On the Fragmentation of Ni(II) β-Diketonate-Diamine Complexes as Molecular Precursors for NiO Films: A Theoretical and Experimental Investigation
Abstract
1. Introduction
2. Results
2.1. ESI-MS Experiments
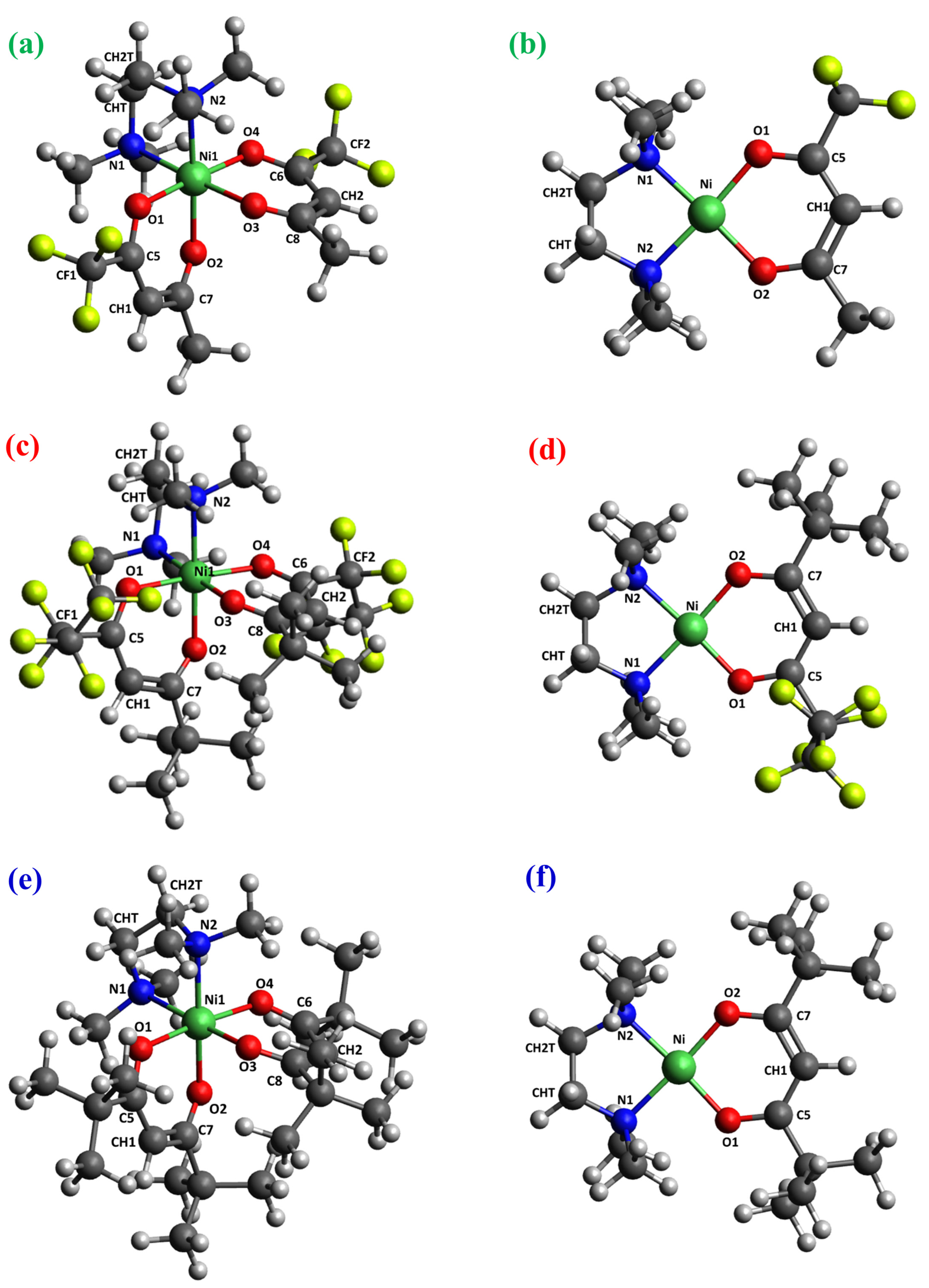
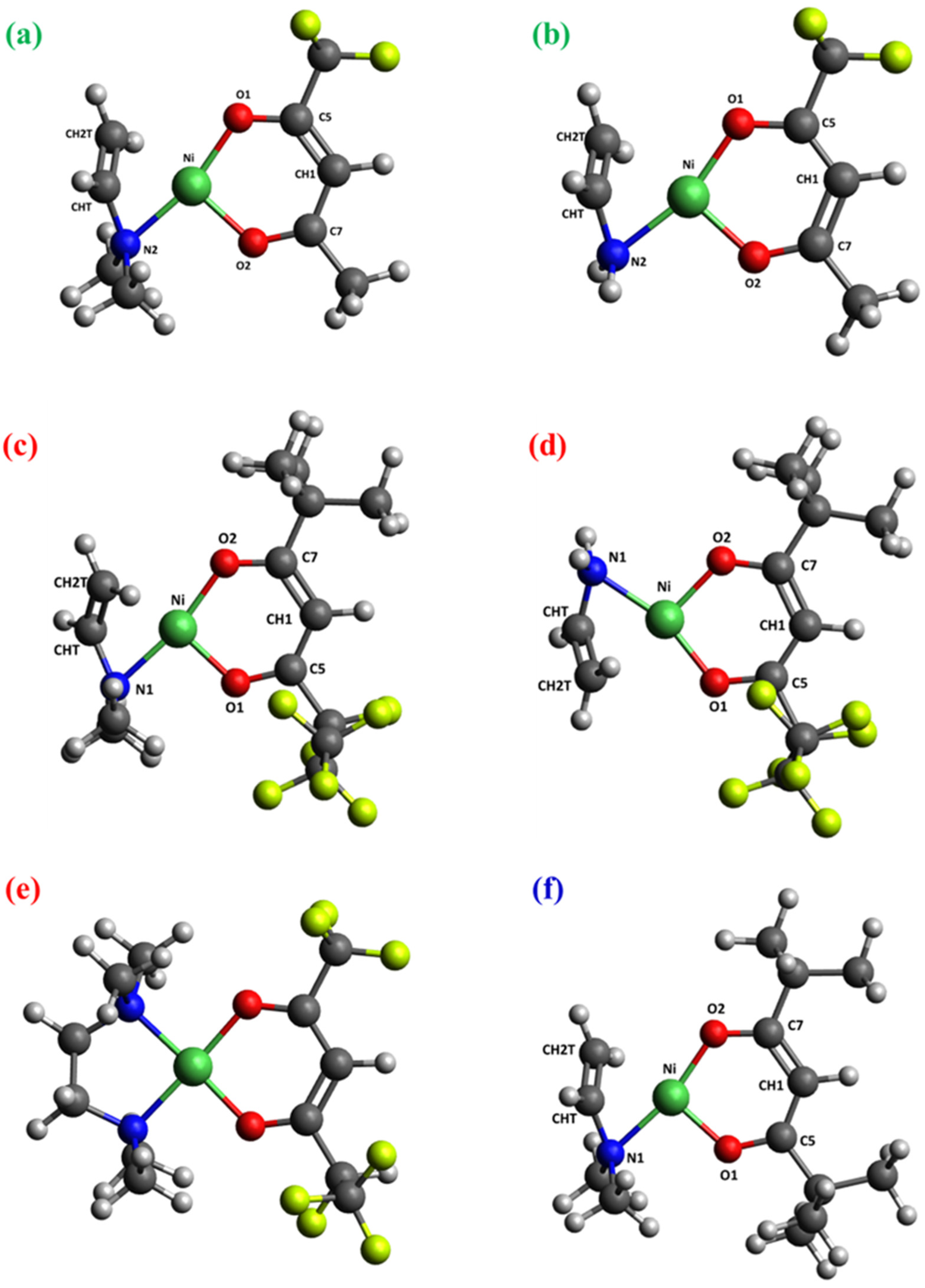
2.2. DFT Calculations
3. Materials and Methods
3.1. Experimental Details
3.2. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nachammai, J.; Perumal, P.; Deivamani, D.; Saravanakumar, S. Effect of concentrations and characterization of nickel oxide thin films prepared by SILAR method. Mater. Today Proc. 2022, 64, 1789–1792. [Google Scholar] [CrossRef]
- Sialvi, M.Z.; Mortimer, R.J.; Wilcox, G.D.; Teridi, A.M.; Varley, T.S.; Wijayantha, K.G.U.; Kirk, C.A. Electrochromic and colorimetric properties of nickel(II) oxide thin films prepared by aerosol-assisted chemical vapor deposition. ACS Appl. Mater. Interfaces 2013, 5, 5675–5682. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Tu, Z.; Ji, L.; Wu, H.; Xu, H.; Liu, C. Electrochromic properties of NiO films prepared by atomic layer deposition. J. Vac. Sci. Technol. A 2023, 41, 062407. [Google Scholar] [CrossRef]
- Khera, E.A.; Ullah, H.; Hussain, F.; Abubakar, S.; Majeed, A.; Tabssum, I.; Batool, Z.; Nazir, A.; Gilanie, G. Characterizing nickel oxide thin films for smart window energy conversion applications: Combined experimental and theoretical analyses. ChemistrySelect 2023, 8, e20230232. [Google Scholar] [CrossRef]
- Brioual, B.; Ghannam, H.; Rossi, Z.; Aouni, A.; El-Habib, A.; Diani, M.; Addou, M.; Matassa, R.; Nottola, S.; Jbilou, M. Effect of In-doping on electrochromic behavior of NiO thin films. Materialia 2023, 30, 101832. [Google Scholar] [CrossRef]
- Carrillo-Delgado, C.; Arano-Martínez, J.A.; Vidales-Hurtado, M.A.; Torres-Torres, D.; Martínez-González, C.L.; Torres-Torres, C. Electrically induced directional self-focusing in electrochromic NiO thin solid films. J. Mater. Sci. Mater. Electron. 2023, 34, 953. [Google Scholar] [CrossRef]
- Tang, Y.; Shen, H.; Wang, T.; Peng, S.; Jin, K.; Qian, Q.; Li, G.; Gan, Z. Study on electrochemical ion behavior and electrochromic properties of NiO thin films prepared by magnetron sputtering with different oxygen levels. Thin Solid Films 2023, 769, 139754. [Google Scholar] [CrossRef]
- Wilson, R.L.; Macdonald, T.J.; Lin, C.-T.; Xu, S.; Taylor, A.; Knapp, C.E.; Guldin, S.; McLachlan, M.A.; Carmalt, C.J.; Blackman, C.S. Chemical vapour deposition (CVD) of nickel oxide using the novel nickel dialkylaminoalkoxide precursor [Ni(dmamp′)2] (dmamp′ = 2-dimethylamino-2-methyl-1-propanolate). RSC Adv. 2021, 11, 22199–22205. [Google Scholar] [CrossRef]
