Traditional Uses, Chemical Constituents and Pharmacological Activities of the Toona sinensis Plant
Abstract
:1. Introduction
2. Traditional Uses
3. Phytochemical Constituents
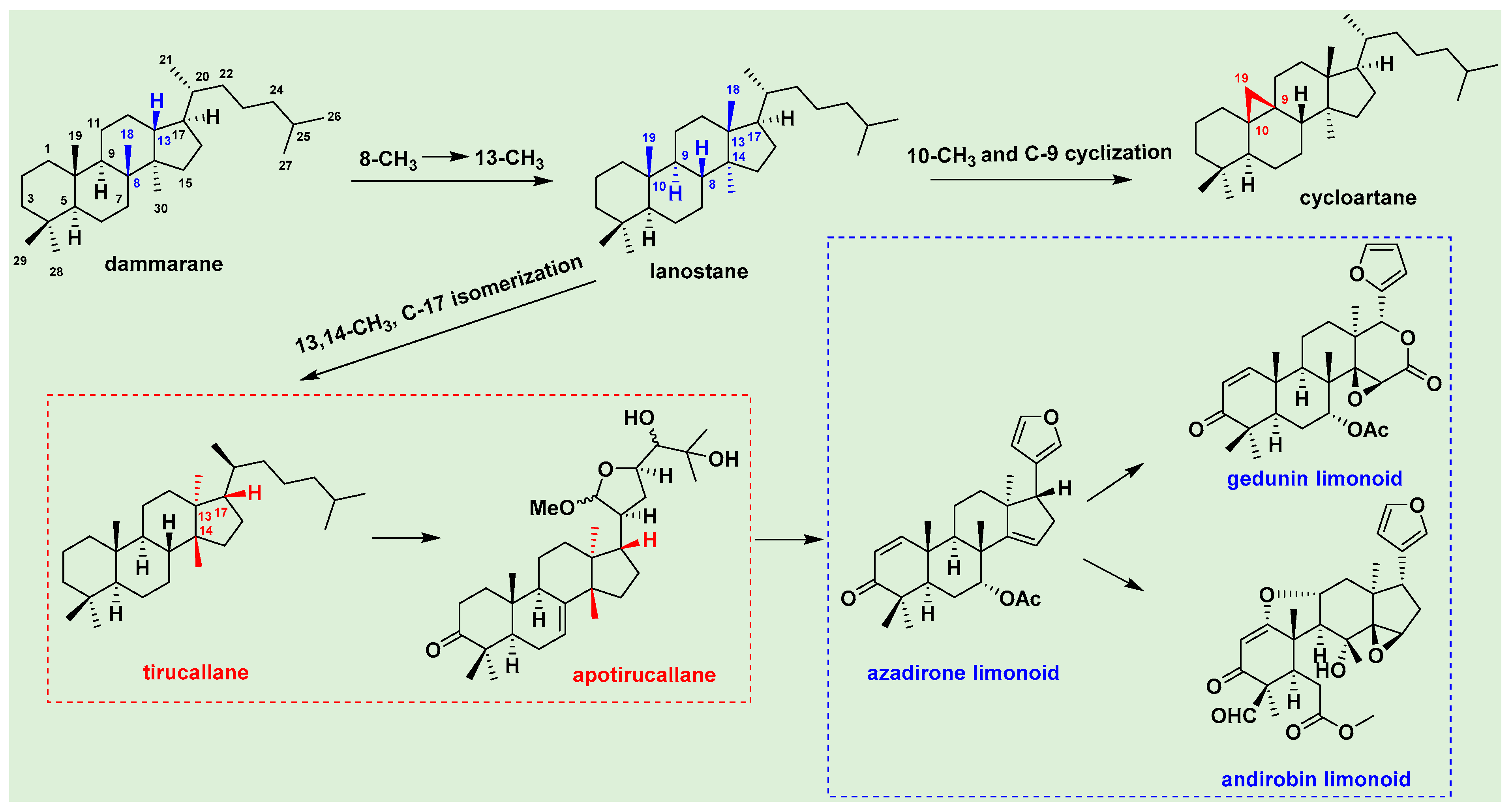
3.1. Triterpenoids
3.2. Dammarane Triterpenoids
3.3. Tirucallane Triterpenoids
3.4. Apo-Tirucallane Triterpenoids
3.5. Limonoid Triterpenoids
3.6. Cycloartane and Other Triterpenoids
3.7. Sesquiterpenoids and Diterpenoids
3.8. Sterols
3.9. Phenols
3.10. Flavonoids
3.11. Phenylpropanoids and Other Compounds
4. Compound Cracking Laws
5. Pharmacological Activities
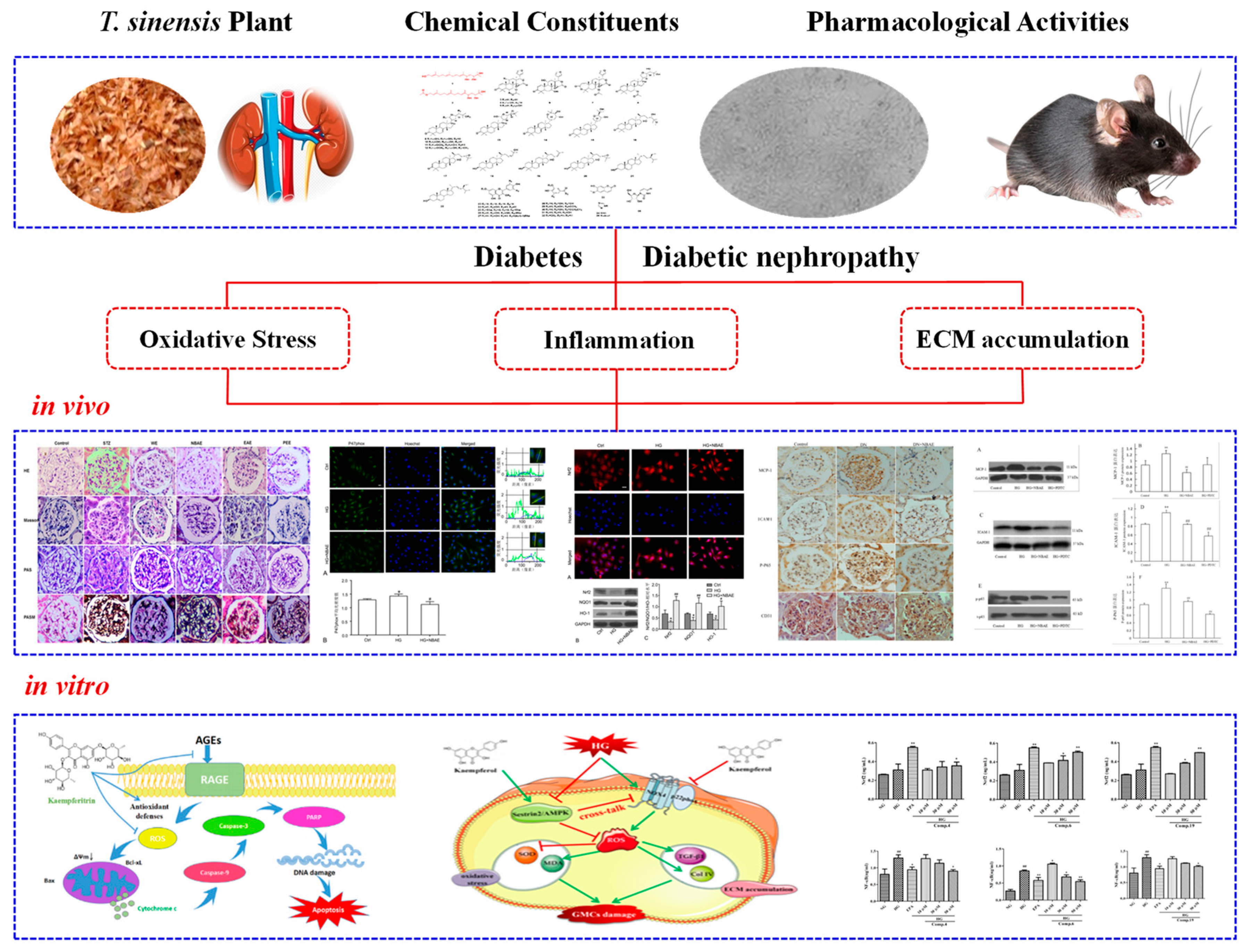
5.1. Antidiabetic Activity
5.2. Antidiabetic Nephropathy Activity
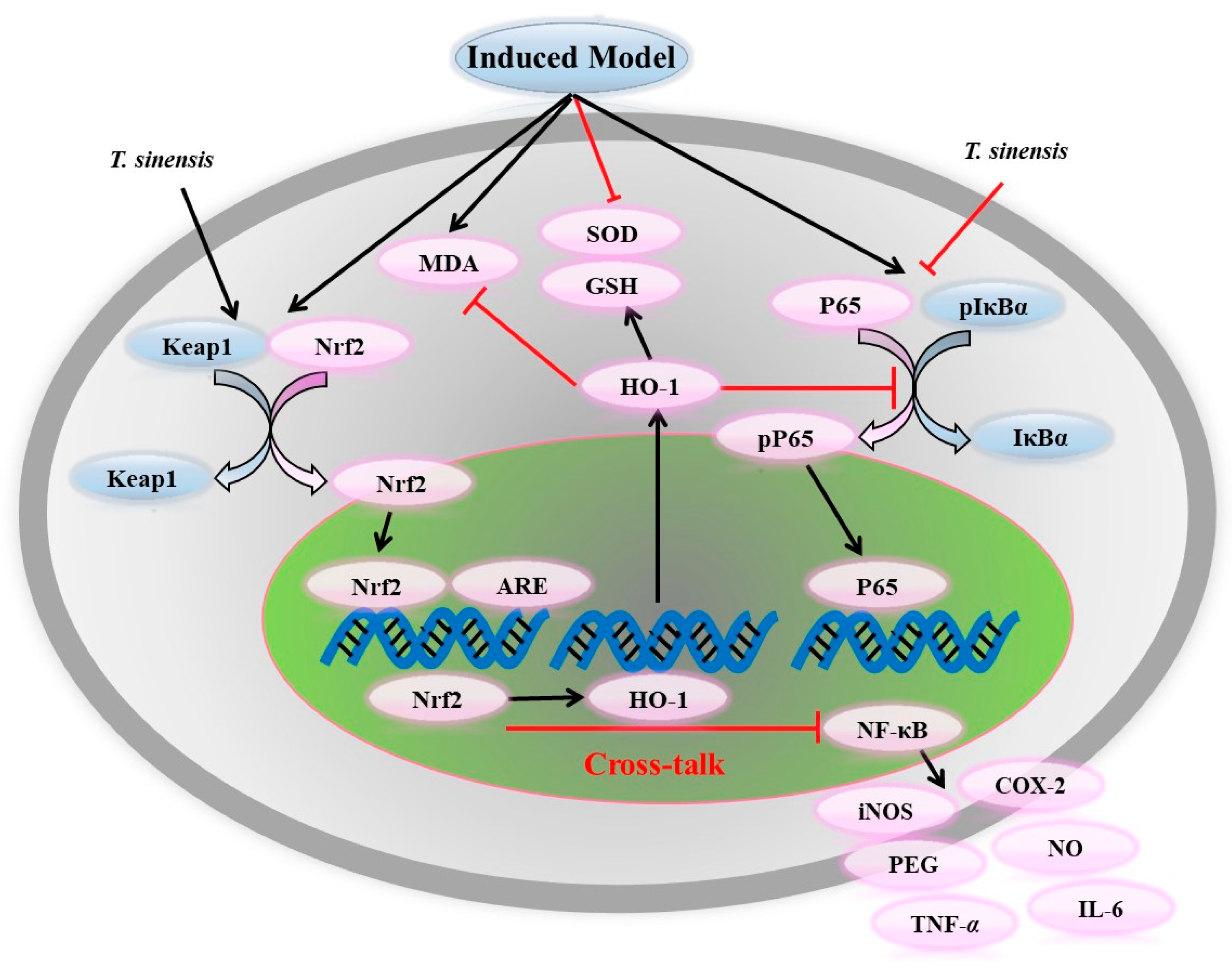
5.3. Antioxidant Activity
5.4. Anti-Inflammatory Activity
5.5. Antitumor Activity
5.6. Hepatoprotective Activity
5.7. Antiviral and Antibacterial Activity
5.8. Immunopotentiation
5.9. Effects on the Male Reproductive System
5.10. Other Aspects
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; Wang, F.; Ji, C.Y.; Liu, D.; Liu, X.X.; Wang, R.S.; Li, W.Z. Chemical constituents of the pericarp of Toona sinensis and their chemotaxonomic significance. Biochem. Syst. Ecol. 2022, 104, 104458. [Google Scholar] [CrossRef]
- Yang, C.C. Study on Mulching Technology of Toon sinensis. Master’s Thesis, Central South University of Forestry and Technology, Changsha, China, 2019. [Google Scholar]
- Zhou, X.Y. Studies on the Genus Toona of China. Master’s Thesis, Nanjing Forestry University, Nanjing, China, 2005. [Google Scholar]
- Jiao, Z.Y.; Zheng, J.W.; Yan, R.C.; Zhang, Y.; Tang, L.L. Summary of main research on multi-functional precious species of Toon sinensis. J. Jiangsu For. Sci. Technol. 2019, 46, 51–56. [Google Scholar]
- He, B.Z.; Yang, H.F. Afforestation technology and benefit analysis of Toon sinensis. South China Agric. 2019, 13, 70–71. [Google Scholar]
- Peng, W.; Liu, Y.; Hu, M.; Zhang, M.; Yang, J.; Liang, F.; Huang, Q.; Wu, C. Toona sinensis: A comprehensive review on its traditional usages, phytochemisty, pharmacology and toxicology. Rev. Bras. Farmacogn. 2019, 29, 111–124. [Google Scholar] [CrossRef]
- Feng, W.; Wang, M.; Cao, J.; Sun, J.; Jiang, W. Regeneration of denaturedpolyphenol oxidase in Toona sinensis (A. Juss.) Roam. Process. Biochem. 2007, 42, 1155–1159. [Google Scholar] [CrossRef]
- Mu, R.; Wang, X.; Liu, S.; Yuan, X.; Wang, S.; Fan, Z. Rapid determination of volatile compounds in Toona sinensis (A. Juss.) Roem. by MAE-HS-SPME followed by GC–MS. Chromatographia 2007, 65, 463–467. [Google Scholar] [CrossRef]
- Hsieh, T.J.; Wang, J.C.; Hu, C.Y.; Li, C.T.; Kuo, C.M.; Hsieh, S.L. Effects of rutin from Toona sinensis on the immune and physiological responses of white shrimp (Litopenaeus vannamei) under Vibrio alginolyticus challenge. Fish Shellfish Immun. 2008, 25, 581–588. [Google Scholar] [CrossRef]
- Wan, X.; Lan, Z.; Yang, S.; Yang, S.; Zhu, Y.; Wang, F.; Yang, W.; Chen, J. Investigation of fragmentation pathways of norpimarane diterpenoids by mass spectrometry combined with computational chemistry. Rapid Commun. Mass Spectrom. 2022, 36, e9269. [Google Scholar] [CrossRef]
- Chen, H.Y.; Lin, Y.C.; Hsieh, C.L. Evaluation of antioxidant activity of aqueousextract of some selected nutraceutical herbs. Food Chem. 2007, 104, 1418–1424. [Google Scholar] [CrossRef]
- Cheng, K.W.; Yang, R.Y.; Tsou, S.C.; Lo, C.S.; Ho, C.T.; Lee, T.C.; Wang, M. Analysisof antioxidant activity and antioxidant constituents of Chinese toon. J. Funct. Foods 2009, 1, 253–259. [Google Scholar] [CrossRef]
- Wu, C.C.; Liu, C.H.; Chang, Y.P.; Hsieh, S.L. Effects of hot-water extract of Toona sinensis on immune response and resistance to Aeromonas hydrophila inoreochromis mossambicus. Fish Shellfish Immun. 2010, 29, 258–263. [Google Scholar] [CrossRef]
- Liao, J.W.; Chung, Y.C.; Yeh, J.Y.; Lin, Y.C.; Lin, Y.G.; Wu, S.M.; Chan, Y.C. Safety evaluation of water extracts of Toona sinensis Roemor leaf. Food Chem. Toxicol. 2007, 45, 1393–1399. [Google Scholar] [CrossRef]
- Liu, D.; Wang, R.S.; Xuan, L.L.; Wang, X.H.; Li, W.Z. Two new apotirucallane-type triterpenoids from the pericarp of Toona sinensis and their ability to reduce oxidative stress in rat glomerular mesangial cells cultured under high-glucose conditions. Molecules 2020, 25, 801. [Google Scholar] [CrossRef]
- Liao, J.W.; Yeh, J.Y.; Lin, Y.C.; Wei, M.M.; Chung, Y.C. Mutagenicity and safety evaluation of water extract of fermented Toona sinensis Roemor leaves. J. Food Sci. 2009, 74, T7–T13. [Google Scholar] [CrossRef]
- Wang, R.S.; Zuo, M.; Ding, S.H.; Liu, B.; Meng, C.; Song, B.; Li, W.Z. Recovery of immune activity by administration of polysaccharides of Toona sinensis and its characterization of major component. Nat. Prod. Res. 2021, 35, 5513–5517. [Google Scholar] [CrossRef]
- Wang, M.X.; Sheng, Z.C.; Wu, M.; Zhang, J.L.; Chen, X.L. Research progress on chemical constituents and pharmacological effects of leaves of Toona sinensis. For. Sci. Technol. 2020, 27, 44–48. [Google Scholar]
- Zhu, Y.F.; Zhou, Q.M.; Feng, G.B.; Song, J.B.; Tao, X.Y. Antimicrobial test in vitro of cortex toonae and cortex ailanthi. Chin. J. Mod. Appl. Pharm. 1999, 16, 19–21. [Google Scholar]
- Li, W.Z.; Han, W.N.; Liu, B.; Ding, S.H.; Zhang, X.K.; Wang, R.S. Extraction of proteins and preliminary characterization of physicochemical properties in Toona sinensis fruit. Genet. Mol. Res. 2017, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, Y.Q.; Li, Y.Q.; Li, W.Z. Research progress of effective components extraction and bioactivity of Toona sinensis seeds. Shandong Chem. Ind. 2018, 47, 55–56. [Google Scholar]
- China Journal of Traditional Chinese medicine. Toona sinensis recipe. Chin. Med. Distance Educ. China 2014, 12, 3.
- Tang, J.; Xu, J.; Zhang, J.; Liu, W.Y.; Xie, N.; Chen, L.; Feng, F.; Qu, W. Novel tirucallane triterpenoids from the stem bark of Toona sinensis. Fitoterapia 2016, 112, 97–103. [Google Scholar] [CrossRef]
