Targeted Anthocyanin Profiling of Fruits from Three Southern Highbush Blueberry Cultivars Propagated in Colombia
Abstract
:1. Introduction
2. Results and Discussion
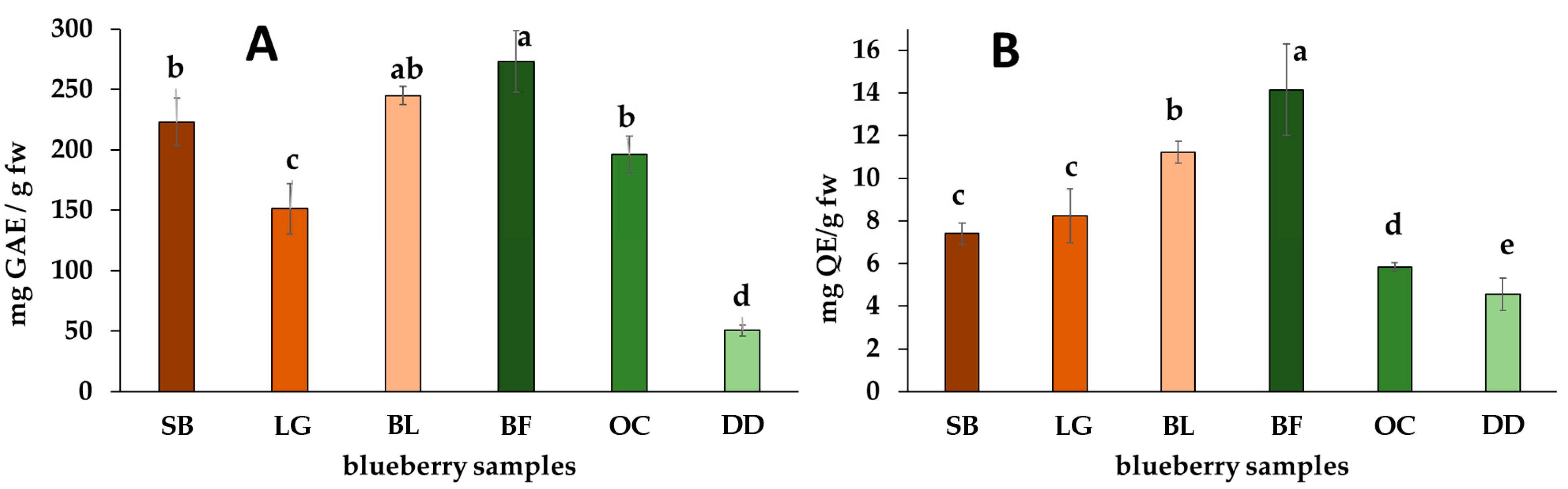
2.1. Total of Phenolics, Flavonoids, and Anthocyanins and Antioxidant Capacity
2.2. Targeted Anthocyanin Targeted Anthocyanin Profiling-Based Differentiation
3. Materials and Methods
3.1. Plant Material
3.2. Sample Preparation
3.3. Total Phenolic Content (TPC)
3.4. Total Flavonoid Content (TFC)
3.5. Total Anthocyanin Content (TAC)
3.6. DPPH· (1,1-Diphenyl-2-picryl-hydrazyl) Radical Scavenging Assay
3.7. HPLC-ESI-MS Analysis
3.8. Statistical Analysis
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cosmulescu, S.; Trandafir, I.; Nour, V. Phenolic Acids and Flavonoids Profiles of Extracts from Edible Wild Fruits and Their Antioxidant Properties. Int. J. Food Prop. 2017, 20, 3124–3134. [Google Scholar] [CrossRef]
- Lee, Y.-M.; Yoon, Y.; Yoon, H.; Park, H.-M.; Song, S.; Yeum, K.-J. Dietary Anthocyanins against Obesity and Inflammation. Nutrients 2017, 9, 1089. [Google Scholar] [CrossRef] [PubMed]
- Merecz-Sadowska, A.; Sitarek, P.; Kowalczyk, T.; Zajdel, K.; Jęcek, M.; Nowak, P.; Zajdel, R. Food Anthocyanins: Malvidin and Its Glycosides as Promising Antioxidant and Anti-Inflammatory Agents with Potential Health Benefits. Nutrients 2023, 15, 3016. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, B.; Gul, K.; Wani, A.A.; Singh, P. Health Benefits of Anthocyanins and Their Encapsulation for Potential Use in Food Systems: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2223–2230. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Chemistry, Pharmacology and Health Benefits of Anthocyanins. Phytother. Res. 2016, 30, 1265–1286. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.C.; Nunes, A.R.; Falcão, A.; Alves, G.; Silva, L.R. Dietary Effects of Anthocyanins in Human Health: A Comprehensive Review. Pharmaceuticals 2021, 14, 690. [Google Scholar] [CrossRef] [PubMed]
- Rashidinejad, A. Chapter 29—Blueberries. In Nutritional Composition and Antioxidant Properties of Fruits and Vegetables; Jaiswal, A.K., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 467–482. ISBN 978-0-12-812780-3. [Google Scholar]
- Song, G.-Q. Blueberry (Vaccinium Corymbosum L.). In Agrobacterium Protocols: Volume 2; Wang, K., Ed.; Springer: New York, NY, USA, 2015; pp. 121–131. ISBN 978-1-4939-1658-0. [Google Scholar]
- Zydlik, Z.; Cieśliński, S.; Kafkas, N.E.; Morkunas, I. Soil Preparation, Running Highbush Blueberry (Vaccinium corymbosum L.) Plantation and Biological Properties of Fruits. In Modern Fruit Industry; Ibrahim, K., Nesibe, E.K., Ayzin, K., Songül, Ç., Eds.; IntechOpen: Rijeka, Croatia, 2019; Chapter 4; ISBN 978-1-78984-731-4. [Google Scholar]
