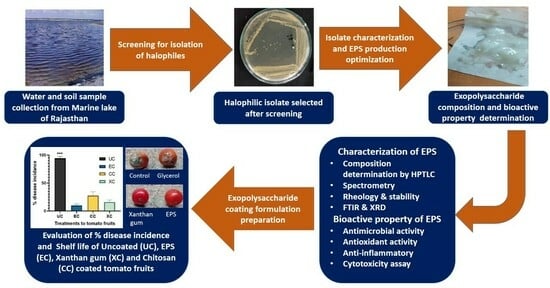
Development of Biological Coating from Novel Halophilic Exopolysaccharide Exerting Shelf-Life-Prolonging and Biocontrol Actions for Post-Harvest Applications
Abstract
:1. Introduction
2. Results and Discussion
2.1. Screening of EPS-Producing Halophiles from Marine Samples
2.2. Characterization of Selected Isolate
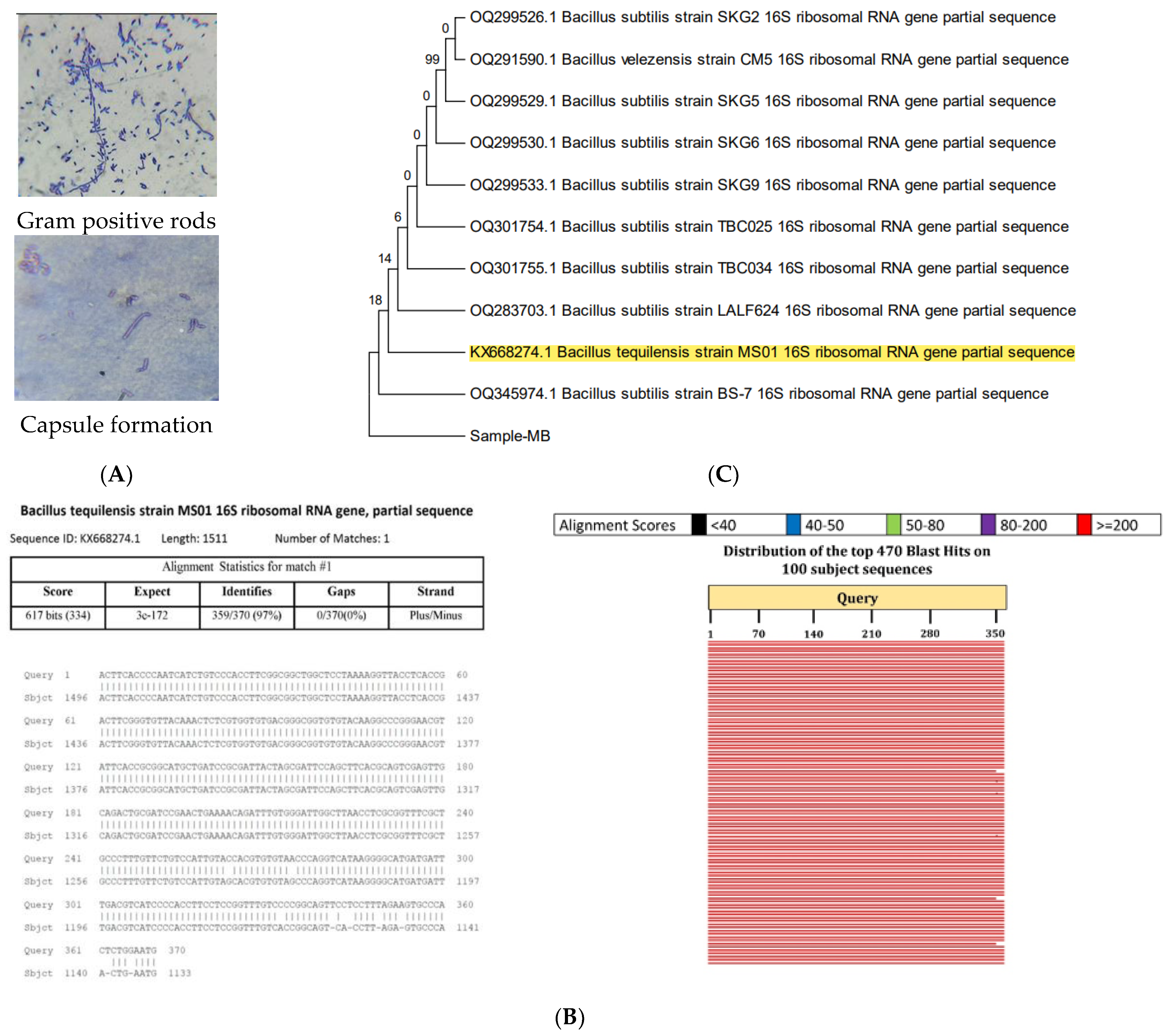
2.2.1. Biochemical Characterization
2.2.2. Molecular Characterization
2.3. Optimization of EPS Production
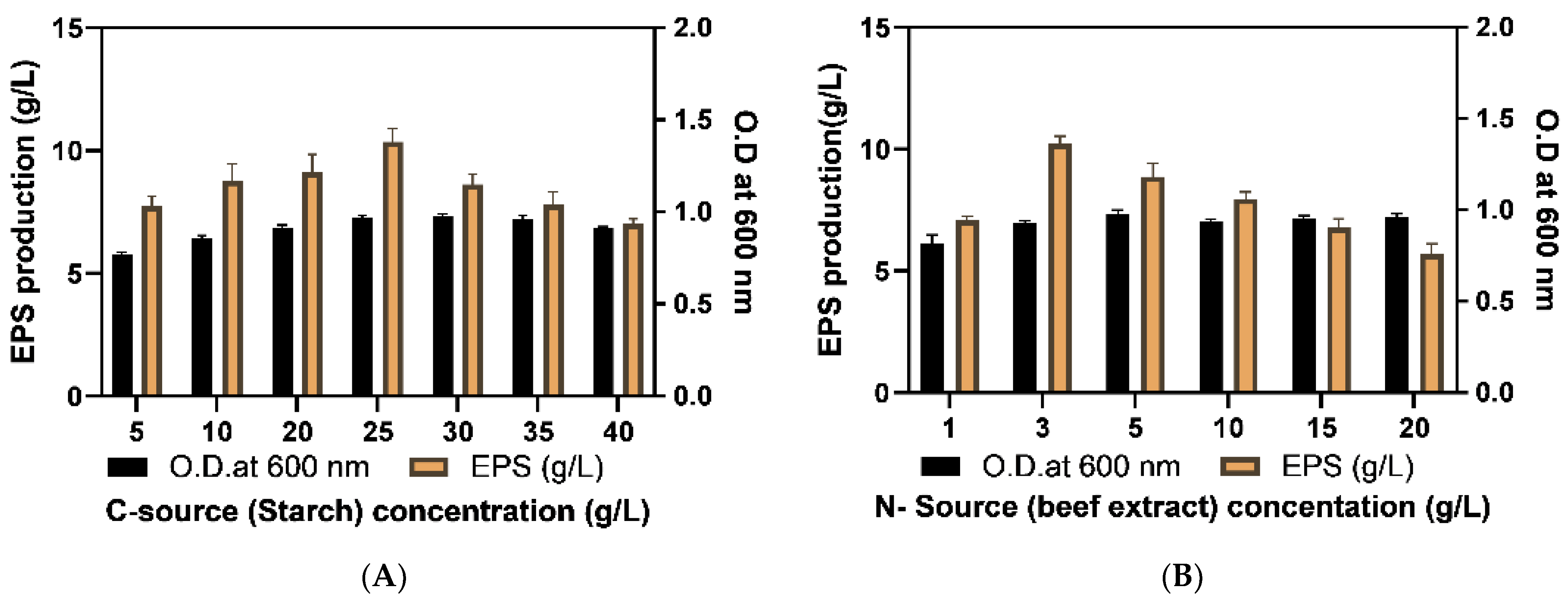
2.3.1. Optimization of Culture Media
2.3.2. Optimization of Physical Growth Parameters
2.3.3. Assessment of the Efficacy of Optimization
2.3.4. Determination of Growth Pattern and Phase of EPS Production for Harvesting
2.4. Characterization of EPS
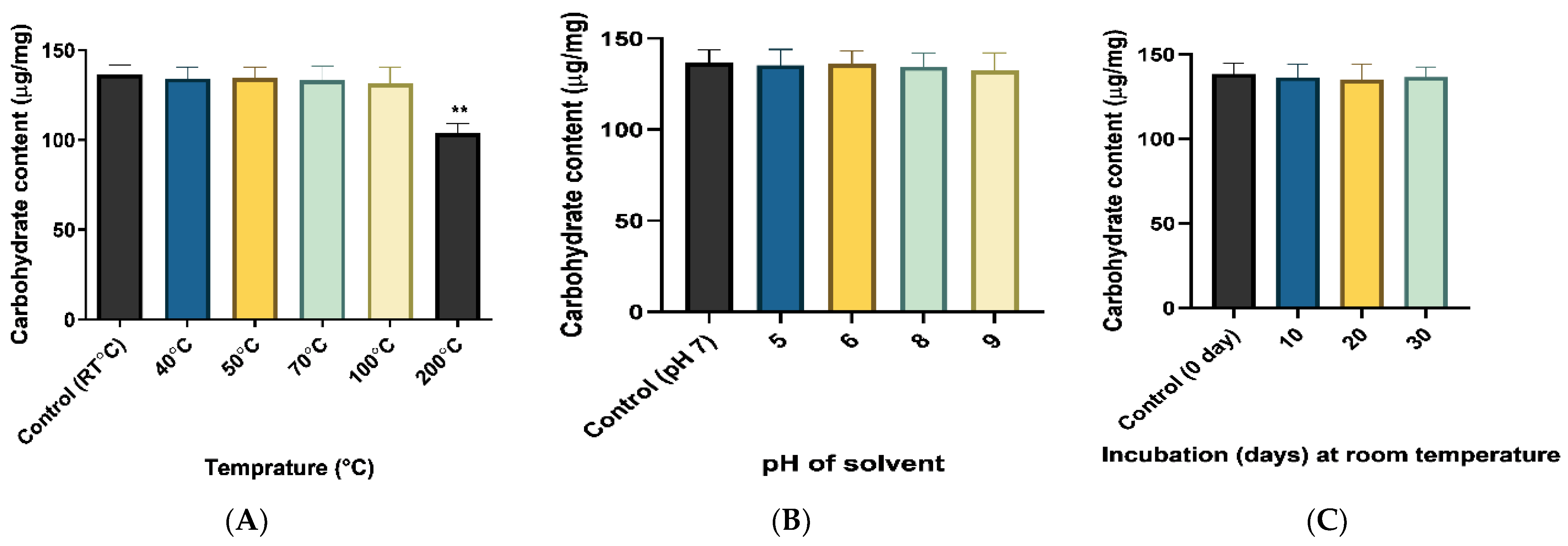
2.4.1. Assessment of Stability of Produced EPS
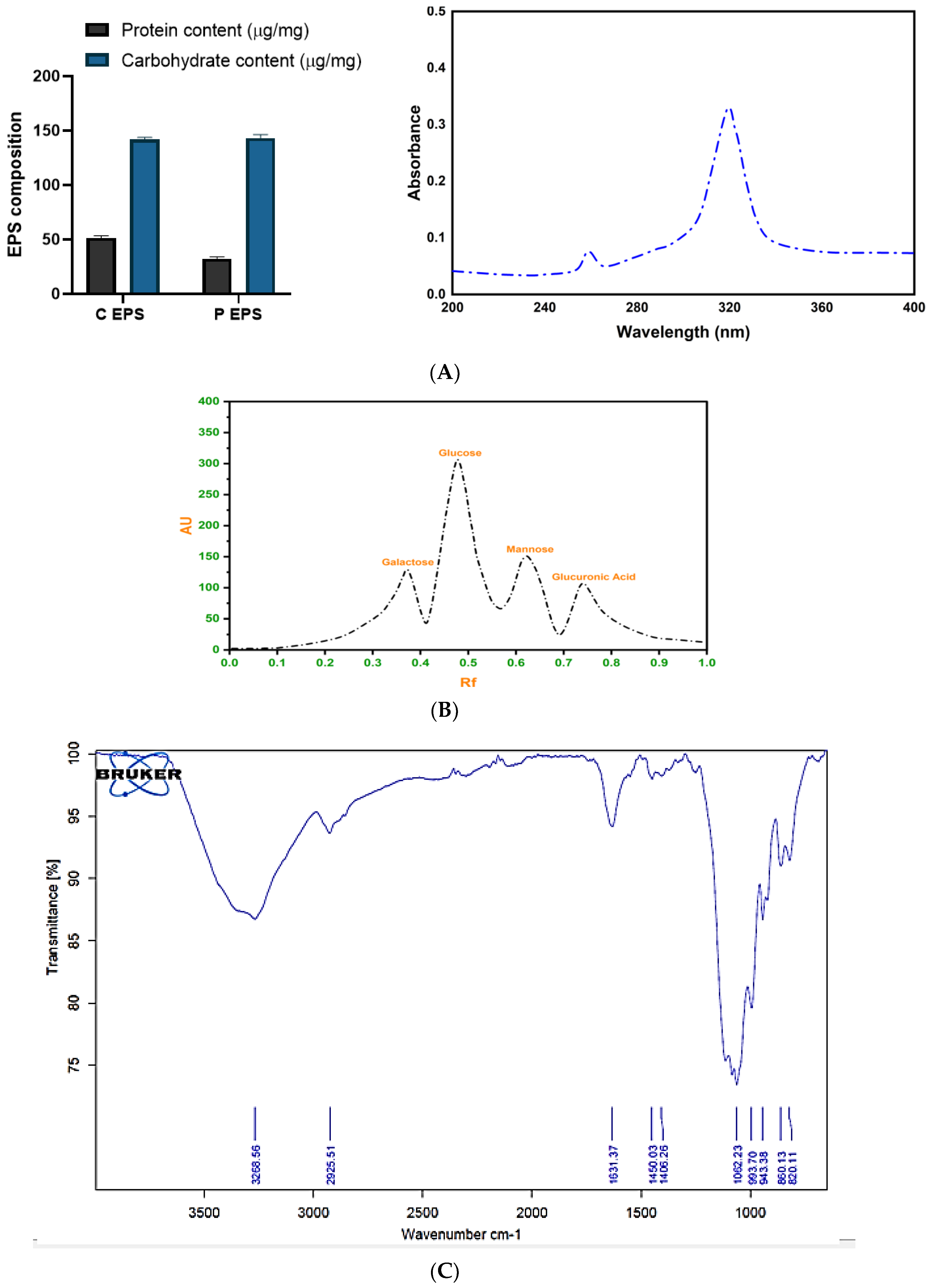
2.4.2. Determination of the Composition of EPS
Biochemical Composition and Spectral Analysis of EPS
HPTLC Analysis
FTIR Analysis
2.5. XRD, Molecular Weight and Rheological Behavior Analysis
2.6. Water Holding Capacity and Water Solubility Index of EPS
2.7. Evaluation of Bioactive Properties of EPS
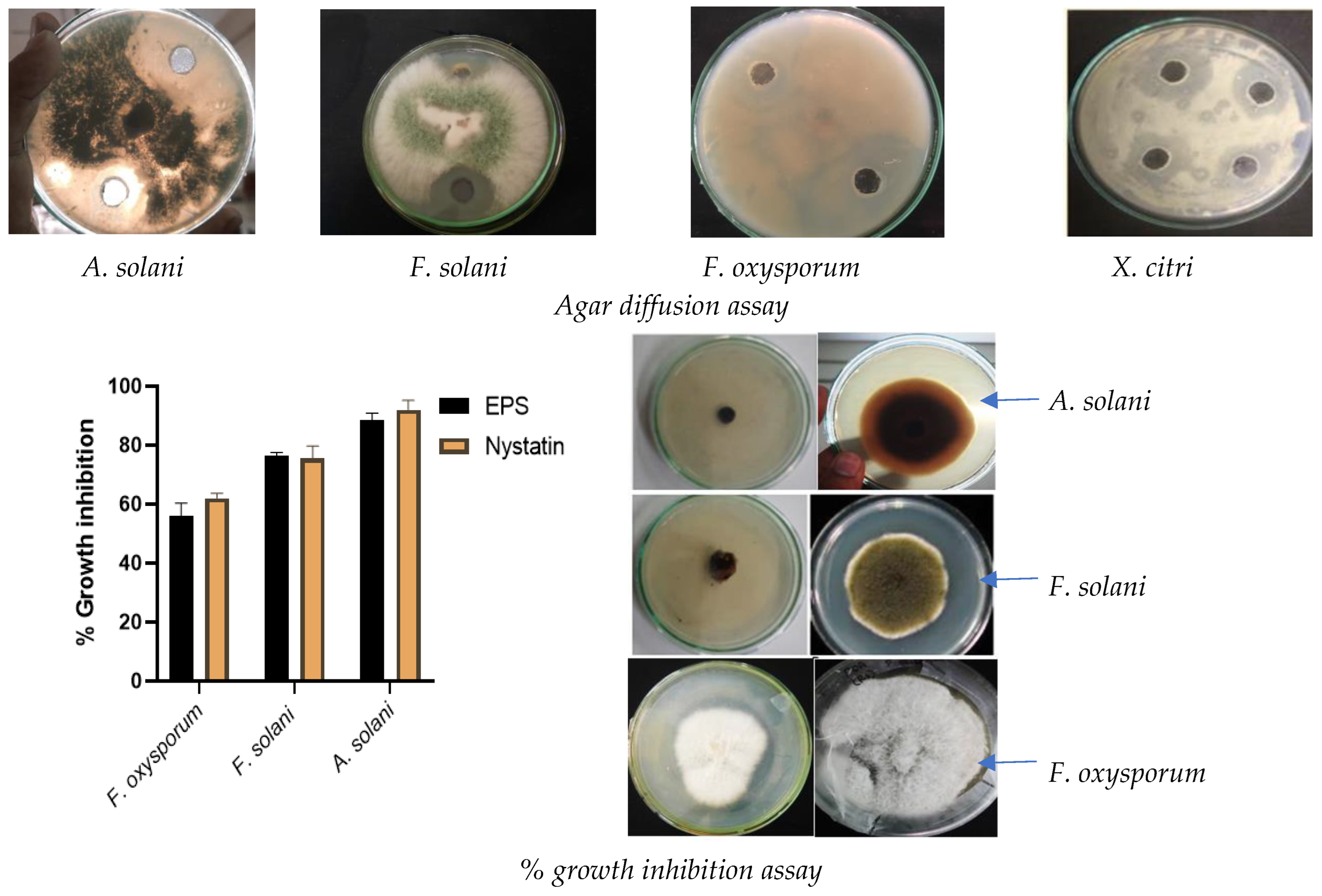
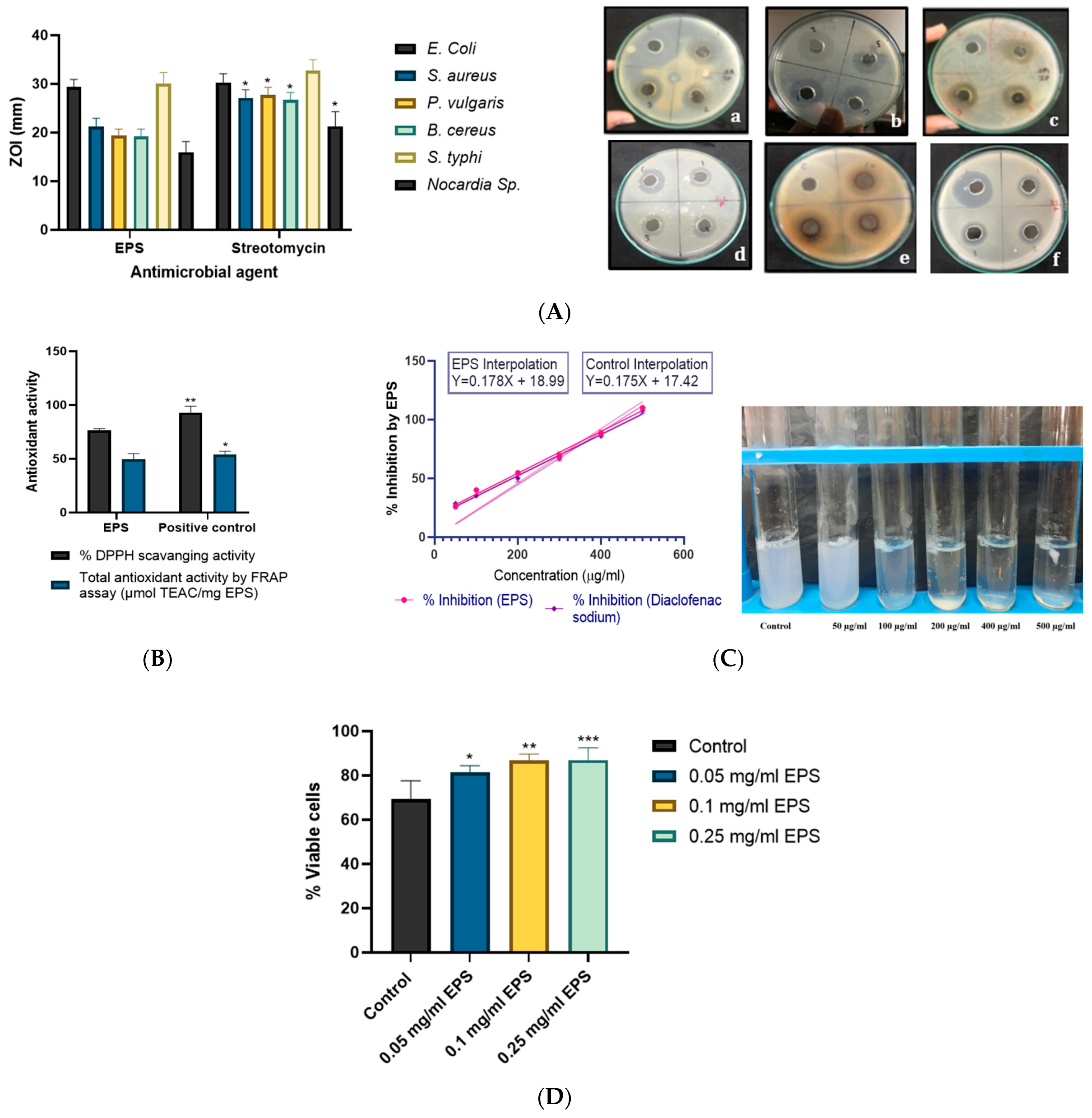
2.7.1. Antimicrobial Activity
2.7.2. Antioxidant Activity of EPS
2.7.3. Anti-Inflammatory and Cytotoxic Activity
2.8. Effect of EPS on the Shelf Life of Tomato Fruits
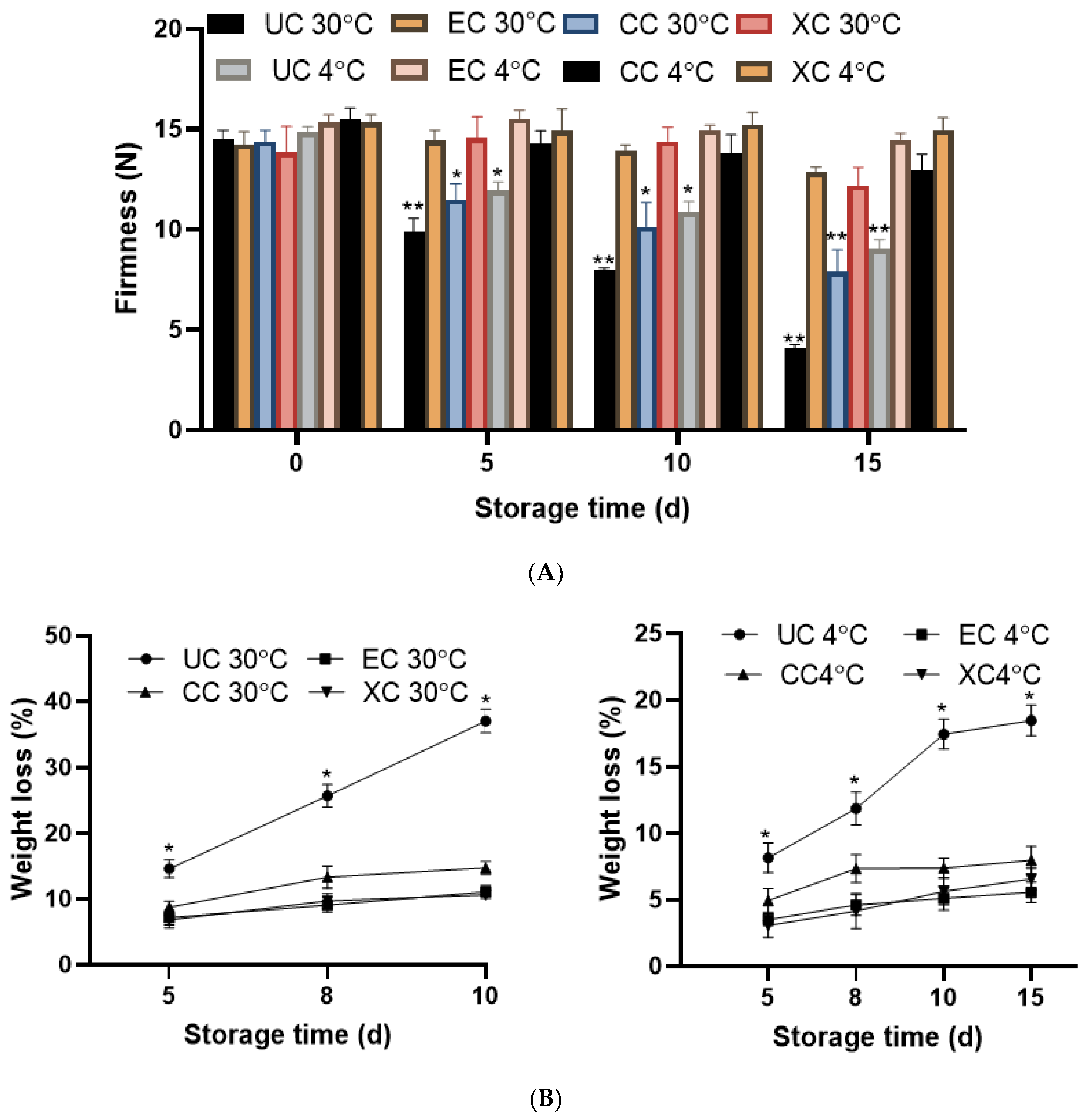
2.8.1. Firmness and Weight Loss (%) Analysis
2.8.2. Analysis of the Shelf Life of Tomato Fruits
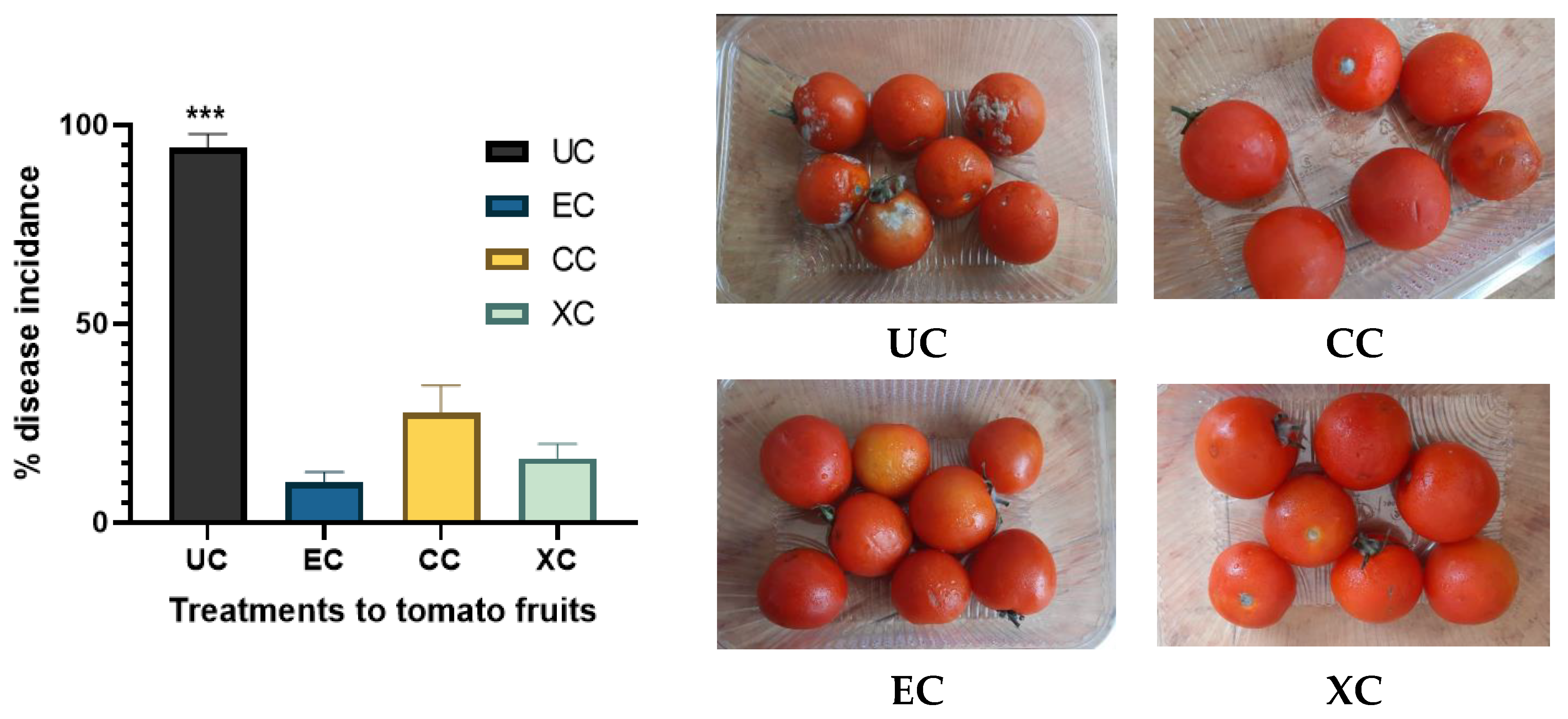
2.9. Effect of EPS on the % Disease Incidence
3. Material and Methods
3.1. Sample Collection
3.2. Screening of Marine Samples for EPS Producing Halophiles
3.3. Selection of Halophile Based on Biocontrol Potential of EPS against Phytopathogens
3.4. Characterization of Selected Isolate
3.5. Optimization of Culture Conditions for EPS Production
3.5.1. Optimization of Media
3.5.2. Optimization of Physical Parameters
3.6. Bacterial Growth Curve, Production, and Extraction of EPS
3.7. Deproteinization and Assessment of the Stability of EPS
3.8. Characterization of EPS
3.8.1. Composition, Spectrophotometric and FTIR Analysis
3.8.2. HPTLC Analysis for Sugar Composition of EPS
3.8.3. XRD Analysis of EPS
3.8.4. Water Solubility Index (WSI) and Water Holding Capacity (WHC) of EPS
3.8.5. Rheological and Viscometric Behavior of EPS
3.8.6. Intrinsic Viscosity and Molecular Weight Determination
3.9. Assessment of Bioactive Properties of EPS
3.9.1. Antimicrobial Activity of EPS
3.9.2. Antioxidant Activity by % DPPH Scavenging and FRAP Assay
3.9.3. In-Vitro Anti-Inflammatory and Cytotoxic Assay
3.10. Preparation of EPS Coating Solution and Uniform Application on Tomato Fruits
3.11. Investigation of Shelf-Life-Prolonging Potential of EPS
3.12. Assessment of the Effect of EPS Coating on Disease Incidence upon Treatment of A. solani
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Salem, D.R.; Sani, R.K. Extremophilic exopolysaccharides: A review and new perspectives on engineering strategies and applications. Carbohydr. Polym. 2019, 205, 8–26. [Google Scholar] [CrossRef]
- Jeewon, R.; Aullybux, A.A.; Puchooa, D.; Nazurally, N.; Alrefaei, A.F.; Zhang, Y. Marine Microbial Polysaccharides: An untapped resource for biotechnological applications. Mar. Drugs 2023, 21, 420. [Google Scholar] [CrossRef]
- Mahmoud, Y.A.G.; El-Naggar, M.E.; Abdel-Megeed, A.; El-Newehy, M. Recent advancements in microbial polysaccharides: Synthesis and applications. Polymers 2021, 13, 4136. [Google Scholar] [CrossRef]
- Nadda, A.K.; Sajna, K.V.; Sharma, S. (Eds.) Microbial Exopolysaccharides as Novel and Significant Biomaterials; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 2–9. [Google Scholar]
- Pradhan, M. Extremophiles: A Versatile Source of Exopolysaccharide. In Microbial Exopolysaccharides as Novel and Significant Biomaterials; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 105–120. [Google Scholar]
