Evaluation of Porous (Poly(lactide-co-glycolide)-co-(ε-caprolactone)) Polyurethane for Use in Orthopedic Scaffolds
Abstract
:1. Introduction
2. Results and Discussion
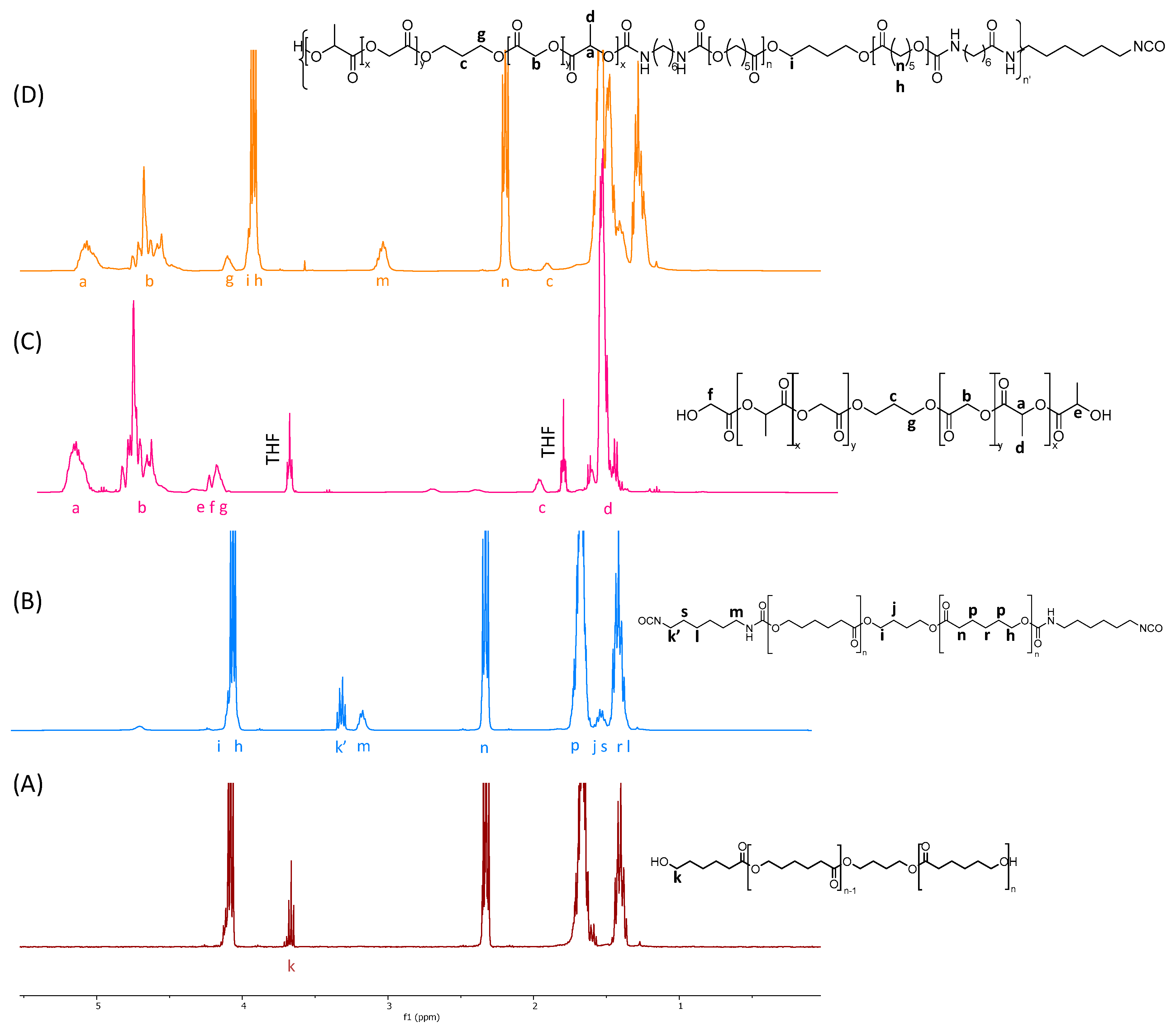
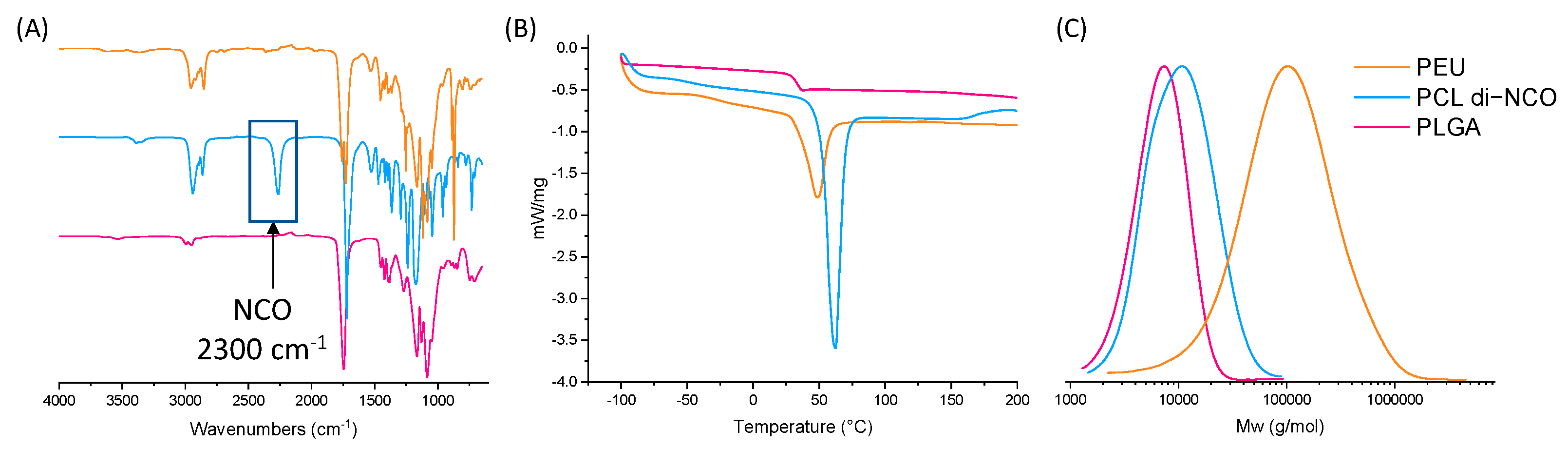
2.1. PLGA Synthesis
2.2. PCL di-NCO Prepolymer Synthesis
2.3. Poly(ester-urethane) Synthesis
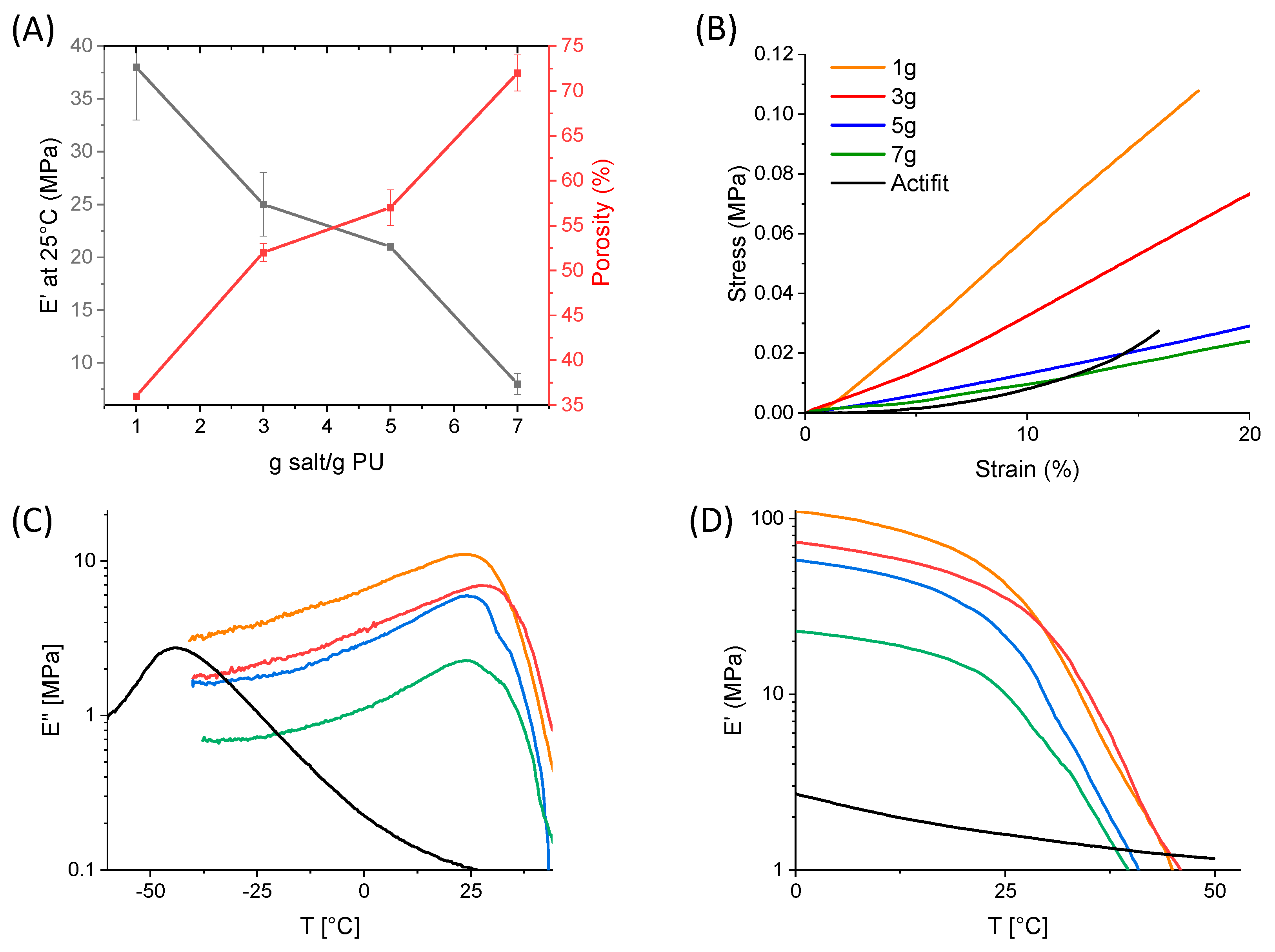
2.4. Scaffold Preparation
2.5. Cytotoxicity
2.6. Degradation
3. Materials and Methods
3.1. Materials
3.2. Nuclear Magnetic Resonance (NMR)
3.3. Fourier Transform Infrared (FTIR)
3.4. Thermogravimetric Analyses (TGA)
3.5. Differential Scanning Calorimetry (DSC)
3.6. Isocyanate Equivalent Weight (IEW) and Hydroxyl Equivalent Weight (HEW)
3.7. Size Exclusion Chromatography Multi-Angle Light Scattering (SEC-MALS)
3.8. Scanning Electron Microscopy (SEM)
3.9. Mechanical Properties
3.10. Dynamic Mechanical Analyses (DMAs)
3.11. MALDI-TOF
3.12. Viscosity
3.13. PLGA Synthesis
3.14. PCL di-NCO Prepolymer Synthesis
3.15. Poly(ester-urethane) Synthesis
3.16. Scaffold Preparation
3.17. Cytotoxicity Study
3.18. In Vitro Degradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tienen, T.G.; Heijkants, R.G.J.C.; Buma, P.; De Groot, J.H.; Pennings, A.J.; Veth, R.P.H. A Porous Polymer Scaffold for Meniscal Lesion Repair—A Study in Dogs. Biomaterials 2003, 24, 2541–2548. [Google Scholar] [CrossRef]
- Bochynska, A.I. Development of Biodegradable Adhesives for Meniscus Repair. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 2016. [Google Scholar]
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA J. Am. Med. Assoc. 2021, 325, 568–578. [Google Scholar] [CrossRef]
- Savannah, S.; Bido, J.; Jamie, C.; Heidi, Y.; Jeffrey, K.; Elena, L. Impact of Preoperative Opioid Use on Total Knee Arthroplasty Outcomes. J. Bone Jt. Surg. Am. Vol. 2017, 99, 803–808. [Google Scholar] [CrossRef]