- Rajesh, M.; Vengatesan, K.; Aly, M.H.; Sitharthan, R.; Dhanabalan, S.S.; Karthikeyan, M. Electrical and optical properties of sol–gel-deposited NiO films and corresponding response to annealing temperature. Opt. Quantum Electron. 2023, 55, 1167. [Google Scholar] [CrossRef]
- Gao, X.; Meng, X.; Li, B. Direct-current-sputtered nickel oxide (NiO) films with improved p-type conduction characteristic: Impact of substrate temperature on structure, optical and electrical properties. Phys. Scr. 2023, 98, 085916. [Google Scholar] [CrossRef]
- Aivalioti, C.; Manidakis, E.G.; Pelekanos, N.T.; Androulidaki, M.; Tsagaraki, K.; Viskadourakis, Z.; Spanakis, E.; Aperathitis, E. Niobium-doped NiO as p-type nanostructured layer for transparent photovoltaics. Thin Solid Films 2023, 778, 139910. [Google Scholar] [CrossRef]
- Jiang, Q.S.; Wu, Y.; Xie, Z.; Wei, M.; Zhao, Y.; Yang, X.; Xun, W.; Cao, S.; Wang, C. Syntheses of efficient nickel oxide film by low-temperature fabrication technique for high-performance perovskite solar cells. Mater. Today Commun. 2023, 35, 106401. [Google Scholar] [CrossRef]
- Rajesh, K.; Pothukanuri, N.; Reddy, M.V.R. Studies on pure and Zn doped NiO nanostructured thin films for enhanced ammonia gas sensing applications. Chem. Phys. Impact 2024, 8, 100397. [Google Scholar] [CrossRef]
- Ambi, R.R.; Mane, A.A.; Tasgaonkar, R.D.; Mane, R.D. Highly porous NiO microstructure for NO2 detection. Physica B 2024, 674, 415567. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, L.; Zhou, Q.; Zhang, Z.; Dong, Z.; Nie, L.; Liu, Q.; Pan, G. In-situ synthesis of needle-like PdO-decorated NiO thin films on Al2O3 substrates for high-performance H2 sensors. Ceram. Int. 2022, 48, 31746–31754. [Google Scholar] [CrossRef]
- Srivastava, S.; Dwivedi, C.; Yadav, A.; Kumar, A.; Gupta, G.; Singh, P. Enhanced H2S gas sensing of Pd functionalized NiO thin films deposited by the magnetron sputtering process. Mater. Lett. 2023, 351, 135040. [Google Scholar] [CrossRef]
- Dastan, D.; Shan, K.; Jafari, A.; Marszalek, T.; Mohammed, M.K.A.; Tao, L.; Shi, Z.; Chen, Y.; Yin, X.-T.; Alharbi, N.D.; et al. Influence of heat treatment on H2S gas sensing features of NiO thin films deposited via thermal evaporation technique. Mater. Sci. Semicond. Process. 2023, 154, 107232. [Google Scholar] [CrossRef]