- Halder, U.; Roy, R.K.; Biswas, R.; Khan, D.; Mazumder, K.; Bandopadhyay, R. Synthesis of copper oxide nanoparticles using capsular polymeric substances produced by Bacillus altitudinis and investigation of its efficacy to kill pathogenic Pseudomonas aeruginosa. Chem. Eng. J. Adv. 2022, 11, 100294. [Google Scholar] [CrossRef]
- Sadanandan, S.; Ramkumar, K.; Pillai, N.P.; Anuvinda, P.; Devika, V.; Ramanunni, K.; Sreejaya, M.M. Biorecognition elements appended gold nanoparticle biosensors for the detection of food-borne pathogens—A review. Food Control 2023, 148, 109510. [Google Scholar] [CrossRef]
- Sun, C.; Xamxidin, M.; Wu, Y.H.; Cheng, H.; Wang, C.S.; Xu, X.W. Alteromonas alba sp. nov., a marine bacterium isolated from seawater of the West Pacific Ocean. Int. J. Syst. Evol. Microbiol. 2019, 69, 278–284. [Google Scholar] [CrossRef]
- Sánchez-León, E.; Bello-Morales, R.; López-Guerrero, J.A.; Poveda, A.; Jiménez-Barbero, J.; Gironès, N.; Abrusci, C. Isolation and characterization of an exopolymer produced by Bacillus licheniformis: In vitro antiviral activity against enveloped viruses. Carbohydr. Polym. 2020, 248, 116737. [Google Scholar] [CrossRef] [PubMed]
- El-Aswar, E.I.; Ramadan, H.; Elkik, H.; Taha, A.G. A comprehensive review on preparation, functionalization and recent applications of nanofiber membranes in wastewater treatment. J. Environ. Manag. 2022, 301, 113908. [Google Scholar] [CrossRef] [PubMed]
- Wilson, Z.E.; Brimble, M.A. Molecules derived from the extremes of life: A decade later. Nat. Prod. Rep. 2021, 38, 24–82. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.A.; Kanekar, P.P. Production of exopolysaccharide by Vagococcus carniphilus MCM B-1018 isolated from alkaline Lonar Lake, India. Ann. Microbiol. 2011, 61, 733–740. [Google Scholar] [CrossRef]
- Biswas, J.; Jana, S.K.; Mandal, S. Biotechnological impacts of Halomonas: A promising cell factory for industrially relevant biomolecules. Biotechnol. Genet. Eng. Rev. 2022, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Adetunji, C.O.; Roli, O.I.; Adetunji, J.B. Exopolysaccharides derived from beneficial microorganisms: Antimicrobial, food, and health benefits. In Innovations in Food Technology: Current Perspectives and Future Goals; Springer: Berlin/Heidelberg, Germany, 2020; pp. 147–160. [Google Scholar]
- Athmika Ghate, S.D.; Arun, A.B.; Rao, S.S.; Kumar, S.A.; Kandiyil, M.K.; Saptami, K.; Rekha, P.D. Genome analysis of a halophilic bacterium Halomonas malpeensis YU-PRIM-29T reveals its exopolysaccharide and pigment producing capabilities. Sci. Rep. 2021, 11, 1749. [Google Scholar] [CrossRef] [PubMed]
- Kaplan Can, H.; Gurbuz, F.; Odabaşı, M. Partial characterization of cyanobacterial extracellular polymeric substances for aquatic ecosystems. Aquat. Ecol. 2019, 53, 431–440. [Google Scholar] [CrossRef]
- Xiao, M.; Ren, X.; Yu, Y.; Gao, W.; Zhu, C.; Sun, H.; Kong, Q.; Fu, X.; Mou, H. Fucose-containing bacterial exopolysaccharides: Sources, biological activities, and food applications. Food Chem. X 2022, 13, 100233. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, C.; Patel, I. Evaluation of mechanism of plant protective attributes of root colonizing bacteria against phytopathogenic fungi. Bulg. J. Agric. Sci. 2022, 28, 238–241. [Google Scholar]
- Morsy, M.K.; Sharoba, A.M.; Khalaf, H.H.; El-Tanahy, H.H.; Cutter, C.N. Efficacy of antimicrobial pullulan-based coating to improve internal quality and shelf-life of chicken eggs during storage. J. Food Sci. 2015, 80, M1066–M1074. [Google Scholar] [CrossRef]
- Davidović, S.; Miljković, M.; Tomić, M.; Gordić, M.; Nešić, A.; Dimitrijević, S. Response surface methodology for optimisation of edible coatings based on dextran from Leuconostoc mesenteroides T3. Carbohydr. Polym. 2018, 184, 207–213. [Google Scholar] [CrossRef]
- Kritchenkov, A.S.; Egorov, A.R.; Volkova, O.V.; Zabodalova, L.A.; Suchkova, E.P.; Yagafarov, N.Z.; Kurasova, M.N.; Dysin, A.P.; Kurliuk, A.V.; Shakola, T.V.; et al. Active antibacterial food coatings based on blends of succinyl chitosan and triazole betaine chitosan derivatives. Food Packag. Shelf Life 2020, 25, 100534. [Google Scholar] [CrossRef]
