One-Dimensional and Two-Dimensional Zn(II) Coordination Polymers with Ditopic Imidazo[1,5-a]pyridine: A Structural and Computational Study
Abstract
1. Introduction
2. Results
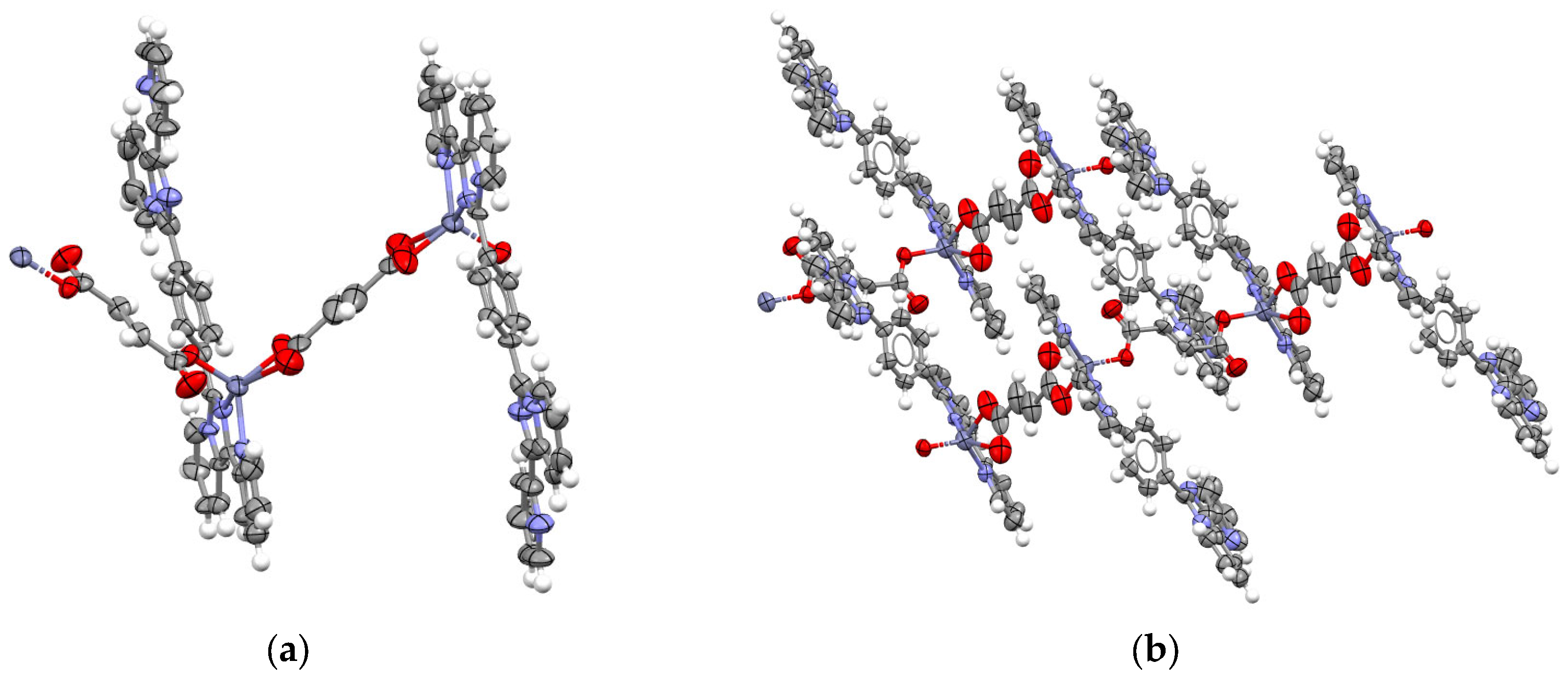
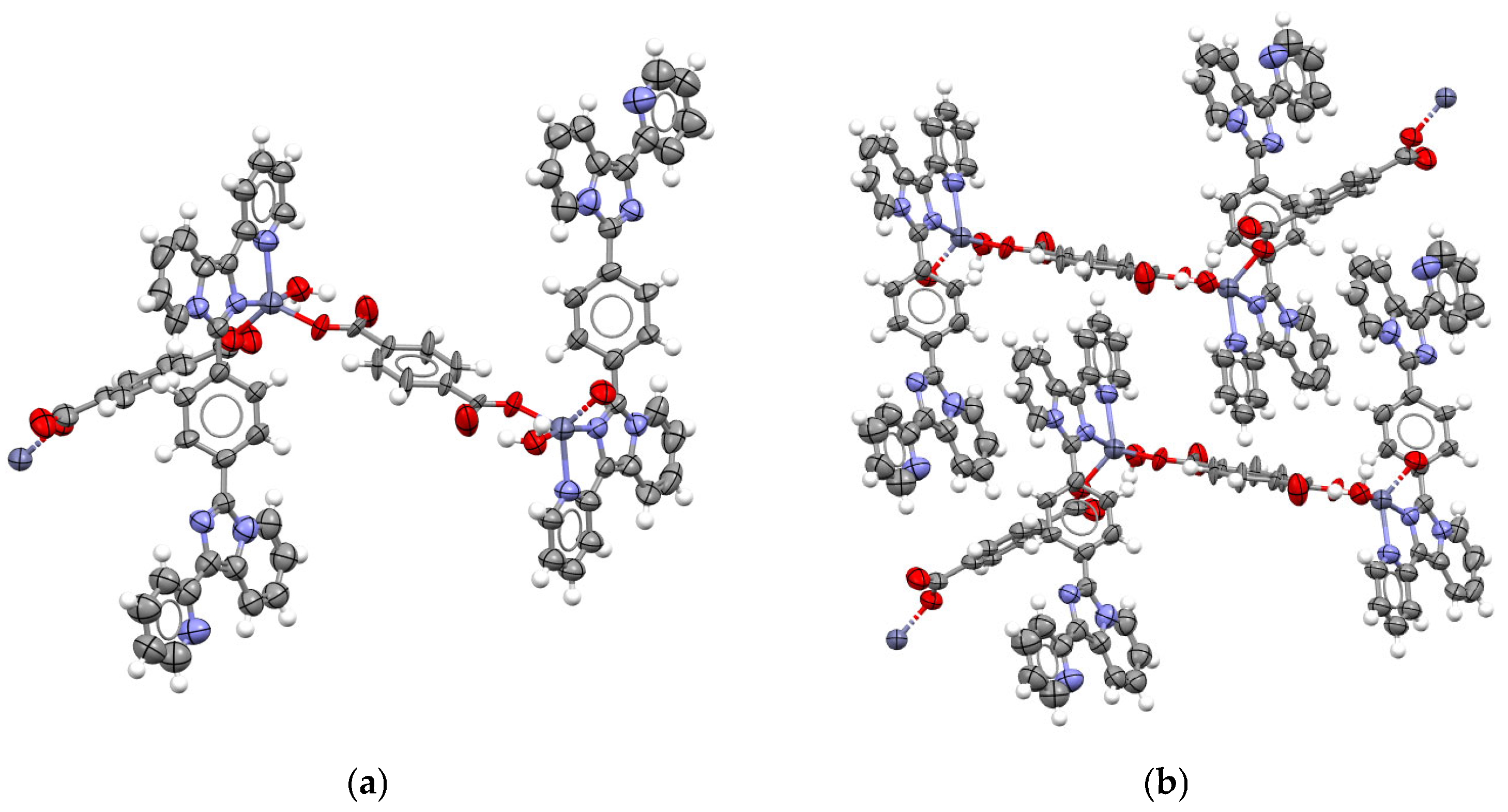
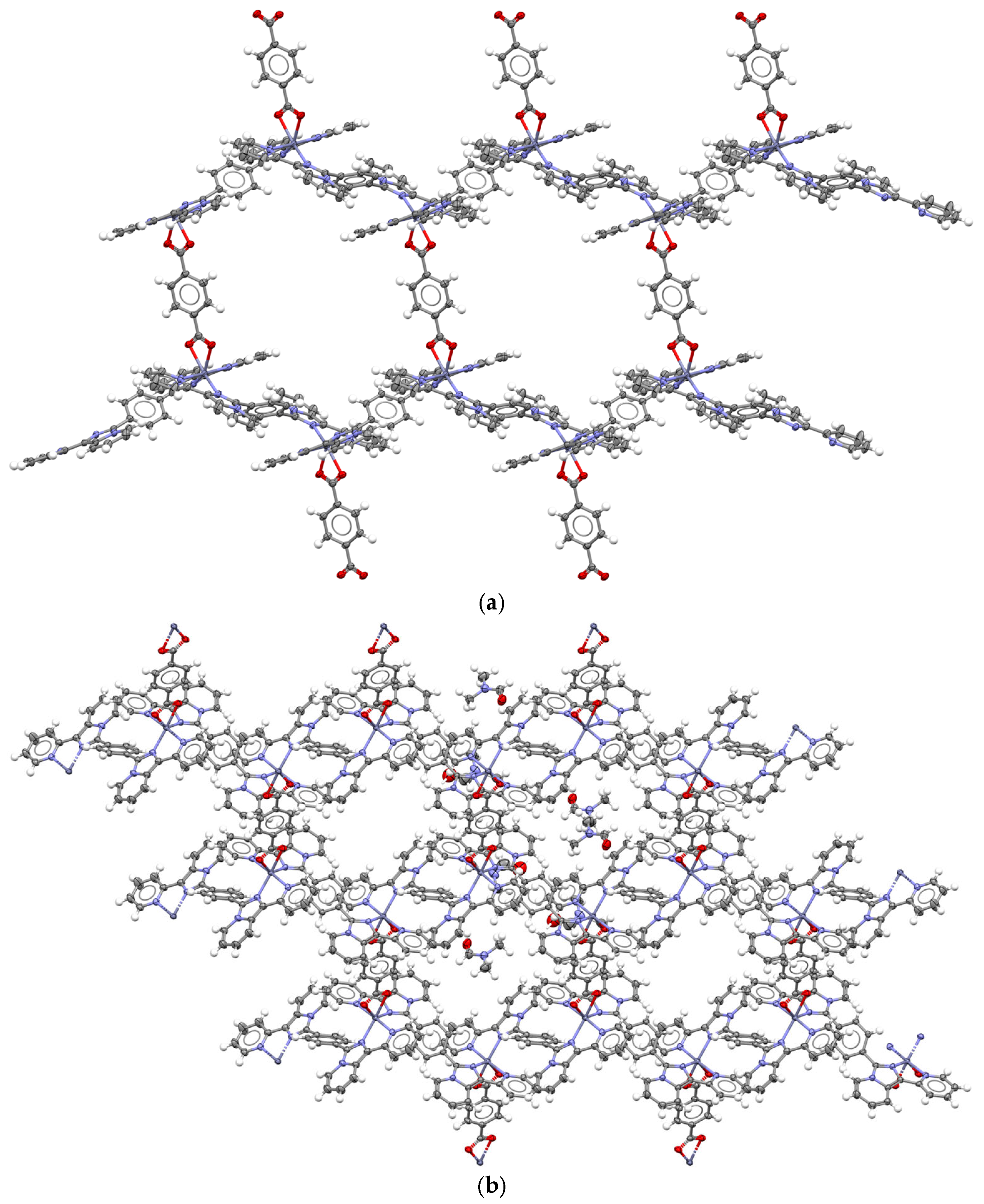
2.1. Synthesis and Structural Description of Compounds 1–3
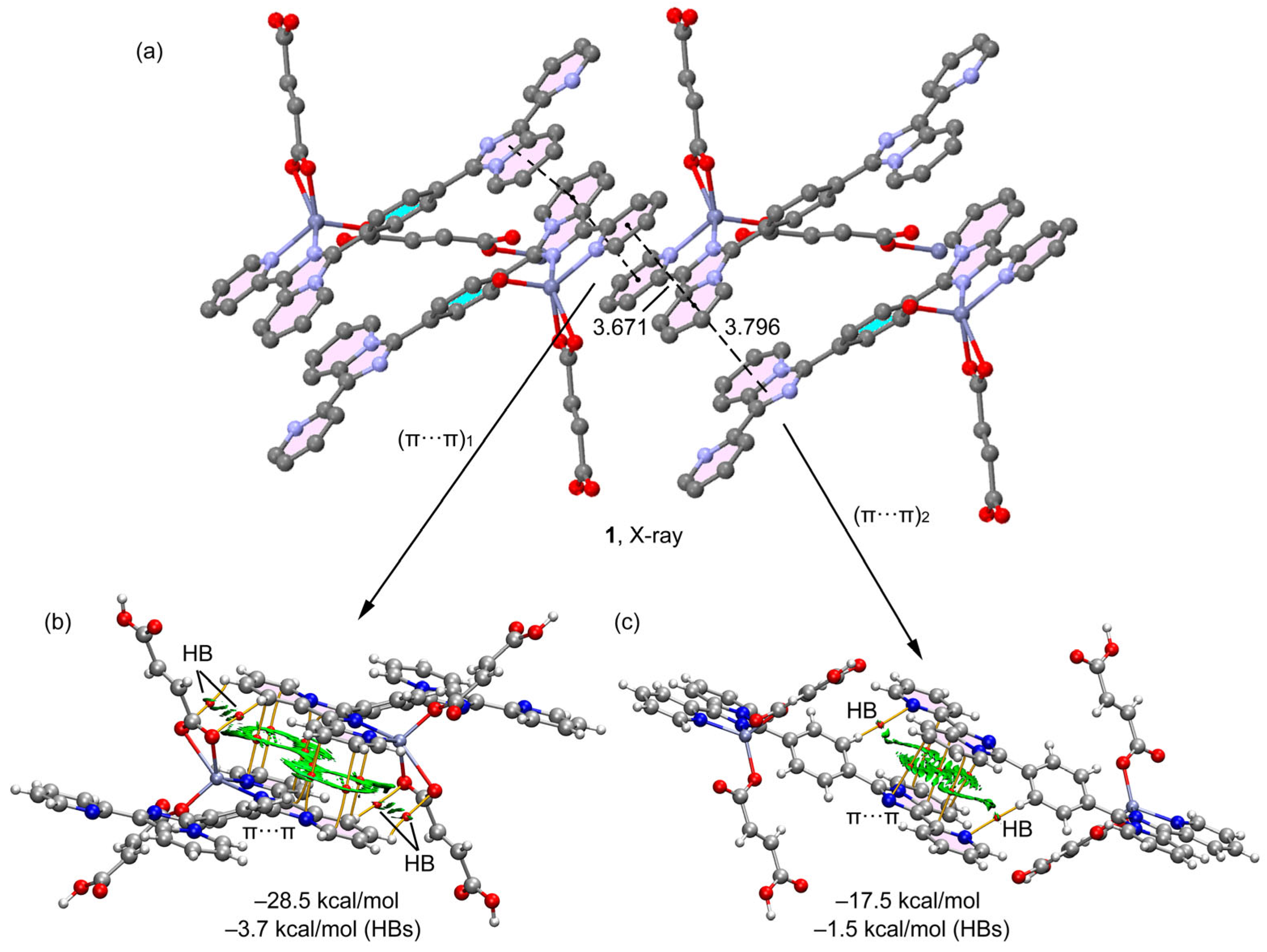
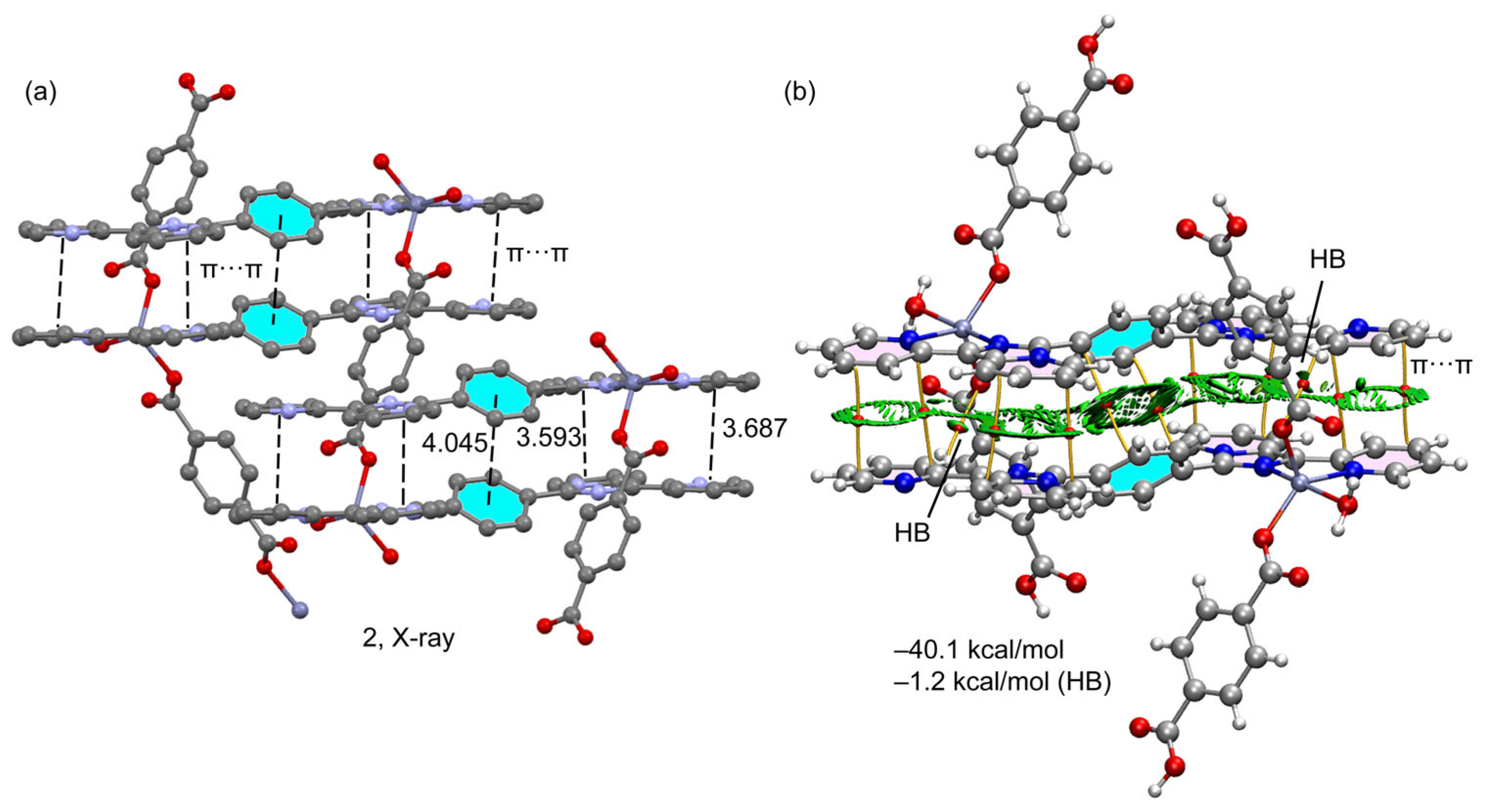
2.2. Theoretical Study
3. Materials and Methods
3.1. Experimental Techniques
3.2. Syntheses
3.3. Theoretical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Y.; Zheng, W.; Wang, W.; Yang, H.-B. When Polymerization Meets Coordination-Driven Self-Assembly: Metallo-Supramolecular Polymers Based on Supramolecular Coordination Complexes. Chem. Soc. Rev. 2021, 50, 7395–7417. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, P.; Fu, J.; Wei, C.; Cai, G. A Mini-Review: Pyridyl-Based Coordination Polymers for Energy Efficient Electrochromic Application. Front. Energy Res. 2021, 9, 620203. [Google Scholar] [CrossRef]
- Morritt, G.H.; Michaels, H.; Freitag, M. Coordination Polymers for Emerging Molecular Devices. Chem. Phys. Rev. 2022, 3, 011306. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, M.; Wu, S.; Zhang, L. Design Strategies for Coordination Polymers as Electrodes and Electrolytes in Rechargeable Lithium Batteries. Coord. Chem. Rev. 2023, 483, 215084. [Google Scholar] [CrossRef]
