katz, J.R.; Derksen, J.C. Abhandlungen Zur Physikalischen Chemie Der Stärke Und Der Brotbereitung. Z. Phys. Chem. 1933, 167A, 129–136. [Google Scholar] [CrossRef]
- Bear, R.S. The Significance of the “V” X-Ray Diffraction Patterns of Starches. J. Am. Chem. Soc. 1942, 64, 1388–1392. [Google Scholar] [CrossRef]
- Meyer, K.H.; Bernfeld, P.; Wolf, E. Recherches Sur l’amidon III. Fractionnement et Purification de l’amylose de Maïs Naturel. Helv. Chim. Acta 1940, 23, 854–864. [Google Scholar] [CrossRef]
- Sarko, A.; Zugenmaier, P. Fiber Diffraction Methods. Am. Chem. Soc. 1980, 141, 459–482. [Google Scholar]
- Rappenecker, G.; Zugenmaier, P. Detailed refinement of the crystal structure of Vh-amylose. Carbohydr Res. 1981, 89, 11–19. [Google Scholar] [CrossRef]
- Murphy, V.G.; Zaslow, B.; French, A.D. The structure of V amylose dehydrate: A combined X-ray and stereochemical approach. Biopolymers 1975, 14, 1487–1501. [Google Scholar] [CrossRef]
- Brisson, J.; Chanzy, H.; Winter, W.T. The crystal and molecular structure of VH amylose by electron diffraction analysis. Int. J. Biol. Macromol. 1991, 13, 31–39. [Google Scholar] [CrossRef]
- Veregin, R.P.; Fyfe, C.A.; Marchessault, R.H. Investigation of the crystalline “V” amylose complexes by high-resolution carbon-13 CP/MAS NMR spectroscopy. Macromolecules 1987, 20, 3007–3012. [Google Scholar] [CrossRef]
- Gidley, M.J.; Bociek, S.M. Carbon-13 CP/MAS NMR studies of amylose inclusion complexes, cyclodextrins, and the amorphous phase of starch granules: Relationships between glycosidic linkage conformation and solid-state carbon-13 chemical shifts. J. Am. Chem. Soc. 1988, 110, 3820–3829. [Google Scholar] [CrossRef]
- Imberty, A.; Chanzy, H.; Pérez, S.; Bulèon, A.; Tran, V. The Double-Helical Nature of the Crystalline Part of A-Starch. J. Mol. Biol. 1988, 201, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Sarko, A.; Wu, H.-C.H. The Crystal Structures of A-, B- and C-Polymorphs of Amylose and Starch. Starch-Starke 1978, 30, 73–78. [Google Scholar] [CrossRef]
- Saenger, W. Inclusion Compounds; Atwood, J.L., Davies, J.E.D., MacNicol, D.D., Eds.; Academic Press: London, UK, 1984; Volume 2. [Google Scholar]
- Harata, K. Comprehensive Supramolecular Chemistry; Atwood, J.L., Davies, J.E.D., MacNicol, D.D., Eds.; Pergamon: Oxford, UK, 1996; Volume 3. [Google Scholar]
- Cohen, R.; Orlova, Y.; Kovalev, V.; Ungar, Y.; Shimoni, E. Structural and Functional Properties of Amylose Complexes with Genistein. J. Agric. Food Chem. 2008, 56, 4212–4218. [Google Scholar] [CrossRef]
- Pesek, S.; Lehene, M.; Brânzanic, A.M.V.; Silaghi-Dumitrescu, R. On the Origin of the Blue Color in The Iodine/Iodide/Starch Supramolecular Complex. Molecules 2022, 27, 8974. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Ali, U. Degree-Based Topological Indices of Polysaccharides: Amylose and Blue Starch-Iodine Complex. J. Chem. 2021, 2021, 6652014. [Google Scholar] [CrossRef]
- Landolt, H. Uber Die Zeitdauer Der Reaction Zwischen Jodsaure Und Schwefliger Saure. Ber. Dtsch. Chem. Ges. 1886, 19, 1317–1365. [Google Scholar] [CrossRef]
- Gilbert, G.A.; Marriott, J.V.R. Starch-Iodine Complexes. Part I. Trans. Faraday Soc. 1948, 44, 84–93. [Google Scholar] [CrossRef]
- Thoma, J.A.; French, D. The Starch-Iodine-Iodide Interaction. Part I. Spectrophotometric Investigations 1. J. Am. Chem. Soc. 1960, 82, 4144–4147. [Google Scholar] [CrossRef]
- Stein, R.S.; Rundle, R.E. On the Nature of the Interaction between Starch and Iodine. J. Chem. Phys. 1948, 16, 195–207. [Google Scholar] [CrossRef]