- Zikmanis, P.; Juhņeviča-Radenkova, K.; Radenkovs, V.; Segliņa, D.; Krasnova, I.; Kolesovs, S.; Orlovskis, Z.; Šilaks, A.; Semjonovs, P. Microbial polymers in edible films and coatings of garden berry and grape: Current and prospective use. Food Bioprocess Technol. 2021, 14, 1432–1445. [Google Scholar] [CrossRef]
- Gan, L.; Li, X.; Wang, H.; Peng, B.; Tian, Y. Structural characterization and functional evaluation of a novel exopolysaccharide from the moderate halophile Gracilibacillus sp. SCU50. Int. J. Biol. Macromol. 2020, 154, 1140–1148. [Google Scholar] [CrossRef]
- Ibrahim, I.M.; Konnova, S.A.; Sigida, E.N.; Lyubun, E.V.; Muratova, A.Y.; Fedonenko, Y.P.; Elbanna, K. Bioremediation potential of a halophilic Halobacillus sp. strain, EG1HP4QL: Exopolysaccharide production, crude oil degradation, and heavy metal tolerance. Extremophiles 2020, 24, 157–166. [Google Scholar] [CrossRef]
- Hu, Q.; Lu, Y.; Luo, Y. Recent advances in dextran-based drug delivery systems: From fabrication strategies to applications. Carbohydr. Polym. 2021, 264, 117999. [Google Scholar] [CrossRef]
- Khan, Z.; Shafique, M.; Nawaz, H.R.; Jabeen, N.; Naz, S.A. Bacillus tequilensis ZMS-2: A novel source of alkaline protease with antimicrobial, anti-coagulant, fibrinolytic and dehairing potentials. Pak. J. Pharm. Sci. 2019, 32, 1913–1918. [Google Scholar]
- Srinivasan, R.; Kannappan, A.; Shi, C.; Lin, X. Marine bacterial secondary metabolites: A treasure house for structurally unique and effective antimicrobial compounds. Mar. Drugs 2021, 19, 530. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Guan, Y.; Dong, Y.; Zhao, L.; Rong, S.; Chen, W.; Lv, M.; Xu, H.; Gao, X.; Chen, R.; et al. Isolation and evaluation of endophytic Bacillus tequilensis GYLH001 with potential application for biological control of Magnaporthe oryzae. PLoS ONE 2018, 13, e0203505. [Google Scholar] [CrossRef] [PubMed]
- Muras, A.; Romero, M.; Mayer, C.; Otero, A. Biotechnological applications of Bacillus licheniformis. Crit. Rev. Biotechnol. 2021, 41, 609–627. [Google Scholar] [CrossRef] [PubMed]
- Franco, W.; Pérez-Díaz, I.M.; Connelly, L.; Diaz, J.T. Isolation of exopolysaccharide-producing yeast and lactic acid bacteria from quinoa (Chenopodium quinoa) sourdough fermentation. Foods 2020, 9, 337. [Google Scholar] [CrossRef] [PubMed]
- Abid, Y.; Azabou, S.; Casillo, A.; Gharsallah, H.; Jemil, N.; Lanzetta, R.; Attia, H.; Corsaro, M.M. Isolation and structural characterization of levan produced by probiotic Bacillus tequilensis-GM from Tunisian fermented goat milk. Int. J. Biol. Macromol. 2019, 133, 786–794. [Google Scholar] [CrossRef]
- Razack, S.A.; Velayutham, V.; Thangavelu, V. Medium optimization for the production of exopolysaccharide by Bacillus subtilis using synthetic sources and agro wastes. Turk. J. Biol. 2013, 37, 280–288. [Google Scholar] [CrossRef]
- Radchenkova, N.; Vassilev, S.; Panchev, I.; Anzelmo, G.; Tomova, I.; Nicolaus, B.; Kuncheva, M.; Petrov, K.; Kambourova, M. Production and properties of two novel exopolysaccharides synthesized by a thermophilic bacterium Aeribacillus pallidus 418. Appl. Biochem. Biotechnol. 2013, 171, 31–43. [Google Scholar] [CrossRef]
- Stautz, J.; Hellmich, Y.; Fuss, M.F.; Silberberg, J.M.; Devlin, J.R.; Stockbridge, R.B.; Hänelt, I. Molecular mechanisms for bacterial potassium homeostasis. J. Mol. Biol. 2021, 433, 166968. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, J.; Guo, H.; Cheng, Q.; Abbas, Z.; Tong, Y.; Yang, T.; Zhou, Y.; Zhang, H.; Wei, X.; et al. Optimization of Exopolysaccharide Produced by Lactobacillus plantarum R301 and Its Antioxidant and Anti-Inflammatory Activities. Foods 2023, 12, 2481. [Google Scholar] [CrossRef]
- Catalão, M.; Fernandes, M.; Galdon, L.; Rodrigues, C.F.; Sobral, R.G.; Gaudêncio, S.P.; Torres, C.A. Exopolysaccharide Production from Marine-Derived Brevundimonas huaxiensis Obtained from Estremadura Spur Pockmarks Sediments Revealing Potential for Circular Economy. Mar. Drugs 2023, 21, 419. [Google Scholar] [CrossRef] [PubMed]