- Mitsui, K.; Saito, H.; Yamamura, R.; Fukaya, H.; Hitotsuyanagi, Y.; Takeya, K. Apotirucallane and tirucallane triterpenoids from Cedrela sinensis. Chem. Pharm. Bull. 2007, 55, 1442–1447. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, H.; Liu, X.X.; Zhou, J.Y.; Jiang, Y.J.; Wang, F.; Wang, R.S.; Li, W.Z. Terpenoids from the seeds of Toona sinensis and their ability to attenuate high glucose-induced oxidative stress and inflammation in rat glomerular mesangial cells. Molecules 2022, 27, 5784. [Google Scholar] [CrossRef]
- Meng, Q.Q.; Peng, X.R.; Lu, S.Y.; Wan, L.S.; Wang, X.; Dong, J.R.; Chu, R.; Zhou, L.; Li, X.N.; Qiu, M.H. Lactam triterpenoids from the bark of Toona sinensis. Nat. Prod. Bioprospecting 2016, 6, 239–245. [Google Scholar] [CrossRef]
- Wang, R.S.; Liu, D.; Liu, X.X.; Liu, F.; Xuan, L.L.; Tang, Y.; Li, W.Z. Cytotoxicity and polyol pathway inhibitory activities of chemical constituents isolated from the pericarp of Toona sinensis. Nat. Prod. Res. 2022, 36, 1593–1598. [Google Scholar] [CrossRef]
- Mitsui, K.; Maejima, M.; Saito, H.; Fukaya, H.; Hitotsuyanagi, Y.; Takeya, K. Triterpenoids from Cedrela sinensis. Tetrahedron 2005, 61, 10569–10582. [Google Scholar] [CrossRef]
- Mitsui, K.; Saito, H.; Yamamura, R.; Fukaya, H.; Hitotsuyanagi, Y.; Takeya, K. Hydroxylated gedunin derivatives from Cedrela sinensis. J. Nat. Prod. 2006, 69, 1310–1314. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Li, Y.; An, F.L.; Zhou, M.M.; Luo, J.; Jian, K.L.; Luo, J.; Kong, L.Y. Limonoids with modified furan rings from root barks of Toona sinensis. Tetrahedron 2016, 72, 7481–7487. [Google Scholar] [CrossRef]
- Hu, J.; Song, Y.; Mao, X.; Wang, Z.J.; Zhao, Q.J. Limonoids isolated from Toona sinensis and their radical scavenging, anti-inflammatory and cytotoxic activities. J. Funct. Foods 2016, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.H.; Xie, Y.T.; Guo, J.M.; Wang, X.P.; Jiang, B.; Zhang, W.; Qiang, L.; Kong, L.Y.; Liu, Y.P. Limonoids from the fresh young leaves and buds of Toona sinensis and their potential neuroprotective effects. J. Agric. Food Chem. 2020, 68, 12326–12335. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.J.; Zhu, Y.F.; Bao, G.H.; Hu, F.L.; Qin, G.W. New limonoids and a dihydrobenzofuran norlignan from the roots of Toona sinensis. Molecules 2013, 18, 2840–2850. [Google Scholar] [CrossRef]
- Luo, X.D.; Wu, S.H.; Ma, Y.B.; Wu, D.G. Limonoids and phytol derivatives from Cedrela sinensis. Fitoterapia 2000, 71, 492–496. [Google Scholar] [CrossRef]
- Mitsui, K.; Maejima, M.; Fukaya, H.; Hitotsuyanagi, Y.; Takeya, K. Limonoids from Cedrela sinensis. Phytochemistry 2004, 65, 3075–3081. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, Q.; Xiang, H.; Liu, M.; Zhang, Q.; Xue, W.; Song, B.; Yang, S. Antiproliferative activity and apoptosis-inducing mechanism of constituents from Toona sinensis on human cancer cells. Cancer Cell Int. 2013, 13, 12. [Google Scholar] [CrossRef]
- Zhou, X.L.; Wang, P.C.; Luo, Q.; Huang, X.; Chen, X.; Li, J.; Liang, C.Q. Study on chemical constituents of bark of Chinese Toona sinensis. Chin. Med. 2017, 40, 1119–1122. [Google Scholar]
- Li, G.C.; Yu, X.X.; Liao, R.F.; Wang, D.Y. Analysis of chemical constituents in bark of Toona sinensis. Chin. Hosp. Pharm. 2006, 26, 949–952. [Google Scholar]
- Wang, K.J.; Yang, C.R.; Zhang, Y.J. Phenolic antioxidants from Chinese toon (fresh young leaves and shoots of Toona sinensis). Food Chem. 2007, 101, 365–371. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, X.W.; Chen, X.B.; Wang, Q.X. α-Glucosidase inhibitory constituents from Toona sinensis. Chem. Nat. Compd. 2009, 45, 244–246. [Google Scholar] [CrossRef]
- Yang, H.; Gu, Q.; Gao, T.; Wang, X.; Chue, P.; Wu, Q.; Jia, X. Flavonols and derivatives of gallic acid from young leaves of Toona sinensis (A. Juss.) Roemer and evaluation of their anti-oxidant capacity by chemical methods. Pharmacogn. Mag. 2014, 10, 185–190. [Google Scholar] [PubMed]
- Kakumu, A.; Ninomiya, M.; Efdi, M.; Adfa, M.; Hayashi, M.; Tanaka, K.; Koketsu, M. Phytochemical analysis and antileukemic activity of polyphenolic constituents of Toona sinensis. Bioorg. Med. Chem. Lett. 2014, 24, 4286–4290. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.X.; Wang, R.S.; Liu, D.; Zuo, M.; Zhao, C.Z.; Zhang, T.L.; Li, W.Z. Protective effects of kaempferitrin on advanced glycation end products induce mesangial cell apoptosis and oxidative stress. Int. J. Mol. Sci. 2018, 19, 3334. [Google Scholar] [CrossRef]
- Lee, I.S.; Kim, H.J.; Youn, U.J.; Chen, Q.C.; Kim, J.P.; Ha, D.T.; Ngoc, T.M. Dihydrobenzofuran norlignans from the leaves of Cedrela sinensis A. Juss. Helv. Chim. Acta. 2010, 93, 272–276. [Google Scholar] [CrossRef]
- Wang, S.W.; Lee, J.; Kwon, H.S.; Lee, K.D.; Nam, S.H.; Park, K.H.; Yang, M.S. Comparison of tyrosinase inhibitory effect of the natural antioxidants from Cedrela sinensis. Agric. Chem. Biotechnol. 2005, 48, 144–147. [Google Scholar]
- Xu, W.J.; Li, J.H.; Zhou, M.M.; Luo, J.; Jian, K.L.; Tian, X.M.; Xia, Y.Z.; Yang, L.; Luo, J.; Kong, L.Y. Toonasindiynes A-F, new polyacetylenes from Toona sinensis with cytotoxic and anti-inflammatory activities. Fitoterapia 2020, 146, 104667. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tang, J. Research progress on limonoids in Xylocarpus and their biological activities. Chin. Tradit. Herb. Drugs 2022, 53, 10. [Google Scholar]
- Ren, W.; Xin, S.K.; Han, L.Y.; Zuo, R.; Li, Y.; Gong, M.X.; Wei, X.L.; Zhou, Y.Y.; He, J.; Wang, H.J.; et al. Comparative metabolism of four limonoids in human liver microsomes using ultra-high-performance liquid chromatography coupled with high-resolution LTQ-Orbitrap mass spectrometry. Rapid Commun. Mass Spectrom. 2015, 29, 2045–2056. [Google Scholar] [CrossRef]
- Suttiarporn, P.; Chumpolsri, W.; Mahatheeranont, S.; Luangkamin, S.; Teepsawang, S.; Leardkamolkarn, V. Structures of phytosterols and triterpenoids with potential anti-cancer activity in bran of black non-glutinous rice. Nutrients 2015, 7, 1672–1687. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, L.; Chen, C.H.; Zhang, Y.Y.; Yang, Y.; Zhang, P.; Bao, G.H. Chemical composition and antibacterial activity of 12 medicinal plant ethyl acetate extracts using LC-MS feature-based molecular networking. Phytochem. Anal. 2022, 33, 473–489. [Google Scholar] [CrossRef]