- Hiromi, K.; Shibaoka, T.; Ono, S. Kinetic Studies of Amylose-Iodine-Iodide Reaction by Stopped-Flow Method. J. Biochem. 1970, 68, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Yajima, H.; Nishimura, T.; Ishii, T.; Handa, T. Effect of Concentration of Iodide on the Bound Species of I2/I−3 in the Amylose-Iodine Complex. Carbohydr. Res. 1987, 163, 155–167. [Google Scholar] [CrossRef]
- Cronan, C.L.; Schneider, F.W. Cooperativity and Composition of the Linear Amylose-Iodine-Iodide Complex. J. Phys. Chem. 1969, 73, 3990–4004. [Google Scholar] [CrossRef]
- Cramer, F.; Herbst, W. Die Lichtabsorption von Jodkettenmolekeln. Naturwissenschaften 1952, 39, 256. [Google Scholar] [CrossRef]
- Bersohn, R.; Isenberg, I. Metallic Nature of the Starch-Iodine Complex. J. Chem. Phys. 1961, 35, 1640–1643. [Google Scholar] [CrossRef]
- Rundle, R.E.; Baldwin, R.R. The Configuration of Starch and Starch-Iodine Complex. I. The Dichroism of Flow of Starch-Iodine Solutions. J. Am. Chem. Soc. 1943, 65, 554–558. [Google Scholar] [CrossRef]
- Rundle, R.E. The Configuration of Starch in the Starch-Iodine Complex. V. Fourier Projections from X-ray Diagrams. J. Am. Chem. Soc. 1947, 69, 1769–1772. [Google Scholar] [CrossRef]
- Noltemeyer, M.; Saenger, W. X-Ray Studies of Linear Polyiodide Chains in α-Cyclodextrin Channels and a Model for the Starch-Iodine Complex. Nature 1976, 259, 629–632. [Google Scholar] [CrossRef]
- Bluhm, T.L.; Zugenmaier, P. Detailed Structure of the Vh-Amylose-Iodine Complex: A Liner Polyiodine Chain. Carbohydr. Res. 1981, 89, 1–10. [Google Scholar] [CrossRef]
- Handa, T.; Yajima, H. Conformation of Amylose-Iodine-Iodide Complex in Aqueous Solution. Biopolymers 1981, 20, 2051–2072. [Google Scholar] [CrossRef]
- Mould, D.L.; Synge, R.M. Separations of Polysaccharides Related to Starch by Electrokinetic Ultrafiltration in Collodion Membranes. Biochem. J. 1954, 58, 571–600. [Google Scholar] [CrossRef] [PubMed]
- Ono, S.; Tsuchihashi, S.; Kuge, T. On the Starch-Iodine Complex. J. Am. Chem. Soc. 1953, 75, 3601–3602. [Google Scholar] [CrossRef]
- Moulay, S. Molecular Iodine/Polymer Complexes. J. Polym. Eng. 2013, 33, 389–443. [Google Scholar] [CrossRef]
- Séne, M.; Thévenot, C.; Prioul, J.L. Simultaneous Spectrophotometric Determination of Amylose and Amylopectin in Starch from Maize Kernel by Multi-Wavelength Analysis. J. Cereal Sci. 1997, 26, 211–221. [Google Scholar] [CrossRef]
- Sashio, M.; Tanaka, M. Thermal Reaction of Poly(Vinyl Alcohol)-Iodine Complex Membranes. J. Polym. Sci. Polym. Chem. Ed. 1985, 23, 905–909. [Google Scholar] [CrossRef]
- Dintzis, F.R. Instability of Solutions of Amylose-Iodine Complex in Concentrated Calcium Chloride. Starch-Stärke 1974, 26, 56–58. [Google Scholar] [CrossRef]
- Tashiro, K.; Gakhutishvili, M. Crystal Structure of Cellulose-Iodine Complex. Polymer 2019, 171, 140–148. [Google Scholar] [CrossRef]
- Konishi, T.; Tanaka, W.; Kawai, T.; Fujikawa, T. Iodine L-Edge XAFS Study of Linear Polyiodide Chains in Amylose and α-Cyclodextrin. J. Synchrotron Radiat. 2001, 8, 737–739. [Google Scholar] [CrossRef]
- Knutson, C.A. Evaluation of Variations in Amylose–Iodine Absorbance Spectra. Carbohydr. Polym. 2000, 42, 65–72. [Google Scholar] [CrossRef]
- Nishimura, T.; Yajima, H.; Ishii, T.; Endo, R. Effect of Molecular Weight of Amylose on the Iodine Coloring Species Responsible for the Optical Properties of Amylose-Iodine Complexes. Kobunshi Ronbunshu 1989, 46, 537–544. [Google Scholar] [CrossRef]