- Vidhyalakshmi, R.; Valli, N.C.; Kumar, G.N.; Sunkar, S. Bacillus circulans exopolysaccharide: Production, characterization and bioactivities. Int. J. Biol. Macromol. 2016, 87, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.R.; Jeong, D.Y.; Baik, S.H. Optimal production of exopolysaccharide by Bacillus licheniformis KS-17 isolated from kimchi. Food Sci. Biotechnol. 2013, 22, 417–423. [Google Scholar] [CrossRef]
- Choi, I.S.; Ko, S.H.; Lee, M.E.; Kim, H.M.; Yang, J.E.; Jeong, S.G.; Lee, K.H.; Chang, J.Y.; Kim, J.C.; Park, H.W. Production, characterization, and antioxidant activities of an exopolysaccharide extracted from spent media wastewater after Leuconostoc mesenteroides WiKim32 fermentation. ACS Omega 2021, 6, 8171–8178. [Google Scholar] [CrossRef] [PubMed]
- Sutthi, N.; Wangkahart, E.; Panase, P.; Karirat, T.; Deeseenthum, S.; Ma, N.L.; Luang-In, V. Dietary Administration Effects of Exopolysaccharide Produced by Bacillus tequilensis PS21 Using Riceberry Broken Rice, and Soybean Meal on Growth Performance, Immunity, and Resistance to Streptococcus agalactiae of Nile tilapia (Oreochromis niloticus). Animals 2023, 13, 3262. [Google Scholar] [CrossRef] [PubMed]
- Karirat, T.; Saengha, W.; Deeseenthum, S.; Ma, N.L.; Sutthi, N.; Wangkahart, E.; Luang-In, V. Data on exopolysaccharides produced by Bacillus spp. from cassava pulp with antioxidant and antimicrobial properties. Data Brief 2023, 50, 109474. [Google Scholar] [CrossRef]
- Malick, A.; Khodaei, N.; Benkerroum, N.; Karboune, S. Production of exopolysaccharides by selected Bacillus strains: Optimization of media composition to maximize the yield and structural characterization. Int. J. Biol. Macromol. 2017, 102, 539–549. [Google Scholar] [CrossRef]
- Rani, R.P.; Anandharaj, M.; Sabhapathy, P.; Ravindran, A.D. Physiochemical and biological characterization of novel exopolysaccharide produced by Bacillus tequilensis FR9 isolated from chicken. Int. J. Biol. Macromol. 2017, 96, 1–10. [Google Scholar] [CrossRef]
- Rani, R.P.; Anandharaj, M.; Ravindran, A.D. Characterization of a novel exopolysaccharide produced by Lactobacillus gasseri FR4 and demonstration of its in vitro biological properties. Int. J. Biol. Macromol. 2018, 109, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, W.; Chen, X.; Feng, M.; Rui, X.; Jiang, M.; Dong, M. Microbiological, physicochemical and rheological properties of fermented soymilk produced with exopolysaccharide (EPS) producing lactic acid bacteria strains. LWT-Food Sci. Technol. 2014, 57, 477–485. [Google Scholar] [CrossRef]
- Chandra, S.; Chatterjee, P.; Dey, P.; Bhattacharya, S. Evaluation of in vitro anti-inflammatory activity of coffee against the denaturation of protein. Asian Pac. J. Trop. Biomed. 2012, 2, S178–S180. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, X.; Cai, L.; Chen, R.; Zhang, Q.; Wang, X. Determination of levan from Bacillus licheniformis by ultraviolet spectrophotometry. Trop. J. Pharm. Res. 2015, 14, 679–685. [Google Scholar] [CrossRef]
- López-Ortega, M.A.; Chavarría-Hernández, N.; del Rocío López-Cuellar, M.; Rodríguez-Hernández, A.I. A review of extracellular polysaccharides from extreme niches: An emerging natural source for the biotechnology. From the adverse to diverse! Int. J. Biol. Macromol. 2021, 177, 559–577. [Google Scholar] [CrossRef]
- Huang-Lin, E.; Sánchez-León, E.; Amils, R.; Abrusci, C. Potential Applications of an Exopolysaccharide Produced by Bacillus xiamenensis RT6 Isolated from an Acidic Environment. Polymers 2022, 14, 3918. [Google Scholar] [CrossRef]
- Bomfim, V.B.; Neto, J.H.P.L.; Leite, K.S.; de Andrade Vieira, É.; Iacomini, M.; Silva, C.M.; dos Santos, K.M.O.; Cardarelli, H.R. Partial characterization and antioxidant activity of exopolysaccharides produced by Lactobacillus plantarum CNPC003. LWT 2020, 127, 109349. [Google Scholar] [CrossRef]
- Hu, X.; Wang, K.; Yu, M.; He, P.; Qiao, H.; Zhang, H.; Wang, Z. Characterization and antioxidant activity of a low-molecular-weight xanthan gum. Biomolecules 2019, 9, 730. [Google Scholar] [CrossRef]