- Bąkowski, P.; Bąkowska-Żywicka, K.; Ciemniewska-Gorzela, K.; Piontek, T. Meniscectomy Is Still a Frequent Orthopedic Procedure: A Pending Need for Education on the Meniscus Treatment Possibilities. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 1430–1435. [Google Scholar] [CrossRef]
- Baratz, M.E.; Fu, F.H.; Mengato, R. Meniscal Tears: The Effect of Meniscectomy and of Repair on Intraarticular Contact Areas Preliminary Report. Am. J. Sports Med. 1986, 14, 270–275. [Google Scholar] [CrossRef]
- Drobnič, M.; Ercin, E.; Gamelas, J.; Papacostas, E.T.; Slynarski, K.; Zdanowicz, U.; Spalding, T.; Verdonk, P. Treatment Options for the Symptomatic Post-Meniscectomy Knee. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 1817–1824. [Google Scholar] [CrossRef]
- Jacquet, C.; Pujol, N.; Pauly, V.; Beaufils, P.; Ollivier, M. Analysis of the Trends in Arthroscopic Meniscectomy and Meniscus Repair Procedures in France from 2005 to 2017. Orthop. Traumatol. Surg. Res. 2019, 105, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Herzog, M.M.; Marshall, S.W.; Lund, J.L.; Pate, V.; Mack, C.D.; Spang, J.T. Trends in Incidence of ACL Reconstruction and Concomitant Procedures Among Commercially Insured Individuals in the United States, 2002–2014. Sports Health 2018, 10, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Buma, P.; van Tienen, T.; Veth, R.P. The Collagen Meniscus Implant. Expert Rev. Med. Devices 2007, 4, 507–516. [Google Scholar] [CrossRef]
- Grassi, A.; Lucidi, G.A.; Agostinone, P.; di Paolo, S.; dal Fabbro, G.; Zaffagnini, S. Lateral Collagen Meniscus Implant (CMI): Techniques and Outcomes—A Narrative Review. Ann. Jt. 2022, 7, 1–9. [Google Scholar] [CrossRef]
- Rodkey, W.G.; Steadman, J.R.; Li, S.T. A Clinical Study of Collagen Meniscus Implants to Restore the Injured Meniscus. Clin. Orthop. Relat. Res. 1999, 367, 281–292. [Google Scholar] [CrossRef]
