Early Diagnosis of Neurodegenerative Diseases: What Has Been Undertaken to Promote the Transition from PET to Fluorescence Tracers
Abstract
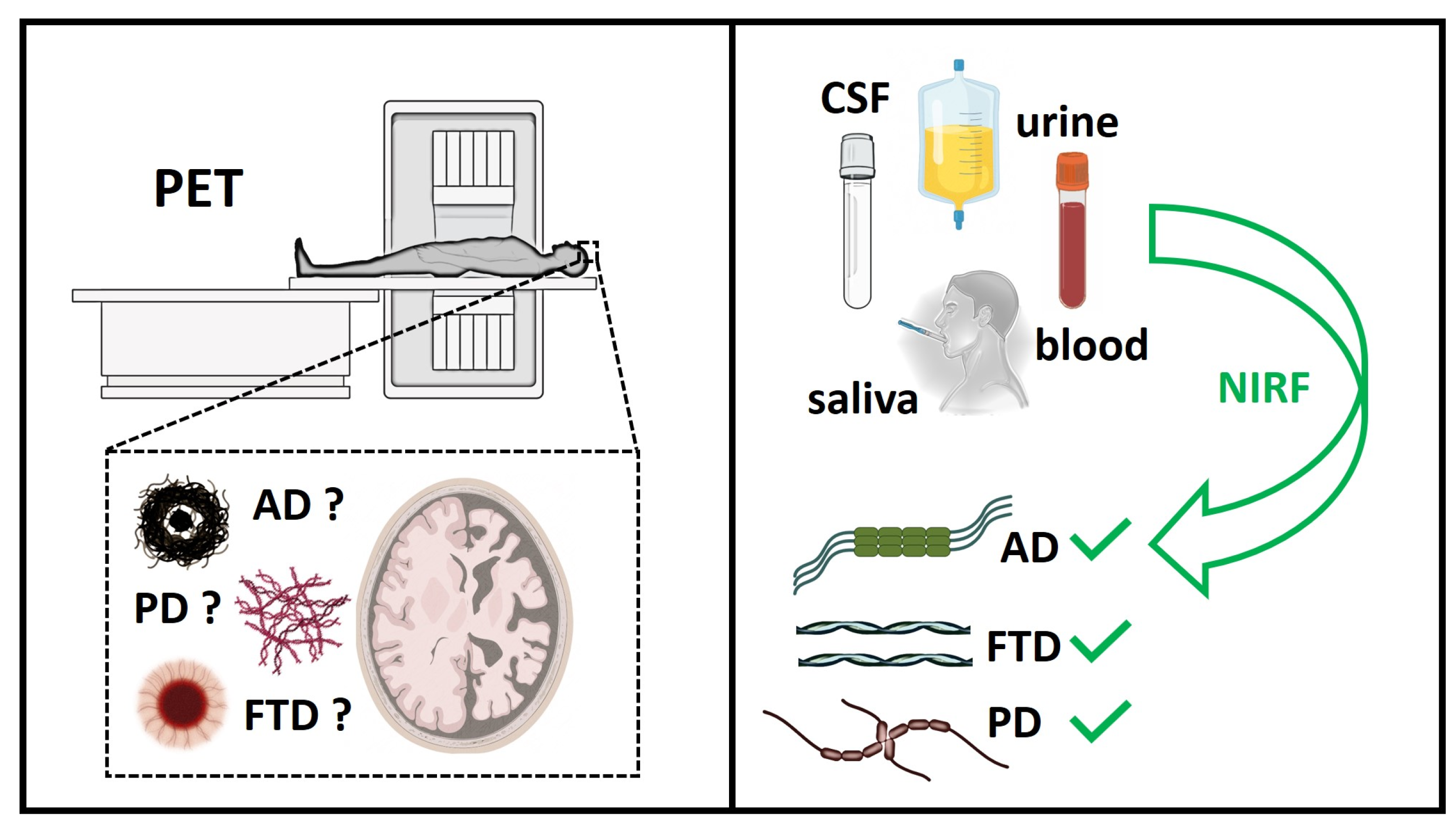
1. Introduction
2. Strategies for the Diagnosis of Pre-Symptomatic Neurodegenerative Diseases
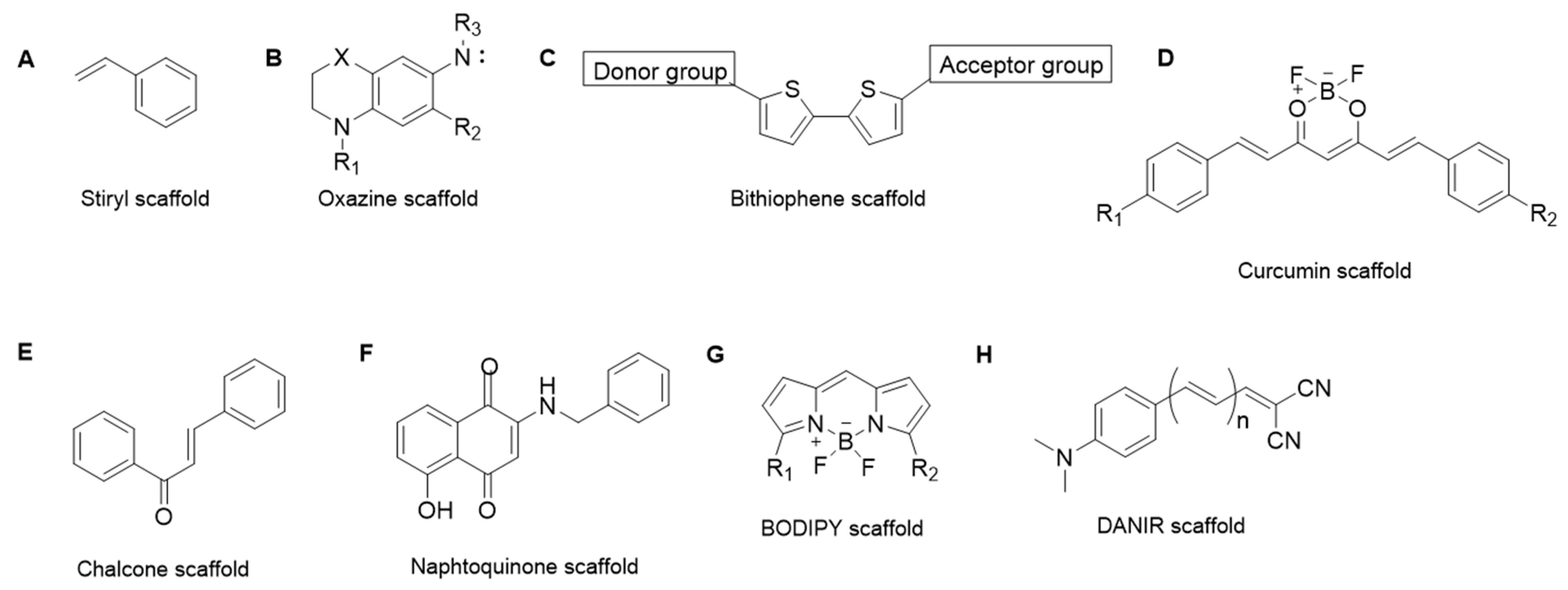
3. Approaches in Aβ1–42, Tau and αSyn Probing
3.1. Aβ1–42 Peptide Probes
3.2. Tau Probes
3.3. α-Synuclein Probes
3.3.1. Florescent Probes
3.3.2. Radiolabeled Probes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Key Figures. Available online: https://institutducerveau-icm.org/en/key-figures/ (accessed on 21 November 2022).