- Uttal, L.J. The Genus Vaccinium L. (Ericaceae) in Virginia. Castanea 1987, 52, 231–255. [Google Scholar]
- Konarska, A. Morphological, Anatomical, and Ultrastructural Changes in Vaccinium corymbosum Fruits during Ontogeny. Botany 2015, 93, 589–602. [Google Scholar] [CrossRef]
- Redpath, L.E.; Gumpertz, M.; Ballington, J.R.; Bassil, N.; Ashrafi, H. Genotype, Environment, Year, and Harvest Effects on Fruit Quality Traits of Five Blueberry (Vaccinium corymbosum L.) Cultivars. Agronomy 2021, 11, 1788. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Veiga, M.; Morais, R.M.; Calhau, C.; Pintado, M. Health Promoting Properties of Blueberries: A Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 181–200. [Google Scholar] [CrossRef]
- Tundis, R.; Tenuta, M.C.; Loizzo, M.R.; Bonesi, M.; Finetti, F.; Trabalzini, L.; Deguin, B. Vaccinium Species (Ericaceae): From Chemical Composition to Bio-Functional Activities. Appl. Sci. 2021, 11, 5655. [Google Scholar] [CrossRef]
- Onuh, J.O.; Dawkins, N.L.; Aluko, R.E. Cardiovascular Disease Protective Properties of Blueberry Polyphenols (Vaccinium corymbosum): A Concise Review. Food Prod. Process. Nutr. 2023, 5, 27. [Google Scholar] [CrossRef]
- Kalt, W.; Dufour, D. Health Functionality of Blueberries. HortTechnology 1997, 7, 216–221. [Google Scholar] [CrossRef]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. 2020, 11, 224–236. [Google Scholar] [CrossRef]
- Sweeney, M.I.; Kalt, W.; MacKinnon, S.L.; Ashby, J.; Gottschall-Pass, K.T. Feeding Rats Diets Enriched in Lowbush Blueberries for Six Weeks Decreases Ischemia-Induced Brain Damage. Nutr. Neurosci. 2002, 5, 427–431. [Google Scholar] [CrossRef]
- Sinelli, N.; Spinardi, A.; Di Egidio, V.; Mignani, I.; Casiraghi, E. Evaluation of Quality and Nutraceutical Content of Blueberries (Vaccinium corymbosum L.) by near and Mid-Infrared Spectroscopy. Postharvest Biol. Technol. 2008, 50, 31–36. [Google Scholar] [CrossRef]
- De Pascual-Teresa, S.; Moreno, D.A.; García-Viguera, C. Flavanols and Anthocyanins in Cardiovascular Health: A Review of Current Evidence. Int. J. Mol. Sci. 2010, 11, 1679–1703. [Google Scholar] [CrossRef] [PubMed]
- Pervaiz, T.; Songtao, J.; Faghihi, F.; Haider, M.S.; Fang, J. Naturally Occurring Anthocyanin, Structure, Functions and BiosyntheticPathway in Fruit Plants. J. Plant Biochem. Physiol. 2017, 5, 1000187. [Google Scholar] [CrossRef]
