Exploration of the Dynamic Variations of the Characteristic Constituents and the Degradation Products of Catalpol during the Process of Radix Rehmanniae
Abstract
1. Introduction
2. Results and Discussions
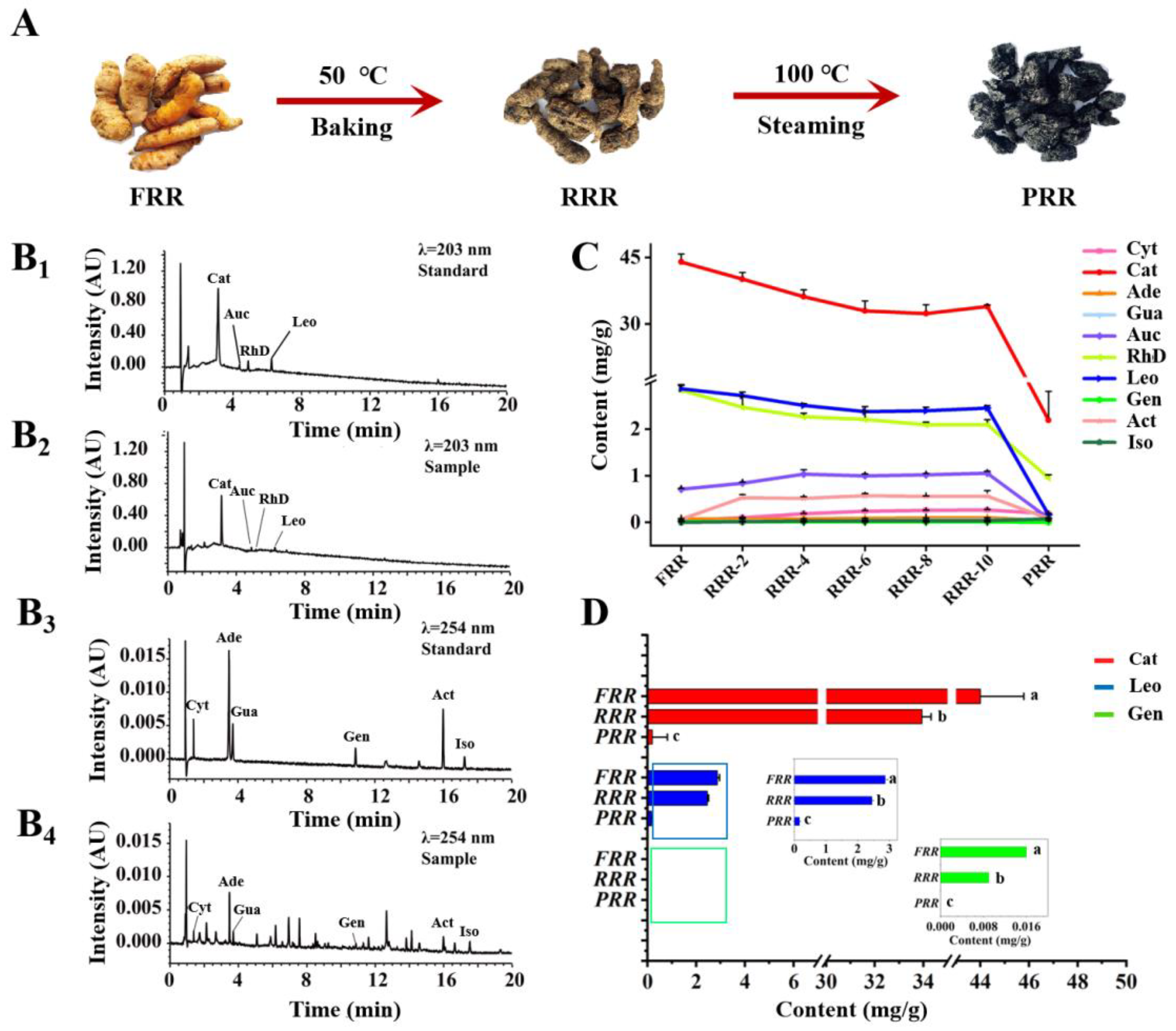
2.1. Methodological Validation of UPLC-PDA Analysis for Quantitation of Ten Compounds in RR
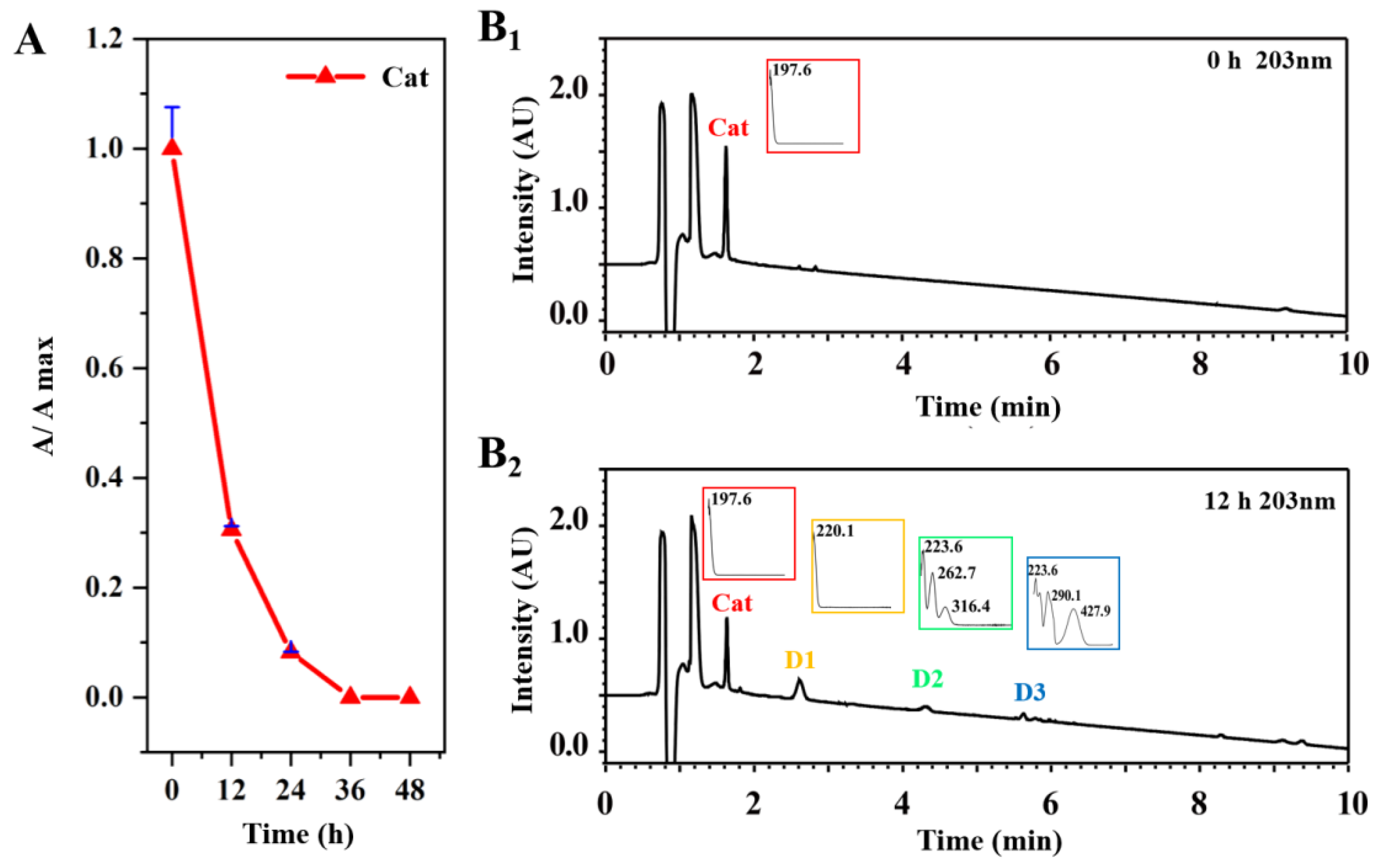
2.2. The Content Variations of Tested Compounds and the Degradation of Cat That Occurred in the Processing of RR
2.3. Characterization of the Degradation Products of Cat
2.3.1. Spectroscopic Analysis of D1
2.3.2. Spectroscopic Analysis of D2
2.3.3. Spectroscopic Analysis of D3
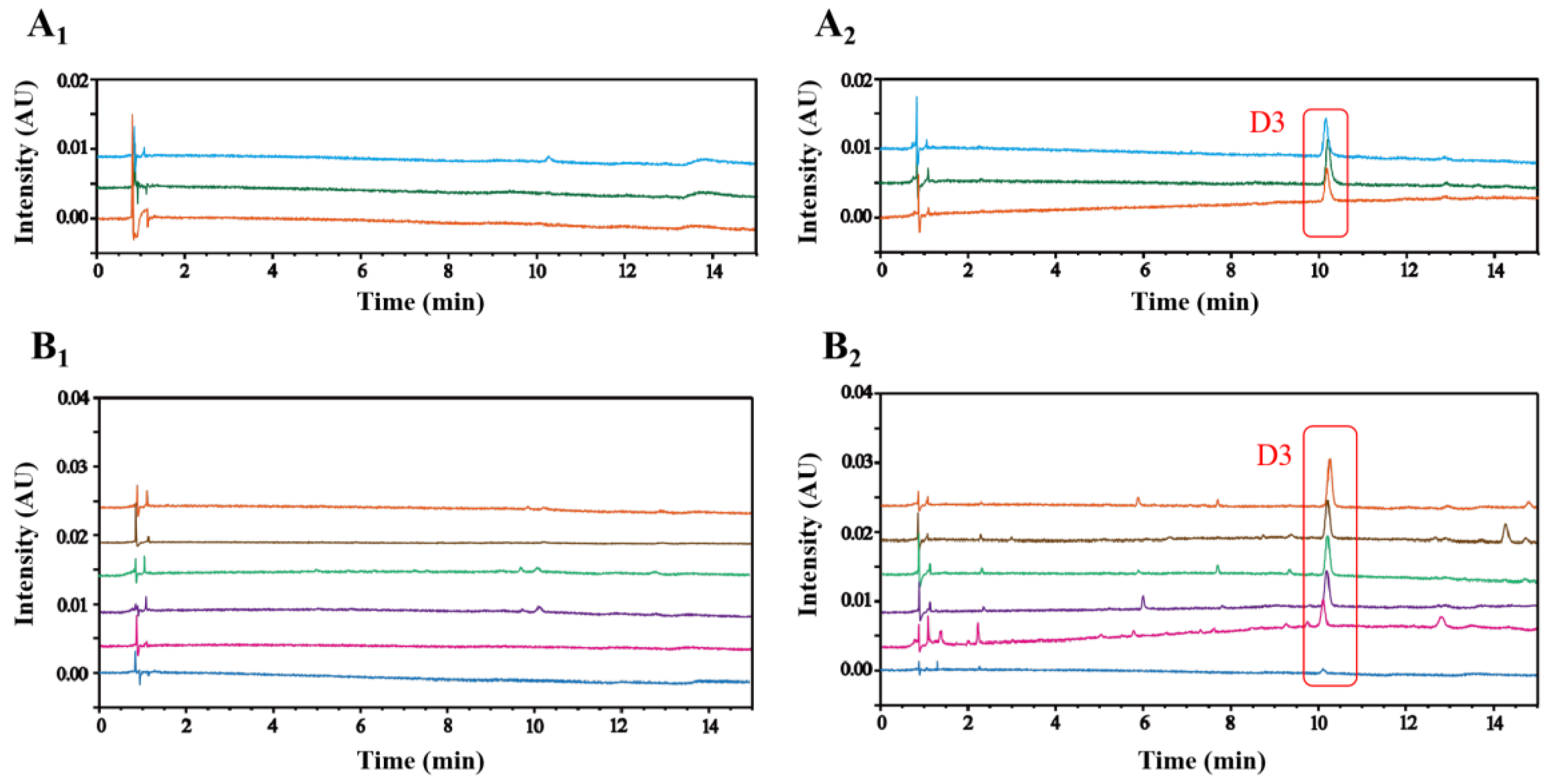
2.4. Specificity of D3 as a Degradation Product of Cat in PRR
3. Materials and Methods
3.1. Reagents and Materials
3.2. Preparation of RRR and PRR
3.3. Preparation of Standard and Sample Solutions
3.3.1. Preparation of Standard and Sample Solutions for Quantitative Analysis
3.3.2. Preparation of Cat Standard Solution for Identification of Degradation Products
3.3.3. Preparation of Sample Solutions for the Validation of Cat Degradation Products
3.4. UPLC-PDA Conditions
3.5. Methodological Validation
3.6. Purification and Enrichment of Degradation Products of Cat
3.6.1. Isolation of Degradation Samples
3.6.2. Purification by Preparative HPLC
3.7. NMR Spectroscopic Analysis
3.8. LC-MS Analysis
3.9. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RR | Radix Rehmanniae |
| R. glutinosa | Rehmannia glutinosa Libosch. |
| TCM | traditional Chinese medicine |
| FRR | fresh Radix Rehmanniae |
| RRR | raw Radix Rehmanniae |
| PRR | processed Radix Rehmanniae |
| Cat | catalpol |
| Leo | leonuride |
| RhD | rehmannioside D |
| ChP | Chinese pharmacopoeia |
| UPLC-PDA | ultra-performance liquid chromatography with photo-diode array detector |