- Ye, G.; Li, G.; Wang, C.; Ling, B.; Yang, R.; Huang, S. Extraction and characterization of dextran from Leuconostoc pseudomesenteroides YB-2 isolated from mango juice. Carbohydr. Polym. 2019, 207, 218–223. [Google Scholar] [CrossRef]
- Asgher, M.; Urooj, Y.; Qamar, S.A.; Khalid, N. Improved exopolysaccharide production from Bacillus licheniformis MS3: Optimization and structural/functional characterization. Int. J. Biol. Macromol. 2020, 151, 984–992. [Google Scholar] [CrossRef]
- Izadi, P.; Izadi, P.; Eldyasti, A. Holistic insights into extracellular polymeric substance (EPS) in anammosx bacterial matrix and the potential sustainable biopolymer recovery: A review. Chemosphere 2021, 274, 129703. [Google Scholar] [CrossRef]
- Berninger, T.; Dietz, N.; González López, Ó. Water-soluble polymers in agriculture: Xanthan gum as eco-friendly alternative to synthetics. Microb. Biotechnol. 2021, 14, 1881–1896. [Google Scholar] [CrossRef]
- Yanti, N.A.; Ahmad, S.W.; Ramadhan, L.O.A.N.; Walhidayah, T. December. Mechanical properties of edible film based bacterial cellulose from sago liquid waste using starch as stabilizer. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 948, No. 1; p. 012063. [Google Scholar]
- Arserim Ucar, D.K.; Konuk Takma, D.; Korel, F. Exopolysaccharides in food processing industrials. In Microbial Exopolysaccharides as Novel and Significant Biomaterials; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 201–234. [Google Scholar]
- Lebeaux, D.; Bergeron, E.; Berthet, J.; Djadi-Prat, J.; Mouniee, D.; Boiron, P.; Lortholary, O.; Rodriguez-Nava, V. Antibiotic susceptibility testing and species identification of Nocardia isolates: A retrospective analysis of data from a French expert laboratory, 2010–2015. Clin. Microbiol. Infect. 2019, 25, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Ayyash, M.; Abu-Jdayil, B.; Itsaranuwat, P.; Almazrouei, N.; Galiwango, E.; Esposito, G.; Hunashal, Y.; Hamed, F.; Najjar, Z. Exopolysaccharide produced by the potential probiotic Lactococcus garvieae C47: Structural characteristics, rheological properties, bioactivities and impact on fermented camel milk. Food Chem. 2020, 333, 127418. [Google Scholar] [CrossRef] [PubMed]
- Salachna, P.; Mizielińska, M.; Soból, M. Exopolysaccharide gellan gum and derived oligo-gellan enhance growth and antimicrobial activity in Eucomis plants. Polymers 2018, 10, 242. [Google Scholar] [CrossRef]
- Riaz Rajoka, M.S.; Mehwish, H.M.; Wu, Y.; Zhao, L.; Arfat, Y.; Majeed, K.; Anwaar, S. Chitin/chitosan derivatives and their interactions with microorganisms: A comprehensive review and future perspectives. Crit. Rev. Biotechnol. 2020, 40, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.D.; Zhu, J.; Wu, L.X.; Qiao, Z.R.; Li, L.; Yan, J.K. Preparation, characterization, rheological and antioxidant properties of ferulic acid-grafted curdlan conjugates. Food Chem. 2019, 300, 125221. [Google Scholar] [CrossRef] [PubMed]
- Luang-In, V.; Saengha, W.; Deeseenthum, S. Characterization and bioactivities of a novel exopolysaccharide produced from lactose by Bacillus tequilensis PS21 isolated from Thai milk kefir. Microbiol. Biotechnol. Lett. 2018, 46, 9–17. [Google Scholar] [CrossRef]
- Luang-In, V.; Deeseenthum, S. Exopolysaccharide-producing isolates from Thai milk kefir and their antioxidant activities. LWT 2016, 73, 592–601. [Google Scholar] [CrossRef]
- Choudhuri, I.; Khanra, K.; Pariya, P.; Maity, G.N.; Mondal, S.; Pati, B.R.; Bhattacharyya, N. Structural characterization of an exopolysaccharide isolated from Enterococcus faecalis, and study on its antioxidant activity, and cytotoxicity against HeLa cells. Curr. Microbiol. 2020, 77, 3125–3135. [Google Scholar] [CrossRef]
- Rahnama Vosough, P.; Habibi Najafi, M.B.; Edalatian Dovom, M.R.; Javadmanesh, A.; Mayo, B. Evaluation of antioxidant, antibacterial and cytotoxicity activities of exopolysaccharide from Enterococcus strains isolated from traditional Iranian Kishk. J. Food Meas. Charact. 2021, 15, 5221–5230. [Google Scholar] [CrossRef]