- Ayvaz, H.; Cabaroglu, T.; Akyildiz, A.; Pala, C.U.; Temizkan, R.; Ağçam, E.; Ayvaz, Z.; Durazzo, A.; Lucarini, M.; Direito, R.; et al. Anthocyanins: Metabolic Digestion, Bioavailability, Therapeutic Effects, Current Pharmaceutical/Industrial Use, and Innovation Potential. Antioxidants 2023, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Kähkönen, M.P.; Heinonen, M. Antioxidant Activity of Anthocyanins and Their Aglycons. J. Agric. Food Chem. 2003, 51, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Correia, P.; Araújo, P.; Ribeiro, C.; Oliveira, H.; Pereira, A.R.; Mateus, N.; de Freitas, V.; Brás, N.F.; Gameiro, P.; Coelho, P.; et al. Anthocyanin-Related Pigments: Natural Allies for Skin Health Maintenance and Protection. Antioxidants 2021, 10, 1038. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fong, S.K.; Singh, A.P.; Vorsa, N.; Johnson-Cicalese, J. Variation of Anthocyanins, Proanthocyanidins, Flavonols, and Organic Acids in Cultivated and Wild Diploid Blueberry Species. HortScience 2019, 54, 576–585. [Google Scholar] [CrossRef]
- Yousef, G.G.; Lila, M.A.; Guzman, I.; Ballington, J.R.; Brown, A.F. Impact of Interspecific Introgression on Anthocyanin Profiles of Southern Highbush Blueberry. J. Am. Soc. Hortic. Sci. 2014, 139, 99–112. [Google Scholar] [CrossRef]
- Spinardi, A.; Cola, G.; Gardana, C.S.; Mignani, I. Variation of Anthocyanin Content and Profile Throughout Fruit Development and Ripening of Highbush Blueberry Cultivars Grown at Two Different Altitudes. Front. Plant Sci. 2019, 10, 1045. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, B.; Ma, Y.; Sun, X.; Lin, Y.; Meng, X. Polyphenols, Anthocyanins, and Flavonoids Contents and the Antioxidant Capacity of Various Cultivars of Highbush and Half-High Blueberries. J. Food Compos. Anal. 2017, 62, 84–93. [Google Scholar] [CrossRef]
- Medeiros, J.G.S.; De Bona, C.M.; Cuquel, F.L.; Biasi, L.A. Performance of Blueberry Cultivars under Mild Winter Conditions. Ciênc. Rural 2017, 47. [Google Scholar] [CrossRef]
- Cortés-Rojas, M.H.; Mesa-Torres, P.A.; Grijalba-Rativa, C.M.; Pérez-Trujillo, M.M. Yield and Fruit Quality of the Blueberry Cultivars Biloxi and Sharpblue in Guasca, Colombia. Agron. Colomb. 2016, 34, 33–41. [Google Scholar] [CrossRef]
- Magnitskiy, S. Native Plants from the Genus Vaccinium in Colombia and Their Potential Uses. A Review. Rev. Colomb. Cienc. Hortícolas 2023, 17, e15503. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, M.; Lei, L.; An, Q.; Zhao, L.; Liu, G.; Wang, H. New Varieties of Blueberry Released by US in 2018 and Analysis of Breeding Trends. Mol. Plant Breed. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Fang, Y.; Nunez, G.H.; Silva, M.N.; Phillips, D.A.; Munoz, P.R. A Review for Southern Highbush Blueberry Alternative Production Systems. Agronomy 2020, 10, 1531. [Google Scholar] [CrossRef]
- Sater, H.; Ferrão, L.F.V.; Olmstead, J.; Munoz, P.R.; Bai, J.; Hopf, A.; Plotto, A. Exploring Environmental and Storage Factors Affecting Sensory, Physical and Chemical Attributes of Six Southern Highbush Blueberry Cultivars. Sci. Hortic. 2021, 289, 110468. [Google Scholar] [CrossRef]