| LOD | the limit of detection |
| LOQ | the limit of quantitation |
| RSD | relative standard deviation |
| Auc | aucubin |
| Gen | geniposide |
| CC | column chromatography |
| preparative HPLC | preparative high-performance liquid chromatography |
| NMR | nuclear magnetic resonance |
| HMBC | heteronuclear multiple bond correlation |
| 1H-1H COSY | 1H-1H correlated spectroscopy |
| LC-MS | liquid chromatography–tandem mass spectrometry |
| DMSO | dimethyl sulfoxide |
| Cyt | cytidine |
| Ade | adenosine |
| Gua | guanosine |
| Act | acteoside |
| Iso | isoacteoside |
| LC-UV | liquid chromatography–ultraviolet |
| S/N | signal-to-noise ratio |
| TMS | tetramethylsilane |
References
- Zhang, R.X.; Li, M.X.; Jia, Z.P. Rehmannia glutinosa: Review of botany, chemistry and pharmacology. J. Ethnopharmacol. 2008, 117, 199–214. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. ChP 2020; China Medical Science Press: Beijing, China, 2020; Volume I and IV. [Google Scholar]
- Li, M.M.; Jiang, H.J.; Hao, Y.L.; Du, K.Q.; Du, H.L.; Ma, C.; Tu, H.; He, Y. A systematic review on botany, processing, application, phytochemistry and pharmacological action of Radix rehmnniae. J. Ethnopharmacol. 2022, 285, 114820. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Xu, J.D.; Wang, Y.; Wu, C.Y.; Zhou, J.; Shen, H.; Zou, Y.T.; Zhu, J.H.; Zhou, S.S.; Li, S.L.; et al. Comparing steamed and wine-stewed Rehmanniae Radix in terms of Yin-nourishing effects via metabolomics and microbiome analysis. J. Ethnopharmacol. 2023, 311, 116424. [Google Scholar] [CrossRef]
- Xia, F.B.; Liu, C.H.; Wan, J.B. Characterization of the cold and hot natures of raw and processed Rehmanniae Radix by integrated metabolomics and network pharmacology. Phytomedicine 2020, 74, 153071. [Google Scholar] [CrossRef]