- Erkkinen, M.G.; Kim, M.-O.; Geschwind, M.D. Clinical Neurology and Epidemiology of the Major Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2018, 10, a033118. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Crowther, R.A.; Jakes, R.; Hasegawa, M.; Goedert, M. A-Synuclein in Filamentous Inclusions of Lewy Bodies from Parkinson’s Disease and Dementia with Lewy Bodies. Proc. Natl. Acad. Sci. USA 1998, 95, 6469–6473. [Google Scholar] [CrossRef]
- Karran, E.; De Strooper, B. The Amyloid Hypothesis in Alzheimer Disease: New Insights from New Therapeutics. Nat. Rev. Drug Discov. 2022, 21, 306–318. [Google Scholar] [CrossRef]
- Xu, M.; Ryan, P.; Rudrawar, S.; Quinn, R.J.; Zhang, H.; Mellick, G.D. Advances in the Development of Imaging Probes and Aggregation Inhibitors for Alpha-Synuclein. Acta Pharmacol. Sin. 2020, 41, 483–498. [Google Scholar] [CrossRef]
- Morris, E.; Chalkidou, A.; Hammers, A.; Peacock, J.; Summers, J.; Keevil, S. Diagnostic Accuracy of 18F Amyloid PET Tracers for the Diagnosis of Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 374–385. [Google Scholar] [CrossRef]
- Herholz, K.; Ebmeier, K. Clinical Amyloid Imaging in Alzheimer’s Disease. Lancet Neurol. 2011, 10, 667–670. [Google Scholar] [CrossRef]
- Stenh, C.; Englund, H.; Lord, A.; Johansson, A.-S.; Almeida, C.G.; Gellerfors, P.; Greengard, P.; Gouras, G.K.; Lannfelt, L.; Nilsson, L.N.G. Amyloid-β Oligomers Are Inefficiently Measured by Enzyme-Linked Immunosorbent Assay. Ann. Neurol. 2005, 58, 147–150. [Google Scholar] [CrossRef]
- Hong, M.C.; Kim, Y.K.; Choi, J.Y.; Yang, S.Q.; Rhee, H.; Ryu, Y.H.; Choi, T.H.; Cheon, G.J.; An, G.I.; Kim, H.Y.; et al. Synthesis and Evaluation of Stilbene Derivatives as a Potential Imaging Agent of Amyloid Plaques. Bioorganic Med. Chem. 2010, 18, 7724–7730. [Google Scholar] [CrossRef]
- Sehlin, D.; Söllvander, S.; Paulie, S.; Brundin, R.; Ingelsson, M.; Lannfelt, L.; Pettersson, F.E.; Englund, H. Interference from Heterophilic Antibodies in Amyloid-β Oligomer ELISAs. J. Alzheimer’s Dis. JAD 2010, 21, 1295–1301. [Google Scholar] [CrossRef]
- Mattsson, N.; Lönneborg, A.; Boccardi, M.; Blennow, K.; Hansson, O. Geneva Task Force for the Roadmap of Alzheimer’s Biomarkers Clinical Validity of Cerebrospinal Fluid Aβ42, Tau, and Phospho-Tau as Biomarkers for Alzheimer’s Disease in the Context of a Structured 5-Phase Development Framework. Neurobiol. Aging 2017, 52, 196–213. [Google Scholar] [CrossRef]
- Shahnawaz, M.; Mukherjee, A.; Pritzkow, S.; Mendez, N.; Rabadia, P.; Liu, X.; Hu, B.; Schmeichel, A.; Singer, W.; Wu, G.; et al. Discriminating α-Synuclein Strains in Parkinson’s Disease and Multiple System Atrophy. Nature 2020, 578, 273–277. [Google Scholar] [CrossRef]
- Schöll, M.; Maass, A.; Mattsson, N.; Ashton, N.J.; Blennow, K.; Zetterberg, H.; Jagust, W. Biomarkers for Tau Pathology. Mol. Cell. Neurosci. 2019, 97, 18–33. [Google Scholar] [CrossRef]
- Schraen-Maschke, S.; Sergeant, N.; Dhaenens, C.-M.; Bombois, S.; Deramecourt, V.; Caillet-Boudin, M.-L.; Pasquier, F.; Maurage, C.-A.; Sablonnière, B.; Vanmechelen, E.; et al. Tau as a Biomarker of Neurodegenerative Diseases. Biomark. Med. 2008, 2, 363–384. [Google Scholar] [CrossRef]
- Blennow, K.; Hampel, H. CSF Markers for Incipient Alzheimer’s Disease. Lancet Neurol. 2003, 2, 605–613. [Google Scholar] [CrossRef]
- Wallin, Å.K.; Blennow, K.; Zetterberg, H.; Londos, E.; Minthon, L.; Hansson, O. CSF Biomarkers Predict a More Malignant Outcome in Alzheimer Disease. Neurology 2010, 74, 1531–1537. [Google Scholar] [CrossRef]
- Buchhave, P.; Minthon, L.; Zetterberg, H.; Wallin, Å.K.; Blennow, K.; Hansson, O. Cerebrospinal Fluid Levels Ofβ-Amyloid 1–42, but Not of Tau, Are Fully Changed Already 5 to 10 Years Before the Onset of Alzheimer Dementia. Arch. General. Psychiatry 2012, 69, 98–106. [Google Scholar] [CrossRef]
- Luk, C.; Compta, Y.; Magdalinou, N.; Martí, M.J.; Hondhamuni, G.; Zetterberg, H.; Blennow, K.; Constantinescu, R.; Pijnenburg, Y.; Mollenhauer, B.; et al. Development and Assessment of Sensitive Immuno-PCR Assays for the Quantification of Cerebrospinal Fluid Three- and Four-Repeat Tau Isoforms in Tauopathies. J. Neurochem. 2012, 123, 396–405. [Google Scholar] [CrossRef]
- Barthélemy, N.R.; Fenaille, F.; Hirtz, C.; Sergeant, N.; Schraen-Maschke, S.; Vialaret, J.; Buée, L.; Gabelle, A.; Junot, C.; Lehmann, S.; et al. Tau Protein Quantification in Human Cerebrospinal Fluid by Targeted Mass Spectrometry at High Sequence Coverage Provides Insights into Its Primary Structure Heterogeneity. J. Proteome Res. 2016, 15, 667–676. [Google Scholar] [CrossRef]
- Ashton, N.J.; Hye, A.; Rajkumar, A.P.; Leuzy, A.; Snowden, S.; Suárez-Calvet, M.; Karikari, T.K.; Schöll, M.; La Joie, R.; Rabinovici, G.D.; et al. An Update on Blood-Based Biomarkers for Non-Alzheimer Neurodegenerative Disorders. Nat. Rev. Neurol. 2020, 16, 265–284. [Google Scholar] [CrossRef]
- Karikari, T.K.; Ashton, N.J.; Brinkmalm, G.; Brum, W.S.; Benedet, A.L.; Montoliu-Gaya, L.; Lantero-Rodriguez, J.; Pascoal, T.A.; Suárez-Calvet, M.; Rosa-Neto, P.; et al. Blood Phospho-Tau in Alzheimer Disease: Analysis, Interpretation, and Clinical Utility. Nat. Rev. Neurol. 2022, 18, 400–418. [Google Scholar] [CrossRef]
- Kac, P.R.; Gonzalez-Ortiz, F.; Simrén, J.; Dewit, N.; Vanmechelen, E.; Zetterberg, H.; Blennow, K.; Ashton, N.J.; Karikari, T.K. Diagnostic Value of Serum versus Plasma Phospho-Tau for Alzheimer’s Disease. Alzheimer’s Res. Ther. 2022, 14, 65. [Google Scholar] [CrossRef]
- Karikari, T.K.; Pascoal, T.A.; Ashton, N.J.; Janelidze, S.; Benedet, A.L.; Rodriguez, J.L.; Chamoun, M.; Savard, M.; Kang, M.S.; Therriault, J.; et al. Blood Phosphorylated Tau 181 as a Biomarker for Alzheimer’s Disease: A Diagnostic Performance and Prediction Modelling Study Using Data from Four Prospective Cohorts. Lancet Neurol. 2020, 19, 422–433. [Google Scholar] [CrossRef]
- Janelidze, S.; Mattsson, N.; Palmqvist, S.; Smith, R.; Beach, T.G.; Serrano, G.E.; Chai, X.; Proctor, N.K.; Eichenlaub, U.; Zetterberg, H.; et al. Plasma P-Tau181 in Alzheimer’s Disease: Relationship to Other Biomarkers, Differential Diagnosis, Neuropathology and Longitudinal Progression to Alzheimer’s Dementia. Nat. Med. 2020, 26, 379–386. [Google Scholar] [CrossRef]
- Mielke, M.M.; Hagen, C.E.; Xu, J.; Chai, X.; Vemuri, P.; Lowe, V.J.; Airey, D.C.; Knopman, D.S.; Roberts, R.O.; Machulda, M.M.; et al. Plasma Phospho-Tau181 Increases with Alzheimer’s Disease Clinical Severity and Is Associated with Tau-PET and Amyloid-PET. Alzheimer’s Dement. 2018, 14, 989–997. [Google Scholar] [CrossRef]
- Ashton, N.J.; Pascoal, T.A.; Karikari, T.K.; Benedet, A.L.; Lantero-Rodriguez, J.; Brinkmalm, G.; Snellman, A.; Schöll, M.; Troakes, C.; Hye, A.; et al. Plasma P-Tau231: A New Biomarker for Incipient Alzheimer’s Disease Pathology. Acta Neuropathol. 2021, 141, 709–724. [Google Scholar] [CrossRef]
- Thijssen, E.H.; La Joie, R.; Wolf, A.; Strom, A.; Wang, P.; Iaccarino, L.; Bourakova, V.; Cobigo, Y.; Heuer, H.; Spina, S.; et al. Diagnostic Value of Plasma Phosphorylated Tau181 in Alzheimer’s Disease and Frontotemporal Lobar Degeneration. Nat. Med. 2020, 26, 387–397. [Google Scholar] [CrossRef]
- Palmqvist, S.; Janelidze, S.; Quiroz, Y.T.; Zetterberg, H.; Lopera, F.; Stomrud, E.; Su, Y.; Chen, Y.; Serrano, G.E.; Leuzy, A.; et al. Discriminative Accuracy of Plasma Phospho-Tau217 for Alzheimer Disease vs Other Neurodegenerative Disorders. JAMA 2020, 324, 772–781. [Google Scholar] [CrossRef]