- Han, S.W.; Kim, I.H.; Kim, D.H.; Park, K.J.; Park, E.J.; Jeong, M.-G.; Kim, Y.D. Temperature regulated-chemical vapor deposition for incorporating NiO nanoparticles into mesoporous media. Appl. Surf. Sci. 2016, 385, 597–604. [Google Scholar] [CrossRef]
- Kitchamsetti, N.; Ramteke, M.S.; Rondiya, S.R.; Mulani, S.R.; Patil, M.S.; Cross, R.W.; Dzade, N.Y.; Devan, R.S. DFT and experimental investigations on the photocatalytic activities of NiO nanobelts for removal of organic pollutants. J. Alloys Compd. 2021, 855, 157337. [Google Scholar] [CrossRef]
- Zywitzki, D.; Taffa, D.H.; Lamkowski, L.; Winter, M.; Rogalla, D.; Wark, M.; Devi, A. Tuning coordination geometry of nickel ketoiminates and its influence on thermal characteristics for chemical vapor deposition of nanostructured NiO electrocatalysts. Inorg. Chem. 2020, 59, 10059–10070. [Google Scholar] [CrossRef] [PubMed]
- An, W.-J.; Thimsen, E.; Biswas, P. Aerosol-chemical vapor deposition method for synthesis of nanostructured metal oxide thin films with controlled morphology. J. Phys. Chem. Lett. 2010, 1, 249–253. [Google Scholar] [CrossRef]
- Weidler, N.; Schuch, J.; Knaus, F.; Stenner, P.; Hoch, S.; Maljusch, A.; Schäfer, R.; Kaiser, B.; Jaegermann, W. X-ray photoelectron spectroscopic investigation of plasma-enhanced chemical vapor deposited NiOx, NiOx(OH)y, and CoNiOx(OH)y: Influence of the chemical composition on the catalytic activity for the oxygen evolution reaction. J. Phys. Chem. C 2017, 121, 6455–6463. [Google Scholar] [CrossRef]
- Hussain, N.; Yang, W.; Dou, J.; Chen, Y.; Qian, Y.; Xu, L. Ultrathin mesoporous F-doped α-Ni(OH)2 nanosheets as an efficient electrode material for water splitting and supercapacitors. J. Mater. Chem. A 2019, 7, 9656–9664. [Google Scholar] [CrossRef]
- Jellal, I.; Daoudi, O.; Nouneh, K.; Boutamart, M.; Briche, S.; Fahoume, M.; Naja, J. Comparative study on the properties of Al- and Ni-doped ZnO nanostructured thin films grown by SILAR technique: Application to solar photocatalysis. Opt. Quantum Electron. 2023, 55, 620. [Google Scholar] [CrossRef]
- Haghverdi Khamene, S.; van Helvoirt, C.; Tsampas, M.N.; Creatore, M. Electrochemical activation of atomic-layer-deposited nickel oxide for water oxidation. J. Phys. Chem. C 2023, 127, 22570–22582. [Google Scholar] [CrossRef] [PubMed]
- Munawar, K.; Mansoor, M.A.; Naeem, R.; Rizwan, M.; Ahmad, M.S.; Zaharinie, T.; Zubir, M.N.M.; Aspanut, Z. Effect of deposition temperature on topography and electrochemical water oxidation of NiO thin films. Thin Solid Films 2023, 782, 140031. [Google Scholar] [CrossRef]