- Qian, Y.J.; Pi, W.X.; Zhu, G.F.; Wei, W.; Lu, T.L.; Mao, C.Q. Quality evaluation of raw and processed Corni Fructus by UHPLC-QTOF-MS and HPLC coupled with color determination. J. Pharmaceut. Biomed. 2022, 218, 114842. [Google Scholar] [CrossRef]
- Hsieh, T.J.; Tsai, Y.H.; Liao, M.C.; Du, Y.C.; Lien, P.J.; Sun, C.C.; Chang, F.R.; Wu, Y.C. Anti-diabetic properties of non-polar Toona sinensis Roem extract prepared by supercritical-CO2 fluid. Food Chem. Toxicol. 2012, 50, 779–789. [Google Scholar] [CrossRef]
- Wang, P.H.; Tsai, M.J.; Hsu, C.Y.; Wang, C.Y.; Hsu, H.K.; Weng, C.F. Toona sinensis Roem (Meliaceae) leaf extract alleviates hyperglycemia via altering adipose glucose transporter 4. Food Chem. Toxicol. 2008, 46, 2554–2560. [Google Scholar] [CrossRef]
- Yang, Y.C.; Hsu, H.K.; Hwang, J.H.; Hong, S.J. Enhancement of glucose uptake in 3T3-L1 adipocytes by Toona sinensis leaf extract. Kaohsiung J. Med. Sci. 2003, 19, 327–333. [Google Scholar] [CrossRef]
- Liu, H.W.; Huang, W.C.; Yu, W.J.; Chang, S.J. Toona Sinensis ameliorates insulin resistance via AMPK and PPARγ pathways. Food Funct. 2015, 6, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Shih, H.Y.; Chia, Y.C.; Lee, C.H.; Ashida, H.; Lai, Y.K.; Weng, C.F. Rutin potentiates insulin receptor kinase to enhance insulin-dependent glucose transporter 4 translocation. Mol. Nutr. Food Res. 2014, 58, 1168–1176. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, H.; Wang, M.; Zhang, J. Quercetin isolated from Toona sinensis leaves attenuates hyperglycemia and protects hepatocytes in high-carbohydrate/high-fat diet and alloxan induced experimental diabetic mice. J. Diabetes Res. 2016, 2016, 8492780. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Z.; Wang, X.H.; Han, W.N.; Liu, D.M. Protective effect of petroleum ether fraction of Toona sinensis Roem seeds extracts on kidney of diabetic nephropathy rats. Nat. Prod. Res. Dev. 2015, 27, 2035–2039. [Google Scholar]
- Li, W.Z.; Wang, X.H.; Zhang, H.X.; Mao, S.M.; Zhao, C.Z. Protective effect of the n-butanol Toona sinensis seed extract on diabetic nephropathy rat kidneys. Genet. Mol. Res. 2016, 15, 15017403. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.X.; Hu, S.; Wang, T.T.; Zhang, Y.M.; Li, W.Z.; Yu, L.; Liu, Y.Q.; Zhang, H.X. N-butyl alcohol extract of Toona sinensis attenuates the oxidative stress of glomerular endothelial cells induced by high glucose through activating Nrf2. Chin. J. Histochem. Cytochem. 2019, 28, 197–203. [Google Scholar]
- Guo, Y.N.; Zhong, Y.X.; Zheng, X.R.; Yu, L.; Li, W.Z.; Zhang, H.X. Extract of Toona sinensis improves glomerular endothelial cell inflammation in DN and its potential mechanism. Nat. Prod. Res. Dev. 2019, 31, 55–60. [Google Scholar]
- Xuan, L.L.; Li, Y.Q.; Wang, H.J.; Liu, F.; Chen, Y.; Zhao, C.Z.; Wang, R.S.; Li, W.Z. Kaempferol inhibited high glucose-induced oxidative stress and extracellular matrix accumulation in glomerular mesangial cells through regulating AMPK/NOX4 pathway. Nat. Prod. Res. Dev. 2021, 33, 1102–1111. [Google Scholar]
- Liao, J.W.; Hsu, C.K.; Wang, M.F.; Hsu, W.M.; Chan, Y.C. Beneficial effect of Toona sinensis Roemor on improving cognitive performance and brain degeneration in senescence-accelerated mice. Br. J. Nutr. 2006, 96, 400–407. [Google Scholar] [CrossRef]
- Shan, S.R.; Huang, X.M.; Zhang, M.X. Anti-cancer and antioxidant properties of phenolics isolated from Toona sinensis A Juss acetone leaf extract. Trop. J. Pharm. Res. 2016, 15, 1205–1213. [Google Scholar] [CrossRef]
- Yang, H.L.; Chen, S.C.; Lin, K.Y.; Wang, M.T.; Chen, Y.C.; Huang, H.C.; Cho, H.J.; Wang, L.; Kumar, K.J.; Hseu, Y.C. Antioxidant activities of aqueous leaf extracts of Toona sinensis on free radical-induced endothelial cell damage. J. Ethnopharmacol. 2011, 137, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Hseu, Y.C.; Chang, W.H.; Chen, C.S.; Liao, J.W.; Huang, C.J.; Lu, F.J.; Chia, Y.C.; Hsu, H.K.; Wu, J.J.; Yang, H.L. Antioxidant activities of Toona Sinensis leaves extracts using different antioxidant models. Food Chem. Toxicol. 2008, 46, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Bu, Y.G.; Zhao, M.L.; Tao, R.; Luo, J.; Li, Y. Studies on antioxidant and α-glucosidase inhibitory constituents of Chinese toon bud (Toona sinensis). J. Funct. Foods 2020, 73, 104–108. [Google Scholar] [CrossRef]
- Wang, Y.X.; Gu, D.Y.; Liu, C.; Tang, S.S.; Wang, S.; Wang, Y.; Tang, Y. Enrichment, analysis, identification and mechanism of antioxidant components in Toona sinensis. Chin. J. Anal. Chem. 2023, 51, 100198. [Google Scholar] [CrossRef]
- Yang, C.J.; Chen, Y.C.; Tsai, Y.J.; Huang, M.S.; Wang, C.C. Toona sinensis leaf aqueous extract displays activity against sepsis in both in vitro and in vivo models. Kaohsiung J. Med. Sci. 2014, 30, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.A.; Wu, S.Y.; Lee, C.H.; Lai, Y.R.; Lu, C.H.; Chen, P.C.; Cheng, J.H.; Tsai, L.Y.; Yen, K.T.; Tsao, Y.; et al. Toona sinensis modulates autophagy and cytokines in lipopolysaccharide-induced RAW 264.7 macrophages. Biomed. Pharmacother. 2020, 129, 110386. [Google Scholar] [CrossRef]
- Lim, H.J.; Park, I.S.; Jie, E.Y.; Ahn, W.S.; Kim, S.J.; Jeong, S.I.; Yu, K.Y.; Kim, S.W.; Jung, C.H. Anti-inflammatory activities of an extract of in vitro grown adventitious shoots of Toona sinensis in LPS-treated RAW264.7 and propionibacterium acnes-treated HaCaT Cells. Plants 2020, 9, 1701. [Google Scholar] [CrossRef]
- Wang, C.C.; Tsai, Y.J.; Hsieh, Y.C.; Lin, R.J.; Lin, C.L. The aqueous extract from Toona sinensis leaves inhibits microglia-mediated neuroinflammation. Kaohsiung J. Med. Sci. 2014, 30, 73–81. [Google Scholar] [CrossRef]
- Zhuang, W.; Cai, M.; Li, W.; Chen, C.; Wang, Y.; Lv, E.; Fu, W. Polyphenols from Toona sinensiss seeds alleviate neuroinflammation induced by 6-hydroxydopamine through suppressing p38 MAPK signaling pathway in a rat model of parkinson’s disease. Neurochem. Res. 2020, 45, 2052–2064. [Google Scholar] [CrossRef]