- Bayoumy, S.; Verberk, I.M.W.; den Dulk, B.; Hussainali, Z.; Zwan, M.; van der Flier, W.M.; Ashton, N.J.; Zetterberg, H.; Blennow, K.; Vanbrabant, J.; et al. Clinical and Analytical Comparison of Six Simoa Assays for Plasma P-Tau Isoforms P-Tau181, P-Tau217, and P-Tau231. Alzheimer’s Res. Ther. 2021, 13, 198. [Google Scholar] [CrossRef]
- Ossenkoppele, R.; van der Kant, R.; Hansson, O. Tau Biomarkers in Alzheimer’s Disease: Towards Implementation in Clinical Practice and Trials. Lancet Neurol. 2022, 21, 726–734. [Google Scholar] [CrossRef]
- Balhara, N.; Devi, M.; Balda, A.; Phour, M.; Giri, A. Urine; a New Promising Biological Fluid to Act as a Non-Invasive Biomarker for Different Human Diseases. URINE 2023, 5, 40–52. [Google Scholar] [CrossRef]
- Harpole, M.; Davis, J.; Espina, V. Current State of the Art for Enhancing Urine Biomarker Discovery. Expert. Rev. Proteom. 2016, 13, 609–626. [Google Scholar] [CrossRef]
- Kohlhase, K.; Frank, F.; Wilmes, C.; Koerbel, K.; Schaller-Paule, M.A.; Miles, M.; Betz, C.; Steinmetz, H.; Foerch, C. Brain-Specific Biomarkers in Urine as a Non-Invasive Approach to Monitor Neuronal and Glial Damage. Eur. J. Neurol. 2023, 30, 729–740. [Google Scholar] [CrossRef]
- Shi, M.; Sui, Y.-T.; Peskind, E.R.; Li, G.; Hwang, H.; Devic, I.; Ginghina, C.; Edgar, J.S.; Pan, C.; Goodlett, D.R.; et al. Salivary Tau Species Are Potential Biomarkers of Alzheimer’s Disease. J. Alzheimer’s Dis. 2011, 27, 299–305. [Google Scholar] [CrossRef]
- Marksteiner, J.; Defrancesco, M.; Humpel, C. Saliva Tau and Phospho-Tau-181 Measured by Lumipulse in Patients with Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 1014305. [Google Scholar] [CrossRef]
- Pekeles, H.; Qureshi, H.Y.; Paudel, H.K.; Schipper, H.M.; Gornistky, M.; Chertkow, H. Development and Validation of a Salivary Tau Biomarker in Alzheimer’s Disease. Alzheimer’s Dement. 2018, 11, 53–60. [Google Scholar] [CrossRef]
- Ashton, N.J.; Schöll, M.; Heurling, K.; Gkanatsiou, E.; Portelius, E.; Höglund, K.; Brinkmalm, G.; Hye, A.; Blennow, K.; Zetterberg, H. Update on Biomarkers for Amyloid Pathology in Alzheimer’s Disease. Biomark. Med. 2018, 12, 799–812. [Google Scholar] [CrossRef]
- Sabaei, M.; Rahimian, S.; Haj Mohamad Ebrahim Ketabforoush, A.; Rasoolijazi, H.; Zamani, B.; Hajiakhoundi, F.; Soleimani, M.; Shahidi, G.; Faramarzi, M. Salivary Levels of Disease-Related Biomarkers in the Early Stages of Parkinson’s and Alzheimer’s Disease: A Cross-Sectional Study. IBRO Neurosci. Rep. 2023, 14, 285–292. [Google Scholar] [CrossRef]
- Lau, H.-C.; Lee, I.-K.; Ko, P.-W.; Lee, H.-W.; Huh, J.-S.; Cho, W.-J.; Lim, J.-O. Non-Invasive Screening for Alzheimer’s Disease by Sensing Salivary Sugar Using Drosophila Cells Expressing Gustatory Receptor (Gr5a) Immobilized on an Extended Gate Ion-Sensitive Field-Effect Transistor (EG-ISFET) Biosensor. PLoS ONE 2015, 10, e0117810. [Google Scholar] [CrossRef]
- Ashton, N.J.; Ide, M.; Zetterberg, H.; Blennow, K. Salivary Biomarkers for Alzheimer’s Disease and Related Disorders. Neurol. Ther. 2019, 8, 83–94. [Google Scholar] [CrossRef]
- Dame, Z.T.; Aziat, F.; Mandal, R.; Krishnamurthy, R.; Bouatra, S.; Borzouie, S.; Guo, A.C.; Sajed, T.; Deng, L.; Lin, H.; et al. The Human Saliva Metabolome. Metabolomics 2015, 11, 1864–1883. [Google Scholar] [CrossRef]
- Król-Grzymała, A.; Sienkiewicz-Szłapka, E.; Fiedorowicz, E.; Rozmus, D.; Cieślińska, A.; Grzybowski, A. Tear Biomarkers in Alzheimer’s and Parkinson’s Diseases, and Multiple Sclerosis: Implications for Diagnosis (Systematic Review). Int. J. Mol. Sci. 2022, 23, 10123. [Google Scholar] [CrossRef]
- Gijs, M.; Ramakers, I.H.G.B.; Visser, P.J.; Verhey, F.R.J.; van de Waarenburg, M.P.H.; Schalkwijk, C.G.; Nuijts, R.M.M.A.; Webers, C.A.B. Association of Tear Fluid Amyloid and Tau Levels with Disease Severity and Neurodegeneration. Sci. Rep. 2021, 11, 22675. [Google Scholar] [CrossRef]
- Gharbiya, M.; Visioli, G.; Trebbastoni, A.; Albanese, G.M.; Colardo, M.; D’Antonio, F.; Segatto, M.; Lambiase, A. Beta-Amyloid Peptide in Tears: An Early Diagnostic Marker of Alzheimer’s Disease Correlated with Choroidal Thickness. Int. J. Mol. Sci. 2023, 24, 2590. [Google Scholar] [CrossRef]
- Ohm, T.G.; Braak, H. Olfactory Bulb Changes in Alzheimer’s Disease. Acta Neuropathol. 1987, 73, 365–369. [Google Scholar] [CrossRef]
- Attems, J.; Jellinger, K.A. Olfactory Tau Pathology in Alzheimer Disease and Mild Cognitive Impairment. Clin. Neuropathol. 2006, 25, 265–271. [Google Scholar]
- Passali, G.C.; Politi, L.; Crisanti, A.; Loglisci, M.; Anzivino, R.; Passali, D. Tau Protein Detection in Anosmic Alzheimer’s Disease Patient’s Nasal Secretions. Chemosens. Percept. 2015, 8, 201–206. [Google Scholar] [CrossRef]
- Pahrudin Arrozi, A.; Yanagisawa, D.; Kato, T.; Akatsu, H.; Hashizume, Y.; Kaneda, D.; Tooyama, I. Nasal Extracts from Patients with Alzheimer’s Disease Induce Tau Aggregates in a Cellular Model of Tau Propagation. J. Alzheimer’s Dis. Rep. 2021, 5, 263–274. [Google Scholar] [CrossRef]
- Lee, D.; Kim, S.M.; Kim, H.Y.; Kim, Y. Fluorescence Chemicals To Detect Insoluble and Soluble Amyloid-β Aggregates. ACS Chem. Neurosci. 2019, 10, 2647–2657. [Google Scholar] [CrossRef]
- Zhou, Y.; Hua, J.; Ding, D.; Tang, Y. Interrogating Amyloid Aggregation with Aggregation-Induced Emission Fluorescence Probes. Biomaterials 2022, 286, 121605. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Gao, F.; Hu, M.; Wang, X. Recent Developments in the Chemical Biology of Amyloid-β Oligomer Targeting. Org. Biomol. Chem. 2023, 21, 4540–4552. [Google Scholar] [CrossRef]
- Li, Q.; Lee, J.-S.; Ha, C.; Park, C.B.; Yang, G.; Gan, W.B.; Chang, Y.-T. Solid-Phase Synthesis of Styryl Dyes and Their Application as Amyloid Sensors. Angew. Chem. Int. Ed. Engl. 2004, 43, 6331–6335. [Google Scholar] [CrossRef]
- Li, Q.; Min, J.; Ahn, Y.-H.; Namm, J.; Kim, E.M.; Lui, R.; Kim, H.Y.; Ji, Y.; Wu, H.; Wisniewski, T.; et al. Styryl-Based Compounds as Potential in Vivo Imaging Agents for Beta-Amyloid Plaques. Chembiochem 2007, 8, 1679–1687. [Google Scholar] [CrossRef]
- Staderini, M.; Aulić, S.; Bartolini, M.; Tran, H.N.A.; González-Ruiz, V.; Pérez, D.I.; Cabezas, N.; Martínez, A.; Martín, M.A.; Andrisano, V.; et al. A Fluorescent Styrylquinoline with Combined Therapeutic and Diagnostic Activities against Alzheimer’s and Prion Diseases. ACS Med. Chem. Lett. 2013, 4, 225–229. [Google Scholar] [CrossRef]
- Lee, C.-J.; Sheu, C.-N.; Tsai, C.-C.; Wu, Z.-Z.; Lin, W. Direct β-Acylation of 2-Arylidene-1,3-Indandiones with Acyl Chlorides Catalyzed by Organophosphanes. Chem. Commun. 2014, 50, 5304–5306. [Google Scholar] [CrossRef]
- Hintersteiner, M.; Enz, A.; Frey, P.; Jaton, A.-L.; Kinzy, W.; Kneuer, R.; Neumann, U.; Rudin, M.; Staufenbiel, M.; Stoeckli, M.; et al. In Vivo Detection of Amyloid-Beta Deposits by near-Infrared Imaging Using an Oxazine-Derivative Probe. Nat. Biotechnol. 2005, 23, 577–583. [Google Scholar] [CrossRef]
- Nesterov, E.E.; Skoch, J.; Hyman, B.T.; Klunk, W.E.; Bacskai, B.J.; Swager, T.M. In Vivo Optical Imaging of Amyloid Aggregates in Brain: Design of Fluorescent Markers. Angew. Chem. Int. Ed. 2005, 44, 5452–5456. [Google Scholar] [CrossRef]