- Vignes, H.; Conzatti, G.; Hua, G.; Benkirane-Jessel, N. Meniscus Repair: From In Vitro Research to Patients. Organoids 2022, 1, 116–134. [Google Scholar] [CrossRef]
- Bulgheroni, E.; Grassi, A.; Campagnolo, M.; Bulgheroni, P.; Mudhigere, A.; Gobbi, A. Comparative Study of Collagen versus Synthetic-Based Meniscal Scaffolds in Treating Meniscal Deficiency in Young Active Population. Cartilage 2016, 7, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Martinek, V.; Ueblacker, P.; Bräun, K.; Nitschke, S.; Mannhardt, R.; Specht, K.; Gansbacher, B.; Imhoff, A.B. Second Generation of Meniscus Transplantation: In-Vivo Study with Tissue Engineered Meniscus Replacement. Arch. Orthop. Trauma Surg. 2006, 126, 228–234. [Google Scholar] [CrossRef]
- Verdonk, P.; Beaufils, P.; Bellemans, J.; Djian, P.; Heinrichs, E.-L.; Huysse, W.; Laprell, H.; Siebold, R.; Verdonk, R.; Colombet, P.; et al. Successful Treatment of Painful Irreparable Partial Meniscal Defects With a Polyurethane Scaffold. Am. J. Sports Med. 2012, 40, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Van Tienen, T.G.; Heijkants, R.G.J.C.; Buma, P.; De Groot, J.H.; Pennings, A.J.; Veth, R.P.H. Tissue Ingrowth and Degradation of Two Biodegradable Porous Polymers with Different Porosities and Pore Sizes. Biomaterials 2002, 23, 1731–1738. [Google Scholar] [CrossRef]
- de Groot, J. Actifit, Polyurethane Meniscus Implant: Basic Science. In The Meniscus; Springer: Berlin/Heidelberg, Germany, 2010; pp. 383–387. [Google Scholar]
- Zhang, H.; Zhou, L.; Zhang, W. Control of Scaffold Degradation in Tissue Engineering: A Review. Tissue Eng. Part B Rev. 2014, 20, 492–502. [Google Scholar] [CrossRef]
- Kucinska-Lipka, J.; Marzec, M.; Gubanska, I.; Janik, H. Porosity and Swelling Properties of Novel Polyurethane-Ascorbic Acid Scaffolds Prepared by Different Procedures for Potential Use in Bone Tissue Engineering. J. Elastomers Plast. 2017, 49, 440–456. [Google Scholar] [CrossRef]