- Pena, E.S.; Lifshits, L.M.; Eckshtain-Levi, M.; Bachelder, E.M.; Ainslie, K.M. Metal–Organic Coordination Polymers for Delivery of Immunomodulatory Agents, and Infectious Disease and Cancer Vaccines. Wiley Interdiscip. Rev.-Nanomed. Nanobiotechnol. 2023, 15, e1877. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Li, P. The Recent Development of Two-Dimensional Conjugated Coordination Polymers. ChemNanoMat 2023, 9, e202300171. [Google Scholar] [CrossRef]
- Vasile Scaeteanu, G.; Maxim, C.; Badea, M.; Olar, R. Zinc(II) Carboxylate Coordination Polymers with Versatile Applications. Molecules 2023, 28, 1132. [Google Scholar] [CrossRef] [PubMed]
- Erxleben, A. Structures and Properties of Zn(II) Coordination Polymers. Coord. Chem. Rev. 2003, 246, 203–228. [Google Scholar] [CrossRef]
- Teng, X.; Lu, J.; Niu, Y.; Gong, S.; Xu, M.; Meyer, T.J.; Chen, Z. Selective CO2 Reduction to Formate on a Zn-Based Electrocatalyst Promoted by Tellurium. Chem. Mater. 2022, 34, 6036–6047. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Ning, S.; Kang, Q. Zinc-Based Materials for Photoelectrochemical Reduction of Carbon Dioxide. Energy Fuels 2022, 36, 11380–11393. [Google Scholar] [CrossRef]
- Manohar, A.; Vijayakanth, V.; Vattikuti, S.V.P.; Reddy, G.R.; Kim, K.H. A Brief Review on Zn—Based Materials and Nanocomposites for Supercapacitor Applications. J. Energy Storage 2023, 68, 107674. [Google Scholar] [CrossRef]
- Mutailipu, M.; Li, F.; Jin, C.; Yang, Z.; Poeppelmeier, K.R.; Pan, S. Strong Nonlinearity Induced by Coaxial Alignment of Polar Chain and Dense [BO3] Units in CaZn2(BO3)2. Angew. Chem.-Int. Edit. 2022, 61, e202202096. [Google Scholar] [CrossRef] [PubMed]
- Gaul, D.A.; Rees, W.S. True Blue Inorganic Optoelectronic Devices. Adv. Mater. 2000, 12, 935–946. [Google Scholar] [CrossRef]