- Raymond, S.B.; Skoch, J.; Hills, I.D.; Nesterov, E.E.; Swager, T.M.; Bacskai, B.J. Smart Optical Probes for Near-Infrared Fluorescence Imaging of Alzheimer’s Disease Pathology. Eur. J. Nucl. Med. Mol. Imaging 2008, 35 (Suppl. 1), S93–S98. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, Y.; Li, Z.; Tian, X.; Sun, H.; Liu, H.; Moore, A.; Ran, C. Design and Synthesis of Curcumin Analogues for in Vivo Fluorescence Imaging and Inhibiting Copper-Induced Cross-Linking of Amyloid Beta Species in Alzheimer’s Disease. J. Am. Chem. Soc. 2013, 135, 16397–16409. [Google Scholar] [CrossRef]
- Liu, K.; Guo, T.L.; Chojnacki, J.; Lee, H.-G.; Wang, X.; Siedlak, S.L.; Rao, W.; Zhu, X.; Zhang, S. Bivalent Ligand Containing Curcumin and Cholesterol as Fluorescence Probe for Aβ Plaques in Alzheimer’s Disease. ACS Chem. Neurosci. 2012, 3, 141–146. [Google Scholar] [CrossRef]
- Ran, C.; Xu, X.; Raymond, S.B.; Ferrara, B.J.; Neal, K.; Bacskai, B.J.; Medarova, Z.; Moore, A. Design, Synthesis, and Testing of Difluoroboron-Derivatized Curcumins as Near-Infrared Probes for in Vivo Detection of Amyloid-β Deposits. J. Am. Chem. Soc. 2009, 131, 15257–15261. [Google Scholar] [CrossRef]
- Ono, M.; Ishikawa, M.; Kimura, H.; Hayashi, S.; Matsumura, K.; Watanabe, H.; Shimizu, Y.; Cheng, Y.; Cui, M.; Kawashima, H.; et al. Development of Dual Functional SPECT/Fluorescent Probes for Imaging Cerebral Beta-Amyloid Plaques. Bioorganic Med. Chem. Lett. 2010, 20, 3885–3888. [Google Scholar] [CrossRef]
- Jung, S.-J.; Park, S.-H.; Lee, E.J.; Park, J.H.; Kong, Y.B.; Rho, J.K.; Hur, M.G.; Yang, S.D.; Park, Y.D. Development of Fluorescent Probes That Bind and Stain Amyloid Plaques in Alzheimer’s Disease. Arch. Pharmacal Res. 2015, 38, 1992–1998. [Google Scholar] [CrossRef]
- Neo Shin, N.; Jeon, H.; Jung, Y.; Baek, S.; Lee, S.; Yoo, H.C.; Bae, G.H.; Park, K.; Yang, S.-H.; Han, J.M.; et al. Fluorescent 1,4-Naphthoquinones To Visualize Diffuse and Dense-Core Amyloid Plaques in APP/PS1 Transgenic Mouse Brains. ACS Chem. Neurosci. 2019, 10, 3031–3044. [Google Scholar] [CrossRef]
- Ono, M.; Watanabe, H.; Kimura, H.; Saji, H. BODIPY-Based Molecular Probe for Imaging of Cerebral β-Amyloid Plaques. ACS Chem. Neurosci. 2012, 3, 319–324. [Google Scholar] [CrossRef]
- Ulrich, G.; Ziessel, R.; Harriman, A. The Chemistry of Fluorescent Bodipy Dyes: Versatility Unsurpassed. Angew. Chem. Int. Ed. 2008, 47, 1184–1201. [Google Scholar] [CrossRef] [PubMed]
- Boens, N.; Leen, V.; Dehaen, W. Fluorescent Indicators Based on BODIPY. Chem. Soc. Rev. 2012, 41, 1130–1172. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.W.; Alonso, A.; Brown, C.M.; Dzyuba, S.V. Triazole-Containing BODIPY Dyes as Novel Fluorescent Probes for Soluble Oligomers of Amyloid Abeta1–42 Peptide. Biochem. Biophys. Res. Commun. 2010, 391, 1455–1458. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Zhang, J.; Peng, C.; Xiang, H.; Chen, J.; Peng, C.; Zhu, W.; Huang, R.; Zhang, H.; Hu, Y. Fluorescent Imaging of β-Amyloid Using BODIPY Based Near-Infrared Off-On Fluorescent Probe. Bioconjugate Chem. 2018, 29, 3459–3466. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Cui, M.; Zhao, L.; Tu, P.; Zhou, K.; Dai, J.; Liu, B. Highly Sensitive Near-Infrared Fluorophores for in Vivo Detection of Amyloid-β Plaques in Alzheimer’s Disease. J. Med. Chem. 2015, 58, 6972–6983. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Tu, P.; Zhao, L.; Dai, J.; Liu, B.; Cui, M. Amyloid-β Deposits Target Efficient Near-Infrared Fluorescent Probes: Synthesis, in Vitro Evaluation, and in Vivo Imaging. Anal. Chem. 2016, 88, 1944–1950. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Ono, M.; Watanabe, H.; Kimura, H.; Liu, B.; Saji, H. Smart Near-Infrared Fluorescence Probes with Donor–Acceptor Structure for in Vivo Detection of β-Amyloid Deposits. J. Am. Chem. Soc. 2014, 136, 3388–3394. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Moon, H.; Baik, S.H.; Singha, S.; Jun, Y.W.; Wang, T.; Kim, K.H.; Park, B.S.; Jung, J.; Mook-Jung, I.; et al. Two-Photon Absorbing Dyes with Minimal Autofluorescence in Tissue Imaging: Application to in Vivo Imaging of Amyloid-β Plaques with a Negligible Background Signal. J. Am. Chem. Soc. 2015, 137, 6781–6789. [Google Scholar] [CrossRef] [PubMed]
- Nicole, O.; Hadzibegovic, S.; Gajda, J.; Bontempi, B.; Bem, T.; Meyrand, P. Soluble Amyloid Beta Oligomers Block the Learning-Induced Increase in Hippocampal Sharp Wave-Ripple Rate and Impair Spatial Memory Formation. Sci. Rep. 2016, 6, 22728. [Google Scholar] [CrossRef] [PubMed]
- Larson, M.E.; Lesné, S.E. Soluble Aβ Oligomer Production and Toxicity. J. Neurochem. 2012, 120 (Suppl. 1), 125–139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tian, Y.; Zhang, C.; Tian, X.; Ross, A.W.; Moir, R.D.; Sun, H.; Tanzi, R.E.; Moore, A.; Ran, C. Near-Infrared Fluorescence Molecular Imaging of Amyloid Beta Species and Monitoring Therapy in Animal Models of Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2015, 112, 9734–9739. [Google Scholar] [CrossRef] [PubMed]
- Teoh, C.L.; Su, D.; Sahu, S.; Yun, S.-W.; Drummond, E.; Prelli, F.; Lim, S.; Cho, S.; Ham, S.; Wisniewski, T.; et al. Chemical Fluorescent Probe for Detection of Aβ Oligomers. J. Am. Chem. Soc. 2015, 137, 13503–13509. [Google Scholar] [CrossRef] [PubMed]
- Jameson, L.P.; Dzyuba, S.V. Aza-BODIPY: Improved Synthesis and Interaction with Soluble Aβ1–42 Oligomers. Bioorganic Med. Chem. Lett. 2013, 23, 1732–1735. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, J.; Zeng, F.; Li, X.; Ran, C.; Xu, Y.; Li, Y. Highly Specific Detection of Aβ Oligomers in Early Alzheimer’s Disease by a near-Infrared Fluorescent Probe with a “V-Shaped” Spatial Conformation. Chem. Commun. 2020, 56, 583–586. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Li, Y.; Chen, X.; Zhang, W.; Zhao, Y.; Liu, G.; Pan, J. Detection of Aβ Oligomers in Early Alzheimer’s Disease Diagnose by in Vivo NIR-II Fluorescence Imaging. Sens. Actuators B Chem. 2022, 358, 131481. [Google Scholar] [CrossRef]
- Li, Y.; Xu, D.; Sun, A.; Ho, S.-L.; Poon, C.-Y.; Chan, H.-N.; Ng, O.T.W.; Yung, K.K.L.; Yan, H.; Li, H.-W.; et al. Fluoro-Substituted Cyanine for Reliable in Vivo Labelling of Amyloid-β Oligomers and Neuroprotection against Amyloid-β Induced Toxicity. Chem. Sci. 2017, 8, 8279–8284. [Google Scholar] [CrossRef]
- Weingarten, M.D.; Lockwood, A.H.; Hwo, S.Y.; Kirschner, M.W. A Protein Factor Essential for Microtubule Assembly. Proc. Natl. Acad. Sci. USA 1975, 72, 1858–1862. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Wischik, C.M.; Crowther, R.A.; Walker, J.E.; Klug, A. Cloning and Sequencing of the CDNA Encoding a Core Protein of the Paired Helical Filament of Alzheimer Disease: Identification as the Microtubule-Associated Protein Tau. Proc. Natl. Acad. Sci. USA 1988, 85, 4051–4055. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.; Win, K.M.; Busciglio, J. Tau Isoform Expression and Regulation in Human Cortical Neurons. FASEB J. 2008, 22, 2357–2367. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Noble, W.; Hanger, D.P. Roles of Tau Protein in Health and Disease. Acta Neuropathol. 2017, 133, 665–704. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.K.; Honea, R.A.; Vidoni, E.D.; Swerdlow, R.H.; Burns, J.M. Is Alzheimer’s Disease a Systemic Disease? Biochim. Biophys. Acta 2014, 1842, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Tabeshmehr, P.; Eftekharpour, E. Tau; One Protein, So Many Diseases. Biology 2023, 12, 244. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-Z.; Liu, F. Microtubule-Associated Protein Tau in Development, Degeneration and Protection of Neurons. Prog. Neurobiol. 2008, 85, 148–175. [Google Scholar] [CrossRef]