- Cui, W.; Zheng, X.M.; Li, H.X.; Feng, Y.F.; Xu, Z. Chemical constituents of Fresh Rehmannia glutinosa. Zhongcaoyao 2022, 45, 2369–2375. [Google Scholar] [CrossRef]
- Liu, Y.F.; Liang, D.; Luo, H.; Hao, Z.Y.; Yu, D.Q. Chemical constituents from root tubers of Rehmannia glutinosa. Zhongcaoyao 2014, 45, 16–22. [Google Scholar]
- Yue, C.; Gao, J.; Shi, S.M.; Zhang, W.T. Simultaneous determination of three components in crude and processed Rehmanniae Radix by HPLC. Zhongguo Shiyan Fangjixue Zazhi 2015, 21, 71–74. [Google Scholar] [CrossRef]
- Youn, U.J.; Gu, B.S.; Kim, K.H.; Ha, C.; Jung, I.C. Variation of main components according to the number of steaming and drying of Rehmanniae radix preparata. J. Pharmacopunct. 2018, 21, 112–119. [Google Scholar] [CrossRef]
- Liu, J.Y.; Zhang, D.J. Amelioration by catalpol of atherosclerotic lesions in hypercholesterolemic rabbits. Planta Med. 2015, 81, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Ma, R.F.; Wang, L.L.; Zhu, R.Y.; Liu, H.X.; Guo, Y.B.; Zhao, B.S.; Zhao, S.G.; Tang, J.F.; Li, Y.Y.; et al. Rehmanniae radix in osteoporosis: A review of traditional Chinese medicinal uses, phytochemistry, pharmacokinetics and pharmacology. J. Ethnopharmacol. 2017, 198, 351–362. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhao, R.S.; Wang, Y.H.; Liu, H.B.; Wang, X.H. Research progress on catalpol as treatment for atherosclerosis. Front. Pharmacol. 2021, 12, 716125. [Google Scholar] [CrossRef]
- Han, Y.; Shen, M.; Tang, L.Y.; Tan, G.; Yang, Q.C.; Ye, L.; Ye, L.H.; Jiang, N.; Gao, G.P.; Shao, Y. Antiangiogenic effects of catalpol on rat corneal neovascularization. Mol. Med. Rep. 2018, 17, 2187–2194. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.M.; Wang, B.W.; Fang, W.J.; Pan, C.M.; Shyu, K.G.; Hou, S.W. Catalpol ameliorates neointimal hyperplasia in diabetic rats. Planta Med. 2019, 85, 406–411. [Google Scholar] [CrossRef]