- Mishra, S.; Daniele, S. Metal–organic derivatives with fluorinated ligands as precursors for inorganic nanomaterials. Chem. Rev. 2015, 115, 8379–8448. [Google Scholar] [CrossRef]
- Bekermann, D.; Barreca, D.; Gasparotto, A.; Maccato, C. Multi-component oxide nanosystems by Chemical Vapor Deposition and related routes: Challenges and perspectives. CrystEngComm 2012, 14, 6347–6358. [Google Scholar] [CrossRef]
- Mishra, S.; Daniele, S. Molecular engineering of metal alkoxides for solution phase synthesis of high-tech metal oxide nanomaterials. Chem. Eur. J. 2020, 26, 9292–9303. [Google Scholar] [CrossRef]
- Barreca, D.; Fois, E.; Gasparotto, A.; Maccato, C.; Oriani, M.; Tabacchi, G. The early steps of molecule-to-material conversion in chemical vapor deposition (CVD): A case study. Molecules 2021, 26, 1988. [Google Scholar] [CrossRef]
- Klotzsche, M.; Barreca, D.; Bigiani, L.; Seraglia, R.; Gasparotto, A.; Vanin, L.; Jandl, C.; Pöthig, A.; Roverso, M.; Bogialli, S.; et al. Facile preparation of a cobalt diamine diketonate adduct as a potential vapor phase precursor for Co3O4 films. Dalton Trans. 2021, 50, 10374–10385. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Bigiani, L.; Klotzsche, M.; Gasparotto, A.; Seraglia, R.; Jandl, C.; Pöthig, A.; Fois, E.; Vanin, L.; Tabacchi, G.; et al. A versatile Fe(II) diketonate diamine adduct: Preparation, characterization and validation in the chemical vapor deposition of iron oxide nanomaterials. Mater. Chem. Phys. 2022, 277, 125534. [Google Scholar] [CrossRef]
- Stienen, C.; Grahl, J.; Wölper, C.; Schulz, S.; Bendt, G. Fluorinated β-diketonate complexes M(tfac)2(TMEDA) (M = Fe, Ni, Cu, Zn) as precursors for the MOCVD growth of metal and metal oxide thin films. RSC Adv. 2022, 12, 22974–22983. [Google Scholar] [CrossRef]
- Utke, I.; Swiderek, P.; Höflich, K.; Madajska, K.; Jurczyk, J.; Martinović, P.; Szymańska, I.B. Coordination and organometallic precursors of group 10 and 11: Focused electron beam induced deposition of metals and insight gained from chemical vapour deposition, atomic layer deposition, and fundamental surface and gas phase studies. Coord. Chem. Rev. 2022, 458, 213851. [Google Scholar] [CrossRef]
- Utriainen, M.; Kröger-Laukkanen, M.; Johansson, L.-S.; Niinistö, L. Studies of metallic thin film growth in an atomic layer epitaxy reactor using M(acac)2 (M=Ni, Cu, Pt) precursors. Appl. Surf. Sci. 2000, 157, 151–158. [Google Scholar] [CrossRef]