- Yun, Z.; Li, T.; Gao, H.; Zhu, H.; Gupta, V.K.; Jiang, Y.; Duan, X. Integrated transcriptomic, proteomic, and metabolomics analysis reveals peel ripening of harvested banana under natural condition. Biomolecules 2019, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, A.; Manjarres, J.J.; Ramírez, C.; Bolívar, G. Use of an exopolysaccharide-based edible coating and lactic acid bacteria with antifungal activity to preserve the postharvest quality of cherry tomato. LWT 2021, 151, 112225. [Google Scholar] [CrossRef]
- Park, G.T.; Go, R.E.; Lee, H.M.; Lee, G.A.; Kim, C.W.; Seo, J.W.; Hong, W.K.; Choi, K.C.; Hwang, K.A. Potential anti-proliferative and immunomodulatory effects of marine microalgal exopolysaccharide on various human cancer cells and lymphocytes in vitro. Mar. Biotechnol. 2017, 19, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Maqbool, M.; Ramachandran, S.; Alderson, P.G. Gum arabic as a novel edible coating for enhancing shelf-life and improving postharvest quality of tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Technol. 2010, 58, 42–47. [Google Scholar] [CrossRef]
- Zaikova, E.; Hawley, A.; Walsh, D.A.; Hallam, S.J. Seawater sampling and collection. JoVE J. Vis. Exp. 2009, 28, e1159. [Google Scholar]
- Patel, H.; Jadav, C.; Kalaria, R.K. Biochemical and molecular profiling of novel halophiles isolated from coastal region of South Gujarat. Trends Biosci. 2017, 10, 368–378. [Google Scholar]
- Gangalla, R.; Sampath, G.; Beduru, S.; Sarika, K.; Govindarajan, R.K.; Ameen, F.; Alwakeel, S.; Thampu, R.K. Optimization and characterization of exopolysaccharide produced by Bacillus aerophilus rk1 and its in vitro antioxidant activities. J. King Saud Univ.-Sci. 2021, 33, 101470. [Google Scholar] [CrossRef]
- Chambi, D.; Lundqvist, J.; Nygren, E.; Romero-Soto, L.; Marin, K.; Gorzsás, A.; Hedenström, M.; Carlborg, M.; Broström, M.; Sundman, O.; et al. Production of exopolysaccharides by cultivation of halotolerant Bacillus atrophaeus BU4 in glucose-and xylose-based synthetic media and in hydrolysates of quinoa stalks. Fermentation 2022, 8, 79. [Google Scholar] [CrossRef]
- Singh, R.P.; Shukla, M.K.; Mishra, A.; Kumari, P.; Reddy, C.R.K.; Jha, B. Isolation and characterization of exopolysaccharides from seaweed associated bacteria Bacillus licheniformis. Carbohydr. Polym. 2011, 84, 1019–1026. [Google Scholar] [CrossRef]
- Saravanan, C.; Shetty, P.K.H. Isolation and characterization of exopolysaccharide from Leuconostoc lactis KC117496 isolated from idli batter. Int. J. Biol. Macromol. 2016, 90, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ren, Y.; Li, L. The relationship between charge intensity and bioactivities/processing characteristics of exopolysaccharides from lactic acid bacteria. LWT 2022, 153, 112345. [Google Scholar] [CrossRef]
- Khan, S.Y.; Yusuf, M.; Sardar, N. Studies on rheological behavior of xanthan gum solutions in presence of additives. Pet Petrochem. Eng. J 2018, 2, 000165. [Google Scholar]
- Yiğit, Y.; Kilislioğlu, A.; Karakuş, S.; Baydoğan, N. Determination of the intrinsic viscosity and molecular weight of poly (methyl methacrylate) blends. J. Investig. Eng. Technol. 2019, 2, 34–39. [Google Scholar]
- Upadhyaya, C.; Upadhyaya, T.; Patel, I. Exposure effects of non-ionizing radiation of radio waves on antimicrobial potential of medicinal plants. J. Radiat. Res. Appl. Sci. 2022, 15, 1–10. [Google Scholar] [CrossRef]
- Upadhyaya, C.; Upadhyaya, T.; Patel, I. Attributes of non-ionizing radiation of 1800 MHz frequency on plant health and antioxidant content of Tomato (Solanum lycopersicum) plants. J. Radiat. Res. Appl. Sci. 2022, 15, 54–68. [Google Scholar] [CrossRef]
- Avelar-Freitas, B.A.; Almeida, V.G.; Pinto, M.C.X.; Mourão, F.A.G.; Massensini, A.R.; Martins-Filho, O.A.; Rocha-Vieira, E.; Brito-Melo, G.E.A. Trypan blue exclusion assay by flow cytometry. Braz. J. Med. Biol. Res. 2014, 47, 307–315. [Google Scholar] [CrossRef]
- Villota, R.; Hawkes, J.G. Reaction kinetics in food systems. In Handbook of Food Engineering; CRC Press: Boca Raton, FL, USA, 2018; pp. 225–484. [Google Scholar]