- Thadavirul, N.; Pavasant, P.; Supaphol, P. Development of Polycaprolactone Porous Scaffolds by Combining Solvent Casting, Particulate Leaching, and Polymer Leaching Techniques for Bone Tissue Engineering. J. Biomed. Mater. Res A 2014, 102, 3379–3392. [Google Scholar] [CrossRef]
- Janik, H.; Marzec, M. A Review: Fabrication of Porous Polyurethane Scaffolds. Mater. Sci. Eng. C 2015, 48, 586–591. [Google Scholar] [CrossRef]
- Gorna, K.; Gogolewski, S. Biodegradable Porous Polyurethane Scaffolds for Tissue Repair and Regeneration. J. Biomed. Mater. Res. A 2006, 79A, 128–138. [Google Scholar] [CrossRef]
- Al Nakib, R.; Toncheva, A.; Fontaine, V.; Vanheuverzwijn, J.; Raquez, J.; Meyer, F. Thermoplastic Polyurethanes for Biomedical Application: A Synthetic, Mechanical, Antibacterial, and Cytotoxic Study. J. Appl. Polym. Sci. 2022, 139, 51666. [Google Scholar] [CrossRef]
- Bruin, P.; Veenstra, G.J.; Nijenhuis, A.J.; Pennings, A.J. Design and Synthesis of Biodegradable Poly(Ester-Urethane) Elastomer Networks Composed of Non-Toxic Building Blocks. Makromol. Chem. Rapid Commun. 1988, 9, 589–594. [Google Scholar] [CrossRef]
- Dempsey, D.K.; Robinson, J.L.; Iyer, A.V.; Parakka, J.P.; Bezwada, R.S.; Cosgriff-Hernandez, E.M. Characterization of a Resorbable Poly(Ester Urethane) with Biodegradable Hard Segments. J. Biomater. Sci. Polym. Ed. 2014, 25, 535–554. [Google Scholar] [CrossRef]
- Skarja, G.A.; Woodhouse, K.A. Synthesis and Characterization of Degradable Polyurethane Elastomers Containing an Amino Acid-Based Chain Extender. J. Biomater. Sci. Polym. Ed. 1998, 9, 271–295. [Google Scholar] [CrossRef]
- Borkenhagen, M.; Stoll, R.C.; Neuenschwander, P.; Suter, U.W.; Aebischer, P. In Vivo Performance of a New Biodegradable Polyester Urethane System Used as a Nerve Guidance Channel. Biomaterials 1998, 19, 2155–2165. [Google Scholar] [CrossRef]