- Wrobel, D.; Graja, A. Photoinduced Electron Transfer Processes in Fullerene-Organic Chromophore Systems. Coord. Chem. Rev. 2011, 255, 2555–2577. [Google Scholar] [CrossRef]
- Dalapati, G.K.; Sharma, H.; Guchhait, A.; Chakrabarty, N.; Bamola, P.; Liu, Q.; Saianand, G.; Krishna, A.M.S.; Mukhopadhyay, S.; Dey, A.; et al. Tin Oxide for Optoelectronic, Photovoltaic and Energy Storage Devices: A Review. J. Mater. Chem. A 2021, 9, 16621–16684. [Google Scholar] [CrossRef]
- Zhang, T.; Li, M.; Chen, J.; Wang, Y.; Miao, L.; Lu, Y.; He, Y. Multi-Component ZnO Alloys: Bandgap Engineering, Hetero-Structures, and Optoelectronic Devices. Mater. Sci. Eng. R-Rep. 2022, 147, 100661. [Google Scholar] [CrossRef]
- Pan, X.; Ou, M.; Lu, Y.; Nie, Q.; Dai, X.; Liu, O. Immunomodulatory Zinc-Based Materials for Tissue Regeneration. Biomater. Adv. 2023, 152, 213503. [Google Scholar] [CrossRef]
- Bahrani, S.; Hashemi, S.A.; Mousavi, S.M.; Azhdari, R. Zinc-Based Metal–Organic Frameworks as Nontoxic and Biodegradable Platforms for Biomedical Applications: Review Study. Drug Metab. Rev. 2019, 51, 356–377. [Google Scholar] [CrossRef]
- Chaudhran, P.A.; Sharma, A. Progress in the Development of Imidazopyridine-Based Fluorescent Probes for Diverse Applications. Crit. Rev. Anal. Chem. 2022, 1–18. [Google Scholar] [CrossRef]
- Volpi, G.; Rabezzana, R. Imidazo[1,5-a]Pyridine Derivatives: Useful, Luminescent and Versatile Scaffolds for Different Applications. New J. Chem. 2021, 45, 5737–5743. [Google Scholar] [CrossRef]
- Vanda, D.; Zajdel, P.; Soural, M. Imidazopyridine-Based Selective and Multifunctional Ligands of Biological Targets Associated with Psychiatric and Neurodegenerative Diseases. Eur. J. Med. Chem. 2019, 181, 111569. [Google Scholar] [CrossRef]