- Lindahl, E.; Ottosson, M.; Carlsson, J.-O. Atomic layer deposition of NiO by the Ni(thd)2/H2O precursor combination. Chem. Vap. Depos. 2009, 15, 186–191. [Google Scholar] [CrossRef]
- Lindahl, E.; Lu, J.; Ottosson, M.; Carlsson, J.O. Epitaxial NiO (100) and NiO (111) films grown by atomic layer deposition. J. Cryst. Growth 2009, 311, 4082–4088. [Google Scholar] [CrossRef]
- Utriainen, M.; Kröger-Laukkanen, M.; Niinistö, L. Studies of NiO thin film formation by atomic layer epitaxy. Mater. Sci. Eng. B 1998, 54, 98–103. [Google Scholar] [CrossRef]
- Chandrakala, M.; Raj Bharath, S.; Maiyalagan, T.; Arockiasamy, S. Synthesis, crystal structure and vapour pressure studies of novel nickel complex as precursor for NiO coating by metalorganic chemical vapour deposition technique. Mater. Chem. Phys. 2017, 201, 344–353. [Google Scholar] [CrossRef]
- Emmenegger, F.; Schlaepfer, C.W.; Stoeckli-Evans, H.; Piccand, M.; Piekarski, H. Chelate effect in the gas phase. The complexes of Ni(2,2,6,6-tetramethyl-3,5-heptanedionate)2 with bidentate ligands. Inorg. Chem. 2001, 40, 3884–3888. [Google Scholar] [CrossRef]
- Maccato, C.; Bigiani, L.; Carraro, G.; Gasparotto, A.; Seraglia, R.; Kim, J.; Devi, A.; Tabacchi, G.; Fois, E.; Pace, G.; et al. Molecular engineering of MnII diamine diketonate precursors for the vapor deposition of manganese oxide nanostructures. Chem. Eur. J. 2017, 23, 17954–17963. [Google Scholar] [CrossRef]
- Barreca, D.; Carraro, G.; Devi, A.; Fois, E.; Gasparotto, A.; Seraglia, R.; Maccato, C.; Sada, C.; Tabacchi, G.; Tondello, E.; et al. β-Fe2O3 nanomaterials from an iron(II) diketonate-diamine complex: A study from molecular precursor to growth process. Dalton Trans. 2012, 41, 149–155. [Google Scholar] [CrossRef]
- Bandoli, G.; Barreca, D.; Gasparotto, A.; Maccato, C.; Seraglia, R.; Tondello, E.; Devi, A.; Fischer, R.A.; Winter, M. A cobalt(II) hexafluoroacetylacetonate ethylenediamine complex as a CVD molecular source of cobalt oxide nanostructures. Inorg. Chem. 2009, 48, 82–89. [Google Scholar] [CrossRef]
- Fois, E.; Tabacchi, G.; Barreca, D.; Gasparotto, A.; Tondello, E. “Hot” surface activation of molecular complexes: Insight from modeling studies. Angew. Chem. Int. Ed. 2010, 49, 1944–1948. [Google Scholar] [CrossRef]
- Barreca, D.; Fois, E.; Gasparotto, A.; Seraglia, R.; Tondello, E.; Tabacchi, G. How does CuII convert into CuI? An unexpected ring-mediated single-electron reduction. Chem. Eur. J. 2011, 17, 10864–10870. [Google Scholar] [CrossRef] [PubMed]