- Henry, J.A.; Simonet, M.; Pandit, A.; Neuenschwander, P. Characterization of a Slowly Degrading Biodegradable Polyesterurethane for Tissue Engineering Scaffolds. J. Biomed. Mater. Res. A 2007, 82A, 669–679. [Google Scholar] [CrossRef]
- Saez, V.; Cerruti, R.; Ramón, J.A.; Santos, E.R.F.; Silva, D.Z.; Pinto, J.C.; Souza, F.G. Quantification of Oxaliplatin Encapsulated into PLGA Microspheres by TGA. Macromol. Symp. 2016, 368, 116–121. [Google Scholar] [CrossRef]
- Silva, A.T.C.R.; Cardoso, B.C.O.; Silva, M.E.S.R.e.; Freitas, R.F.S.; Sousa, R.G. Synthesis, Characterization, and Study of PLGA Copolymer in Vitro Degradation. J. Biomater. Nanobiotechnol. 2015, 6, 8–19. [Google Scholar] [CrossRef]
- Heijkants, R.G.J.C.; Van Tienen, T.G.; De Groot, J.H.; Pennings, A.J.; Buma, P.; Veth, R.P.H.; Schouten, A.J. Preparation of a Polyurethane Scaffold for Tissue Engineering Made by a Combination of Salt Leaching and Freeze-Drying of Dioxane. J. Mater. Sci. 2006, 41, 2423–2428. [Google Scholar] [CrossRef]
- Spaans, C.J.; Belgraver, V.W.; Rienstra, O.; De Groot, J.H.; Veth, R.P.H.; Pennings, A.J. Solvent-Free Fabrication of Micro-Porous Polyurethane Amide and Polyurethane-Urea Scaffolds for Repair and Replacement of the Knee-Joint Meniscus. Biomaterials 2000, 21, 2453–2460. [Google Scholar] [CrossRef]
- Heijkants, R.G.J.C.; van Calck, R.V.; de Groot, J.H.; Pennings, A.J.; Schouten, A.J.; van Tienen, T.G.; Ramrattan, N.; Buma, P.; Veth, R.P.H. Design, Synthesis and Properties of a Degradable Polyurethane Scaffold for Meniscus Regeneration. J. Mater. Sci. Mater. Med. 2004, 15, 423–427. [Google Scholar] [CrossRef]
- Spaans, C.J.; De Groot, J.H.; Belgraver, V.W.; Pennings, A.J. A New Biomedical Polyurethane with a High Modulus Based on 1,4-Butanediisocyanate and ε-Caprolactone. J. Mater. Sci. Mater. Med. 1998, 9, 675–678. [Google Scholar] [CrossRef]