- Chen, J.Y.; Zhu, G.Y.; Su, X.H.; Wang, R.; Liu, J.; Liao, K.; Ren, R.; Li, T.; Liu, L. 7-deacetylgedunin suppresses inflammatory responses through activation of Keap1/Nrf2/HO-1 signaling. Oncotarget 2017, 8, 55051–55063. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, G.; Sun, Y.; Wu, Y.; Wu, B.; Zheng, W.; Ma, X.; Zheng, Y. 7-deacetyl-gedunin suppresses proliferation of Human rheumatoid arthritis synovial fibroblast through activation of Nrf2/ARE signaling. Int. Immunopharmacol. 2022, 107, 108557. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Tang, P.; Zhang, P.; Cui, L.; Li, Y.; Li, J.; Kong, L.; Luo, J. Inhibition of the P2X7/NLRP3 inflammasome signaling pathway by deacetylgedunin from Toona sinensis. J. Nat. Prod. 2022, 85, 1388–1397. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.L.; Ko, S.Y.; Jun, M.; Jeong, W.S. Quercitrin from Toona sinensis (Juss.) M. Roem. attenuates acetaminophen-induced acute liver toxicity in HepG2 cells and mice through induction of antioxidant machinery and inhibition of inflammation. Nutrients 2016, 8, 431. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Li, C.J.; Tai, I.C.; Lin, X.H.; Hsu, H.K.; Ho, M.L. The fractionated Toona sinensis leaf extract induces apoptosis of human osteosarcoma cells and inhibits tumor growth in a murine xenograft model. Integr. Cancer Ther. 2017, 16, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Thiyagarajan, V.; Liao, J.W.; Chu, Y.L.; Chang, C.T.; Huang, P.J.; Hsu, C.J.; Hseu, Y.C. Toona sinensis inhibits murine leukemia WEHI-3 Cells and promotes immune response in vivo. Integr. Cancer Ther. 2017, 16, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Chang, W.H.; Chia, Y.C.; Huang, C.J.; Lu, F.J.; Hsu, H.K.; Hseu, Y.C. Toona sinensis extracts induces apoptosis via reactive oxygen species in human premyelocytic leukemia cells. Food Chem. Toxicol. 2006, 44, 1978–1988. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.C.; Hung, W.C.; Huang, M.S.; Hsu, H.K. Extract from the leaves of Toona sinensis roemor exerts potent antiproliferative effect on human lung cancer cells. Am. J. Chin. Med. 2002, 30, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Lin, K.H.; Yang, C.J.; Tsai, J.R.; Hung, J.Y.; Wang, P.H.; Hsu, H.K.; Huang, M.S. Toona sinensis extracts induced cell cycle arrest and apoptosis in the human lung large cell carcinoma. Kaohsiung J. Med. Sci. 2010, 26, 68–75. [Google Scholar] [CrossRef]
- Yang, C.J.; Huang, Y.J.; Wang, C.Y.; Wang, P.H.; Hsu, H.K.; Tsai, M.J.; Chen, Y.C.; Bharath Kumar, V.; Huang, M.S.; Weng, C.F. Antiproliferative effect of Toona sinensis leaf extract on non-small-cell lung cancer. Transl. Res. 2010, 155, 305–314. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chien, L.H.; Huang, B.M.; Chia, Y.C.; Chiu, H.F. Aqueous extracts of Toona sinensis leaves inhibit renal carcinoma cell growth and migration through JAK2/stat3, Akt, MEK/ERK, and mTOR/HIF-2α Pathways. Nutr. Cancer 2016, 68, 654–666. [Google Scholar] [CrossRef]
- Chang, H.L.; Hsu, H.K.; Su, J.H.; Wang, P.H.; Chung, Y.F.; Chia, Y.C.; Tsai, L.Y.; Wu, Y.C.; Yuan, S.S. The fractionated Toona sinensis leaf extract induces apoptosis of human ovarian cancer cells and inhibits tumor growth in a murine xenograft model. Gynecol. Oncol. 2006, 102, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.C.; Chen, C.Y.; Hsu, H.K.; Lin, L.M.; Chen, Y.K. Chemopreventive effect of Toona sinensis leaf extract on 7,12-dimethylbenz[a]anthracene-induced hamster buccal pouch squamous cell carcinogenesis. Arch. Oral Biol. 2016, 70, 130–142. [Google Scholar] [CrossRef]
- Liu, J.; You, L.; Wang, C.; Liu, R. Antioxidization and antiproliferation of extract from leaves of Toona sinensis. Med. Sci. 2012, 37, 42–47. [Google Scholar]
- Chen, H.M.; Wu, Y.C.; Chia, Y.C.; Chang, F.R.; Hsu, H.K.; Hsieh, Y.C.; Chen, C.C.; Yuan, S.S. Gallic acid, a major component of Toona sinensis leaf extracts, contains a ROS-mediated anti-cancer activity in human prostate cancer cells. Cancer Lett. 2009, 286, 161–171. [Google Scholar] [CrossRef]
- Chia, Y.C.; Rajbanshi, R.; Calhoun, C.; Chiu, R.H. Anti-neoplastic effects of gallic acid, a major component of Toona sinensis leaf extract, on oral squamous carcinoma cells. Molecules 2010, 15, 8377–8389. [Google Scholar] [CrossRef]
- Fan, S.; Chen, H.N.; Wang, C.J.; Tseng, W.C.; Hsu, H.K.; Weng, C.F. Toona sinensis Roem (Meliaceae) leaf extract alleviates liver fibrosis via reducing TGFbeta1 and collagen. Food Chem. Toxicol. 2007, 45, 2228–2236. [Google Scholar] [CrossRef]
- Cao, J.J.; Lv, Q.Q.; Zhang, B.; Chen, H.Q. Structural characterization and hepatoprotective activities of polysaccharides from the leaves of Toona sinensis (A. Juss) Roem. Carbohydr. Polym. 2019, 212, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Chen, H.J.; Huang, B.M.; Chen, Y.C.; Chang, C.F. Polyphenol-rich extracts from Toona sinensis bark and fruit ameliorate free fatty acid-induced lipogenesis through AMPK and LC3 pathways. J. Clin. Med. 2019, 8, 1664. [Google Scholar] [CrossRef]
- Chen, C.J.; Michaelis, M.; Hsu, H.K.; Tsai, C.C.; Yang, K.D.; Wu, Y.C.; Cinatl, J., Jr.; Doerr, H.W. Toona sinensis Roem tender leaf extract inhibits SARS coronavirus replication. J. Ethnopharmacol. 2008, 120, 108–111. [Google Scholar] [CrossRef] [PubMed]
- You, H.L.; Chen, C.J.; Eng, H.L.; Liao, P.L.; Huang, S.T. The effectiveness and mechanism of Toona sinensis extract inhibit attachment of pandemic influenza A (H1N1) Virus. Evid.-Based Complement. Altern. 2013, 2013, 479718. [Google Scholar]
- Wu, J.G.; Peng, W.; Yi, J.; Wu, Y.B.; Chen, T.Q.; Wong, K.H.; Wu, J.Z. Chemical composition, antimicrobial activity against Staphylococcus aureus and a pro-apoptotic effect in SGC-7901 of the essential oil from Toona sinensis (A. Juss.) Roem. leaves. J. Ethnopharmacol. 2014, 154, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.C.; Yu, W.J.; Huang, C.Y.; Chen, Y.H.; Tsai, Y.C.; Chang, C.C.; Chang, S.J. Toona sinensis leaf aqueous extract improves the functions of sperm and testes via regulating testicular proteins in rats under oxidative stress. Evid.-Based Complement. Altern. 2012, 2012, 681328. [Google Scholar]