- Benedet, M.; Barreca, D.; Fois, E.; Seraglia, R.; Tabacchi, G.; Roverso, M.; Pagot, G.; Invernizzi, C.; Gasparotto, A.; Heidecker, A.A.; et al. Interplay between coordination sphere engineering and properties of nickel diketonate–diamine complexes as vapor phase precursors for the growth of NiO thin films. Dalton Trans. 2023, 52, 10677–10688. [Google Scholar] [CrossRef] [PubMed]
- Benedet, M.; Maccato, C.; Pagot, G.; Invernizzi, C.; Sada, C.; Di Noto, V.; Rizzi, G.A.; Fois, E.; Tabacchi, G.; Barreca, D. Growth of NiO thin films in the presence of water vapor: Insights from experiments and theory. J. Phys. Chem. C 2023, 127, 22304–22314. [Google Scholar] [CrossRef]
- Kruve, A.; Kaupmees, K.; Liigand, J.; Oss, M.; Leito, I. Sodium adduct formation efficiency in ESI source. J. Mass Spectrom. 2013, 48, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Fenn, J.B.; Mann, M.; Meng, C.K.; Wong, S.F.; Whitehouse, C.M. Electrospray ionization–principles and practice. Mass Spectrom. Rev. 1990, 9, 37–70. [Google Scholar] [CrossRef]
- Fenn, J.B.; Mann, M.; Meng, C.K.; Wong, S.F.; Whitehouse, C.M. Electrospray ionization for mass spectrometry of large biomolecules. Science 1989, 246, 64–71. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Wang, Y.; Verma, P.; Jin, X.; Truhlar, D.G.; He, X. Revised M06 density functional for main-group and transition-metal chemistry. Proc. Natl. Acad. Sci. USA 2018, 115, 10257–10262. [Google Scholar] [CrossRef] [PubMed]
- Tabacchi, G.; Fois, E.; Barreca, D.; Gasparotto, A. CVD precursors for transition metal oxide nanostructures: Molecular properties, surface behavior and temperature effects. Phys. Status Solidi A 2014, 211, 251–259. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Martin, J.M.L.; Sundermann, A. Correlation consistent valence basis sets for use with the Stuttgart–Dresden–Bonn relativistic effective core potentials: The atoms Ga–Kr and In–Xe. J. Chem. Phys. 2001, 114, 3408–3420. [Google Scholar] [CrossRef]
- Dunning, T.H., Jr.; Hay, P.J. Modern Theoretical Chemistry; Plenum: New York, NY, USA, 1977; Volume 3, pp. 1–28. [Google Scholar]
- Reed, A.E.; Weinhold, F. Natural localized molecular orbitals. J. Chem. Phys. 1985, 83, 1736–1740. [Google Scholar] [CrossRef]