- Yuan, H.X.; Ni, X.Q.; Zheng, M.; Han, X.M.; Song, Y.C.; Yu, M.F. Effect of catalpol on behavior and neurodevelopment in an ADHD rat model. Biomed. Pharmacother. 2019, 118, 109033. [Google Scholar] [CrossRef]
- Liu, Z.G.; Zhu, P.T.; Zhang, L.; Xiong, B.; Tao, J.H.; Guan, W.; Li, C.L.; Chen, C.; Gu, J.Y.; Duanmu, J.X.; et al. Autophagy inhibition attenuates the induction of anti-inflammatory effect of catalpol in liver fibrosis. Biomed. Pharmacother. 2018, 103, 1262–1271. [Google Scholar] [CrossRef]
- Qiu, J.G.; Zhang, R.X.; Jia, Z.P.; Li, M.X.; Zhang, Q.L.; Zhou, J.; Wei, L.L. Change of oligosaccharide and catalpol contents in Rehmanniae Radix at various processing phases. Zhongcaoyao 2011, 42, 2434–2437. [Google Scholar] [CrossRef]
- Wei, G.D.; Wen, X.S. Characteristics and kinetics of catalpol degradation and the effect of its degradation products on free radical scavenging. Pharmacogn. Mag. 2014, 10, S122–S129. [Google Scholar] [CrossRef]
- Du, Y.P.; Wang, M.; Li, L.Y.; Zhou, K.; Bai, Y.J.; Li, Y.; Wang, S.W.; Wang, M.; Zhao, Y.; Zheng, X.H. Research progress on effect of processing on properties and efficacy of traditional Chinese medicine containing iridoid terpenoids based on stability of compounds. Zhongcaoyao 2021, 52, 5039–5051. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, C. Discussion on the processing technology and quality standards of Rehmanniae Radix Praeparata. Pharm. Clin. Chin. Mater. Medica 2017, 8, 21–25. [Google Scholar]
- Ji, Y.H.; Wang, W.L.; Yang, Z.; Gan, L.F.; Xu, J.; Deng, T.; Zhang, Z.P. Study on the difference comparison of Rehmanniae Radix and Rehmanniae Radix Praeparata based on characteristic spectrum and color image technology. Zhongyaocai 2023, 46, 1912–1918. [Google Scholar] [CrossRef]
