Fluacrypyrim Protects Hematopoietic Stem and Progenitor Cells against Irradiation via Apoptosis Prevention
Abstract
:1. Introduction
2. Results
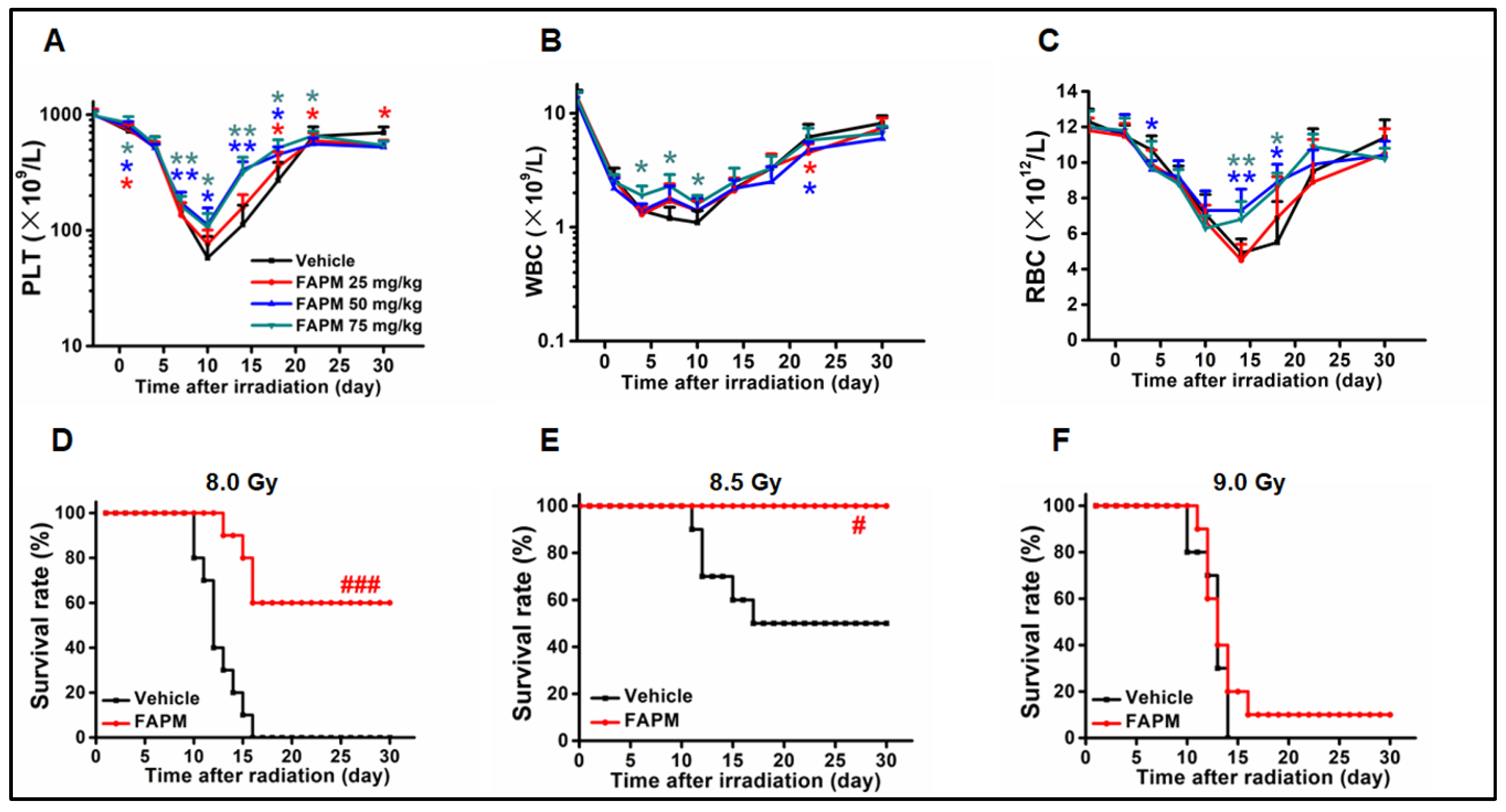
2.1. FAPM Administration Could Benefit the Hematological Recovery after Irradiation
2.2. FAPM Improved the Survival Rate of Mice after Lethal Irradiation
2.3. FAPM Alleviated Irradiation-Induced Injury to BM
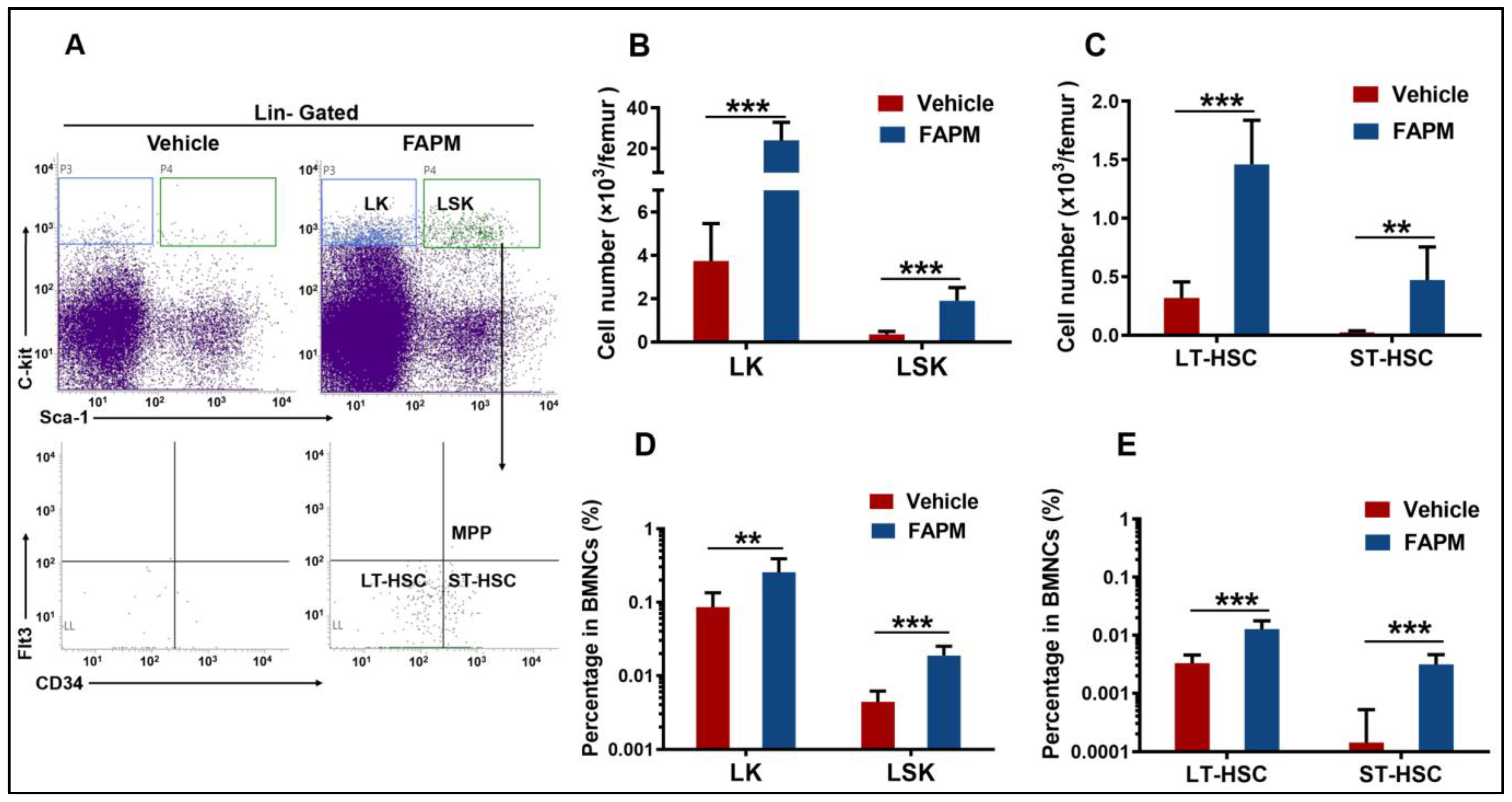
2.4. FAPM Accelerated the Recovery of HSPCs after Irradiation Exposure
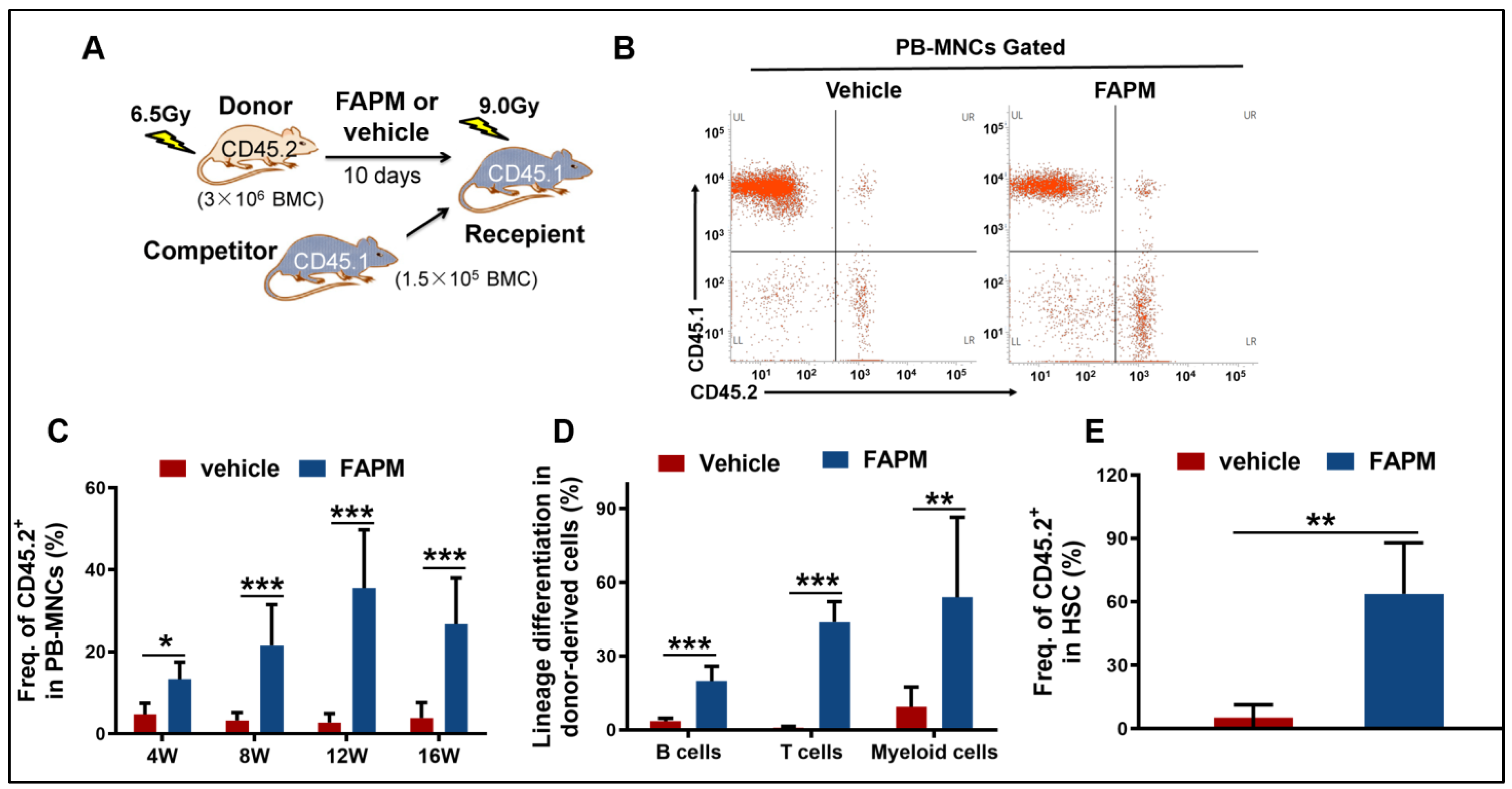
2.5. FAPM Enhanced the Self-Renewal Capacity of HSCs after Irradiation
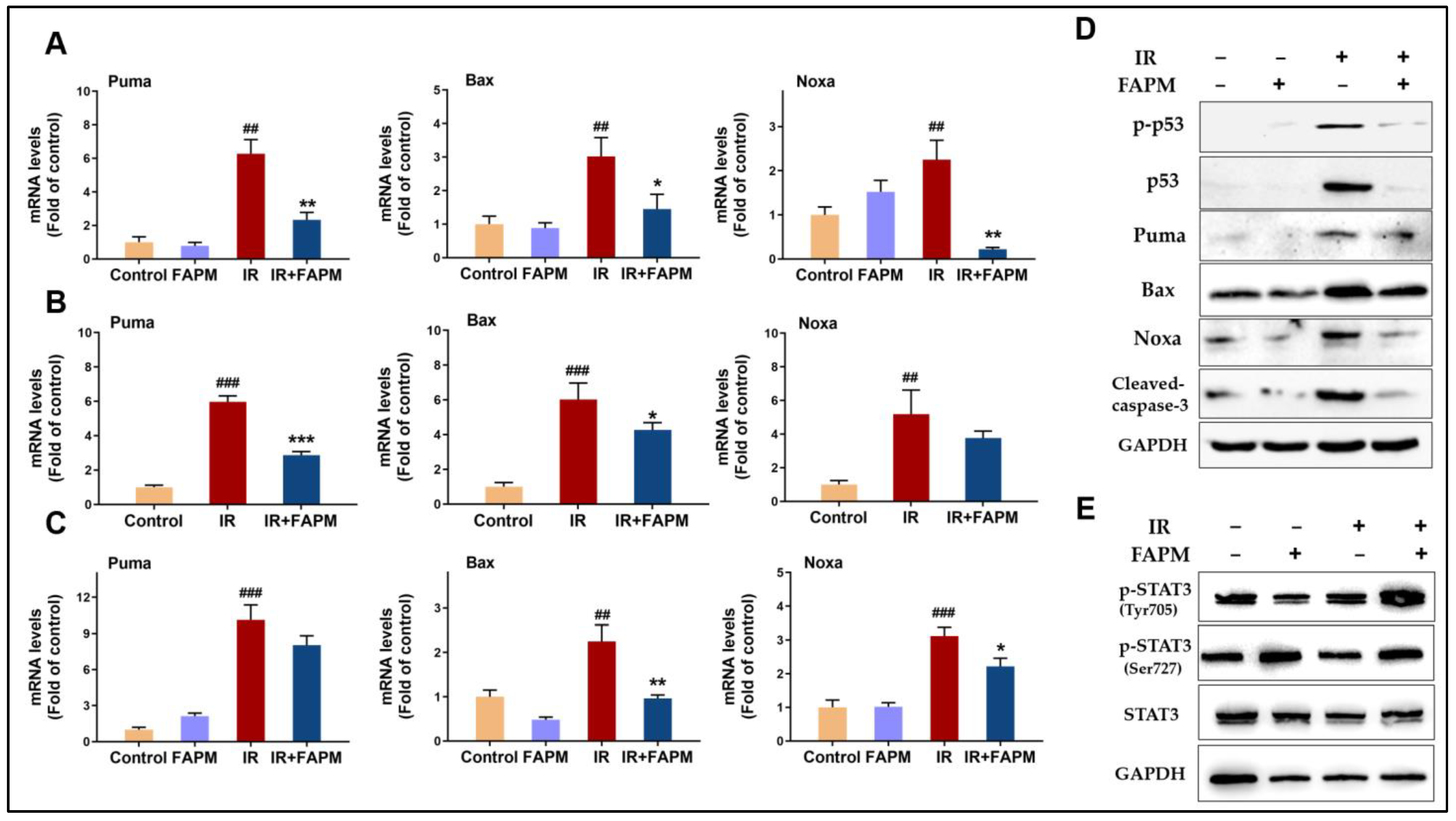
2.6. FAPM Mitigated Cell Apoptosis Both In Vitro and In Vivo
2.7. FAPM Decreased Cell Apoptosis by Regulating the p53-PUMA Pathway
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Mice
4.3. Irradiation and FAPM Administration
4.4. Peripheral Blood Cell Counts
4.5. BM Cellularity and BM-Derived Clonogenic Activity Assay
4.6. Mouse Competitive Repopulation Assay
4.7. Flow Cytometry Analysis
4.8. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.9. Western Blotting
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shao, L.; Luo, Y.; Zhou, D. Hematopoietic stem cell injury induced by ionizing radiation. Antioxid. Redox Signal. 2014, 20, 1447–1462. [Google Scholar] [CrossRef]
- Qian, L.; Cen, J. Hematopoietic Stem Cells and Mesenchymal Stromal Cells in Acute Radiation Syndrome. Oxid. Med. Cell. Longev. 2020, 2020, 8340756. [Google Scholar] [CrossRef]