- Kolarova, M.; García-Sierra, F.; Bartos, A.; Ricny, J.; Ripova, D. Structure and Pathology of Tau Protein in Alzheimer Disease. Int. J. Alzheimer’s Dis. 2012, 2012, 731526. [Google Scholar] [CrossRef]
- Crespo-Biel, N.; Theunis, C.; Van Leuven, F. Protein Tau: Prime Cause of Synaptic and Neuronal Degeneration in Alzheimer’s Disease. Int. J. Alzheimer’s Dis. 2012, 2012, 251426. [Google Scholar] [CrossRef]
- Rajasekhar, K.; Govindaraju, T. Current Progress, Challenges and Future Prospects of Diagnostic and Therapeutic Interventions in Alzheimer’s Disease. RSC Adv. 2018, 8, 23780–23804. [Google Scholar] [CrossRef] [PubMed]
- Soeda, Y.; Takashima, A. New Insights Into Drug Discovery Targeting Tau Protein. Front. Mol. Neurosci. 2020, 13, 590896. [Google Scholar] [CrossRef] [PubMed]
- Pinzi, L.; Tinivella, A.; Rastelli, G. Chemoinformatics Analyses of Tau Ligands Reveal Key Molecular Requirements for the Identification of Potential Drug Candidates against Tauopathies. Molecules 2021, 26, 5039. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, J.; Smeralda, W.; Jouanne, M.; Sopkova-de Oliveira Santos, J.; Catto, M.; Voisin-Chiret, A.S. Tau Protein Aggregation: Key Features to Improve Drug Discovery Screening. Drug Discov. Today 2022, 27, 1284–1297. [Google Scholar] [CrossRef]
- VandeVrede, L.; Boxer, A.L.; Polydoro, M. Targeting Tau: Clinical Trials and Novel Therapeutic Approaches. Neurosci. Lett. 2020, 731, 134919. [Google Scholar] [CrossRef]
- Robbins, M. Therapies for Tau-Associated Neurodegenerative Disorders: Targeting Molecules, Synapses, and Cells. Neural Regen. Res. 2023, 18, 2633–2637. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Dong, H.; Bian, J.; Li, D.; Liu, C. Targeting Amyloid Proteins for Clinical Diagnosis of Neurodegenerative Diseases. Fundam. Res. 2023, 3, 505–519. [Google Scholar] [CrossRef]
- Pinzi, L.; Bisi, N.; Sorbi, C.; Franchini, S.; Tonali, N.; Rastelli, G. Insights into the Structural Conformations of the Tau Protein in Different Aggregation Status. Molecules 2023, 28, 4544. [Google Scholar] [CrossRef]
- Wesseling, H.; Mair, W.; Kumar, M.; Schlaffner, C.N.; Tang, S.; Beerepoot, P.; Fatou, B.; Guise, A.J.; Cheng, L.; Takeda, S.; et al. Tau PTM Profiles Identify Patient Heterogeneity and Stages of Alzheimer’s Disease. Cell 2020, 183, 1699–1713.e13. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, Y.; Ma, L.; Wei, Y.; Li, H. Possible Mechanisms of Tau Spread and Toxicity in Alzheimer’s Disease. Front. Cell Dev. Biol. 2021, 9, 707268. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, K.-M.; Yang, L.; Dong, Q.; Yu, J.-T. Tauopathies: New Perspectives and Challenges. Mol. Neurodegener. 2022, 17, 28. [Google Scholar] [CrossRef]
- Chang, H.-Y.; Sang, T.-K.; Chiang, A.-S. Untangling the Tauopathy for Alzheimer’s Disease and Parkinsonism. J. Biomed. Sci. 2018, 25, 54. [Google Scholar] [CrossRef]
- Wang, Y.T.; Edison, P. Tau Imaging in Neurodegenerative Diseases Using Positron Emission Tomography. Curr. Neurol. Neurosci. Rep. 2019, 19, 45. [Google Scholar] [CrossRef]
- Villemagne, V.L.; Fodero-Tavoletti, M.T.; Masters, C.L.; Rowe, C.C. Tau Imaging: Early Progress and Future Directions. Lancet Neurol. 2015, 14, 114–124. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Goto, T.K.; Leung, W.K. The Changing Landscape of Neuroscience Research, 2006–2015: A Bibliometric Study. Front. Neurosci. 2017, 11, 120. [Google Scholar] [CrossRef]
- James, O.G.; Doraiswamy, P.M.; Borges-Neto, S. PET Imaging of Tau Pathology in Alzheimer’s Disease and Tauopathies. Front. Neurol. 2015, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Kepe, V.; Barrio, J.R.; Small, G.W. The Merits of FDDNP-PET Imaging in Alzheimer’s Disease. J. Alzheimer’s Dis. 2011, 26 (Suppl. 3), 135–145. [Google Scholar] [CrossRef] [PubMed]
- Wood, H. Alzheimer Disease: [11C]PBB3--a New PET Ligand That Identifies Tau Pathology in the Brains of Patients with AD. Nat. Rev. Neurol. 2013, 9, 599. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, M.; Shimada, H.; Suhara, T.; Shinotoh, H.; Ji, B.; Maeda, J.; Zhang, M.-R.; Trojanowski, J.Q.; Lee, V.M.-Y.; Ono, M.; et al. Imaging of Tau Pathology in a Tauopathy Mouse Model and in Alzheimer Patients Compared to Normal Controls. Neuron 2013, 79, 1094–1108. [Google Scholar] [CrossRef] [PubMed]
- Okamura, N.; Suemoto, T.; Furumoto, S.; Suzuki, M.; Shimadzu, H.; Akatsu, H.; Yamamoto, T.; Fujiwara, H.; Nemoto, M.; Maruyama, M.; et al. Quinoline and Benzimidazole Derivatives: Candidate Probes for in Vivo Imaging of Tau Pathology in Alzheimer’s Disease. J. Neurosci. 2005, 25, 10857–10862. [Google Scholar] [CrossRef] [PubMed]
- Fodero-Tavoletti, M.T.; Okamura, N.; Furumoto, S.; Mulligan, R.S.; Connor, A.R.; McLean, C.A.; Cao, D.; Rigopoulos, A.; Cartwright, G.A.; O’Keefe, G.; et al. 18F-THK523: A Novel in Vivo Tau Imaging Ligand for Alzheimer’s Disease. Brain 2011, 134, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Harada, R.; Okamura, N.; Furumoto, S.; Tago, T.; Maruyama, M.; Higuchi, M.; Yoshikawa, T.; Arai, H.; Iwata, R.; Kudo, Y.; et al. Comparison of the Binding Characteristics of [18F]THK-523 and Other Amyloid Imaging Tracers to Alzheimer’s Disease Pathology. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Okamura, N.; Furumoto, S.; Harada, R.; Tago, T.; Yoshikawa, T.; Fodero-Tavoletti, M.; Mulligan, R.S.; Villemagne, V.L.; Akatsu, H.; Yamamoto, T.; et al. Novel 18F-Labeled Arylquinoline Derivatives for Noninvasive Imaging of Tau Pathology in Alzheimer Disease. J. Nucl. Med. 2013, 54, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Villemagne, V.L.; Furumoto, S.; Fodero-Tavoletti, M.T.; Mulligan, R.S.; Hodges, J.; Harada, R.; Yates, P.; Piguet, O.; Pejoska, S.; Doré, V.; et al. In Vivo Evaluation of a Novel Tau Imaging Tracer for Alzheimer’s Disease. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Okamura, N.; Furumoto, S.; Fodero-Tavoletti, M.T.; Mulligan, R.S.; Harada, R.; Yates, P.; Pejoska, S.; Kudo, Y.; Masters, C.L.; Yanai, K.; et al. Non-Invasive Assessment of Alzheimer’s Disease Neurofibrillary Pathology Using 18F-THK5105 PET. Brain 2014, 137, 1762–1771. [Google Scholar] [CrossRef] [PubMed]
- Harada, R.; Okamura, N.; Furumoto, S.; Furukawa, K.; Ishiki, A.; Tomita, N.; Hiraoka, K.; Watanuki, S.; Shidahara, M.; Miyake, M.; et al. [18F]THK-5117 PET for Assessing Neurofibrillary Pathology in Alzheimer’s Disease. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Harada, R.; Okamura, N.; Furumoto, S.; Furukawa, K.; Ishiki, A.; Tomita, N.; Tago, T.; Hiraoka, K.; Watanuki, S.; Shidahara, M.; et al. 18F-THK5351: A Novel PET Radiotracer for Imaging Neurofibrillary Pathology in Alzheimer Disease. J. Nucl. Med. 2016, 57, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Commissioner, O. Of the FDA Approves First Drug to Image Tau Pathology in Patients Being Evaluated for Alzheimer’s Disease. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-image-tau-pathology-patients-being-evaluated-alzheimers-disease (accessed on 25 November 2023).