- Kaufmann, P.M.; Heimrath, S.; Kim, B.S.; Mooney, D.J. Highly Porous Polymer Matrices as a Three-Dimensional Culture System for Hepatocytes. Cell Transplant. 1997, 6, 463–468. [Google Scholar] [CrossRef]
- Heijkants, R.G.J.C.; van Calck, R.V.; van Tienen, T.G.; de Groot, J.H.; Pennings, A.J.; Buma, P.; Veth, R.P.H.; Schouten, A.J. Polyurethane Scaffold Formation via a Combination of Salt Leaching and Thermally Induced Phase Separation. J. Biomed. Mater. Res. A 2008, 87A, 921–932. [Google Scholar] [CrossRef]
- Hasirci, V.; Berthiaume, F.; Bondre, S.P.; Gresser, J.D.; Trantolo, D.J.; Toner, M.; Wise, D.L. Expression of Liver-Specific Functions by Rat Hepatocytes Seeded in Treated Poly(Lactic-Co-Glycolic) Acid Biodegradable Foams. Tissue Eng. 2001, 7, 385–394. [Google Scholar] [CrossRef]
- De Groot, J.H.; Zijlstra, F.M.; Kuipers, H.W.; Pennings, A.J.; Klompmaker, J.; Veth, R.P.H.; Jansen, H.W.B. Meniscal Tissue Regeneration in Porous 50/50 Copoly(L-Lactide/ε-Caprolactone) Implants. Biomaterials 1997, 18, 613–622. [Google Scholar] [CrossRef]
- Bil, M.; Ryszkowska, J.; Kurzydłowski, K.J. Effect of Polyurethane Composition and the Fabrication Process on Scaffold Properties. J. Mater. Sci. 2009, 44, 1469–1476. [Google Scholar] [CrossRef]
- Bakar, M.; Białkowska, A. Preparation and Properties Evaluation of Nonisocyanate Condensation Polyurethanes Based on Phenol Sulfonic and Hydroxybenzoic Acids. J. Plast. Film Sheeting 2012, 28, 257–272. [Google Scholar] [CrossRef]
- Bruin, P.; Smedinga, J.; Pennings, A.J.; Jonkman, M.F. Biodegradable Lysine Diisocyanate-Based Poly(Glycolide-Co-ϵ-Caprolactone)-Urethane Network in Artificial Skin. Biomaterials 1990, 11, 291–295. [Google Scholar] [CrossRef]
- Yang, D.; Xiao, J.; Wang, B.; Li, L.; Kong, X.; Liao, J. The Immune Reaction and Degradation Fate of Scaffold in Cartilage/Bone Tissue Engineering. Mater. Sci. Eng. C 2019, 104, 109927. [Google Scholar] [CrossRef] [PubMed]