- Gan, J.; Meng, F.; Zhou, X.; Li, C.; He, Y.; Zeng, X.; Jiang, X.; Liu, J.; Zeng, G.; Tang, Y. Hematopoietic recovery of acute radiation syndrome by human superoxide dismutase-expressing umbilical cord mesenchymal stromal cells. Cytotherapy 2015, 17, 403–417. [Google Scholar] [CrossRef]
- Gale, R.P.; Armitage, J.O. Use of molecularly-cloned haematopoietic growth factors in persons exposed to acute high-dose, high-dose rate whole-body ionizing radiations. Blood Rev. 2021, 45, 100690. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, H.; Li, D.; Song, N.; Yang, F.; Xu, W. MST1/2 inhibitor XMU-MP-1 alleviates the injury induced by ionizing radiation in haematopoietic and intestinal system. J. Cell Mol. Med. 2022, 26, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.L.; Han, X.D.; Li, Y.; Chu, X.F.; Miao, W.M.; Zhang, J.L.; Fan, S.J. Astaxanthin attenuates total body irradiation-induced hematopoietic system injury in mice via inhibition of oxidative stress and apoptosis. Stem Cell Res. Ther. 2017, 8, 7. [Google Scholar] [CrossRef]
- Singh, V.K.; Shafran, R.L.; Inal, C.E.; Jackson, W.E., 3rd; Whitnall, M.H. Effects of whole-body gamma irradiation and 5-androstenediol administration on serum G-CSF. Immunopharmacol. Immunotoxicol. 2005, 27, 521–534. [Google Scholar] [CrossRef]
- Eaves, C.J. Hematopoietic stem cells: Concepts, definitions, and the new reality. Blood 2015, 125, 2605–2613. [Google Scholar] [CrossRef] [PubMed]