- Barthel, H. First Tau PET Tracer Approved: Toward Accurate In Vivo Diagnosis of Alzheimer Disease. J. Nucl. Med. 2020, 61, 1409–1410. [Google Scholar] [CrossRef]
- Tian, M.; Civelek, A.C.; Carrio, I.; Watanabe, Y.; Kang, K.W.; Murakami, K.; Garibotto, V.; Prior, J.O.; Barthel, H.; Zhou, R.; et al. International Consensus on the Use of Tau PET Imaging Agent 18F-Flortaucipir in Alzheimer’s Disease. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 895–904. [Google Scholar] [CrossRef]
- Lowe, V.J.; Lundt, E.S.; Albertson, S.M.; Min, H.-K.; Fang, P.; Przybelski, S.A.; Senjem, M.L.; Schwarz, C.G.; Kantarci, K.; Boeve, B.; et al. Tau-Positron Emission Tomography Correlates with Neuropathology Findings. Alzheimer’s Dement. 2020, 16, 561–571. [Google Scholar] [CrossRef]
- Xia, C.-F.; Arteaga, J.; Chen, G.; Gangadharmath, U.; Gomez, L.F.; Kasi, D.; Lam, C.; Liang, Q.; Liu, C.; Mocharla, V.P.; et al. [(18)F]T807, a Novel Tau Positron Emission Tomography Imaging Agent for Alzheimer’s Disease. Alzheimer’s Dement. 2013, 9, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Marquié, M.; Normandin, M.D.; Vanderburg, C.R.; Costantino, I.M.; Bien, E.A.; Rycyna, L.G.; Klunk, W.E.; Mathis, C.A.; Ikonomovic, M.D.; Debnath, M.L.; et al. Validating Novel Tau Positron Emission Tomography Tracer [F-18]-AV-1451 (T807) on Postmortem Brain Tissue. Ann. Neurol. 2015, 78, 787–800. [Google Scholar] [CrossRef]
- Fleisher, A.S.; Pontecorvo, M.J.; Devous, M.D.; Lu, M.; Arora, A.K.; Truocchio, S.P.; Aldea, P.; Flitter, M.; Locascio, T.; Devine, M.; et al. Positron Emission Tomography Imaging With [18F]Flortaucipir and Postmortem Assessment of Alzheimer Disease Neuropathologic Changes. JAMA Neurol. 2020, 77, 829–839. [Google Scholar] [CrossRef]
- Vermeiren, C.; Motte, P.; Viot, D.; Mairet-Coello, G.; Courade, J.-P.; Citron, M.; Mercier, J.; Hannestad, J.; Gillard, M. The Tau Positron-Emission Tomography Tracer AV-1451 Binds with Similar Affinities to Tau Fibrils and Monoamine Oxidases. Mov. Disord. 2018, 33, 273–281. [Google Scholar] [CrossRef]
- Cassinelli Petersen, G.; Roytman, M.; Chiang, G.C.; Li, Y.; Gordon, M.L.; Franceschi, A.M. Overview of Tau PET Molecular Imaging. Curr. Opin. Neurol. 2022, 35, 230–239. [Google Scholar] [CrossRef]
- Brendel, M.; Schönecker, S.; Höglinger, G.; Lindner, S.; Havla, J.; Blautzik, J.; Sauerbeck, J.; Rohrer, G.; Zach, C.; Vettermann, F.; et al. [18F]-THK5351 PET Correlates with Topology and Symptom Severity in Progressive Supranuclear Palsy. Front. Aging Neurosci. 2018, 9, 440. [Google Scholar] [CrossRef]
- Coakeley, S.; Cho, S.S.; Koshimori, Y.; Rusjan, P.; Harris, M.; Ghadery, C.; Kim, J.; Lang, A.E.; Wilson, A.; Houle, S.; et al. Positron Emission Tomography Imaging of Tau Pathology in Progressive Supranuclear Palsy. J. Cereb. Blood Flow. Metab. 2017, 37, 3150–3160. [Google Scholar] [CrossRef]
- Leuzy, A.; Chiotis, K.; Lemoine, L.; Gillberg, P.-G.; Almkvist, O.; Rodriguez-Vieitez, E.; Nordberg, A. Tau PET Imaging in Neurodegenerative Tauopathies-Still a Challenge. Mol. Psychiatry 2019, 24, 1112–1134. [Google Scholar] [CrossRef] [PubMed]
- Wolters, E.E.; Dodich, A.; Boccardi, M.; Corre, J.; Drzezga, A.; Hansson, O.; Nordberg, A.; Frisoni, G.B.; Garibotto, V.; Ossenkoppele, R. Clinical Validity of Increased Cortical Uptake of [18F]Flortaucipir on PET as a Biomarker for Alzheimer’s Disease in the Context of a Structured 5-Phase Biomarker Development Framework. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2097–2109. [Google Scholar] [CrossRef] [PubMed]
- Harada, R.; Lerdsirisuk, P.; Shimizu, Y.; Yokoyama, Y.; Du, Y.; Kudo, K.; Ezura, M.; Ishikawa, Y.; Iwata, R.; Shidahara, M.; et al. Preclinical Characterization of the Tau PET Tracer [18F]SNFT-1: Comparison of Tau PET Tracers. J. Nucl. Med. 2023, 64, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Malarte, M.-L.; Gillberg, P.-G.; Kumar, A.; Bogdanovic, N.; Lemoine, L.; Nordberg, A. Discriminative Binding of Tau PET Tracers PI2620, MK6240 and RO948 in Alzheimer’s Disease, Corticobasal Degeneration and Progressive Supranuclear Palsy Brains. Mol. Psychiatry 2023, 28, 1272–1283. [Google Scholar] [CrossRef] [PubMed]
- Pascoal, T.A.; Mathotaarachchi, S.; Shin, M.; Benedet, A.L.; Mohades, S.; Wang, S.; Beaudry, T.; Kang, M.S.; Soucy, J.; Labbe, A.; et al. Synergistic Interaction between Amyloid and Tau Predicts the Progression to Dementia. Alzheimer’s Dement. 2017, 13, 644–653. [Google Scholar] [CrossRef]
- Mueller, A.; Bullich, S.; Barret, O.; Madonia, J.; Berndt, M.; Papin, C.; Perrotin, A.; Koglin, N.; Kroth, H.; Pfeifer, A.; et al. Tau PET Imaging with 18F-PI-2620 in Patients with Alzheimer Disease and Healthy Controls: A First-in-Humans Study. J. Nucl. Med. 2020, 61, 911–919. [Google Scholar] [CrossRef]
- Leuzy, A.; Smith, R.; Ossenkoppele, R.; Santillo, A.; Borroni, E.; Klein, G.; Ohlsson, T.; Jögi, J.; Palmqvist, S.; Mattsson-Carlgren, N.; et al. Diagnostic Performance of RO948 F 18 Tau Positron Emission Tomography in the Differentiation of Alzheimer Disease From Other Neurodegenerative Disorders. JAMA Neurol. 2020, 77, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Rowe, C.C.; Doré, V.; Krishnadas, N.; Burnham, S.; Lamb, F.; Mulligan, R.; Bozinovski, S.; Laws, S.; Tyrell, R.; Huang, K.; et al. Tau Imaging with 18F-MK6240 across the Alzheimer’s Disease Spectrum. medRxiv 2022. medRxiv:2022.02.13.22270894. [Google Scholar]
- Xu, X.; Ruan, W.; Liu, F.; Gai, Y.; Liu, Q.; Su, Y.; Liang, Z.; Sun, X.; Lan, X. 18F-APN-1607 Tau Positron Emission Tomography Imaging for Evaluating Disease Progression in Alzheimer’s Disease. Front. Aging Neurosci. 2022, 13, 789054. [Google Scholar] [CrossRef] [PubMed]
- Merz, G.E.; Chalkley, M.J.; Tan, S.K.; Tse, E.; Lee, J.; Prusiner, S.B.; Paras, N.A.; DeGrado, W.F.; Southworth, D.R. Stacked Binding of a PET Ligand to Alzheimer’s Tau Paired Helical Filaments. Nat. Commun. 2023, 14, 3048. [Google Scholar] [CrossRef] [PubMed]
- Seidler, P.M.; Murray, K.A.; Boyer, D.R.; Ge, P.; Sawaya, M.R.; Hu, C.J.; Cheng, X.; Abskharon, R.; Pan, H.; DeTure, M.A.; et al. Structure-Based Discovery of Small Molecules That Disaggregate Alzheimer’s Disease Tissue Derived Tau Fibrils in Vitro. Nat. Commun. 2022, 13, 5451. [Google Scholar] [CrossRef] [PubMed]
- Leuzy, A.; Smith, R.; Cullen, N.C.; Strandberg, O.; Vogel, J.W.; Binette, A.P.; Borroni, E.; Janelidze, S.; Ohlsson, T.; Jögi, J.; et al. Biomarker-Based Prediction of Longitudinal Tau Positron Emission Tomography in Alzheimer Disease. JAMA Neurol. 2022, 79, 149–158. [Google Scholar] [CrossRef]
- Rojo, L.E.; Alzate-Morales, J.; Saavedra, I.N.; Davies, P.; Maccioni, R.B. Selective Interaction of Lansoprazole and Astemizole with Tau Polymers: Potential New Clinical Use in Diagnosis of Alzheimer’s Disease. J. Alzheimer’s Dis. 2010, 19, 573–589. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.K.; Shepelytskyi, Y.; Grynko, V.; Albert, M.S. Molecular Imaging of Fluorinated Probes for Tau Protein and Amyloid-β Detection. Molecules 2020, 25, 3413. [Google Scholar] [CrossRef]
- Ni, R. Magnetic Resonance Imaging in Tauopathy Animal Models. Front. Aging Neurosci. 2022, 13, 791679. [Google Scholar] [CrossRef] [PubMed]
- Hane, F.T.; Robinson, M.; Lee, B.Y.; Bai, O.; Leonenko, Z.; Albert, M.S. Recent Progress in Alzheimer’s Disease Research, Part 3: Diagnosis and Treatment. J. Alzheimer’s Dis. 2017, 57, 645–665. [Google Scholar] [CrossRef] [PubMed]