- Scalzo, J.; Stevenson, D.; Hedderley, D. Blueberry Estimated Harvest from Seven New Cultivars: Fruit and Anthocyanins. Food Chem. 2013, 139, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Ehlenfeldt, M.K.; Rowland, L.J.; Ogden, E.L.; Vinyard, B.T. Cold-Hardiness, Acclimation, and Deacclimation among Diverse Blueberry Genotypes. J. Am. Soc. Hortic. Sci. J. Am. Soc. Hortic. Sci. 2012, 137, 31–37. [Google Scholar] [CrossRef]
- Stevenson, D.; Scalzo, J. Anthocyanin Composition and Content of Blueberries from around the World. J. Berry Res. 2012, 2, 179–189. [Google Scholar] [CrossRef]
- Smith, E.D. Cold Hardiness and Options for the Freeze Protection of Southern Highbush Blueberry. Agriculture 2019, 9, 9. [Google Scholar] [CrossRef]
- Yang, H.; Wu, Y.; Zhang, C.; Wu, W.; Lyu, L.; Li, W. Comprehensive Resistance Evaluation of 15 Blueberry Cultivars under High Soil pH Stress Based on Growth Phenotype and Physiological Traits. Front. Plant Sci. 2022, 13, 1072621. [Google Scholar] [CrossRef]
- Olas, B. Berry Phenolic Antioxidants—Implications for Human Health? Front. Pharmacol. 2018, 9, 78. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive Compounds and Antioxidant Activity in Different Types of Berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef]
- Battino, M.; Beekwilder, J.; Denoyes-Rothan, B.; Laimer, M.; McDougall, G.J.; Mezzetti, B. Bioactive Compounds in Berries Relevant to Human Health. Nutr. Rev. 2009, 67, S145–S150. [Google Scholar] [CrossRef]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for Analysis of Plant Phenolic Compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef]
- Haminiuk, C.W.I.; Maciel, G.M.; Plata-Oviedo, M.S.V.; Peralta, R.M. Phenolic Compounds in Fruits—An Overview. Int. J. Food Sci. Technol. 2012, 47, 2023–2044. [Google Scholar] [CrossRef]
- Kwok, B.H.L.; Hu, C.; Durance, T.; Kitts, D.D. Dehydration Techniques Affect Phytochemical Contents and Free Radical Scavenging Activities of Saskatoon Berries (Amelanchier alnifolia Nutt.). J. Food Sci. 2004, 69, SNQ122–SNQ126. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Khanal, R.C.; Howard, L.R.; Prior, R.L. Effect of Heating on the Stability of Grape and Blueberry Pomace Procyanidins and Total Anthocyanins. Food Res. Int. 2010, 43, 1464–1469. [Google Scholar] [CrossRef]
- Chiabrando, V.; Giacalone, G.; Rolle, L. Mechanical Behaviour and Quality Traits of Highbush Blueberry during Postharvest Storage. J. Sci. Food Agric. 2009, 89, 989–992. [Google Scholar] [CrossRef]
- Lafarga, T.; Aguiló-Aguayo, I.; Bobo, G.; Chung, A.V.; Tiwari, B.K. Effect of Storage on Total Phenolics, Antioxidant Capacity, and Physicochemical Properties of Blueberry (Vaccinium corymbosum L.) Jam. J. Food Process. Preserv. 2018, 42, e13666. [Google Scholar] [CrossRef]