- Golan, K.; Singh, A.K.; Kollet, O.; Bertagna, M.; Althoff, M.J.; Khatib-Massalha, E.; Petrovich-Kopitman, E.; Wellendorf, A.M.; Massalha, H.; Levin-Zaidman, S. Bone marrow regeneration requires mitochondrial transfer from donor Cx43-expressing hematopoietic progenitors to stroma. Blood 2020, 136, 2607–2619. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Yin, X.; Zhang, Y.; Pang, A.; Xie, X.; Yang, S.; Zhu, C.; Li, Y.; Zhang, B.; Huang, Y. Radiation-induced bystander effects impair transplanted human hematopoietic stem cells via oxidative DNA damage. Blood 2021, 137, 3339–3350. [Google Scholar] [CrossRef]
- Kernagis, D.N.; Balcer-Kubiczek, E.; Bazyar, S.; Orschell, C.M.; Jackson, I.L. Medical countermeasures for the hematopoietic-subsyndrome of acute radiation syndrome in space. Life Sci. Space Res. 2022, 35, 36–43. [Google Scholar] [CrossRef]
- Abu-Khudir, R.; Habieb, M.E.; Mohamed, M.A.; Hawas, A.M.; Mohamed, T.M. Anti-apoptotic role of spermine against lead and/or gamma irradiation-induced hepatotoxicity in male rats. Environ. Sci. Pollut. Res. Int. 2017, 24, 24272–24283. [Google Scholar] [CrossRef]
- Yu, Z.Y.; Huang, R.; Xiao, H.; Sun, W.F.; Shan, Y.J.; Wang, B.; Zhao, T.T.; Dong, B.; Zhao, Z.H.; Liu, X.L. Fluacrypyrim, a novel STAT3 activation inhibitor, induces cell cycle arrest and apoptosis in cancer cells harboring constitutively-active STAT3. Int. J. Cancer 2010, 127, 1259–1270. [Google Scholar] [CrossRef]
- Li, Z.T.; Wang, L.M.; Cong, Y.; Guo, L.; Lin, X.L.; Yu, Z.Y.; Wu, X.G.; Dong, J.X.; Yang, R.R.; Cong, Y.W. Flucrypyrim, a novel uterine relaxant, has antinociceptive and anti-inflammatory effects in vivo. Sci. Rep. 2017, 7, 42040. [Google Scholar] [CrossRef]