- Durini, S.; Ardizzoia, G.A.; Therrien, B.; Brenna, S. Tuning the Fluorescence Emission in Mononuclear Heteroleptic Trigonal Silver(I) Complexes. New J. Chem. 2017, 41, 3006–3014. [Google Scholar] [CrossRef]
- Ardizzoia, G.A.; Ghiotti, D.; Therrien, B.; Brenna, S. Homoleptic Complexes of Divalent Metals Bearing N,O-Bidentate Imidazo[1,5-a]Pyridine Ligands: Synthesis, X-Ray Characterization and Catalytic Activity in the Heck Reaction. Inorg. Chim. Acta 2018, 471, 384–390. [Google Scholar] [CrossRef]
- Giordano, M.; Volpi, G.; Bonomo, M.; Mariani, P.; Garino, C.; Viscardi, G. Methoxy-Substituted Copper Complexes as Possible Redox Mediators in Dye-Sensitized Solar Cells. New J. Chem. 2021, 45, 15303–15311. [Google Scholar] [CrossRef]
- Cerrato, V.; Volpi, G.; Priola, E.; Giordana, A.; Garino, C.; Rabezzana, R.; Diana, E. Mono-, Bis-, and Tris-Chelate Zn(II) Complexes with Imidazo[1,5-a]Pyridine: Luminescence and Structural Dependence. Molecules 2023, 28, 3703. [Google Scholar] [CrossRef]
- Blanco-Rodríguez, A.M.; Kvapilova, H.; Sykora, J.; Towrie, M.; Nervi, C.; Volpi, G.; Zalis, S.; Vlcek, A. Photophysics of Singlet and Triplet Intraligand Excited States in [ReCl(CO)3(1-(2-Pyridyl)-Imidazo[1,5-a]Pyridine)] Complexes. J. Am. Chem. Soc. 2014, 136, 5963–5973. [Google Scholar] [CrossRef]
- Volpi, G.; Garino, C.; Salassa, L.; Fiedler, J.; Hardcastle, K.I.; Gobetto, R.; Nervi, C. Cationic Heteroleptic Cyclometalated Iridium Complexes with 1-Pyridylimidazo[1,5-a]Pyridine Ligands: Exploitation of an Efficient Intersystem Crossing. Chem.-Eur. J. 2009, 15, 6415–6427. [Google Scholar] [CrossRef] [PubMed]