- Martín-Gómez, J.; Varo, M.Á.; Mérida, J.; Serratosa, M.P. Influence of Drying Processes on Anthocyanin Profiles, Total Phenolic Compounds and Antioxidant Activities of Blueberry (Vaccinium corymbosum). LWT 2020, 120, 108931. [Google Scholar] [CrossRef]
- Muñoz-Fariña, O.; López-Casanova, V.; García-Figueroa, O.; Roman-Benn, A.; Ah-Hen, K.; Bastias-Montes, J.M.; Quevedo-León, R.; Ravanal-Espinosa, M.C. Bioaccessibility of Phenolic Compounds in Fresh and Dehydrated Blueberries (Vaccinium corymbosum L.). Food Chem. Adv. 2023, 2, 100171. [Google Scholar] [CrossRef]
- Cavalcanti, R.N.; Santos, D.T.; Meireles, M.A.A. Non-Thermal Stabilization Mechanisms of Anthocyanins in Model and Food Systems—An Overview. Food Res. Int. 2011, 44, 499–509. [Google Scholar] [CrossRef]
- Kalt, W.; McDonald, J.E.; Donner, H. Anthocyanins, Phenolics, and Antioxidant Capacity of Processed Lowbush Blueberry Products. J. Food Sci. 2000, 65, 390–393. [Google Scholar] [CrossRef]
- Connor, A.M.; Luby, J.J.; Tong, C.B.S.; Finn, C.E.; Hancock, J.F. Genotypic and Environmental Variation in Antioxidant Activity, Total Phenolic Content, and Anthocyanin Content among Blueberry Cultivars. J. Am. Soc. Hortic. Sci. Jashs 2002, 127, 89–97. [Google Scholar] [CrossRef]
- Jung, Y.S.; Kwak, I.A.; Lee, S.G.; Cho, H.-S.; Cho, Y.-S.; Kim, D.-O. Influence of Production Systems on Phenolic Characteristics and Antioxidant Capacity of Highbush Blueberry Cultivars. J. Food Sci. 2021, 86, 2949–2961. [Google Scholar] [CrossRef] [PubMed]
- Sellappan, S.; Akoh, C.C.; Krewer, G. Phenolic Compounds and Antioxidant Capacity of Georgia-Grown Blueberries and Blackberries. J. Agric. Food Chem. 2002, 50, 2432–2438. [Google Scholar] [CrossRef] [PubMed]
- Arellanes-Juarez, N.; Benito-Bautista, P.; Zarate-Nicolas, B.H. Phenolic Compound Content in “Biloxi” Blueberry Grown in the Sierra Norte of Oaxaca, Mexico, Harvested in Warm Season. In Proceedings of the Acta Horticulturae; International Society for Horticultural Science (ISHS), Leuven, Belgium, 31 January 2023; pp. 207–212. [Google Scholar]
- Bernal, F.A.; Orduz-Diaz, L.L.; Coy-Barrera, E. Exploitation of the Complexation Reaction of Ortho-Dihydroxylated Anthocyanins with Aluminum(III) for Their Quantitative Spectrophotometric Determination in Edible Sources. Food Chem. 2015, 185, 84–89. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; Pacheco-Hernández, M.d.L.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical Studies of Anthocyanins: A Review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Pękal, A.; Pyrzynska, K. Effect of pH and Metal Ions on DPPH Radical Scavenging Activity of Tea. Int. J. Food Sci. Nutr. 2015, 66, 58–62. [Google Scholar] [CrossRef]