- Poon, S.L.; Leu, S.F.; Hsu, H.K.; Liu, M.Y.; Huang, B.M. Regulatory mechanism of Toona sinensis on mouse leydig cell steroidogenesis. Life Sci. 2005, 76, 1473–1487. [Google Scholar] [CrossRef]
- Yu, W.J.; Yu, B.C.; Yang, F.Y.; Chang, C.C.; Huang, C.Y.; Chang, S.J. Toona sinensis affects reproductive physiology of male. J. Food Drug Anal. 2012, 20, 53. [Google Scholar] [CrossRef]
- Su, Y.F.; Yang, Y.C.; Hsu, H.K.; Hwang, S.L.; Lee, K.S.; Lieu, A.S.; Chan, T.F.; Lin, C.L. Toona sinensis leaf extract has antinociceptive effect comparable with non-steroidal anti-inflammatory agents in mouse writhing test. BMC Complem. Altern. Med. 2015, 15, 70. [Google Scholar] [CrossRef]
- Duan, D.; Chen, L.; Yang, X.; Tu, Y.; Jiao, S. Antidepressant-like effect of essential oil isolated from Toona ciliata Roem. var. yunnanensis. J. Nat. Med. 2014, 69, 191–197. [Google Scholar] [CrossRef]
- Jiang, X. Research progress of T. sinensis. Heilongjiang Sci. Technol. Inf. 2009, 9, 100. [Google Scholar]

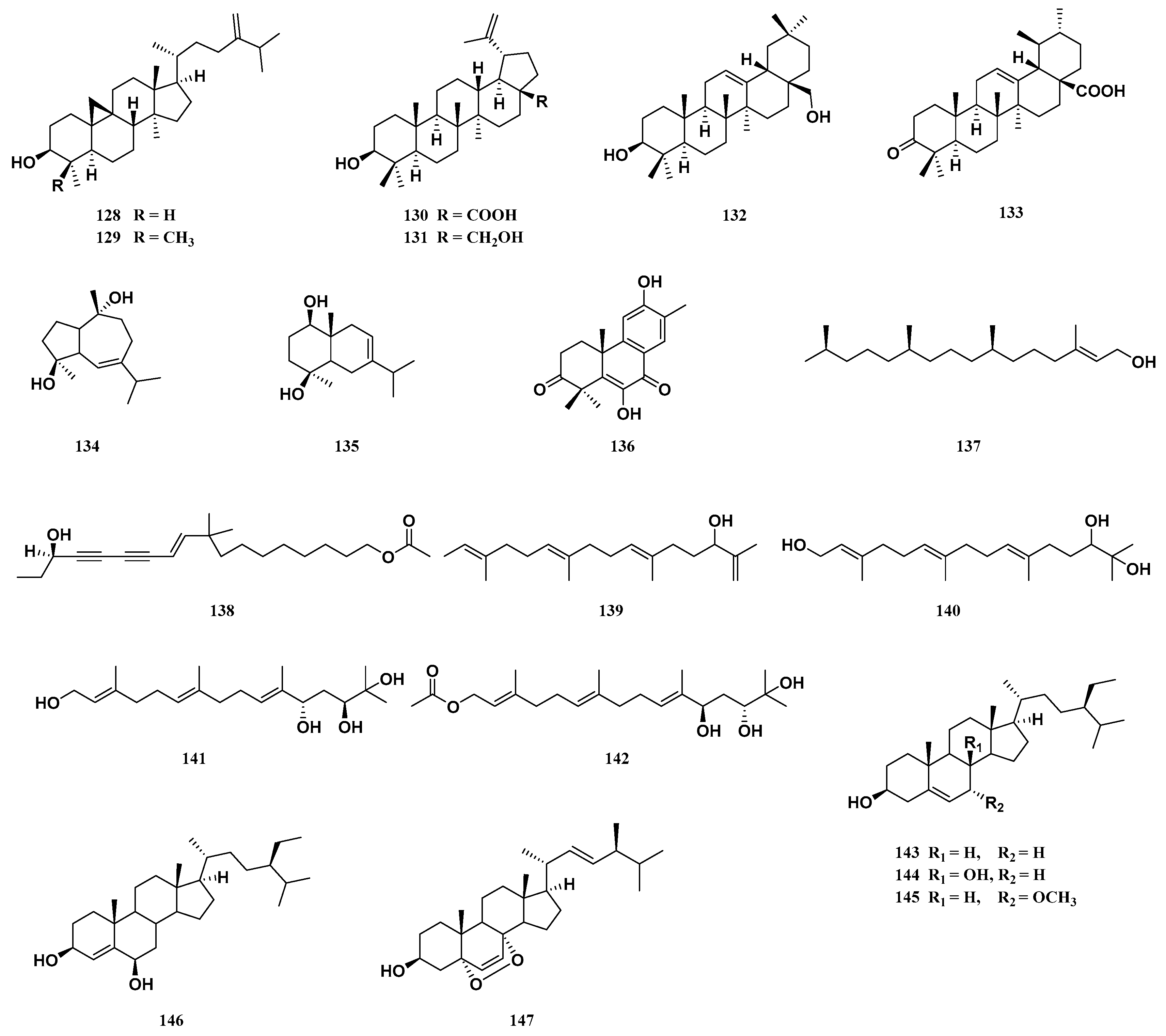
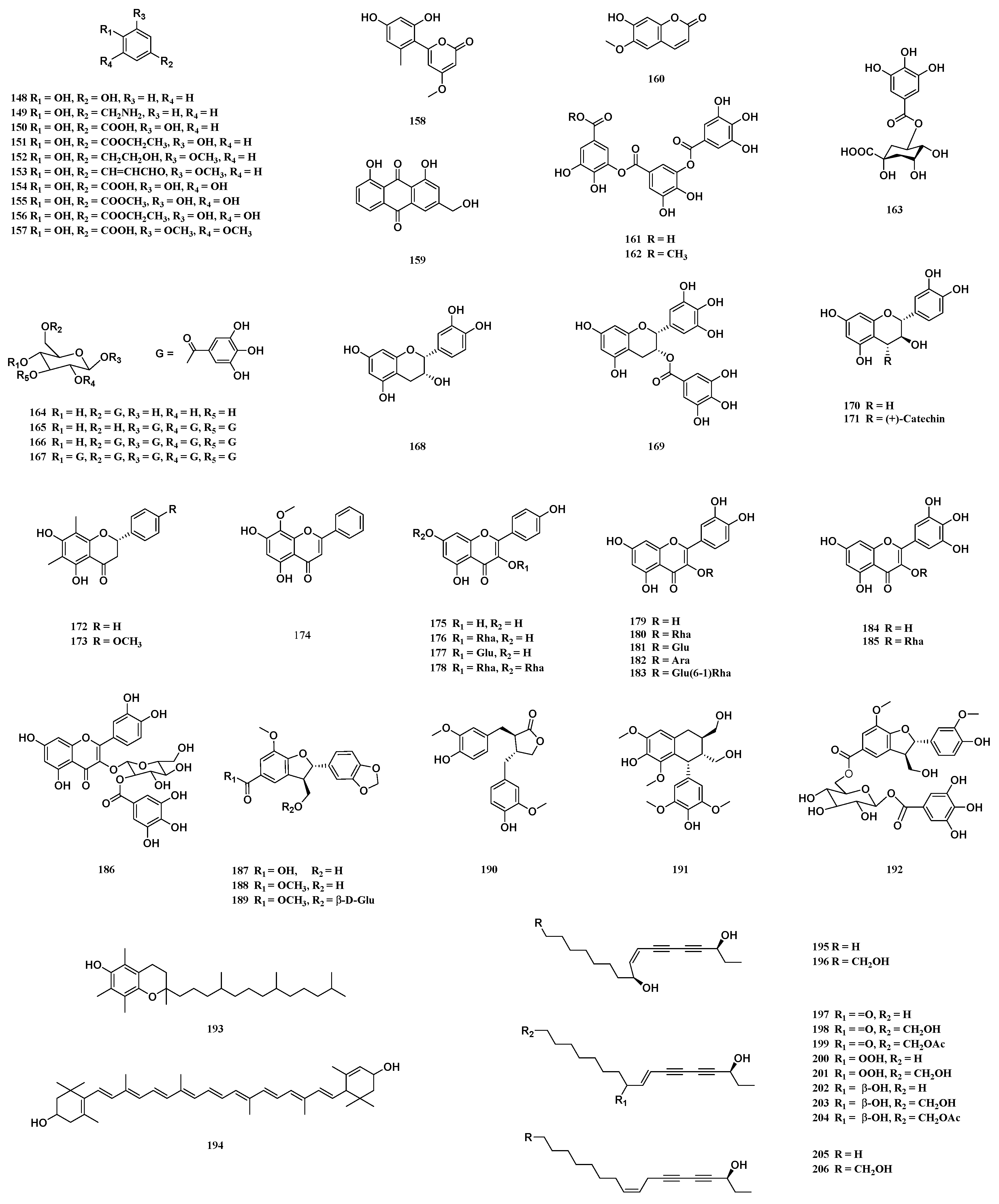

| Comp. | Name | Type | Sources | Ref. |
|---|---|---|---|---|
| triterpenoids | ||||
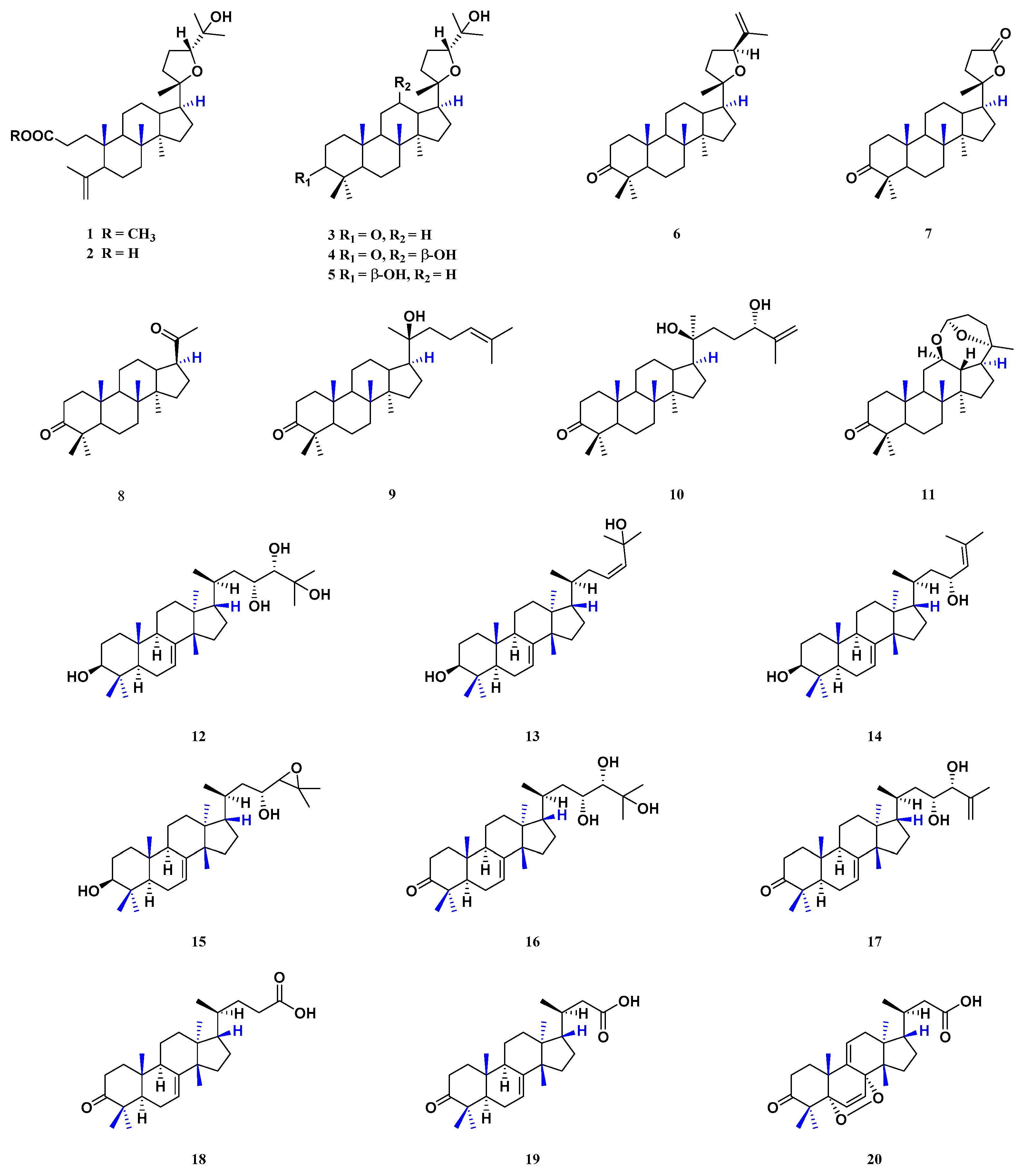
| 1 | methyl shoreate | dammarane | stem barks | [23] |
| 2 | shoreic acid | dammarane | stem barks | [23] |
| 3 | ocotillone | dammarane | stem barks | [23] |
| 4 | (20S, 24R)-epoxydammarane-12, 25-diol-3-one | dammarane | stem barks | [23] |
| 5 | (20S, 24R)-epoxydammarane-3β, 25-diol-marane-3β, 25-diol | dammarane | stem barks | [23] |
| 6 | richenone | dammarane | stem barks | [23] |
| 7 | cabralealactone | dammarane | stem barks | [23] |
| 8 | hollongdione | dammarane | stem barks | [23] |
| 9 | 20-hydroxy-24-dammaren-3-one | dammarane | stem barks | [23] |
| 10 | (20S, 24S)-dihydroxydammar-25-en-3-one | dammarane | stem barks | [23] |
| 11 | cylindrictone D | dammarane | stem barks | [23] |
| 12 | hispidol B | tirucallane | barks | [24] |
| 13 | 3β, 25-dihydroxy-tirucalla-7, 23-diene | tirucallane | seeds | [25] |
| 14 | 3β, 23-dihydroxy-tirucalla-7, 24-diene | tirucallane | seeds | [25] |
| 15 | 24, 25-epoxy-3β, 23-dihydroxy-7-tirucallene | tirucallane | seeds | [25] |
| 16 | piscidinol | tirucallane | barks | [24] |
| 17 | bourjotinolone B | tirucallane | barks | [26] |
| 18 | (20S)-3-oxo-tirucalla-25-nor-7-en-24-oic acid | tirucallane | stem barks | [23] |
| 19 | 4, 4, 14-trimethyl-3-oxo-24-nor-5α,13α,14β,17α, 20S-chol-7-en-23-oic acid | tirucallane | stem barks | [23] |
| 20 | (20S)-5α, 8α-epidioxy-3-oxo-24-nor-6,9 (11)-dien-23-oic acid | tirucallane | stem barks | [23] |
| 21 | Comp. 1 of [24] | apo-tirucallane | barks | [24] |
| 22 | Comp. 2 of [24] | apo-tirucallane | barks | [24] |
| 23 | Comp. 7 of [24] | apo-tirucallane | barks | [24] |
| 24 | Comp. 8 of [24] | apo-tirucallane | barks | [24] |
| 25 | 21α-O-methylmelianodiol | apo-tirucallane | pericarps | [1,15,27] |
| 26 | 21β-O-methylmelianodiol | apo-tirucallane | pericarps | [1,15,27] |
| 27 | Comp. 9 of [24] | apo-tirucallane | barks | [24] |
| 28 | sapelin E acetate | apo-tirucallane | barks | [24] |
| 29 | grandifoliolenone | apo-tirucallane | barks | [24] |
| 30 | bourjotinolone A | apo-tirucallane | barks | [24] |
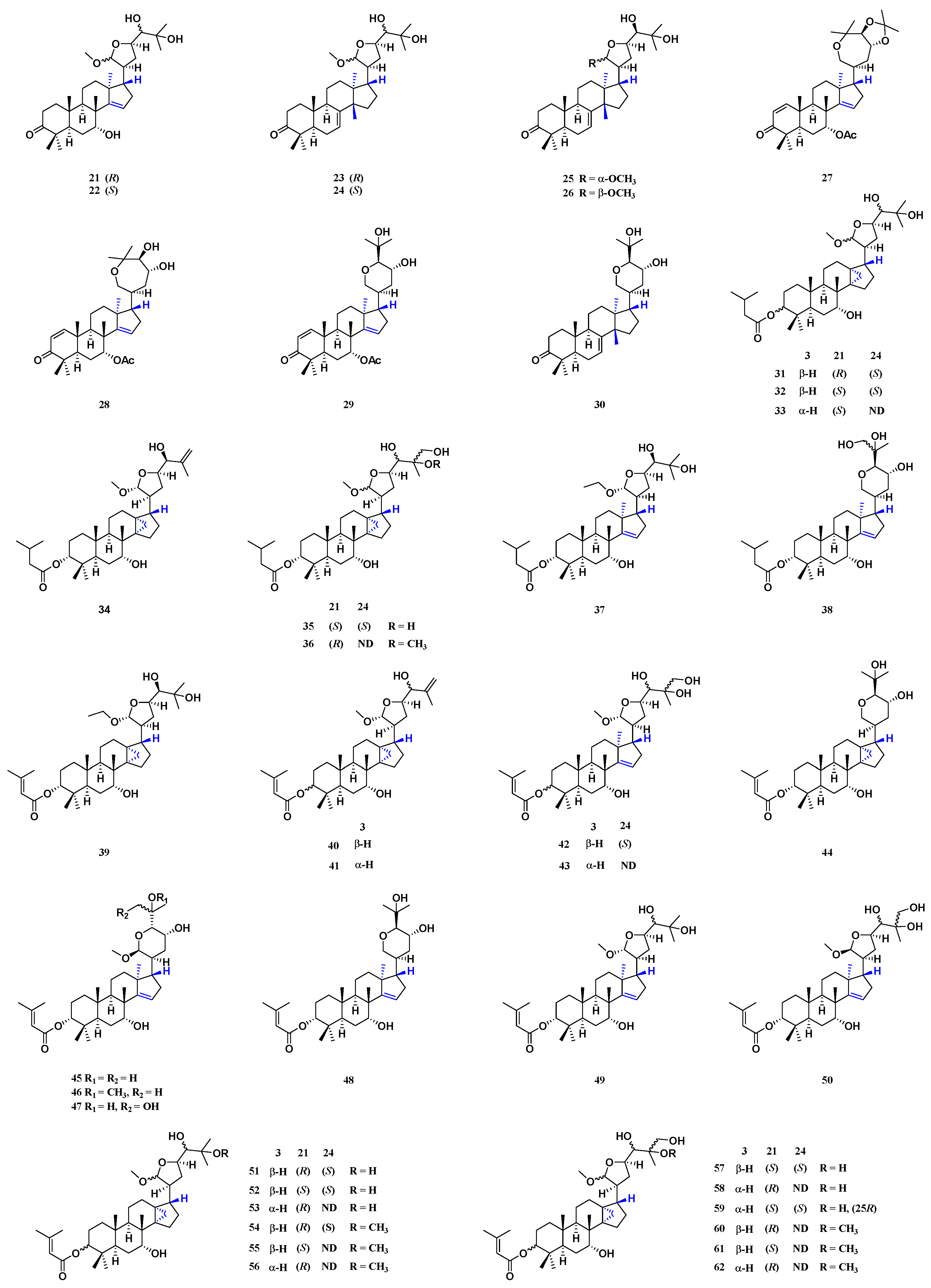
| 31 | Comp. 4 of [28] | apo-tirucallane | seeds, stems | [28] |
| 32 | Comp. 5 of [28] | apo-tirucallane | stems | [28] |
| 33 | Comp. 6 of [28] | apo-tirucallane | stems | [28] |
| 34 | toonasinensin E | apo-tirucallane | seeds, pericarps | [1,15,18,27] |
| 35 | Comp. 13 of [28] | apo-tirucallane | stems | [28] |
| 36 | Comp. 17 of [28] | apo-tirucallane | stems | [28] |
| 37 | toonasinensin A | apo-tirucallane | pericarps | [1,15,27] |
| 38 | Comp. 6 of [24] | apo-tirucallane | barks | [24] |
| 39 | toonasinensin B | apo-tirucallane | pericarps | [1,15,27] |
| 40 | Comp. 18 of [28] | apo-tirucallane | leaves, stems | [28] |
| 41 | toonasinensin D | apo-tirucallane | seeds, pericarps | [1,15,27,28] |
| 42 | Comp. 22 of [28] | apo-tirucallane | leaves | [28] |
| 43 | toonasinensin C | apo-tirucallane | leaves, barks, pericarps | [1,15,24,27,28] |
| 44 | Comp. 21 of [28] | apo-tirucallane | leaves | [28] |
| 45 | Comp. 1a of [28] | apo-tirucallane | seeds | [28] |
| 46 | Comp. 7a of [28] | apo-tirucallane | stems | [28] |
| 47 | Comp. 10a of [28] | apo-tirucallane | leaves | [28] |
| 48 | Comp. 5 of [24] | apo-tirucallane | barks | [24] |
| 49 | Comp. 3 of [24] | apo-tirucallane | barks | [24] |
| 50 | Comp. 4 of [24] | apo-tirucallane | barks | [24] |
| 51 | Comp. 1 of [28] | apo-tirucallane | seeds, leaves, stems | [28] |
| 52 | Comp. 2 of [28] | apo-tirucallane | leaves, stems | [28] |
| 53 | Comp. 3 of [28] | apo-tirucallane | leaves, stems | [28] |
| 54 | Comp. 7 of [28] | apo-tirucallane | leaves, stems | [28] |
| 55 | Comp. 8 of [28] | apo-tirucallane | leaves | [28] |
| 56 | Comp. 9 of [28] | apo-tirucallane | stems | [28] |
| 57 | Comp. 10 of [28] | apo-tirucallane | leaves | [28] |
| 58 | Comp. 11 of [28] | apo-tirucallane | leaves | [28] |
| 59 | Comp. 12 of [28] | apo-tirucallane | leaves | [28] |
| 60 | Comp. 14 of [28] | apo-tirucallane | leaves | [28] |
| 61 | Comp. 15 of [28] | apo-tirucallane | leaves | [28] |
| 62 | Comp. 16 of [28] | apo-tirucallane | leaves | [28] |
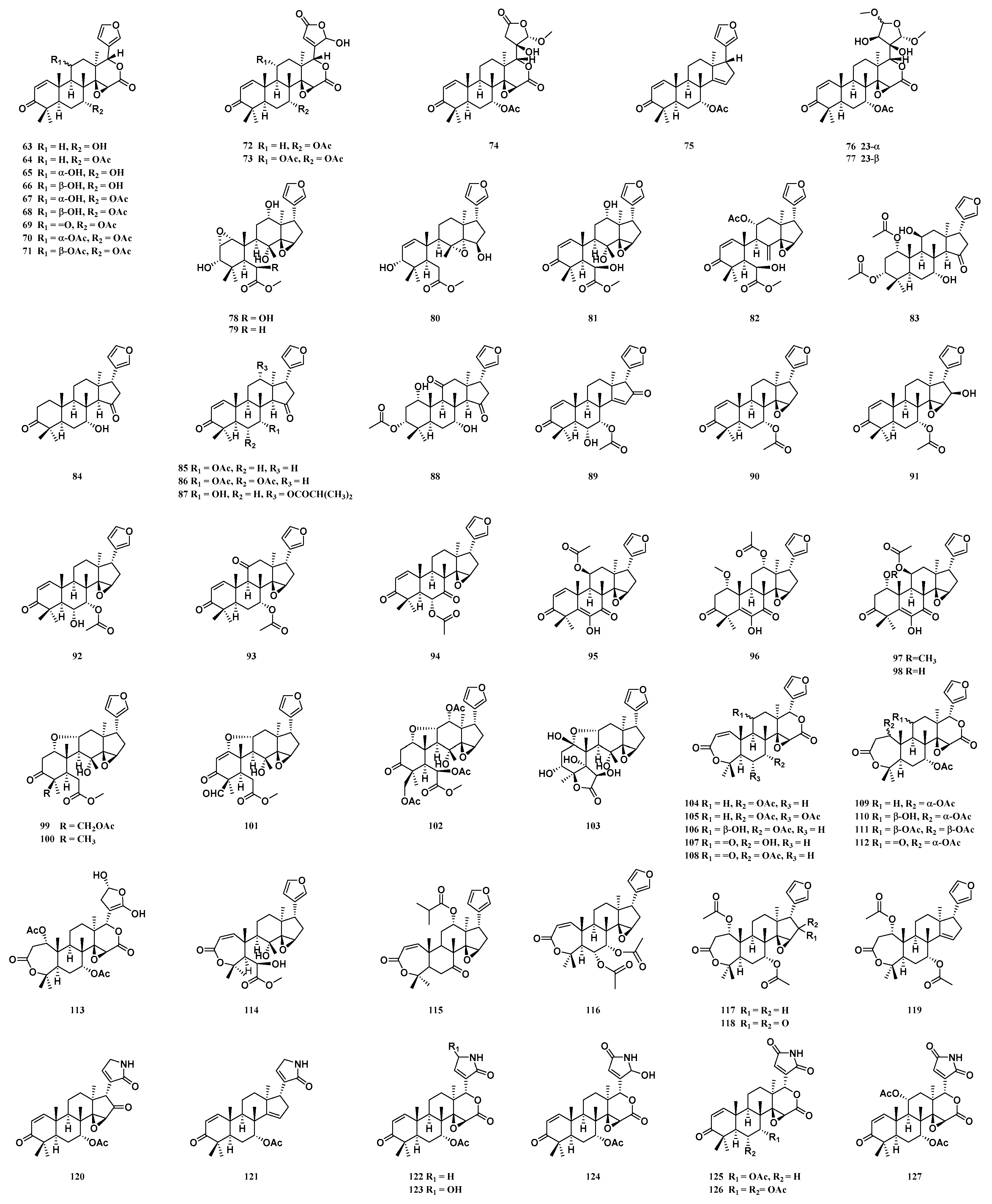
| 63 | 7-deacetoxy-7α-hydroxygedunin | limonoids | barks | [29] |
| 64 | gedunin | limonoids | barks | [29] |
| 65 | 7-deacetoxy-7α, 11α-dihydroxygedunin | limonoids | barks | [29] |
| 66 | 7-deacetoxy-7α, 11β-dihydroxygedunin | limonoids | barks | [29] |
| 67 | 11α-hydroxygedunin | limonoids | barks | [29] |
| 68 | 11β-hydroxygedunin | limonoids | barks | [29] |
| 69 | 11-oxogedunin | limonoids | barks | [29] |
| 70 | 11α-acetoxygedunin | limonoids | barks | [29 |
| 71 | 11β-acetoxygedunin | limonoids | barks | [29] |
| 72 | photogedunin | limonoids | barks | [26] |
| 73 | toonasinemine I | limonoids | root barks | [30] |
| 74 | toonasinemine J | limonoids | root barks | [30] |
| 75 | azadirone | limonoids | barks | [24] |
| 76 | toonasinemine K | limonoids | root barks | [30] |