- Yu, H.; Shen, H.; Yuan, Y.; XuFeng, R.; Hu, X.; Garrison, S.P.; Zhang, L.; Yu, J.; Zambetti, G.P.; Cheng, T. Deletion of Puma protects hematopoietic stem cells and confers long-term survival in response to high-dose gamma-irradiation. Blood 2010, 115, 3472–3480. [Google Scholar] [CrossRef] [PubMed]
- Lotem, J.; Sachs, L. Hematopoietic cells from mice deficient in wild-type p53 are more resistant to induction of apoptosis by some agents. Blood 1993, 82, 1092–1096. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Seed, T.M. A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: Part I. Radiation sub-syndromes, animal models and FDA-approved countermeasures. Int. J. Radiat. Biol. 2017, 93, 851–869. [Google Scholar] [CrossRef] [PubMed]
- Pestina, T.I.; Cleveland, J.L.; Yang, C.; Zambetti, G.P.; Jackson, C.W. Mpl ligand prevents lethal myelosuppression by inhibiting p53-dependent apoptosis. Blood 2001, 98, 2084–2090. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Garcia, M.; Seed, T.M. A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: Part II. Countermeasures for limited indications, internalized radionuclides, emesis, late effects, and agents demonstrating efficacy in large animals with or without FDA IND status. Int. J. Radiat. Biol. 2017, 93, 870–884. [Google Scholar] [PubMed]