- Reitz, C.; Mayeux, R. Alzheimer Disease: Epidemiology, Diagnostic Criteria, Risk Factors and Biomarkers. Biochem. Pharmacol. 2014, 88, 640–651. [Google Scholar] [CrossRef] [PubMed]
- Frisoni, G.B.; Fox, N.C.; Jack, C.R.; Scheltens, P.; Thompson, P.M. The Clinical Use of Structural MRI in Alzheimer Disease. Nat. Rev. Neurol. 2010, 6, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, M.; Iwata, N.; Matsuba, Y.; Sato, K.; Sasamoto, K.; Saido, T.C. 19F and 1H MRI Detection of Amyloid β Plaques In Vivo. Nat. Neurosci. 2005, 8, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Amatsubo, T.; Yanagisawa, D.; Morikawa, S.; Taguchi, H.; Tooyama, I. Amyloid Imaging Using High-Field Magnetic Resonance. Magn. Reson. Med. Sci. 2010, 9, 95–99. [Google Scholar] [CrossRef]
- Yanagisawa, D.; Ibrahim, N.F.; Taguchi, H.; Morikawa, S.; Kato, T.; Hirao, K.; Shirai, N.; Sogabe, T.; Tooyama, I. Fluorine-19 Magnetic Resonance Imaging Probe for the Detection of Tau Pathology in Female RTg4510 Mice. J. Neurosci. Res. 2018, 96, 841–851. [Google Scholar] [CrossRef]
- Badachhape, A.; Parekh, P.A.; Mu, Q.; Bhavane, R.; Srivastava, M.; Stupin, I.; Bhandari, P.; Devkota, L.; Tanifum, E.; Ghaghada, K.; et al. A Novel MRI Contrast Agent for Identifying Hyperphosphorylative Neurons as a Marker of Future Tau Pathology. Alzheimer’s Dement. 2020, 16, e041080. [Google Scholar] [CrossRef]
- Yamanakkanavar, N.; Choi, J.Y.; Lee, B. MRI Segmentation and Classification of Human Brain Using Deep Learning for Diagnosis of Alzheimer’s Disease: A Survey. Sensors 2020, 20, 3243. [Google Scholar] [CrossRef]
- Li, C.; Liu, M.; Xia, J.; Mei, L.; Yang, Q.; Shi, F.; Zhang, H.; Shen, D. Predicting Brain Amyloid-β PET Phenotypes with Graph Convolutional Networks Based on Functional MRI and Multi-Level Functional Connectivity. J. Alzheimer’s Dis. 2021, 86, 1679–1693. [Google Scholar] [CrossRef] [PubMed]
- Ten Kate, M.; Redolfi, A.; Peira, E.; Bos, I.; Vos, S.J.; Vandenberghe, R.; Gabel, S.; Schaeverbeke, J.; Scheltens, P.; Blin, O.; et al. MRI Predictors of Amyloid Pathology: Results from the EMIF-AD Multimodal Biomarker Discovery Study. Alzheimer’s Res. Ther. 2018, 10, 100. [Google Scholar] [CrossRef]
- Kang, S.H.; Cheon, B.K.; Kim, J.-S.; Jang, H.; Kim, H.J.; Park, K.W.; Noh, Y.; Lee, J.S.; Ye, B.S.; Na, D.L.; et al. Machine Learning for the Prediction of Amyloid Positivity in Amnestic Mild Cognitive Impairment. J. Alzheimer’s Dis. 2021, 80, 143–157. [Google Scholar] [CrossRef]
- Lew, C.O.; Zhou, L.; Mazurowski, M.A.; Doraiswamy, P.M.; Petrella, J.R.; Alzheimer’s Disease Neuroimaging Initiative. MRI-Based Deep Learning Assessment of Amyloid, Tau, and Neurodegeneration Biomarker Status across the Alzheimer Disease Spectrum. Radiology 2023, 309, e222441. [Google Scholar] [CrossRef]
- Barghorn, S.; Biernat, J.; Mandelkow, E. Purification of Recombinant Tau Protein and Preparation of Alzheimer-Paired Helical Filaments in Vitro. Methods Mol. Biol. 2005, 299, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Sui, D.; Liu, M.; Kuo, M.-H. In Vitro Aggregation Assays Using Hyperphosphorylated Tau Protein. J. Vis. Exp. 2015, 95, e51537. [Google Scholar] [CrossRef]
- Elbatrawy, A.A.; Hyeon, S.J.; Yue, N.; Osman, E.E.A.; Choi, S.H.; Lim, S.; Kim, Y.K.; Ryu, H.; Cui, M.; Nam, G. “Turn-On” Quinoline-Based Fluorescent Probe for Selective Imaging of Tau Aggregates in Alzheimer’s Disease: Rational Design, Synthesis, and Molecular Docking. ACS Sens. 2021, 6, 2281–2289. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Li, Z.; Luo, K.; Gong, Q.; Tian, X. Application of Biomarker-Derived Fluorescent Probes for the Detection of Alzheimer’s Disease. TrAC Trends Anal. Chem. 2023, 169, 117369. [Google Scholar] [CrossRef]
- Verwilst, P.; Kim, H.S.; Kim, S.; Kang, C.; Kim, J.S. Shedding Light on Tau Protein Aggregation: The Progress in Developing Highly Selective Fluorophores. Chem. Soc. Rev. 2018, 47, 2249–2265. [Google Scholar] [CrossRef]
- Verwilst, P.; Kim, H.-R.; Seo, J.; Sohn, N.-W.; Cha, S.-Y.; Kim, Y.; Maeng, S.; Shin, J.-W.; Kwak, J.H.; Kang, C.; et al. Rational Design of in Vivo Tau Tangle-Selective Near-Infrared Fluorophores: Expanding the BODIPY Universe. J. Am. Chem. Soc. 2017, 139, 13393–13403. [Google Scholar] [CrossRef]
- Zhao, Y.; Tietz, O.; Kuan, W.-L.; Haji-Dheere, A.K.; Thompson, S.; Vallin, B.; Ronchi, E.; Tóth, G.; Klenerman, D.; Aigbirhio, F.I. A Fluorescent Molecular Imaging Probe with Selectivity for Soluble Tau Aggregated Protein. Chem. Sci. 2020, 11, 4773–4778. [Google Scholar] [CrossRef]
- Åslund, A.; Sigurdson, C.J.; Klingstedt, T.; Grathwohl, S.; Bolmont, T.; Dickstein, D.L.; Glimsdal, E.; Prokop, S.; Lindgren, M.; Konradsson, P.; et al. Novel Pentameric Thiophene Derivatives for in Vitro and in Vivo Optical Imaging of a Plethora of Protein Aggregates in Cerebral Amyloidoses. ACS Chem. Biol. 2009, 4, 673–684. [Google Scholar] [CrossRef]
- Oh, Y.; Lee, T.; Kim, M.K.; Chong, Y. Thiophene-π-Cyanoacetamides Show Intense and Tau-Selective Turn-on Fluorescence in the Near-Infrared Region. Bull. Korean Chem. Soc. 2021, 42, 1285–1288. [Google Scholar] [CrossRef]
- Soloperto, A.; Quaglio, D.; Baiocco, P.; Romeo, I.; Mori, M.; Ardini, M.; Presutti, C.; Sannino, I.; Ghirga, S.; Iazzetti, A.; et al. Rational Design and Synthesis of a Novel BODIPY-Based Probe for Selective Imaging of Tau Tangles in Human IPSC-Derived Cortical Neurons. Sci. Rep. 2022, 12, 5257. [Google Scholar] [CrossRef]
- Fields, C.R.; Bengoa-Vergniory, N.; Wade-Martins, R. Targeting Alpha-Synuclein as a Therapy for Parkinson’s Disease. Front. Mol. Neurosci. 2019, 12, 299. [Google Scholar] [CrossRef]
- Shvadchak, V.V.; Afitska, K.; Yushchenko, D.A. Inhibition of α-Synuclein Amyloid Fibril Elongation by Blocking Fibril Ends. Angew. Chem. Int. Ed. 2018, 57, 5690–5694. [Google Scholar] [CrossRef]
- Veys, L.; Vandenabeele, M.; Ortuño-Lizarán, I.; Baekelandt, V.; Cuenca, N.; Moons, L.; De Groef, L. Retinal α-Synuclein Deposits in Parkinson’s Disease Patients and Animal Models. Acta Neuropathol. 2019, 137, 379–395. [Google Scholar] [CrossRef]
- Deeg, A.A.; Reiner, A.M.; Schmidt, F.; Schueder, F.; Ryazanov, S.; Ruf, V.C.; Giller, K.; Becker, S.; Leonov, A.; Griesinger, C.; et al. Anle138b and Related Compounds Are Aggregation Specific Fluorescence Markers and Reveal High Affinity Binding to α-Synuclein Aggregates. Biochim. Biophys. Acta 2015, 1850, 1884–1890. [Google Scholar] [CrossRef]
- Martinez Hernandez, A.; Urbanke, H.; Gillman, A.L.; Lee, J.; Ryazanov, S.; Agbemenyah, H.Y.; Benito, E.; Jain, G.; Kaurani, L.; Grigorian, G.; et al. The Diphenylpyrazole Compound Anle138b Blocks Aβ Channels and Rescues Disease Phenotypes in a Mouse Model for Amyloid Pathology. EMBO Mol. Med. 2018, 10, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Krauss, S.; Shi, S.; Ryazanov, S.; Steffen, J.; Miklitz, C.; Leonov, A.; Kleinknecht, A.; Göricke, B.; Weishaupt, J.H.; et al. Reducing Tau Aggregates with Anle138b Delays Disease Progression in a Mouse Model of Tauopathies. Acta Neuropathol. 2015, 130, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Ono, M.; Ariyoshi, T.; Katayanagi, R.; Saji, H. Novel Benzothiazole Derivatives as Fluorescent Probes for Detection of β-Amyloid and α-Synuclein Aggregates. ACS Chem. Neurosci. 2017, 8, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ge, P.; Murray, K.A.; Sheth, P.; Zhang, M.; Nair, G.; Sawaya, M.R.; Shin, W.S.; Boyer, D.R.; Ye, S.; et al. Cryo-EM of Full-Length α-Synuclein Reveals Fibril Polymorphs with a Common Structural Kernel. Nat. Commun. 2018, 9, 3609. [Google Scholar] [CrossRef]