| 77 | toonasinemine L | limonoids | root barks | [30] |
| 78 | toonasinenine F | limonoids | root barks | [30] |
| 79 | toonacilianin D | limonoids | leaves | [31] |
| 80 | toonasinenine H | limonoids | leaves | [31] |
| 81 | toonasinenine G | limonoids | leaves | [31] |
| 82 | toonasinenine E | limonoids | leaves | [31] |
| 83 | toonasinenoids A | limonoids | leaves, buds | [32] |
| 84 | walsurin D | limonoids | leaves, buds | [32] |
| 85 | walsurin E | limonoids | leaves, buds | [32] |
| 86 | toonaciliatone F | limonoids | leaves, buds | [32] |
| 87 | toonayunnanin B | limonoids | leaves, buds | [32] |
| 88 | toonasinenoids B | limonoids | leaves, buds | [32] |
| 89 | 6α-hydroxyazadiradione | limonoids | leaves, buds | [32] |
| 90 | trichilenone acetate | limonoids | leaves, buds | [32] |
| 91 | toonasinenoid E | limonoids | leaves, buds | [32] |
| 92 | 14, 15-epoxynimonol | limonoids | leaves, buds | [32] |
| 93 | toonasinenoids D | limonoids | leaves, buds | [32] |
| 94 | toonaciliatone B | limonoids | leaves, buds | [32] |
| 95 | walsunoid H | limonoids | leaves, buds | [32] |
| 96 | 1α-methoxy-12α-acetoxydihydrocedrelone | limonoids | leaves, buds | [32] |
| 97 | dysoxylumosin G | limonoids | leaves, buds | [32] |
| 98 | toonasinenoids C | limonoids | leaves, buds | [32] |
| 99 | toonasinenine A | limonoids | leaves | [31] |
| 100 | toonafolin | limonoids | leaves | [31] |
| 101 | toonasinenine B | limonoids | leaves | [31] |
| 102 | toonasinenine C | limonoids | leaves | [31] |
| 103 | toonasinenine D | limonoids | leaves | [31] |
| 104 | proceranone | limonoids | root barks | [33] |
| 105 | 6-acetoxyobacunol acetate | limonoids | leaves | [34] |
| 106 | 11β-hydroxy-7α-obacunyl acetate | limonoids | leaves | [35] |
| 107 | 11-oxo-7α-obacunol | limonoids | leaves | [35] |
| 108 | 11-oxo-7α-obacunyl acetate | limonoids | leaves | [35] |
| 109 | 7α-acetoxydihydronomilin | limonoids | leaves | [34] |
| 110 | 11β-hydroxycneorin G | limonoids | leaves | [35] |
| 111 | toonins A | limonoids | root barks | [33] |
| 112 | 11-oxocneorin G | limonoids | leaves | [35] |
| 113 | cedrellin | limonoids | leaves | [34] |
| 114 | toonasinenine I | limonoids | leaves | [31] |
| 115 | toonasinenine J | limonoids | leaves | [31] |
| 116 | surenin | limonoids | root barks | [33] |
| 117 | toonins B | limonoids | root barks | [33] |
| 118 | carapolide H | limonoids | root barks | [33] |
| 119 | carapolide I | limonoids | root barks | [33] |
| 120 | toonasinemine A | limonoids | root barks | [30] |
| 121 | toonasinemine B | limonoids | root barks | [30] |
| 122 | toonasinemine C | limonoids | root barks | [30] |
| 123 | toonasinemine F | limonoids | barks | [26] |
| 124 | toonasinemine G | limonoids | root barks | [30] |
| 125 | toonasinemine D | limonoids | barks | [26] |
| 126 | toonasins B | limonoids | barks | [26] |
| 127 | toonasinemine E | limonoids | root barks | [30] |
| 128 | cycloeucalenol | cycloartane | pericarps | [1,15,27] |
| 129 | 24-methylenecycloartanol | cycloartane | pericarps | [1,15,27] |
| 130 | betulinic acid | other | barks | [26,36] |
| 131 | betulin | other | barks | [26] |
| 132 | erythrodiol | other | barks | [26] |
| 133 | 3-oxours-12-en-28-oic acid | other | roots | [36] |
| 134 | alismoxide | sesquiterpenoids | pericarps | [1,27] |
| 135 | oplodiol | sesquiterpenoids | pericarps | [1,27] |
| 136 | gossweilone | diterpenoids | barks | [26] |
| 137 | phytol | diterpenoids | leaves | [34] |
| 138 | (9S, 10E, 16R)-9, 16-dihydroxyoctadec-10-ene-12, 14-diyn-1-yl acetate | diterpenoids | barks | [37] |
| 139 | 2, 6, 10, 15-phytatetraene-14-ol | diterpenoids | leaves | [34] |
| 140 | 2, 6, 10-phytatriene-1, 14, 15-triol | diterpenoids | leaves | [34] |
| 141 | 15-tetrahydroxy-3,7, 11, 15, 15-pentamethyl-2, 6, 10-hexadecatriene | diterpenoids | seeds | [25] |
| 142 | 1-O-acetyl-12, 14, 15-trihydroxy-3, 7, 11, 15, 15-pentamethyl-2, 6, 10-hexadecatriene | diterpenoids | seeds | [25] |
| 143 | β-sitosterol | sterols | pericarps, barks, roots | [1,27,33,38] |
| 144 | lawsaritol A | sterols | pericarps | [1,27] |
| 145 | (3β, 7α)-7-methoxystigmast-5-en-3-ol | sterols | pericarps | [1,27] |
| 145 | stigmast-4-ene-3β, 6β-diol | sterols | pericarps | [1,27] |
| 147 | 5α, 8α-epidioxy-(22E, 24R)-ergosta-6, 22-dien-3β-ol | sterols | pericarps | [1,27] |
| 148 | hydroquinone | phenols | pericarps | [1] |
| 149 | 4-hydroxybenzylamine | phenols | pericarps | [1,27] |
| 150 | protocatechuic acid | phenols | pericarps | [1] |
| 151 | 3, 4-dihydroxybenzoic acid ethyl ester | phenols | pericarps | [1,27] |
| 152 | 3-methoxy-4-hydroxy phenylethanol | phenols | pericarps, roots | [1,27,33] |
| 153 | coniferyl aldehyde | phenols | pericarps | [1,27] |
| 154 | gallic acid | phenols | pericarps | [1] |
| 155 | methyl gallate | phenols | young leaves, pericarps | [1,39] |
| 156 | ethyl gallate | phenols | young leaves, leaves, stems, fruits, pericarps | [25,34,40,41] |
| 157 | syringic acid | phenols | roots | [33] |
| 158 | 4-methoxy-6-(2′, 4′-dihydroxy-6′-methylphenyl)-pyran-2-one | phenols | roots | [33] |
| 159 | aloeemodin | phenols | roots | [33] |
| 160 | isoscopoletin | phenols | roots | [33] |
| 161 | trigallic acid | phenols | pericarps | [1] |
| 162 | 7-methoxy trigallic acid | phenols | pericarps | [1] |
| 163 | 5-O-galloylquinic acid | phenols | leaves | [12] |
| 164 | 6-O-galloyl-D-glucose | phenols | leaves, shoots | [39] |
| 165 | 1, 2, 3-tri-O-galloyl-β-D-glucopyranose | phenols | leaves, shoots | [39] |
| 166 | 1, 2, 3, 6-tetra-O-galloyl-β-D-glucopyranose | phenols | leaves, shoots | [39] |
| 167 | 1, 2, 3, 4, 6-penta-O-galloyl-β-D-glucose | phenols | pericarps, young leaves | [1,39] |
| flavonoids | ||||
| 168 | (-)-epicatechin | flavan-3-ols | stems | [40] |
| 169 | (-)-epigallocatechin gallate | flavan-3-ols | leaves | [40] |
| 170 | (+)-catechin | flavan-3-ols | leaves, woods | [42] |
| 171 | procyanidin B3 | flavan-3-ols | leaves, woods | [42] |
| 172 | demethoxymatteucinol | flavanones | stems | [40] |
| 173 | matteucinol | flavanones | stems | [40] |