- Wiberg, K.B. Application of the pople-santry-segal CNDO method to the cyclopropylcarbinyl and cyclobutyl cation and to bicyclobutane. Tetrahedron 1968, 24, 1083–1096. [Google Scholar] [CrossRef]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Tang, W.; Sanville, E.; Henkelman, G. A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens. Matter 2009, 21, 084204. [Google Scholar] [CrossRef] [PubMed]
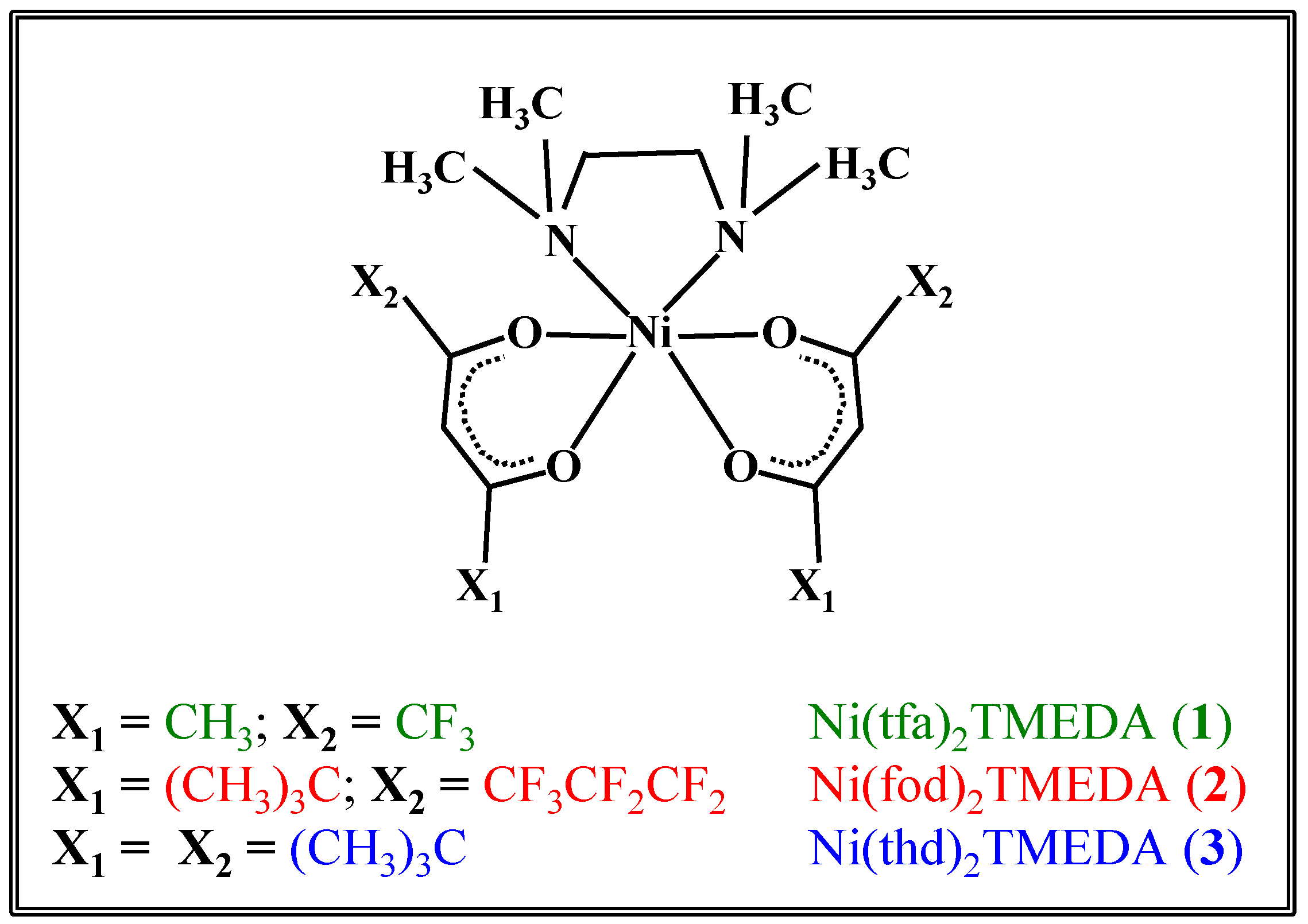
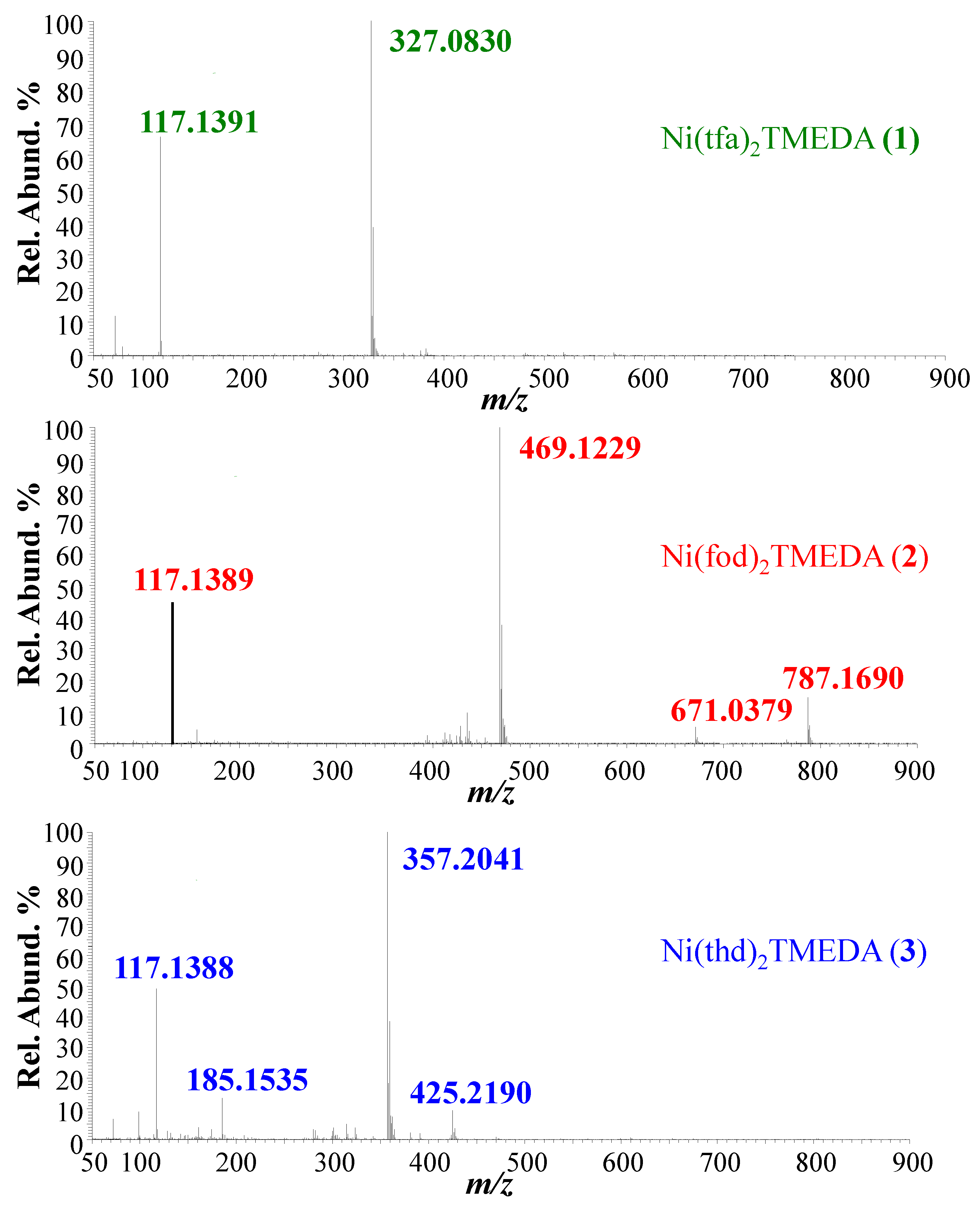

| 1 | 2 | 3 | |
|---|---|---|---|
| Ionic Species | m/z (%) | m/z (%) | m/z (%) |
| [M+Na]+ | --- | 787.1690 (25) | --- |
| [M−TMEDA+Na]+ | --- | 671.0379 (12) | --- |
| [M−TMEDA+H]+ | --- | --- | 425.2190 (15) |
| [M−L]+ | 327.0830 (100) | 469.1229 (100) | 357.2041 (100) |
| [HL+H]+ | --- | --- | 185.1535 (18) |
| [TMEDA+H]+ | 117.1391 (70) | 117.1389 (50) | 117.1388 (55) |
| 1 | 2 | 3 | ||||
|---|---|---|---|---|---|---|
| M | [M-tfa]+ | M | [M-fod]+ | M | [M-thd]+ | |
| BL BO | BL BO | BL BO | BL BO | BL BO | BL BO | |
| Ni-O1 | 2.024 0.284 | 1.831 0.471 | 2.035 0.242 | 1.833 0.454 | 2.018 0.241 | 1.828 0.476 |
| Ni-O4 | 2.024 0.284 | - | 2.035 0.242 | - | 2.018 0.241 | - |
| Ni-O2 | 2.041 0.294 | 1.848 0.469 | 2.023 0.258 | 1.842 0.452 | 2.009 0.256 | 1.828 0.476 |
| Ni-O3 | 2.041 0.294 | - | 2.023 0.258 | - | 2.010 0.256 | - |
| Ni-N1 | 2.147 0.230 | 1.946 0.405 | 2.153 0.201 | 1.946 0.393 | 2.165 0.178 | 1.957 0.368 |
| Ni-N2 | 2.147 0.230 | 1.946 0.399 | 2.153 0.201 | 1.947 0.387 | 2.165 0.178 | 1.957 0.368 |
| O1-C5 | 1.260 1.362 | 1.276 1.285 | 1.260 1.363 | 1.276 1.279 | 1.267 1.352 | 1.284 1.267 |
| O2-C7 | 1.257 1.427 | 1.274 1.318 | 1.258 1.418 | 1.276 1.316 | 1.263 1.376 | 1.284 1.267 |
| O4-C6 | 1.260 1.362 | - | 1.260 1.363 | - | 1.267 1.352 | - |
| O3-C8 | 1.257 1.427 | - | 1.258 1.418 | - | 1.263 1.376 | - |
| C5-CH1 | 1.392 1.423 | 1.380 1.450 | 1.393 1.421 | 1.381 1.451 | 1.411 1.348 | 1.400 1.367 |
| C7-CH1 | 1.424 1.264 | 1.413 1.300 | 1.425 1.260 | 1.413 1.288 | 1.414 1.325 | 1.400 1.367 |
| C6-CH2 | 1.392 1.423 | - | 1.393 1.421 | - | 1.411 1.348 | - |
| C8-CH2 | 1.424 1.264 | - | 1.425 1.260 | - | 1.414 1.325 | - |
| CHT-CH2T | 1.520 1.021 | 1.508 1.027 | 1.518 1.021 | 1.507 1.027 | 1.520 1.020 | 1.508 1.026 |
| 1 | 2 | 3 | ||||
|---|---|---|---|---|---|---|
| M | [M-tfa]+ | M | [M-fod]+ | M | [M-thd]+ | |
| Ni | 1.00 | 0.73 | 1.08 | 0.75 | 1.12 | 0.75 |
| L | −0.65 | −0.35 | −0.68 | −0.36 | −0.69 | −0.32 |
| TMEDA | 0.30 | 0.62 | 0.28 | 0.61 | 0.26 | 0.57 |
| 1 | 2 | 3 | ||||
|---|---|---|---|---|---|---|
| [M-tfa]+- NH(CH3)2 | [M-tfa]+- CH3CH2N(CH3)2 | [M-fod]+- NH(CH3)2 | [M-fod]+- CH3CH2N(CH3)2 | [M-fod]+- -(CH3)2C=CH2 | [M-thd]+- NH(CH3)2 | |