- Alam, P.; Bousset, L.; Melki, R.; Otzen, D.E. α-Synuclein Oligomers and Fibrils: A Spectrum of Species, a Spectrum of Toxicities. J. Neurochem. 2019, 150, 522–534. [Google Scholar] [CrossRef]
- Zeng, Q.; Cui, M. Current Progress in the Development of Probes for Targeting α-Synuclein Aggregates. ACS Chem. Neurosci. 2022, 13, 552–571. [Google Scholar] [CrossRef]
- Bagchi, D.P.; Yu, L.; Perlmutter, J.S.; Xu, J.; Mach, R.H.; Tu, Z.; Kotzbauer, P.T. Binding of the Radioligand SIL23 to α-Synuclein Fibrils in Parkinson Disease Brain Tissue Establishes Feasibility and Screening Approaches for Developing a Parkinson Disease Imaging Agent. PLoS ONE 2013, 8, e55031. [Google Scholar] [CrossRef]
- Korat, Š.; Bidesi, N.S.R.; Bonanno, F.; Di Nanni, A.; Hoàng, A.N.N.; Herfert, K.; Maurer, A.; Battisti, U.M.; Bowden, G.D.; Thonon, D.; et al. Alpha-Synuclein PET Tracer Development—An Overview about Current Efforts. Pharmaceuticals 2021, 14, 847. [Google Scholar] [CrossRef]
- Chu, W.; Zhou, D.; Gaba, V.; Liu, J.; Li, S.; Peng, X.; Xu, J.; Dhavale, D.; Bagchi, D.P.; d’Avignon, A.; et al. Design, Synthesis, and Characterization of 3-(Benzylidene)Indolin-2-One Derivatives as Ligands for α-Synuclein Fibrils. J. Med. Chem. 2015, 58, 6002–6017. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Doi, Y.; Watanabe, H.; Ihara, M.; Ozaki, A.; Saji, H. Structure–Activity Relationships of Radioiodinated Diphenyl Derivatives with Different Conjugated Double Bonds as Ligands for α-Synuclein Aggregates. RSC Adv. 2016, 6, 44305–44312. [Google Scholar] [CrossRef]
- Verdurand, M.; Levigoureux, E.; Zeinyeh, W.; Berthier, L.; Mendjel-Herda, M.; Cadarossanesaib, F.; Bouillot, C.; Iecker, T.; Terreux, R.; Lancelot, S.; et al. In Silico, in Vitro, and in Vivo Evaluation of New Candidates for α-Synuclein PET Imaging. Mol. Pharm. 2018, 15, 3153–3166. [Google Scholar] [CrossRef]
- Maurer, A.; Leonov, A.; Ryazanov, S.; Herfert, K.; Kuebler, L.; Buss, S.; Schmidt, F.; Weckbecker, D.; Linder, R.; Bender, D.; et al. 11C Radiolabeling of Anle253b: A Putative PET Tracer for Parkinson’s Disease That Binds to A-Synuclein Fibrils in Vitro and Crosses the Blood-Brain Barrier. ChemMedChem 2020, 15, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Kuebler, L.; Buss, S.; Leonov, A.; Ryazanov, S.; Schmidt, F.; Maurer, A.; Weckbecker, D.; Landau, A.M.; Lillethorup, T.P.; Bleher, D.; et al. [11C]MODAG-001-towards a PET Tracer Targeting α-Synuclein Aggregates. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1759–1772. [Google Scholar] [CrossRef] [PubMed]
- Kaide, S.; Watanabe, H.; Shimizu, Y.; Iikuni, S.; Nakamoto, Y.; Hasegawa, M.; Itoh, K.; Ono, M. Identification and Evaluation of Bisquinoline Scaffold as a New Candidate for α-Synuclein-PET Imaging. ACS Chem. Neurosci. 2020, 11, 4254–4261. [Google Scholar] [CrossRef] [PubMed]

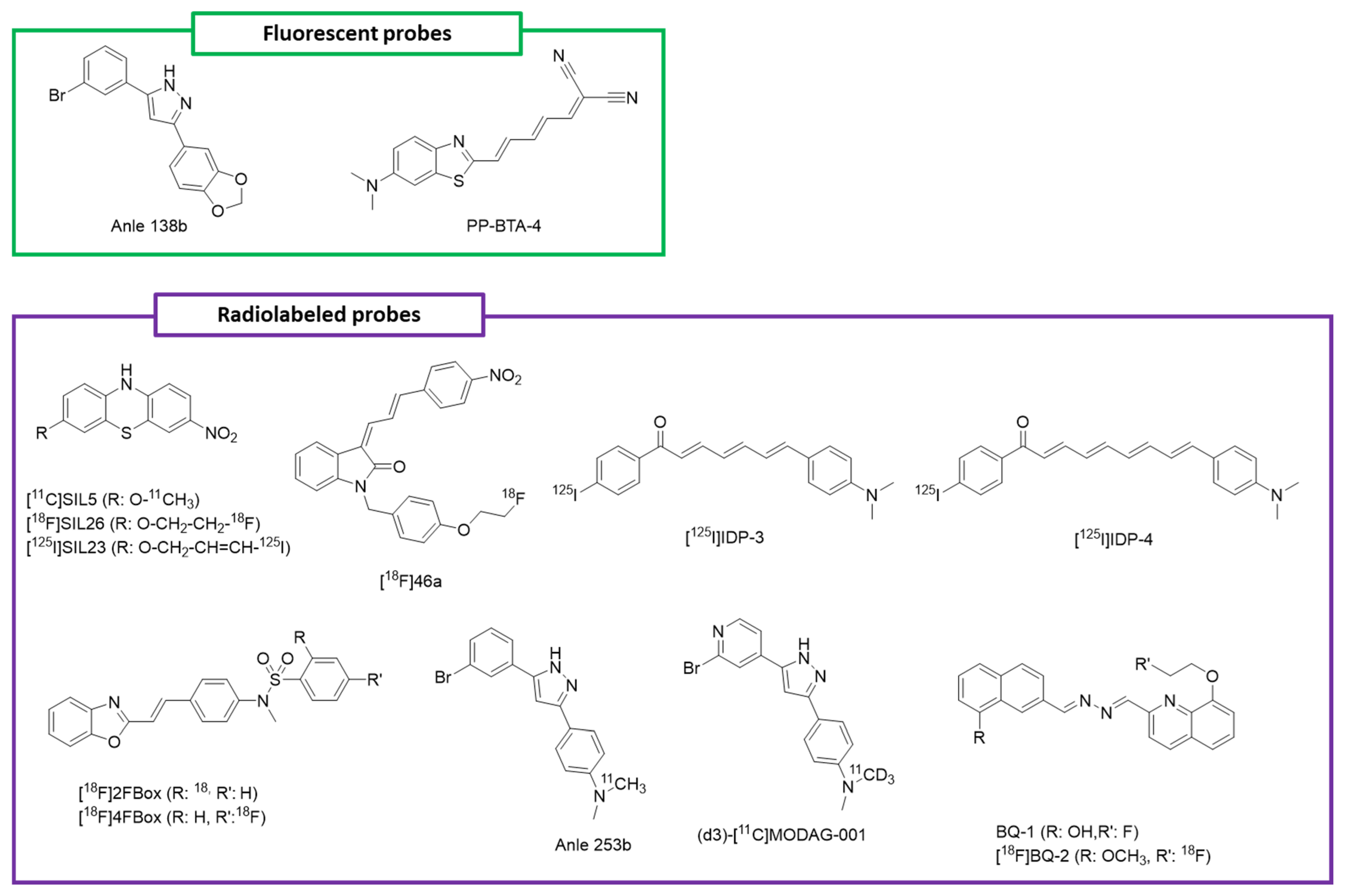
| Compounds | Aggregate Selectivity | Amyloid Selectivity | Mechanism of Interaction |
|---|---|---|---|
| Styryl derivatives | None (Aβ1–42 aggregates) | Aβ1–42 | Possible intercalation in β-sheet-rich structures |
| Oxazine dyes | None (Aβ1–42 aggregates) | Aβ1–42 | Possible intercalation in β-sheet-rich structures |
| 2,2′-bithiophene derivatives | None (Aβ1–42 fibrils, amyloid aggregates) | None | Binding to amyloid fibrils surface |
| Curcumin derivatives | Oligomers and fibrils | None (αSyn, Aβ1–42, Tau) | Not specified |
| Chalcone derivatives | Aβ1–42 plaques | Aβ1–42 | Not specified |
| 1,4-napthoquinones | None (Aβ1–42 aggregates and plaques) | Aβ1–42 | Not specified |
| BODIPY dies | Oligomers and fibrils | Aβ1–42 | Binding to hydrophobic surfaces |
| CRANAD-3 | None (different oligomers, monomers) | Aβ1–42 | Not specified |
| BD-Oligo probe | Oligomers | Aβ1–42 | Similar to BODIPY dies |
| BODIPY-6 and aza-BODIPY | Oligomers and fibrils | Aβ1–42 | Similar to BODIPY dies |
| PTO-29 | Oligomers | Aβ1–42 | Not specified |
| F-SLOH | Oligomers | Aβ1–42 | Not specified |
| [18F]-FDDNP | None (Tau aggregates) | Aβ1–42, Tau | Not specified |
| [11C]-PBB3 | None (Tau aggregates) | Aβ1–42, Tau | Not specified |
| THK compounds ([18F]-THK-523, [18F]-THK-5105, [18F]-THK-5116, [18F]-THK-5117 and [18F]-THK5351) | None (Tau aggregates) | Tau | Not specified |
| Flortaucipir (18F) | Tau fibrils (PHF) | Tau | Not specified |
| [18F]-GTP1 | Neurofibrillary tangles (NFT) | Tau | Not specified |
| [18F]-JNJ069 | Neurofibrillary tangles (NFT) | Tau | Not specified |
| [18F]-JNJ311 | Neurofibrillary tangles (NFT) | Tau | Not specified |
| [18F]/[3H]-MK-6240 | Neurofibrillary tangles (NFT) | Tau | Not specified |
| [18F]/[3H]-PI-2620 | Neurofibrillary tangles (NFT) | Tau | Not specified |
| [18F]/[3H]-RO-948 | Neurofibrillary tangles (NFT) | Tau | Not specified |
| Shiga-X34 | Tau NFT | Tau | Not specified |
| Shiga-X35 | None (Tau NFT and other aggregates) | Tau | Not specified |
| Quinoline-based probes (Q-tau1 and Q-tau4) | None (Tau aggregates) | Tau | Not specified |
| pTP-TFE | None (Tau soluble aggregates) | Tau | Not specified |
| BODIPY-derived probes (BT1) | Tau oligomers (phosphorylated) | Tau | Similar to BODIPY dies |
| Anle138b | Oligomers and fibrils | None (αSyn, Aβ1–42, Tau) | Possible methyl bridge disruption leading to free hydroxyl (H-bond interaction), intercalation in β-sheet-rich structures |
| PP-BTA-4 | Fibrils | αSyn, Aβ1–42 | Not specified |
| [11C]SIL5 | Fibrils | αSyn | Not specified |
| [125I]SIL23 | Fibrils | αSyn | Not specified |
| [18F]SIL26 | Fibrils | αSyn | Not specified |
| [18F]46a | Fibrils | αSyn | Not specified |
| [125I] IDP-3 | None (αSyn aggregates in LBs) | αSyn | Not specified |
| [125I] IDP-4 | None (αSyn aggregates in LBs) | αSyn | Not specified |
| [18F] 2FBox | None | αSyn and Aβ1–42 | Not specified |
| [18F] 4FBox | None | αSyn and Aβ1–42 | Not specified |
| Anle253b | Fibrils | αSyn | Not specified, probably similar to Anle138b |
| (d3)-[11C]MODAG-001 | Fibrils | αSyn | Not specified |
| BQ-1 | None | αSyn | Not specified |
| [18F]BQ-2 | None | αSyn | Not specified |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bisi, N.; Pinzi, L.; Rastelli, G.; Tonali, N. Early Diagnosis of Neurodegenerative Diseases: What Has Been Undertaken to Promote the Transition from PET to Fluorescence Tracers. Molecules 2024, 29, 722. https://doi.org/10.3390/molecules29030722
Bisi N, Pinzi L, Rastelli G, Tonali N. Early Diagnosis of Neurodegenerative Diseases: What Has Been Undertaken to Promote the Transition from PET to Fluorescence Tracers. Molecules. 2024; 29(3):722. https://doi.org/10.3390/molecules29030722
Chicago/Turabian StyleBisi, Nicolò, Luca Pinzi, Giulio Rastelli, and Nicolò Tonali. 2024. "Early Diagnosis of Neurodegenerative Diseases: What Has Been Undertaken to Promote the Transition from PET to Fluorescence Tracers" Molecules 29, no. 3: 722. https://doi.org/10.3390/molecules29030722
APA StyleBisi, N., Pinzi, L., Rastelli, G., & Tonali, N. (2024). Early Diagnosis of Neurodegenerative Diseases: What Has Been Undertaken to Promote the Transition from PET to Fluorescence Tracers. Molecules, 29(3), 722. https://doi.org/10.3390/molecules29030722