- Pobiega, K.; Przybył, J.L.; Żubernik, J.; Gniewosz, M. Prolonging the shelf life of cherry tomatoes by pullulan coating with ethanol extract of propolis during refrigerated storage. Food Bioprocess Technol. 2020, 13, 1447–1461. [Google Scholar] [CrossRef]
- Salazar, B.; Ortiz, A.; Keswani, C.; Minkina, T.; Mandzhieva, S.; Pratap Singh, S.; Rekadwad, B.; Borriss, R.; Jain, A.; Singh, H.B.; et al. Bacillus spp. as bio-factories for antifungal secondary metabolites: Innovation beyond whole organism formulations. Microb. Ecol. 2023, 86, 1–24. [Google Scholar] [CrossRef]







| Halophilic Bacteria | EPS Properties | Application | Reference |
|---|---|---|---|
| Bacillus altitudinis | Antimicrobial and antioxidant activity | Antagonism of pathogenic Pseudomonas arugenosa | [6] |
| Pseudomonas stuzeri | Antibiofilm forming and anti-biofouling potential | Treatment of antibiotic resistant infections and pervert biofouling in marine environment | [7] |
| Alteromonas sp. | Heavy metal binding capacity | Heavy metal remediation from polluted sites | [8] |
| Geobacillus, B. licheniformis | Antiviral and immune stimulatory actions | Therapy of viral infections viz. herpes | [9] |
| Halomonas maura | Electrospnning potential | Biomedical sector for sustained bioimaging, drug delivery via nano particles | [10] |
| Halomonas alkaliantarctica | High emulsification capability | Oil recovery | [11] |
| Vagococcus carniphilus | Potent flocculating activity comparable to xanthan gum | Industrial waste water flocculants | [12] |
| Halomonas xianhensis | Anticancer activity | Biomedical applications | [13] |
| Marine Bacillus cereus and Brachybacterium sp. | Antimicrobial activity | Potential use as antimicrobial agent | [14] |
| Halomonas malpeensis YU-PRIM-29 | Emulsification activity, antioxidant activity | Potential petroleum hydrocarbon emulsification | [15] |
| Marine cynobacteria | Acidic nature and presence of uronic acid | Potential use in inhibiting the growth of toxic cyanobacteria in aquatic environment | [16] |
| Halomonas strains | High viscosity, Fucose present, Pseudoplastic behavior | Potential use in food industry for food preservation | [17] |
| (a) | ||||
|---|---|---|---|---|
| Isolate | EPS Content (g/L) | Optical Density | Dry Cell Mass (g/L) | |
| SWIS01 | 5.69 ± 0.27 | 0.84 ± 0.02 | 0.93 ± 0.04 | |
| SWIS02 | 13.76 ± 0.25 | 0.99 ± 0.01 | 0.86 ± 0.02 | |
| SSIS01 | 3.88 ± 0.26 | 1.05 ± 0.13 | 0.97 ± 0.13 | |
| SSIS02 | 9.64 ± 0.37 | 1.83 ± 0.02 | 0.69 ± 0.10 | |
| KSIS01 | 5.68 ± 0.33 | 1.04 ± 0.17 | 0.87 ± 0.09 | |
| KSIS02 | 11.04 ± 0.26 | 1.04 ± 0.05 | 0.87 ± 0.02 | |
| KWIS01 | 3.13 ± 0.27 | 1.17 ± 0.18 | 0.85 ± 0.12 | |
| KWIS02 | 13.68 ± 0.34 | 2.02 ± 0.03 | 0.52 ± 0.12 | |
| TSIS01 | 12.84 ± 0.18 | 0.87 ± 0.02 | 0.83 ± 0.02 | |
| TSIS02 | 6.17 ± 0.29 | 0.96 ± 0.04 | 0.70 ± 0.03 | |
| TWIS01 | 4.83 ± 0.27 | 0.68 ± 0.05 | 0.61 ± 0.03 | |
| UWIS01 | 3.88 ± 0.39 | 1.45 ± 0.19 | 0.75 ± 0.07 | |
| UWIS02 | 5.58 ± 0.54 | 1.00 ± 0.09 | 0.84 ± 0.06 | |
| UWIS03 | 8.14 ± 0.36 | 1.18 ± 0.12 | 0.82 ± 0.05 | |
| UWIS04 | 11.57 ± 0.29 | 0.78 ± 0.02 | 0.86 ± 0.02 | |
| DWIS01 | 2.35 ± 0.62 | 0.85 ± 0.06 | 0.57 ± 0.10 | |
| DWIS02 | 4.07 ± 0.29 | 0.74 ± 0.06 | 0.61 ± 0.04 | |
| DWIS03 | 10.28 ± 1.18 | 1.57 ± 0.01 | 0.69 ± 0.02 | |
| DUIS01 | 4.44 ± 0.41 | 1.46 ± 0.25 | 0.66 ± 0.04 | |
| KAIS01 | 3.55 ± 0.61 | 1.30 ± 0.27 | 0.64 ± 0.04 | |
| KAIS02 | 6.84 ± 0.58 | 1.31 ± 0.33 | 0.74 ± 0.07 | |
| KEIS01 | 3.40 ± 0.66 | 1.03 ± 0.25 | 0.52 ± 0.11 | |
| (b) | ||||
| Halophilic EPS | Zone of Inhibition (mm) against Selected Phytopathogens | |||
| F. oxysporum | F. solani | A. solani | X. citri | |
| SWIS02 | 12.3 ± 1.09 | 11.82 ± 1.19 | 12.74 ± 0.85 | 11.8 ± 1.16 |
| TSIS01 | 16.44 ± 1.48 | 18.32 ± 1.10 | 23.78 ± 1.25 | 21.04 ± 1.59 |
| KSIS02 | 11.56 ± 0.83 | 12.20 ± 0.47 | 14.08 ± 1.35 | 14.76 ± 1.18 |
| KWIS02 | 10.78 ± 0.43 | 12.50 ± 1.15 | 15.32 ± 0.69 | 14.46 ± 1.02 |
| UWIS04 | 13.32 ± 1.22 | 14.56 ± 0.59 | 15.52 ± 1.15 | 18.18 ± 1.45 |