- Priola, E.; Volpi, G.; Rabezzana, R.; Borfecchia, E.; Garino, C.; Benzi, P.; Martini, A.; Operti, L.; Diana, E. Bridging Solution and Solid-State Chemistry of Dicyanoaurate: The Case Study of Zn–Au Nucleation Units. Inorg. Chem. 2020, 59, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Ardizzoia, G.A.; Brenna, S.; Durini, S.; Therrien, B. Synthesis and Characterization of Luminescent Zinc(II) Complexes with a N,N-Bidentate 1-Pyridylimidazo[1,5-a]Pyridine Ligand. Polyhedron 2015, 90, 214–220. [Google Scholar] [CrossRef]
- Volpi, G.; Priola, E.; Garino, C.; Daolio, A.; Rabezzana, R.; Benzi, P.; Giordana, A.; Diana, E.; Gobetto, R. Blue Fluorescent Zinc(II) Complexes Based on Tunable Imidazo[1,5-a]Pyridines. Inorg. Chim. Acta 2020, 509, 119662. [Google Scholar] [CrossRef]
- Ardizzoia, G.A.; Brenna, S.; Durini, S.; Therrien, B.; Veronelli, M. Synthesis, Structure, and Photophysical Properties of Blue-Emitting Zinc(II) Complexes with 3-Aryl-Substituted 1-Pyridylimidazo[1,5-a]Pyridine Ligands. Eur. J. Inorg. Chem. 2014, 24, 4310–4319. [Google Scholar] [CrossRef]
- Murai, T.; Nagaya, E.; Shibahara, F.; Maruyama, T.; Nakazawa, H. Rhodium(I) and Iridium(I) Imidazo[1,5-a]Pyridine-1-Ylalkylalkoxy Complexes: Synthesis, Characterization and Application as Catalysts for Hydrosilylation of Alkynes. J. Organomet. Chem. 2015, 794, 76–80. [Google Scholar] [CrossRef]
- Volpi, G.; Garino, C.; Conterosito, E.; Barolo, C.; Gobetto, R.; Viscardi, G. Facile Synthesis of Novel Blue Light and Large Stoke Shift Emitting Tetradentate Polyazines Based on Imidazo[1,5-a]Pyridine. Dyes Pigment. 2016, 128, 96–100. [Google Scholar] [CrossRef]