- SenGupta, U.K.; MuKherjee, A.K.; SenGupta, K.K. Spectrophotometric Studies on Amylose-Iodine and Amylopectin-Iodine Complexes. Kolloid-Z. Z. Polym. 1966, 208, 32–34. [Google Scholar] [CrossRef]
- Sakajiri, T.; Kikuchi, T.; Simon, I.; Uchida, K.; Yamamura, T.; Ishii, T.; Yajima, H. Molecular Dynamics Approach to Study the Discrepancies in the Thermal Behavior of Amylose and Chitosan Conformations. J. Mol. Struct. THEOCHEM 2006, 764, 133–140. [Google Scholar] [CrossRef]
- Szejtli, J.; Augustat, S.; Richter, M. Molecular Configuration of Amylose and Its Complexes in Aqueous Solutions. Part III. Investigation of the DP Distribution of Helical Segments in Amylose-Iodine Complexes. Biopolymers 1967, 5, 17–26. [Google Scholar] [CrossRef]
- McMullan, R.K.; Saenger, W.; Fayos, J.; Mootz, D. Topography of Cyclodextrin Inclusion Complexes. Carbohydr. Res. 1973, 31, 211–227. [Google Scholar] [CrossRef]
- Baldwin, R.R.; Bear, R.S.; Rundle, R.E. The Relation of Starch—Iodine Absorption Spectra to the Structure of Starch and Starch Components 1. J. Am. Chem. Soc. 1944, 66, 111–115. [Google Scholar] [CrossRef]
- Davis, H.; Khan, A. Determining the Chromophore in the Amylopectin–Iodine Complex by Theoretical and Experimental Studies. J. Polym. Sci. A Polym. Chem. 1994, 32, 2257–2265. [Google Scholar] [CrossRef]
- Rendleman, J.A. The Reaction of Starch with Iodine Vapor. Determination of Iodide-Ion Content of Starch–Iodine Complexes. Carbohydr. Polym. 2003, 51, 191–202. [Google Scholar] [CrossRef]
- Yu, X.; Houtman, C.; Atalla, R.H. The Complex of Amylose and Iodine. Carbohydr. Res. 1996, 292, 129–141. [Google Scholar] [CrossRef]
- Abe, T. The Visible and Ultraviolet Absorption Spectra of Cellulose- and Amylose-Iodine Complexes. Bull. Chem. Soc. Jpn. 1958, 31, 661–662. [Google Scholar] [CrossRef]
- Takahashi, Y.J. Binding Properties of Alginic Acid and Chitin. Inclus. Phenom. 1978, 5, 525–534. [Google Scholar] [CrossRef]
- Yajima, H.; Morita, M.; Hashimoto, M.; Sashiwa, H.; Kikuchi, T.; Ishii, T. Complex Formation of Chitosan with Iodine and Its Strucutre and Spectroscopic Properties—Molecular Assembly and Thermal Hysteresis Behavior. Int. J. Thermophys. 2001, 22, 1265–1283. [Google Scholar] [CrossRef]
- Gunasekaran, M. Physiological Studies on Phymatotrichum Omnivorum II. Physiocochemical Properties of Glycogen. Arch. Mikrobiol. 1972, 84, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Roman, A.; Khan, A. Chromophore and Spectrum of the Glycogen-Iodine Complex. J. Polym. Sci. Part A Polym. Chem. 1996, 34, 2975–2980. [Google Scholar] [CrossRef]
- Lecker, D.N.; Kumari, S.; Khan, A. Iodine Binding Capacity and Iodine Binding Energy of Glycogen. J. Polym. Sci. Part A Polym. Chem. 1997, 35, 1409–1412. [Google Scholar] [CrossRef]
- Aterman, K. A Historical Note on the Iodine-Sulphuric Acid Reaction of Amyloid. Histochemistry 1976, 49, 131–143. [Google Scholar] [CrossRef]
- Dzwolak, W. Insulin Amyloid Fibrils Form an Inclusion Complex with Molecular Iodine: A Misfolded Protein as a Nanoscale Scaffold. Biochemistry 2007, 46, 1568–1572. [Google Scholar] [CrossRef]
- Pritchard, J.G.; Serra, F.T. Complexation of Polyvinyl Acetate with Iodine. Talanta 1973, 20, 541–546. [Google Scholar] [CrossRef]
- Hughes, J. Analytical Behaviour of Poly(Vinyl Acetate) and Its Hydrolysis Products with Iodine. Talanta 1979, 26, 1161–1163. [Google Scholar] [CrossRef]