| 174 | 5, 7-dihydroxy-8-methoxy flavone | flavones | barks | [38] |
| 175 | kaempferol | flavonols | young leaves | [41] |
| 176 | kaempferol-3-O-α-rhamopyranoside | flavonols | young leaves | [41] |
| 177 | astragalin | flavonols | young leaves | [41] |
| 178 | kaempferitrin | flavonols | seeds | [43] |
| 179 | quercetin | flavonols | young leaves | [41] |
| 180 | quercetin-3-rhamnoside | flavonols | pericarps, young leaves | [1,39] |
| 181 | quercetin 3-glucoside | flavonols | pericarps | [25] |
| 182 | quercetin-3-O-α-L-arabinopyranoside | flavonols | pericarps | [1] |
| 183 | rutin | flavonols | leaves, shoots | [39] |
| 184 | myricetin | flavonols | barks | [38] |
| 185 | myricitrin | flavonols | barks | [38] |
| 186 | quercetin 3-O-(2″-O-galloyl)-β-D-glucopyranoside | flavonols | leaves | [12] |
| 187 | cedralins A | phenylpropanoids | leaves | [44] |
| 188 | toonin C | phenylpropanoids | roots, pericarps | [1,27] |
| 189 | cedralins B | phenylpropanoids | leaves | [44] |
| 190 | matairesinol | phenylpropanoids | root barks | [33] |
| 191 | lyoniresinol | phenylpropanoids | root barks | [33] |
| 192 | punicatannin C | phenylpropanoids | pericarps | [1,27] |
| 193 | α-tocopherol | others | leaves | [45] |
| 194 | lutein | others | leaves | [45] |
| 195 | toonasindiyne A | others | root barks | [46] |
| 196 | toonasindiyne B | others | root barks | [46] |
| 197 | toonasindiyne C | others | root barks | [46] |
| 198 | toonasindiyne D | others | root barks | [46] |
| 199 | toonasindiyne E | others | root barks | [46] |
| 200 | toonasindiyne F | others | root barks | [46] |
| 201 | Comp. 7 of [46] | others | root barks | [46] |
| 202 | Comp. 8 of [46] | others | root barks | [46] |
| 203 | Comp. 9 of [46] | others | root barks | [46] |
| 204 | Comp. 10 of [46] | others | root barks | [46] |
| 205 | Comp. 11 of [46] | others | root barks | [46] |
| 206 | Comp. 12 of [46] | others | root barks | [46] |
| Active Constituents | Extraction Solvent | Experimental Model | Regulatory Mechanism a | Ref. |
|---|---|---|---|---|
| Antidiabetic activity | ||||
| leaves extracts | supercritical-CO2 fluid | in vivo: STZ induced mice | triglyceride levels↑, adiponectin levels↓ | [52] |
| leaves extracts | water | in vivo: alloxan-induced diabetic Long-Even rats | GLUT4 mRNA (RT-PCR)↑, GLUT4 protein↑ | [53] |
| leaves extracts | 50% alcohol/water | in vitro: 3T3-L1 adipocytes treated by calphostin C | cellular glucose uptake↓ | [54] |
| leaves extracts | 95% ethanol | in vivo and in vitro | stimulating glucose uptake, ameliorating insulin resistance | [55] |
| rutin (183, leaves) | water | in vivo: insulin-resistant type 2 diabetes mouse model | IRK activity↑, glucose uptake↑ | [56] |
| quercetin (179, leaves) | ethyl acetate | in vivo: diabetic mice induced by HFD and alloxan | p65/NF-κB↓, ERK1/2/MAPK↓, caspase-9↓, caspase-3↓ | [57] |
| Antidiabetic nephropathy activity | ||||
| seeds extracts | petroleum ether | in vivo: STZ-induced DN rats | TGF-β1↓, Col IV↓, CTGF↓ | [58] |
| seeds extracts | n-butanol | in vivo: STZ-induced DN rats | blood glucose↓, urinary albumin↓, kidney index↓, oxidative stress index↓, serum creatinine↓, urea nitrogen levels↓, oxidative stress↓, TGF-β1↓, Col IV↓, CTGF↓ | [59] |
| seeds extracts | n-butyl alcohol | in vitro: HG-induced GMCs | ROS↓, p47phox↓, Nrf2↑,NQO1↑, HO-1↑ | [60] |
| seeds extracts | n-butyl alcohol | in vivo: STZ-induced DN rats in vitro: HG-induced human renal glomerular endothelial cells | MCP-1↓, ICAM-1↓, p65↓ | [61] |
| kaempferitrin (178, seeds) | in vitro: AGEs-induced GMCs | SOD↑, MDA↓, ROS↓, protecte against OS | [43] | |
| kaempferol (175, seeds) | in vitro: HG-induced GMCs | ROS↓, MDA↓, SOD↑, TGF-β1↓, Col IV↓, NOX4↓, p22phox↓, Sestrin2↑, AMPK↑ | [62] | |
| toonasinensin B (39), toonasinensin D (41), 21α-O-methylmelianodiol (25), 21β-O-methylmelianodiol (26) (pericarps) | in vitro: HG-induced GMCs | NADPH↓, sorbitol↓ | [27] | |
| two acyclic diterpenoids (seeds) | in vitro: HG-induced GMCs | Nrf2/HO-1↑, NF-κB↓, TNF-α↓, IL-6↓ | [25] | |
| Antioxidant activity | ||||
| leaves, roots, barks extracts | water | in vivo: senescence-accelerated mice in vitro: DPPH· | TBARS↓, SOD↑, CAT↑, GSH-Px↑, DPPH↓ DPPH free-radical activity | [63] |
| leaves extracts | acetone | in vitro: ORAC, PSC, HepG2 cells, CAA | anti-proliferative effect, antioxidant properties | [64] |
| leaves extracts, gallic acid (154) | water | in vitro: AAPH inducedhuman umbilical vein endothelial cells | ROS↓, MDA↓, SOD/CAT↑, reverse Bax/Bcl-2 dysregulation | [65] |
| leaves extracts, gallic acid (154) | water | in vitro: various oxidative systems, AAPH-induced human erythrocytes | oxidative hemolysis↓, lipid peroxidation↓, SOD↓ | [66] |
| flavonoids, methyl gallate (155) (buds) | 70% methanol | in vitro: ABTS·+, DPPH· | ABTS and DPPH free-radical activity | [67] |
| PGG (167), EG (156) (young leaves) | liquid-liquid refined extraction | in vitro: ABTS·+, DPPH· | ABTS and DPPH free-radical activity | [68] |
| five flavonols, three derivatives of gallic acid (young leaves) | 95% ethanol | in vitro: four chemical-induced oxidative models | significant antioxidant properties | [41] |
| toonasinenine D (103), E (82), G (81), H (80), I (114) and J (115) (leaves) | 95% ethanol | in vitro: ABTS·+, DPPH· | strong scavenging activities | [31] |
| Anti-inflammatory activity | ||||
| leaves extracts | water | in vitro: LPS-induced macrophage | HO-1↑, TNF-α↓ | [69] |
| leaves extracts | water | in vitro: RAW264.7 cells treated with LPS | GSH↑, GSH/GSSG↑, reverse the effects of IL-6 and IL-10 | [70] |
| adventitious shoots extracts | in vitro: LPS treated RAW 264.7 cells and propionibacterium acnes-treated HaCaT cells | suppress MAPK pathways | [71] | |
| leaves extracts | water | in vitro: LPS-induced microglial | NO↓, TNF-α↓, iNOS↓ | [72] |
| polyphenols (seeds) | 50% acetone | in vivo: a rat model of Parkinson’s disease | p38 MAPK↓, protein levels of infammatory mediators↓ | [73] |
| 7-DGD (63) | in vivo: LPS-induced septic shock models in vitro: macrophages | activate Keap1/Nrf2/HO-1 signaling | [74] | |