- Volpi, G.; Garino, C.; Priola, E.; Diana, E.; Gobetto, R.; Buscaino, R.; Viscardi, G.; Barolo, C. Facile Synthesis of Novel Blue Light and Large Stoke Shift Emitting Tetradentate Polyazines Based on Imidazo[1,5-a]Pyridine—Part 2. Dyes Pigment. 2017, 143, 284–290. [Google Scholar] [CrossRef]
- Guckian, A.L.; Doering, M.; Ciesielski, M.; Walter, O.; Hjelm, J.; O’Boyle, N.M.; Henry, W.; Browne, W.R.; McGarvey, J.J.; Vos, J.G. Assessment of Intercomponent Interaction in Phenylene Bridged Dinuclear Ruthenium(II) and Osmium(II) Polypyridyl Complexes. Dalton Trans. 2004, 23, 3943–3949. [Google Scholar] [CrossRef] [PubMed]
- Priola, E.; Conterosito, E.; Giordana, A.; Volpi, G.; Garino, C.; Andreo, L.; Diana, E.; Barolo, C.; Milanesio, M. Polymorphism and Solid State Peculiarities in Imidazo[1,5-a]Pyridine Core Deriving Compounds: An Analysis of Energetic and Structural Driving Forces. J. Mol. Struct. 2022, 1253, 132175. [Google Scholar] [CrossRef]
- Bhattacharyya, M.K.; Dutta, K.K.; Sharma, P.; Gomila, R.M.; Barceló-Oliver, M.; Frontera, A. Structure Guiding Supramolecular Assemblies in Metal-Organic Multi-Component Compounds of Mn(II): Experimental and Theoretical Studies. Crystals 2023, 13, 837. [Google Scholar] [CrossRef]
- Sharma, P.; Sarma, P.; Frontera, A.; Hussain, S.; Verma, A.K.; Bhattacharyya, M.K. Energetically Significant Anti-Parallel π-Stacking and Unconventional Anion-π Interactions in Phenanthroline Based Ni(II) and Cu(II) Coordination Compounds: Antiproliferative Evaluation and Theoretical Studies. Inorg. Chim. Acta 2021, 516, 120082. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New Features for the Visualization and Investigation of Crystal Structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Stock, N.; Biswas, S. Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef]