- Schulz, R.C.; Fleischer, D.; Henglein, A.; Bössler, H.M.; Trisnadi, J.; Tanaka, H. Addition Compounds and Complexes with Polymers and Models. Pure Appl. Chem. 1974, 38, 227–247. [Google Scholar] [CrossRef]
- Chernov’yants, M.S.; Burykin, I.V.; Pisanov, R.V.; Shalu, O.A. Synthesis and Antimicrobial Activity of Poly(N-Methyl-4-Vinylpyridinium Triiodide). Pharm. Chem. J. 2010, 44, 61–63. [Google Scholar] [CrossRef]
- Pal, S.K.; Krishnan, A.; Das, P.K.; Samuelson, A.G. Schiff Base Linked Ferrocenyl Complexes for Second-Order Nonlinear Optics. J. Organomet. Chem. 2000, 604, 248–259. [Google Scholar] [CrossRef]
- Liu, W.-J.; Xiong, G.-X.; Zeng, D.-H. Synthesis and Electrical Properties of Three Novel Poly (Ferrocenyl-Schiff Bases) and Their Charge Transfer Complexes with Iodine. J. Inorg. Organomet. Polym. 2010, 20, 97–103. [Google Scholar] [CrossRef]
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.F.; Heeger, A.J. Synthesis of Electrically Conducting Organic Polymers: Halogen Derivatives of Polyacetylene, (CH)x. J. Chem. Soc. Commun. 1977, 578–580. [Google Scholar] [CrossRef]
- Bakueva, L.; Matheson, D.; Musikhin, S.; Sargent, E.H. Luminescence of Pure and Iodine Doped PPV: Internal Energetic Structure Revealed through Spectral Signatures. Synth. Met. 2002, 126, 207–211. [Google Scholar] [CrossRef]
- Fasahat, P.; Rahman, S.; Ratnam, W. Genetic Controls on Starch Amylose Content in Wheat and Rice Grains. J. Genet. 2014, 93, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Ashogbon, A.O.; Akintayo, E.T.; Oladebeye, A.O.; Oluwafemi, A.D.; Akinsola, A.F.; Imanah, O.E. Developments in the Isolation, Composition, and Physicochemical Properties of Legume Starches. Crit. Rev. Food Sci. Nutr. 2021, 61, 2938–2959. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, L.; Zhao, J.; Li, C.; Wu, H.; Zhao, H.; Wu, Q. Granule-Bound Starch Synthase in Plants: Towards an Understanding of Their Evolution, Regulatory Mechanisms, Applications, and Perspectives. Plant Sci. 2023, 336, 111843. [Google Scholar] [CrossRef]
- Guo, K.; Liang, W.; Wang, S.; Guo, D.; Liu, F.; Persson, S.; Herburger, K.; Petersen, B.L.; Liu, X.; Blennow, A.; et al. Strategies for Starch Customization: Agricultural Modification. Carbohydr. Polym. 2023, 321, 121336. [Google Scholar] [CrossRef]
- Chiaramonte, E.; Rhazi, L.; Aussenac, T.; White, D.R. Amylose and Amylopectin in Starch by Asymmetric Flow Field-Flow Fractionation with Multi-Angle Light Scattering and Refractive Index Detection (AF4–MALS–RI). J. Cereal Sci. 2012, 56, 457–463. [Google Scholar] [CrossRef]
- Ulbrich, M.; Scholz, F.; Flöter, E. Chromatographic Study of High Amylose Corn Starch Genotypes—Investigation of Molecular Properties after Specific Enzymatic Digestion. Starch-Stärke 2022, 74, 2100303. [Google Scholar] [CrossRef]
- Tutorskii, I.A.; Sokolova, L.V. Mechanism of the Reaction of Polybutadiene with Molecular Iodine. Polym. Sci. U.S.S.R. 1977, 19, 176–183. [Google Scholar] [CrossRef]
- Sreeja, R.; Najidha, S.; Remya Jayan, S.; Predeep, P.; Mazur, M.; Sharma, P.D. Electro-Optic Materials from Co-Polymeric Elastomer–Acrylonitrile Butadiene Rubber (NBR). Polymer 2006, 47, 617–623. [Google Scholar] [CrossRef]
- Vippa, P.; Rajagopalan, H.; Thakur, M. Electrical and Optical Properties of a Novel Nonconjugated Conductive Polymer, Poly (β-pinene). J. Polym. Sci. Part B Polym. Phys. 2005, 43, 3695–3698. [Google Scholar] [CrossRef]
- Nagelli, W. Beitrage Zur Naheren Kenntniss Der Starkegruppe. Ann. Chem. 1874, 173, 218–227. [Google Scholar] [CrossRef]