- Gao, G.Z.; Zhou, J.W.; Wang, H.Q.; Rao, P.F.; Ke, L.J. Analysis of Amino Acids in Rehmannia glutinosa Libosch. during Heating Process. Anjisuan He Shengwu Ziyuan 2010, 32, 52–54. [Google Scholar] [CrossRef]
- Xue, S.J.; Fu, Y.; Sun, X.Y.; Chen, S.Q. Changes in the Chemical Components of Processed Rehmanniae Radix Distillate during Different Steaming Times. Evid. Based Complement. Alternat. Med. 2022, 2022, 3382333. [Google Scholar] [CrossRef]
- Takashi, M.; Hiroaki, N.; Hiroshi, S.; Masao, C.; Ko, S.; Takao, K.; Hiroshi, M. Five cyclopentanoid monoterpenes from Rehmannia glutinosa. Phytochemistry 1989, 28, 2385–2391. [Google Scholar] [CrossRef]
- Machida, K.; Ikeda, C.; Kakuda, R.; Yaoita, Y.; Kikuchi, Y. Studies on the constituents of Catalpa species. v. iridoids from catalpae fructus. Nat. Med. 2001, 55, 61–63. [Google Scholar] [CrossRef]
- Joshi, K.C.; Singh, P.; Taneja, S.; Cox, P.J.; Howie, R.A.; Thomson, R.H. New terpenoid aldehydes from Kigelia pinnata: Crystal structure of pinnatal. Tetrahedron 1982, 38, 2703–2708. [Google Scholar] [CrossRef]
- Liu, C.L.; Cheng, L.; Kwok, H.F.; Ko, C.H.; Lau, T.W.; Koon, C.M.; Zhao, M.; Lau, C.P.; Lau, K.M.; Wong, C.W.; et al. Bioassay-guided isolation of norviburtinal from the root of Rehmannia glutinosa, exhibited angiogenesis effect in zebrafish embryo model. J. Ethnopharmacol. 2011, 137, 1323–1327. [Google Scholar] [CrossRef]
- Meng, X.L.; Wang, B.; Zhang, X.Y.; Lyu, C.Z.; Su, X.J.; Ning, C.X.; Zhang, S.S. The dynamic changes and mechanisms of Rehmanniae radix processing based on Maillard reaction. Tradit. Med. Res. 2021, 6, 63–73. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Dong, X.Y.; Zhang, R.H.; Hao, D.Z.; Zhang, J.X.; Shen, Y.H.; Chai, X.; Wang, H.X.; Wang, Y.F.; Wang, Y.F. Unraveling the mechanism of the supramolecular self-assembly during the in vivo metabolism of geniposide from Chinese medicine. Mater. Des. 2023, 225, 111546. [Google Scholar] [CrossRef]