| 7-DGD (63) | in vitro: human rheumatoid arthritis synovial fibroblast | activate Nrf2/ARE signaling | [75] | |
| DAG (63) | in vitro: LPS treated RAW 264.7 cells | K+ efflux↓, ROS↓ | [76] | |
| toonasinenine A (99), B (101), C (102), D (103), toonafolin (100) (leaves) | ethanol | in vitro | COX-1↓, COX-2↓ | [31] |
| toonasinemine A (120), B (121), F (123), I (73) (root barks) | CH2Cl2 | in vitro: LPS-activated RAW 264.7 macrophages | NO↓ | [30] |
| two acyclic diterpenoids (141, 142) (seeds) | in vivo: HG- induced GMCs | Nrf-2/HO-1↑, NF-κB↓, TNF-α↓, IL-6↓ | [25] | |
| quercitrin (180, leaves) | 95% ethanol | in vitro: APAP-treated HepG2 cell | iNOS↓, COX-2↓, IL-1β↓ | [77] |
| polyacetylenes | CH2Cl2 | in vitro: LPS treated RAW 264.7 cells | NO↓ | [46] |
| Antitumor activity | ||||
| leaves extracts | water | in vitro: osteosarcoma cells | inhibit the activity of MG-63, Saos-2 and U2OS osteosarcoma cells. | [78] |
| leaves extracts | water | in vitro: WEHI-3 cells | WEHI-3 cells viability↓, cytochrome C↑, caspase-3↑, Bax↑, Bcl-2↓ | [79] |
| leaves extracts | water | in vitro: HL-60 cells | induce cytochrome C translocation, caspase 3 activation, degradation of PARP, dysregulation of Bcl-2 and Bax | [80] |
| leaves extracts | water | in vitro: A549 lung cancer cells | cyclin D1 and cyclin E↓ | [81] |
| leaves extracts | in vitro: H441 and H661 cells | cyclin D1 and CDK4↓, block the cell cycle in G1 phase, Bcl2↓, Bax↑ | [82,83] | |
| leaves extracts | water | in vitro: ccRCC cells | cyclin D1↓, CDK2↓, CDK4↓, p53 ↑, FOXO3a ↑ | [84] |
| leaves extracts | in vitro: ovarian cancer cells | arrest SKOV3 ovarian cancer cells at the G2/M phase | [85] | |
| leaves extracts | water | in vitro: DMBA-induced hamster cheek pouch squamous cells | survivin, XIAP, PCNA, iNOS, and COX-2 proteins↓ | [86] |
| the total phenolic (leaves) | 60% ethanol | in vitro: Caco-2, HepG2, MCF-7 | inhibit proliferation | [87] |
| gallic acid (154, leaves) | in vitro: DU145 cells | ROS↑, cytotoxic to DU145cells | [88] | |
| gallic acid (154, leaves) | in vitro: HOSCC cells | TNF-α↑, TP53BP2↑, GADD45A↑, Survivin↓, cIAP1↓, induces cell death | [89] | |
| betulinic acid (130), 3-oxours-12-en-28-oic acid (133) (roots) | in vitro: MGC-803 and PC3 cells | inhibite proliferation, led to apoptosis | [36] | |
| toonasinenine A (99), B (101), C (102), D (103), toonafolin (100) (leaves) | 95% ethanol | in vitro: tumor cell lines | significant effects on all tumor cell lines except glioma cell lines | [31] |
| Hepatoprotective activity | ||||
| leaves extracts | water | in vivo: TAA treated liver injury rats | collagen formation↓, TGF-β1↓ | [90] |
| polysaccharide (leaves) | water | in vivo: the liver injury induced by CCl4 in mice | ALT↓, AST↓, MDA↓, SOD↑, GSH-Px↑, CAT↑, GSH↑, TNF-α↓, IL-6↓ | [91] |
| polyphenols (barks and fruits) | water | in vitro: FFA-treated HepG2 cells | lipoprotein↓, activating AMPK pathway, lipid metabolism↑, lipid accumulation↓ | [92] |
| quercetin (179, leaves) | 70% ethanol | in vivo: diabetic mice induced by HFD and alloxouracil | ameliorating oxidative stress in the liver, protects hepatocytes | [57] |
| quercitrin (180, leaves) | 95% ethanol | in vivo:APAP-treated HepG2 cell in vitro: APAP-treated animal models | activation of defensive genes and the inhibition of pro-inflammatory genes via the suppressions of JNK and p38 signaling | [77] |
| Antiviral and antibacterial activity | ||||
| tender leaves extracts | in vitro | anti-SARS coronavirus | [93] | |
| tender leaves extracts | water | in vitro | anti-influenza A virus (H1N1) | [94] |
| sesquiterpene from essential oil (leaves) | n-hexane | in vitro | antimicrobial activity against MSSA and MRSA strains | [95] |
| polyphenols, glycosides, terpenoids contained in shoots extracts | ethyl acetate | in vitro | inhibitory activities against Staphylococcus aureus, Shigella dysenteriae and Escherichia coli | [50] |
| Immunopotentiation | ||||
| leaves extracts | water | in vivo: tilapia | improve the immune response and resistance of tilapia to hydrophilic bacteroides infection | [13] |
| polysaccharide TSP-3a (seeds) | water | in vivo: CY induced immunodeficiency mice model | significant immune restoring activity and enhance phagocytosis | [17] |
| rutin (183, leaves) | methanol | in vivo | enhance immunity of shrimp | [9] |
| Effects on the male reproductive system | ||||
| leaves extracts | water | in vivo: rats | ROS↓, aintained MMP, restored the sperm motility | [96] |
| leaves extracts | water | in vitro: primary mouse Leydig cells | inhibited testosterone production | [97] |
| leaves extracts | ethanol | in vitro: the human spermatozoa treated with H2O2 | ROS↓, cell death↓ | [98] |
| Other aspects | ||||
| leaves extracts | water | in vitro: a visceral pain mouse model | anti-visceral pain properties | [99] |
| essential oil (leaves) | water | in vivo: CMS rats | anti-depression | [100] |
| limonoids (leaves and buds) | ethanol | in vitro: 6-hydroxydopamine-induced SH-SY5Y cells | neuroprotective effects | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Li, H.; Wang, R.; Lan, S.; Wang, Y.; Zhang, Y.; Sui, H.; Li, W. Traditional Uses, Chemical Constituents and Pharmacological Activities of the Toona sinensis Plant. Molecules 2024, 29, 718. https://doi.org/10.3390/molecules29030718
Zhao M, Li H, Wang R, Lan S, Wang Y, Zhang Y, Sui H, Li W. Traditional Uses, Chemical Constituents and Pharmacological Activities of the Toona sinensis Plant. Molecules. 2024; 29(3):718. https://doi.org/10.3390/molecules29030718
Chicago/Turabian StyleZhao, Mengyao, Huiting Li, Rongshen Wang, Shuying Lan, Yuxin Wang, Yuhua Zhang, Haishan Sui, and Wanzhong Li. 2024. "Traditional Uses, Chemical Constituents and Pharmacological Activities of the Toona sinensis Plant" Molecules 29, no. 3: 718. https://doi.org/10.3390/molecules29030718
APA StyleZhao, M., Li, H., Wang, R., Lan, S., Wang, Y., Zhang, Y., Sui, H., & Li, W. (2024). Traditional Uses, Chemical Constituents and Pharmacological Activities of the Toona sinensis Plant. Molecules, 29(3), 718. https://doi.org/10.3390/molecules29030718