- Helbert, W.; Chanzy, H. The Ultrastructure of Starch from Ultrathin Sectioning in Melamine Resin. Starch-Starke 1996, 48, 185–188. [Google Scholar] [CrossRef]
- Atkin, N.J.; Abeysekera, R.M.; Cheng, S.L.; Robards, A.W. An Experimentally-Based Predictive Model for the Separation of Amylopectin Subunits during Starch Gelatinization. Carbohydr. Polym. 1998, 36, 173–192. [Google Scholar] [CrossRef]
- Baker, A.A.; Miles, M.J.; Helbert, W. Internal Structure of the Starch Resistant Granule Revealed by AFM. Carbohydr. Res. 2001, 330, 249–256. [Google Scholar] [CrossRef]
- Baldwin, P.M.; Adler, J.; Davies, M.; Melia, D. High Resolution Imaging of Starch Granule Surfaces by Atomic Force Microscopy. J. Cereal Sci. 1998, 27, 255–265. [Google Scholar] [CrossRef]
- Dang, J.M.C.; Copeland, L. Imaging Rice Grains Using Atomic Force Microscopy. J. Cereal Sci. 2003, 37, 165–170. [Google Scholar] [CrossRef]
- Ohtani, T.; Yoshimo, T.; Hagiwara, S.; Maekawa, T. High-Resolution Imaging of Starch Granule Structure Using Atomic Force Microscopy. Starch-Starke 2000, 52, 150–153. [Google Scholar] [CrossRef]
- Park, H.; Xu, S.; Seetharaman, S. A Novel in Situ Atomic Force Microscopy Imaging Technique to Probe Surface Morphological Features of Starch Granules. Carbohydr. Res. 2011, 346, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Ridout, M.J.; Gunning, A.P.; Parker, M.L.; Wilson, R.H.; Morris, V.J. Using AFM to Image the Internal Structure of Starch Grsnules. Carbohydr. Polym. 2002, 50, 123–132. [Google Scholar] [CrossRef]
- Szymonska, J.; Krok, F. Potato Starch Granule Nanostructure Studied by Highresolution Non-Contact AFM. Int. J. Biol. Macromol. 2003, 33, 1–7. [Google Scholar] [CrossRef]
- Waduge, R.N.; Xu, S.; Seetharaman, S. Iodine Absorption Properties and Its Effect on the Crystallinity of Developing Wheat Starch Granules. Carbohydr. Polym. 2010, 82, 786–794. [Google Scholar] [CrossRef]
- Gallant, D.J.; Bouchet, B.; Baldwin, P.M. Microscopy of Starch: Evidence of a New Level of Granule Organization. Carbohydr. Polym. 1997, 32, 177–191. [Google Scholar] [CrossRef]
- Doutch, J.; Gilbert, E.P. Characterisation of Large Scale Structures in Starch Granules via Small-Angle Neutron and X-ray Scattering Techniques. Carbohydr. Polym. 2013, 91, 444–451. [Google Scholar] [CrossRef]
- Barrett, A.J.; Barrett, K.L.; Khan, A. Effects of Acetone, Ethanol, Isopropanol, and Dimethyl Sulfoxide on Amylose-Iodine Complex. J. Macromol. Sci. Part A 1998, 35, 711–722. [Google Scholar] [CrossRef]
- Fonslick, J.; Khan, A. Thermal Stability and Composition of the Amylose–Iodine Complex. J. Polym. Sci. A Polym. Chem. 1989, 27, 4161–4167. [Google Scholar] [CrossRef]
- Rundle, R.E.; French, D. The Configuration of Starch and the Starch—Iodine Complex. II. Optical Properties of Crystalline Starch Fractions 1. J. Am. Chem. Soc. 1943, 65, 558–561. [Google Scholar] [CrossRef]
- Rundle, R.E.; French, D. The Configuration of Starch in the Starch—Iodine Complex. III. X-ray Diffraction Studies of the Starch—Iodine Complex 1. J. Am. Chem. Soc. 1943, 65, 1707–1710. [Google Scholar] [CrossRef]
- Cramer, F.; Windel, H. Über Einschlußverbindungen, X. Mitteil.: Die Blauen Jodverbindungen Der Cumarine Und Anderer Verwandter Verbindungen. Chem. Ber. 1956, 89, 354–365. [Google Scholar] [CrossRef]
- Saenger, W. The Structure of the Blue Starch-Iodine Complex. Naturwissenschaften 1984, 71, 31–36. [Google Scholar] [CrossRef]
- Immel, S.; Lichtenthaler, F.W. The Hydrophobic Topographies of Amylose and Its Blue Iodine Complex. Starch-Stärke 2000, 52, 1–8. [Google Scholar] [CrossRef]