| Compounds | Linear Regression | LODs | LOQs | Precision (RSD,%) | Repeatability | Stability | Recovery | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Regression Equation | r2 | Linear Range (μg/mL) | (μg/mL) | (μg/mL) | Intra-Day (n = 6) | Inter-Day (n = 3) | (n = 6, RSD, %) | (n = 7, RSD, %) | (n = 6, Mean ± SD, %) | |
| Cyt | y = 4204.9x − 297.17 | 0.9997 | 0.0992–2.008 | 0.0331 | 0.0992 | 2.0 | 1.9 | 0.6 | 2.3 | 101.58 ± 1.7 |
| Cat | y = 1326.1x + 7233.5 | 0.9996 | 84.00–1701 | 9.3334 | 28.000 | 1.3 | 1.0 | 0.9 | 0.8 | 98.30 ± 3.0 |
| Ade | y = 22932x + 324.90 | 1.0000 | 0.1242–2.515 | 0.0138 | 0.0414 | 2.6 | 2.9 | 2.0 | 2.8 | 98.31 ± 3.6 |
| Gua | y = 18807x + 203.55 | 0.9996 | 0.04347–0.8803 | 0.0145 | 0.0435 | 2.0 | 2.7 | 1.5 | 2.1 | 96.66 ± 1.0 |
| Auc | y = 16688x − 2154.9 | 0.9996 | 1.196–24.21 | 0.3985 | 1.1956 | 2.3 | 2.8 | 2.4 | 2.5 | 99.36 ± 3.3 |
| RhD | y = 5738.1x + 19552 | 0.9993 | 5.985–121.2 | 1.9951 | 5.9852 | 1.1 | 2.3 | 2.8 | 2.7 | 99.90 ± 3.2 |
| Leo | y = 8574.4x + 29466 | 0.9990 | 5.931–120.1 | 0.6590 | 1.9770 | 0.8 | 2.2 | 1.5 | 1.6 | 92.87 ± 4.5 |
| Gen | y = 13584x − 8.9794 | 0.9999 | 0.06210–1.258 | 0.0207 | 0.0621 | 2.6 | 2.4 | 1.8 | 2.0 | 91.49 ± 2.8 |
| Act | y = 13794x + 307.19 | 1.0000 | 0.3148–6.375 | 0.0350 | 0.1049 | 2.1 | 3.0 | 2.9 | 1.8 | 93.41 ± 4.9 |
| Iso | y = 13299x + 431.85 | 0.9996 | 0.07422–1.503 | 0.0247 | 0.0742 | – | – | – | – | 96.92 ± 1.2 |
| Position | D1 (in DMSO-d6) | D2 (in CD3OD) | D3 (in CD3OD) | |||
|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | |
| 2 | 7.52 (1H, s) | 136.0 | – | 157.5 | 8.11 (1H, d, 4.8) | 146.5 |
| 3 | – | 130.5 | 7.27 (1H, s) | 115.0 | 7.56 (1H, d, 4.8) | 112.3 |
| 4 | 4.60 (1H, d, 4.8) | 65.9 | – | 137.9 | – | 139.0 |