- Biemmi, E.; Christian, S.; Stock, N.; Bein, T. High-Throughput Screening of Synthesis Parameters in the Formation of the Metal-Organic Frameworks MOF-5 and HKUST-1. Microporous Mesoporous Mater. 2009, 117, 111–117. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward Reliable Density Functional Methods without Adjustable Parameters: The PBE0 Model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-Fitting Basis Sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Ahlrichs, R.; Bär, M.; Häser, M.; Horn, H.; Kölmel, C. Electronic Structure Calculations on Workstation Computers: The Program System Turbomole. Chem. Phys. Lett. 1989, 162, 165–169. [Google Scholar] [CrossRef]
- Caldeweyher, E.; Ehlert, S.; Hansen, A.; Neugebauer, H.; Spicher, S.; Bannwarth, C.; Grimme, S. A Generally Applicable Atomic-Charge Dependent London Dispersion Correction. J. Chem. Phys. 2019, 150, 154122. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.F.W. A Quantum Theory of Molecular Structure and Its Applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Contreras-García, J.; Johnson, E.R.; Keinan, S.; Chaudret, R.; Piquemal, J.-P.; Beratan, D.N.; Yang, W. NCIPLOT: A Program for Plotting Noncovalent Interaction Regions. J. Chem. Theory Comput. 2011, 7, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen Bond Strengths Revealed by Topological Analyses of Experimentally Observed Electron Densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Volpi, G. Luminescent Imidazo[1,5-a]Pyridine Scaffold: Synthetic Heterocyclization Strategies-Overview and Promising Applications. Asian J. Org. Chem. 2022, 11, e202200171. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sozzi, M.; Chierotti, M.R.; Gobetto, R.; Gomila, R.M.; Marzaroli, V.; Priola, E.; Volpi, G.; Zago, S.; Frontera, A.; Garino, C. One-Dimensional and Two-Dimensional Zn(II) Coordination Polymers with Ditopic Imidazo[1,5-a]pyridine: A Structural and Computational Study. Molecules 2024, 29, 653. https://doi.org/10.3390/molecules29030653
Sozzi M, Chierotti MR, Gobetto R, Gomila RM, Marzaroli V, Priola E, Volpi G, Zago S, Frontera A, Garino C. One-Dimensional and Two-Dimensional Zn(II) Coordination Polymers with Ditopic Imidazo[1,5-a]pyridine: A Structural and Computational Study. Molecules. 2024; 29(3):653. https://doi.org/10.3390/molecules29030653
Chicago/Turabian StyleSozzi, Mattia, Michele R. Chierotti, Roberto Gobetto, Rosa M. Gomila, Vittoria Marzaroli, Emanuele Priola, Giorgio Volpi, Stefano Zago, Antonio Frontera, and Claudio Garino. 2024. "One-Dimensional and Two-Dimensional Zn(II) Coordination Polymers with Ditopic Imidazo[1,5-a]pyridine: A Structural and Computational Study" Molecules 29, no. 3: 653. https://doi.org/10.3390/molecules29030653
APA StyleSozzi, M., Chierotti, M. R., Gobetto, R., Gomila, R. M., Marzaroli, V., Priola, E., Volpi, G., Zago, S., Frontera, A., & Garino, C. (2024). One-Dimensional and Two-Dimensional Zn(II) Coordination Polymers with Ditopic Imidazo[1,5-a]pyridine: A Structural and Computational Study. Molecules, 29(3), 653. https://doi.org/10.3390/molecules29030653