- Minick, M.; Fotta, K.; Khan, A. Polyiodine Units in Starch-Iodine Complex: INDO CI Study of Spectra and Comparison with Experiments. Biopolymers 1991, 31, 57–63. [Google Scholar] [CrossRef]
- Zaslow, B.; Miller, R.L. Hydration of the “V” Amylose Helix 1. J. Am. Chem. Soc. 1961, 83, 4378–4381. [Google Scholar] [CrossRef]
- Hirai, M.; Hirai, T.; Ueki, T. Effect of Branching of Amylopectin on Complexation with Iodine as Steric Hindrance. Polymer 1994, 35, 2222–2225. [Google Scholar] [CrossRef]
- Dintzis, F.R.; Beckwith, A.C.; Babcock, G.E.; Tobin, R. Amylose-Iodine Complex. I. Sedimentation Behavior. Macromolecules 1976, 9, 471–478. [Google Scholar] [CrossRef]
- Moulik, S.P.; Gupta, S. Effects of Solvents on the Spectrophotometric and Hydrodynamic Behavior of Amylose and Its Iodine Complex. Carbohydr. Res. 1980, 81, 131–143. [Google Scholar] [CrossRef]
- Senior, M.B.; Hamori, E. Investigation of the Effect of Amylose/Iodine Complexation on the Conformation of Amylose in Aqueous Solution. Biopolymers 1973, 12, 65–78. [Google Scholar] [CrossRef]
- Vladimirov, A.V.; Volkova, T.V.; Agafonov, A.V. Temperature Dependence of Stability Constants of the Iodine-Iodide-Amylose Complexes. Russ. J. Phys. Chem. A 2003, 77, 612–615. [Google Scholar]
- Zhang, Q.; Lu, Z.; Hu, H.; Yang, W.; Marszalek, P.E. Direct Detection of the Formation of V-Amylose Helix by Single Molecule Force Spectroscopy. J. Am. Chem. Soc. 2006, 128, 9387–9393. [Google Scholar] [CrossRef] [PubMed]
- Dintzis, F.R.; Tobin, R.; Beckwith, A.C. Amylose-Iodine Complex. II. Molecular Weight Estimates. Macromolecules 1976, 9, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Mikus, F.F.; Hixon, R.M.; Rundle, R.E. The Complexes of Fatty Acids with Amylose 1. J. Am. Chem. Soc. 1946, 68, 1115–1123. [Google Scholar] [CrossRef]
- Calabrese, V.T.; Khan, A. Amylose-Iodine Complex Formation without KI: Evidence for Absence of Iodide Ions within the Complex. J. Polym. Sci. A Polym. Chem. 1999, 37, 2711–2717. [Google Scholar] [CrossRef]
- Cesaro, A.; Benegas, J.C.; Ripoll, D.R. Molecular Model of the Cooperative Amylose-Iodine-Triiodide Complex. J. Phys. Chem. 1986, 90, 2787–2791. [Google Scholar] [CrossRef]
- Kuge, T.; Ono, S. Amylose-Iodine Complex. III. Potentiometric and Spectrophotometric Studies. Bull. Chem. Soc. Jpn. 1960, 33, 1273–1278. [Google Scholar] [CrossRef]
- Schulz, W.; Sklenar, H.; Hinrichs, W.; Saenger, W. The Structure of the Left-Handed Antiparallel Amylose Double Helix: Theoretical Studies. Biopolymers 1993, 33, 363–375. [Google Scholar] [CrossRef]
- Moulik, S.P.; Gupta, S. Environment-Induced, Physicochemical Behavior of Amylose-Iodine Complexes. Carbohydr. Res. 1979, 71, 251–264. [Google Scholar] [CrossRef]
- Szejtli, J.; Richter, M.; Augustat, S. Molecular Configuration of Amylose and Its Complexes in Aqueous Solutions. Part IV. Determination OfDP of Amylose by Measuring the Concentration of Free Iodine in Solution of Amylose-Iodine Complex. Biopolymers 1968, 6, 27–41. [Google Scholar] [CrossRef]
- Peng, Q.-J.; Perlin, A.S. Observations on N.M.R. Spectra of Starches in Dimethyl Sulfoxide, Iodine-Complexing, and Solvation in Water-Di-Methyl Sulfoxide. Carbohydr. Res. 1987, 160, 57–72. [Google Scholar] [CrossRef]
- Murdoch, K.A. The Amylose-Iodine Complex. Carbohydr. Res. 1992, 233, 161–174. [Google Scholar] [CrossRef]
- Knutson, C.A.; Cluskey, J.E.; Dintzis, F.R. Properties of Amylose-Iodine Complexes Prepared in the Presence of Excess Iodine. Carbohydr. Res. 1982, 101, 117–128. [Google Scholar] [CrossRef]