- Dekeyser, M.A. Acaricide mode of action. Pest Manag. Sci. 2005, 61, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.L.; Cai, Z.F.; Cao, Y.Y.; Du, X.H. Design, Synthesis, and Acaricidal Activity of Phenyl Methoxyacrylates Containing 2-Alkenylthiopyrimidine. Molecules 2020, 25, 3379. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, L.; Xing, S.; Zhang, X.W.; Xiong, G.L.; Cong, Y.W.; Xiao, H.; Wang, X.R.; Yu, Z.Y. Inhibition of pyrimidine biosynthesis by strobilurin derivatives induces differentiation of acute myeloid leukemia cells. Leuk. Lymphoma 2022, 63, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Cameron, B.D.; Sekhar, K.R.; Ofori, M.; Freeman, M.L. The Role of Nrf2 in the Response to Normal Tissue Radiation Injury. Radiat. Res. 2018, 190, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Schulte, B.A.; LaRue, A.C.; Ogawa, M.; Zhou, D. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood 2006, 107, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.J.; Sun, Y.; Zhang, Z.H.; Feng, W.; Gao, Y.X.; Cai, Z.L.; Wang, Z.Z.; Look, A.T.; Wu, W.S. Deletion of proapoptotic Puma selectively protects hematopoietic stem and progenitor cells against high-dose radiation. Blood 2010, 115, 4707–4714. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lu, L.; Zhang, J.; Huang, S.; Xing, Y.; Zhao, M.; Zhou, D.; Li, D.; Meng, A. Granulocyte colony-stimulating factor exacerbates hematopoietic stem cell injury after irradiation. Cell Biosci. 2015, 5, 65. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.M.; Torrice, C.D.; Bell, J.F.; Monahan, K.B.; Jiang, Q.; Wang, Y.; Ramsey, M.R.; Jin, J.; Wong, K.K.; Su, L. Mitigation of hematologic radiation toxicity in mice through pharmacological quiescence induced by CDK4/6 inhibition. J. Clin. Investig. 