- Yousef, G.G.; Brown, A.F.; Funakoshi, Y.; Mbeunkui, F.; Grace, M.H.; Ballington, J.R.; Loraine, A.; Lila, M.A. Efficient Quantification of the Health-Relevant Anthocyanin and Phenolic Acid Profiles in Commercial Cultivars and Breeding Selections of Blueberries (Vaccinium spp.). J. Agric. Food Chem. 2013, 61, 4806–4815. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Salo, H.M.; Nguyen, N.; Alakärppä, E.; Klavins, L.; Hykkerud, A.L.; Karppinen, K.; Jaakola, L.; Klavins, M.; Häggman, H. Authentication of Berries and Berry-Based Food Products. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5197–5225. [Google Scholar] [CrossRef]
- Bondia-Pons, I.; Savolainen, O.; Törrönen, R.; Martinez, J.A.; Poutanen, K.; Hanhineva, K. Metabolic Profiling of Goji Berry Extracts for Discrimination of Geographical Origin by Non-Targeted Liquid Chromatography Coupled to Quadrupole Time-of-Flight Mass Spectrometry. Food Res. Int. 2014, 63, 132–138. [Google Scholar] [CrossRef]
- Carrillo, C.; Tomasevic, I.B.; Barba, F.J.; Kamiloglu, S. Modern Analytical Techniques for Berry Authentication. Chemosensors 2023, 11, 500. [Google Scholar] [CrossRef]
- Tingting, S.; Xiaobo, Z.; Jiyong, S.; Zhihua, L.; Xiaowei, H.; Yiwei, X.; Wu, C. Determination Geographical Origin and Flavonoids Content of Goji Berry Using Near-Infrared Spectroscopy and Chemometrics. Food Anal. Methods 2016, 9, 68–79. [Google Scholar] [CrossRef]
- Hurkova, K.; Uttl, L.; Rubert, J.; Navratilova, K.; Kocourek, V.; Stranska-Zachariasova, M.; Paprstein, F.; Hajslova, J. Cranberries versus Lingonberries: A Challenging Authentication of Similar Vaccinium Fruit. Food Chem. 2019, 284, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Kim, S.-H.; Ahn, H.M.; Lim, S.R.; Oh, J.; Choi, S.; Lee, H.-J.; Auh, J.-H.; Choi, H.-K. Differentiation of Highbush Blueberry (Vaccinium corymbosum L.) Fruit Cultivars by GC–MS-Based Metabolic Profiling. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 21–28. [Google Scholar] [CrossRef]
- Netzel, M.; Netzel, G.; Tian, Q.; Schwartz, S.; Konczak, I. Native Australian Fruits—A Novel Source of Antioxidants for Food. Innov. Food Sci. Emerg. Technol. 2007, 8, 339–346. [Google Scholar] [CrossRef]
- Buitrago, D.; Buitrago-Villanueva, I.; Barbosa-Cornelio, R.; Coy-Barrera, E. Comparative Examination of Antioxidant Capacity and Fingerprinting of Unfractionated Extracts from Different Plant Parts of Quinoa (Chenopodium quinoa) Grown under Greenhouse Conditions. Antioxidants 2019, 8, 238. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the pH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the Gap between Raw Spectra and Functional Insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]





| # a | tR b (min) | [M]+ (m/z) | Aglycone (m/z) | Formula | Accurate Mass | Error c (ppm) | Annotation d |
|---|---|---|---|---|---|---|---|
| 1 | 12.01 | 465.1031 | 303.0530 | C21H21O12 | 465.1034 | 0.432 | delphinidin galactoside |