- Murakami, H. Electronic Structure of the Amylose-Iodine Complex. J. Chem. Phys. 1954, 22, 367–374. [Google Scholar] [CrossRef]
- Nishimura, T.; Yajima, H.; Kubota, S.; Ishii, T.; Endo, R. Polymer Effect on the Iodine Coloring Species Responsible for the Spectroscopic Properties of Amylose-Iodine Complexes. Kobunshi Ronbunshu 1990, 47, 717–725. [Google Scholar] [CrossRef]
- Nishimura, T.; Yajima, H.; Kubota, S.; Ishii, T.; Endo, R. Effect of I− Concentration on the Optical Properties of Amylose-Iodine Complexes. Kobunshi Ronbunshu 1988, 45, 945–952. [Google Scholar] [CrossRef]
- Rawlings, P.K.; Schneider, F.W. Models for Competitive Cooperative Linear Adsorption. The Amylose–Iodine–Iodide Complex. J. Chem. Phys. 1970, 52, 946–952. [Google Scholar] [CrossRef]
- Kuge, T.; Ono, S. Advances in Carbohydrate Chemistry and Biochemistry. Bull. Chem. Sot. Jpn. 1960, 33, 1269–1272. [Google Scholar] [CrossRef]
- Teitelbaum, R.C.; Ruby, S.L.; Marks, T.J. A Resonance Raman/Iodine Moessbauer Investigation of the Starch-Iodine Structure. Aqueous Solution and Iodine Vapor Preparations. J. Am. Chem. Soc. 1980, 102, 3322–3328. [Google Scholar] [CrossRef]
- Cesàro, A.; Jerian, E.; Saule, S. Physicochemical Studies of Amylose and Its Derivatives in Aqueous Solutions: Thermodynamics of the Iodine-Triiodide Complex. Biopolymers 1980, 19, 1491–1506. [Google Scholar] [CrossRef]
- Nishimura, T.; Yajima, H.; Ishii, T.; Endo, R. Study of the Bluing Mechanism of Amylose-Iodine Complexes by CD Stopped-Flow Method. Kobunshi Ronbunshu 1991, 48, 525–528. [Google Scholar] [CrossRef]
- Wolf, R.; Schulz, R.C. Optical Rotatory Dispersion of the Starch Iodine Complex. Part 2. J. Macromol. Sci. Part A Chem. 1968, 2, 821–832. [Google Scholar] [CrossRef]
- Agafonov, A.V.; Vladimirov, A.V.; Volkova, T.V. The Concentration Dependences of the Stability Constants of Iodine-Iodide-Amylose Complexes in Aqueous Solutions of Electrolytes. Russ. J. Phys. Chem. A 2004, 78, 1584–1587. [Google Scholar]
- Yamamoto, M.; Sano, T.; Harada, S.; Yasunaga, T. Interaction of Amylose with Iodine. II. Kinetic Studies of the Complex Formation by the Temperature-Jump Method. Bull. Chem. Soc. Jpn. 1982, 55, 3702–3706. [Google Scholar] [CrossRef]
- Foster, J.F.; Zucker, D. Length of the Amylose–Iodine Complex as Determined by Streaming Dichroism. J. Phys. Chem. 1952, 56, 170–173. [Google Scholar] [CrossRef]
- Noltemeyer, M.; Saenger, W. Topography of Cyclodextrin Inclusion Complexes. 12. Structural Chemistry of Linear.Alpha.-Cyclodextrin-Polyiodide Complexes. X-ray Crystal Structures of (.Alpha.-Cyclodextrin)2.LiI3.I2.8H2O and (.Alpha.-Cyclodextrin)2.Cd0.5).I5.27H2O. Models for the Blue. J. Am. Chem. Soc. 1980, 102, 2710–2722. [Google Scholar] [CrossRef]
- Betzel, C.; Hingerty, B.; Noltemeyer, M.; Weber, G.; Saenger, W.; Hamilton, J.A. (β-Cyclodextrin)2 KI7 9 H2O. Spatial Fitting of a Polyiodide Chain to a given Matrix. J. Incl. Phenom. 1983, 1, 181–191. [Google Scholar] [CrossRef]
- Bowmaker, G. Bonding and Nuclear Quadrupole Coupling in Linear Pentaiodide Ions. Aust. J. Chem. 1978, 31, 2713. [Google Scholar] [CrossRef]
- Nimz, O.; Geßler, K.; Usón, I.; Laettig, S.; Welfle, H.; Sheldrick, G.M.; Saenger, W. X-Ray Structure of the Cyclomaltohexaicosaose Triiodide Inclusion Complex Provides a Model for Amylose–Iodine at Atomic Resolution. Carbohydr. Res. 2003, 338, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Ziegast, G.; Pfannemüller, B. Resonance Raman Studies of Amaylose—Iodine Complexes. Int. J. Biol. Macromol. 1982, 4, 419–424. [Google Scholar] [CrossRef]