| BL BO | BL BO | BL BO | BL BO | BL BO | BL BO | |
| Ni-O1 | 1.811 0.523 | 1.799 0.557 | 1.814 0.506 | 1.794 0.554 | 1.837 0.450 | 1.808 0.535 |
| Ni-O2 | 1.830 0.511 | 1.819 0.541 | 1.816 0.518 | 1.815 0.543 | 1.842 0.453 | 1.803 0.546 |
| Ni-N1 | - | - | 1.929 0.391 | 1.946 0.402 | 1.938 0.415 | 1.941 0.368 |
| Ni-N2 | 1.932 0.393 | 1.943 0.408 | - | - | 1.937 0.414 | - |
| Ni-CHT | 1.972 0.248 | 1.987 0.264 | 1.976 0.239 | 1.986 0.264 | - | 1.974 0.243 |
| Ni-CH2T | 2.096 0.394 | 2.128 0.368 | 2.098 0.393 | 2.128 0.363 | - | 2.108 0.375 |
| O1-C5 | 1.277 1.288 | 1.281 1.267 | 1.280 1.258 | 1.281 1.263 | 1.265 1.342 | 1.288 1.264 |
| O2-C7 | 1.281 1.297 | 1.279 1.308 | 1.279 1.314 | 1.280 1.299 | 1.270 1.318 | 1.287 1.264 |
| C5-CH1 | 1.384 1.444 | 1.381 1.463 | 1.380 1.468 | 1.381 1.458 | 1.400 1.345 | 1.399 1.381 |
| C7-CH1 | 1.409 1.315 | 1.412 1.300 | 1.417 1.281 | 1.413 1.292 | 1.389 1.404 | 1.403 1.361 |
| CHT-CH2T | 1.379 1.566 | 1.373 1.588 | 1.380 1.567 | 1.373 1.590 | 1.508 1.027 | 1.378 1.579 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Invernizzi, C.; Tabacchi, G.; Seraglia, R.; Benedet, M.; Roverso, M.; Maccato, C.; Bogialli, S.; Barreca, D.; Fois, E. On the Fragmentation of Ni(II) β-Diketonate-Diamine Complexes as Molecular Precursors for NiO Films: A Theoretical and Experimental Investigation. Molecules 2024, 29, 642. https://doi.org/10.3390/molecules29030642
Invernizzi C, Tabacchi G, Seraglia R, Benedet M, Roverso M, Maccato C, Bogialli S, Barreca D, Fois E. On the Fragmentation of Ni(II) β-Diketonate-Diamine Complexes as Molecular Precursors for NiO Films: A Theoretical and Experimental Investigation. Molecules. 2024; 29(3):642. https://doi.org/10.3390/molecules29030642
Chicago/Turabian StyleInvernizzi, Cristiano, Gloria Tabacchi, Roberta Seraglia, Mattia Benedet, Marco Roverso, Chiara Maccato, Sara Bogialli, Davide Barreca, and Ettore Fois. 2024. "On the Fragmentation of Ni(II) β-Diketonate-Diamine Complexes as Molecular Precursors for NiO Films: A Theoretical and Experimental Investigation" Molecules 29, no. 3: 642. https://doi.org/10.3390/molecules29030642
APA StyleInvernizzi, C., Tabacchi, G., Seraglia, R., Benedet, M., Roverso, M., Maccato, C., Bogialli, S., Barreca, D., & Fois, E. (2024). On the Fragmentation of Ni(II) β-Diketonate-Diamine Complexes as Molecular Precursors for NiO Films: A Theoretical and Experimental Investigation. Molecules, 29(3), 642. https://doi.org/10.3390/molecules29030642