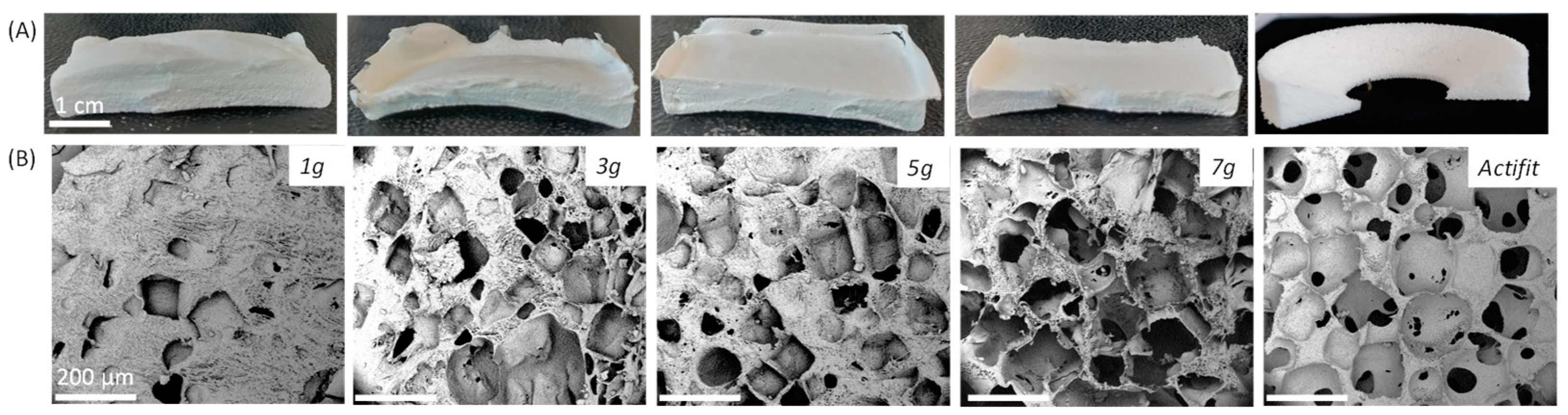


| Salt Quantity (g/1 g PEU) | 1 | 3 | 5 | 7 | Actifit® |
|---|---|---|---|---|---|
| Pores size (µm) | 111 ± 91 | 164 ± 40 | 151 ± 51 | 155 ± 48 | 240 ± 30 |
| Porosity (%) | 36 ± 0 | 52 ±1 | 57 ± 2 | 72 ± 2 | 53 ± 4 |
| Ey (Mpa) at 25 °C | 35 ± 1 | 33 ± 6 | 14 ± 1 | 12 ± 1 | 6 ± 1 |
| E′ (MPa) at 25 °C | 38 ± 5 | 25 ± 3 | 21 ± 0 | 8 ± 1 | 2 |
| E′ (MPa) at 37 °C | 4.5 ± 0.8 | 4.1 ± 2.3 | 2.8 ± 0.2 | 1.5 ± 1.1 | 1.3 |
| E″ (MPa) at 37 °C | 2.5 ± 0.8 | 2.7 ± 1.9 | 1.4 ± 0.1 | 1.0 ± 0.1 | 0.1 |
| Tα (°C) | 24 | 28 | 24 | 25 | −43 |
| Tg (°C) | 45 | 47 | 49 | 49 | −50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savin, G.; Sastourne-Array, O.; Caillol, S.; Bethry, A.; Assor, M.; David, G.; Nottelet, B. Evaluation of Porous (Poly(lactide-co-glycolide)-co-(ε-caprolactone)) Polyurethane for Use in Orthopedic Scaffolds. Molecules 2024, 29, 766. https://doi.org/10.3390/molecules29040766
Savin G, Sastourne-Array O, Caillol S, Bethry A, Assor M, David G, Nottelet B. Evaluation of Porous (Poly(lactide-co-glycolide)-co-(ε-caprolactone)) Polyurethane for Use in Orthopedic Scaffolds. Molecules. 2024; 29(4):766. https://doi.org/10.3390/molecules29040766
Chicago/Turabian StyleSavin, Gaëlle, Océane Sastourne-Array, Sylvain Caillol, Audrey Bethry, Michel Assor, Ghislain David, and Benjamin Nottelet. 2024. "Evaluation of Porous (Poly(lactide-co-glycolide)-co-(ε-caprolactone)) Polyurethane for Use in Orthopedic Scaffolds" Molecules 29, no. 4: 766. https://doi.org/10.3390/molecules29040766
APA StyleSavin, G., Sastourne-Array, O., Caillol, S., Bethry, A., Assor, M., David, G., & Nottelet, B. (2024). Evaluation of Porous (Poly(lactide-co-glycolide)-co-(ε-caprolactone)) Polyurethane for Use in Orthopedic Scaffolds. Molecules, 29(4), 766. https://doi.org/10.3390/molecules29040766