| Antimicrobial agent | 18.3 ± 0.95 | 20.04 ± 1.28 | 25.34 ± 1.15 | 22.8 ± 1.37 |
| Parameter | Optimum Condition |
|---|---|
| Media | Minimal media |
| Carbon source | Starch |
| Nitrogen source | Beef extract |
| Content of carbon source | 25 g/L |
| Content of nitrogen source | 3 g/L |
| K2HPO4 concentration | 6 g/L |
| MgSO4 concentration | 1 g/L |
| NaCl concentration | 7% |
| Temperature | 30 °C |
| pH | 7.0 |
| Incubation time | 72–144 h |
| Static or shaking conditions | Shaking at 150 rpm |
| Water (D/W or sea water) | Sea water |
| % Weight Loss | T (°C) | n = 0 | n = 1 | n = 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2 | Kc | A0 | R2 | Kc | A0 | R2 | Kc | A0 | ||
| UC | 4 | 0.987 | 1.51 | 99.89 | 0.987 | 0.019 | 99.95 | 0.987 | 0.0003 | 100.0 |
| 30 | 0.998 | 4.10 | 99.98 | 0.999 | 0.047 | 100.55 | 0.992 | 0.0002 | 100.0 | |
| EC | 4 | 0.987 | 1.32 | 99.75 | 0.977 | 0.015 | 99.86 | 0.988 | 0.0006 | 102.0 |
| 30 | 0.999 | 2.00 | 99.92 | 0.998 | 0.024 | 100.13 | 0.997 | 0.0004 | 100.0 | |
| CC | 4 | 0.988 | 1.40 | 99.69 | 0.989 | 0.017 | 99.89 | 0.996 | 0.0005 | 100.0 |
| 30 | 0.998 | 3.25 | 99.82 | 0.997 | 0.039 | 100.47 | 0.994 | 0.0003 | 100.0 | |
| XC | 4 | 0.988 | 1.36 | 99.78 | 0.977 | 0.017 | 99.85 | 0.997 | 0.0006 | 100.0 |
| 30 | 0.998 | 2.20 | 99.91 | 0.997 | 0.023 | 100.15 | 0.995 | 0.0004 | 100.0 | |
| Firmness | T °C | n = 0 | n = 1 | n = 2 | ||||||
| R2 | Kc | A0 | R2 | Kc | A0 | R2 | Kc | A0 | ||
| UC | 4 | 0.915 | 0.477 | 12.71 | 0.920 | 0.047 | 13.34 | 0.920 | 0.0051 | 12.45 |
| 30 | 0.826 | 0.682 | 11.74 | 0.876 | 0.071 | 12.73 | 0.911 | 0.0070 | 11.75 | |
| EC | 4 | 0.954 | 0.192 | 12.71 | 0.932 | 0.019 | 13.74 | 0.932 | 0.0020 | 12.91 |
| 30 | 0.895 | 0.453 | 12.19 | 0.922 | 0.052 | 14.00 | 0.923 | 0.0050 | 12.17 | |
| CC | 4 | 0.945 | 0.188 | 12.71 | 0.929 | 0.028 | 13.57 | 0.925 | 0.0030 | 12.81 |
| 30 | 0.879 | 0.436 | 12.00 | 0.913 | 0.064 | 13.89 | 0.918 | 0.0065 | 11.98 | |
| XC | 4 | 0.957 | 0.194 | 12.71 | 0.935 | 0.021 | 13.79 | 0.934 | 0.0020 | 12.87 |
| 30 | 0.890 | 0.457 | 12.73 | 0.919 | 0.050 | 13.98 | 0.921 | 0.0055 | 12.14 | |
| Treatment | 4 °C | 30 °C | ||
|---|---|---|---|---|
| Weight Loss | Firmness | Weight Loss | Firmness | |
| UC | 5 | 13 | 3 | 8 |
| EC | 17 | 35 | 13 | 25 |
| CC | 13 | 29 | 10 | 16 |
| XC | 18 | 37 | 12 | 22 |
| Location | Coordinates |
|---|---|
| Sambhar lake | (26.9456° N, 75.2097° E) |
| Kutch | (23.7337° N, 69.8597° E) |
| Tithal | (20.5890° N, 72.9031° E) |
| Khambhat | (22.3181° N, 72.6190° E) |
| Udvada | (20.4863° N, 72.8722° E) |
| Ubharat | (21.0126° N, 72.7348° E) |
| Dumas | (21.0949° N, 72.7114° E) |
| Treatment | Composition | No. of Replication | Total Number of Tomatoes Treated |
|---|---|---|---|
| Experimental coating | EPS of Bacillus tequilensis (1%) + 1% glycerol + 0.5% Tween 80 + 1% Oleic acid | 5 | 250 |
| Positive control 1 | Xanthan gum (1%) + 1% glycerol + 0.5% Tween 80 + 1% Oleic acid | 5 | 250 |
| Positive control 2 | Chitosan (1%) + 1% glycerol + 0.5% Tween 80 + 1% Oleic acid | 5 | 250 |
| Negative control (Uncoated) | Uncoated tomatoes (immersed in D/W) | 5 | 250 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Upadhyaya, C.; Patel, H.; Patel, I.; Ahir, P.; Upadhyaya, T. Development of Biological Coating from Novel Halophilic Exopolysaccharide Exerting Shelf-Life-Prolonging and Biocontrol Actions for Post-Harvest Applications. Molecules 2024, 29, 695. https://doi.org/10.3390/molecules29030695
Upadhyaya C, Patel H, Patel I, Ahir P, Upadhyaya T. Development of Biological Coating from Novel Halophilic Exopolysaccharide Exerting Shelf-Life-Prolonging and Biocontrol Actions for Post-Harvest Applications. Molecules. 2024; 29(3):695. https://doi.org/10.3390/molecules29030695
Chicago/Turabian StyleUpadhyaya, Chandni, Hiren Patel, Ishita Patel, Parth Ahir, and Trushit Upadhyaya. 2024. "Development of Biological Coating from Novel Halophilic Exopolysaccharide Exerting Shelf-Life-Prolonging and Biocontrol Actions for Post-Harvest Applications" Molecules 29, no. 3: 695. https://doi.org/10.3390/molecules29030695