| 2 | 12.88 | 465.1038 | 303.0526 | C21H21O12 | 465.1034 | 1.073 | delphinidin glucoside |
| 3 | 14.25 | 449.1073 | 287.0584 | C21H21O11 | 449.1084 | 2.419 | cyanidin galactoside |
| 4 | 15.24 | 449.1055 | 287.0576 | C28H17O6 | 449.1026 | 6.427 | cyanidin glucoside |
| 5 | 15.54 | 479.1208 | 317.0691 | C22H23O12 | 479.1190 | 3.859 | petunidin galactoside |
| 6 | 16.49 | 479.1178 | 317.0684 | C22H23O12 | 479.1190 | 2.403 | petunidin glucoside |
| 7 | 17.87 | 449.1057 | 317.0685 | C28H17O6 | 449.1026 | 5.982 | petunidin arabinoside |
| 8 | 18.96 | 493.1328 | 331.0845 | C23H25O12 | 493.1347 | 3.653 | malvidin galactoside |
| 9 | 19.99 | 493.1358 | 331.0845 | C23H25O12 | 493.1347 | 2.431 | malvidin glucoside |
| 10 | 21.50 | 463.121 | 331.0845 | C22H23O11 | 463.1241 | 6.557 | malvidin arabinoside |
| Type a | Anthocyanin b (mg C3G/100 g fw) c | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| SB | 93.8 ± 1.5 C | 40.5 ± 0.7 B | 75.8 ± 1.1 D | 8.8 ± 0.3 D | 36.4 ± 0.4 D | 37.7 ± 0.8 D | 16.9 ± 0.6 D | 31.3 ± 0.4 E | 19.7 ± 0.6 C | 9.5 ± 0.5 D |
| LG | 79.8 ± 3.1 D | 55.3 ± 2.2 A | 73.0 ± 2.9 D | 4.3 ± 0.2 C | 46.4 ± 2.2 B | 55.1 ± 2.8 A | 29.9 ± 1.5 A | 73.1 ± 2.2 B | 56.5 ± 3.2 A | 42.1 ± 2.8 A |
| BL | 96.5 ± 0.7 B | 38.9 ± 0.6 C | 110.5 ± 1.3 A | 13.5 ± 0.3 A | 35.9 ± 0.2 D | 49.7 ± 0.1 B | 20.2 ± 0.5 C | 25.4 ± 1.0 F | 19.1 ± 0.8 C | 7.6 ± 0.1 E |
| BF | 80.6 ± 0.2 D | 28.6 ± 0.1 E | 101.2 ± 0.3 B | 11.0 ± 0.1 B | 41.0 ± 0.2 C | 49.4 ± 0.2 B | 30.3 ± 0.1 A | 61.5 ± 0.2 C | 35.9 ± 0.2 B | 34.3 ± 0.1 B |
| OC | 92.7 ± 3.7 BC | 34.7 ± 1.4 D | 59.7 ± 0.8 E | 4.3 ± 0.1 C | 54.7 ± 2.4 A | 44.1 ± 1.5 C | 22.8 ± 1.0 B | 88.2 ± 1.4 A | 56.5 ± 0.6 A | 35.3 ± 1.7 B |
| DD | 115 ± 6.8 A | n.d. | 90.3 ± 3.8 C | n.d. | 52.3 ± 3.4 A | 15.6 ± 0.5 E | 23.1 ± 0.9 B | 57.2 ± 2.1 D | 0.5 ± 0.1 D | 17.4 ± 1.0 C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prada-Muñoz, J.; Coy-Barrera, E. Targeted Anthocyanin Profiling of Fruits from Three Southern Highbush Blueberry Cultivars Propagated in Colombia. Molecules 2024, 29, 691. https://doi.org/10.3390/molecules29030691
Prada-Muñoz J, Coy-Barrera E. Targeted Anthocyanin Profiling of Fruits from Three Southern Highbush Blueberry Cultivars Propagated in Colombia. Molecules. 2024; 29(3):691. https://doi.org/10.3390/molecules29030691
Chicago/Turabian StylePrada-Muñoz, Jessica, and Ericsson Coy-Barrera. 2024. "Targeted Anthocyanin Profiling of Fruits from Three Southern Highbush Blueberry Cultivars Propagated in Colombia" Molecules 29, no. 3: 691. https://doi.org/10.3390/molecules29030691
APA StylePrada-Muñoz, J., & Coy-Barrera, E. (2024). Targeted Anthocyanin Profiling of Fruits from Three Southern Highbush Blueberry Cultivars Propagated in Colombia. Molecules, 29(3), 691. https://doi.org/10.3390/molecules29030691