| 5 | 3.79 (1H, dd, 4.8, 8.4) | 83.2 | 7.33 (1H, brs) | 123.1 | 6.71 (1H, d, 3.6) | 112.7 |
| 6 | 3.15 (1H, m) | 36.3 | 7.33 (1H, s) | 133.4 | 8.09 (1H, d, 3.6) | 146.5 |
| 7 | – | 130.2 | – | 133.2 | – | 123.2 |
| 8 | 7.17 (1H, s) | 134.3 | 2.98 (1H, dd, 5.4, 13.8) 2.90 (1H, dd, 5.4, 13.8) | 39.6 | – | 124.0 |
| 9 | 2.60 (1H, ddd, 1.8, 9.0, 17.4) 2.85 (1H, ddd, 1.8, 5.4, 17.4) | 45.8 | 4.85 (1H, m, overlapped) | 98.7 | 9.27 (1H, s) | 152.6 |
| 10 | 9.82 (1H, t, 1.8) | 202.9 | 9.85 (1H, s) | 194.0 | 9.66 (1H, s) | 187.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Zhang, L.; Zhang, M.; Yang, M.; Zou, L.; Cui, Y.; Yang, J.; Chai, X.; Wang, Y. Exploration of the Dynamic Variations of the Characteristic Constituents and the Degradation Products of Catalpol during the Process of Radix Rehmanniae. Molecules 2024, 29, 705. https://doi.org/10.3390/molecules29030705
Yang J, Zhang L, Zhang M, Yang M, Zou L, Cui Y, Yang J, Chai X, Wang Y. Exploration of the Dynamic Variations of the Characteristic Constituents and the Degradation Products of Catalpol during the Process of Radix Rehmanniae. Molecules. 2024; 29(3):705. https://doi.org/10.3390/molecules29030705
Chicago/Turabian StyleYang, Jingjing, Lihua Zhang, Mengyue Zhang, Mingxuan Yang, Lin Zou, Ying Cui, Jing Yang, Xin Chai, and Yuefei Wang. 2024. "Exploration of the Dynamic Variations of the Characteristic Constituents and the Degradation Products of Catalpol during the Process of Radix Rehmanniae" Molecules 29, no. 3: 705. https://doi.org/10.3390/molecules29030705
APA StyleYang, J., Zhang, L., Zhang, M., Yang, M., Zou, L., Cui, Y., Yang, J., Chai, X., & Wang, Y. (2024). Exploration of the Dynamic Variations of the Characteristic Constituents and the Degradation Products of Catalpol during the Process of Radix Rehmanniae. Molecules, 29(3), 705. https://doi.org/10.3390/molecules29030705