- Heyde, M.E.; Rimai, L.; Kilponen, R.G.; Gill, D. Resonance-Enhanced Raman Spectra of Iodine Complexes with Amylose and Poly(Vinyl Alcohol), and of Some Iodine-Containing Trihalides. J. Am. Chem. Soc. 1972, 94, 5222–5227. [Google Scholar] [CrossRef]
- Okuda, M.; Hiramatsu, T.; Yasuda, M.; Ishigaki, M.; Ozaki, Y.; Hayashi, M.; Tominaga, K.; Chatani, E. Theoretical Modeling of Electronic Structures of Polyiodide Species Included in α-Cyclodextrin. J. Phys. Chem. B 2020, 124, 4089–4096. [Google Scholar] [CrossRef]
- Mizuno, M.; Tanaka, J.; Harada, I. Electronic Spectra and Structures of Polyiodide Chain Complexes. J. Phys. Chem. 1981, 85, 1789–1794. [Google Scholar] [CrossRef]
- Teitelbaum, R.C.; Ruby, S.L.; Marks, T.J. On the Structure of Starch-Iodine. J. Am. Chem. Soc. 1978, 100, 3215–3217. [Google Scholar] [CrossRef]
- Hach, R.J.; Rundle, R.E. The Structure of Tetramethylammonium Pentaiodide 1,1a. J. Am. Chem. Soc. 1951, 73, 4321–4324. [Google Scholar] [CrossRef]
- Herbstein, F.H.; Kapon, M. Zigzag Chains of Alternating Molecules and Triiodide Ions in Crystalline (Phenacetin)2.HI5. Nat. Phys. Sci. 1972, 239, 153–154. [Google Scholar] [CrossRef]
- Haddock, A.; Steidemann, M.; Readnour, M. Polyiodide Equilibria in Aqueous Solutions of Iodine and Iodide. Synth. React. Inorg. Met. Org. Chem. 1979, 9, 39–56. [Google Scholar] [CrossRef]
- Ramette, R.W.; Sandford, R.W. Thermodynamics of Iodine Solubility and Triiodide Ion Formation in Water and in Deuterium Oxide. J. Am. Chem. Soc. 1965, 87, 5001–5005. [Google Scholar] [CrossRef]
- Sekine, T. Abstracts. Nippon Kagaku Zassi 1969, 90, 951–983. [Google Scholar] [CrossRef]
- Mould, D.L. Potentiometric and Spectrophotometric Studies of Complexes of Hydrolysis Products of Amylose with Iodine and Potassium Iodide. Biochem. J. 1954, 58, 593–600. [Google Scholar] [CrossRef]
- Bhide, S.V.; Kale, N.R. Ligand-Induced Structural Changes in Amylose Partially Complexed with Iodine. Biochim. Biophys. Acta (BBA)—Gen. Subj. 1976, 444, 719–726. [Google Scholar] [CrossRef]
- Benesi, H.A.; Hildebrand, J.H. A Spectrophotometric Investigation of the Interaction of Iodine with Aromatic Hydrocarbons. J. Am. Chem. Soc. 1949, 71, 2703–2707. [Google Scholar] [CrossRef]
- Bernal-Uruchurtu, M.I.; Kerenskaya, G.; Janda, K.C. Structure, Spectroscopy and Dynamics of Halogen Molecules Interacting with Water. Int. Rev. Phys. Chem. 2009, 28, 223–265. [Google Scholar] [CrossRef]
- Kireev, S.V.; Shnyrev, S.L. Study of Molecular Iodine, Iodate Ions, Iodide Ions, and Triiodide Ions Solutions Absorption in the UV and Visible Light Spectral Bands. Laser Phys. 2015, 25, 075602. [Google Scholar] [CrossRef]
- Prasanna; Shrikanth, B.K.; Hegde, M.S. Formation and Structure of Iodine: Water (H2O-I2) Charge-Transfer Complex. J. Chem. Sci. 2021, 133, 51. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pesek, S.; Silaghi-Dumitrescu, R. The Iodine/Iodide/Starch Supramolecular Complex. Molecules 2024, 29, 641. https://doi.org/10.3390/molecules29030641
Pesek S, Silaghi-Dumitrescu R. The Iodine/Iodide/Starch Supramolecular Complex. Molecules. 2024; 29(3):641. https://doi.org/10.3390/molecules29030641
Chicago/Turabian StylePesek, Szilard, and Radu Silaghi-Dumitrescu. 2024. "The Iodine/Iodide/Starch Supramolecular Complex" Molecules 29, no. 3: 641. https://doi.org/10.3390/molecules29030641