2010, 120, 2528–2536. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, L.B.; Pazhanisamy, S.K.; Li, H.L.; Meng, A.; Zhou, D.H. Total body irradiation causes residual bone marrow injury by induction of persistent oxidative stress in murine hematopoietic stem cells. Free Radic. Biol. Med. 2010, 48, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Xufeng, R.; Park, M.R.; Gu, H.; Hu, L.; Kang, J.W.; Ma, S.; Liang, P.H.; Li, Y.; Cheng, H. Hematopoietic stem cell regeneration enhanced by ectopic expression of ROS-detoxifying enzymes in transplant mice. Mol. Ther. 2013, 21, 423–432. [Google Scholar] [CrossRef]
- Maier, P.; Wenz, F.; Herskind, C. Radioprotection of normal tissue cells. Strahlenther. Onkol. 2014, 190, 745–752. [Google Scholar] [CrossRef]
- Lee, J.M.; Bernstein, A. p53 mutations increase resistance to ionizing radiation. Proc. Natl. Acad. Sci. USA 1993, 90, 5742–5746. [Google Scholar] [CrossRef] [PubMed]
- Kemp, C.J.; Wheldon, T.; Balmain, A. p53-deficient mice are extremely susceptible to radiation-induced tumorigenesis. Nat. Genet. 1994, 8, 66–69. [Google Scholar] [CrossRef]
- Niu, G.L.; Wright, K.L.; Ma, Y.H.; Wright, G.M.; Huang, M.; Irby, R.; Briggs, J.; Karras, J.; Cress, W.D.; Pardoll, D. Role of Stat3 in regulating p53 expression and function. Mol. Cell Biol. 2005, 25, 7432–7440. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.J.; Park, B.B.; Kang, Y.J.; Kim, T.M.; Eaves, C.J.; Oh, I.H. Unique effects of Stat3 on the early phase of hematopoietic stem cell regeneration. Blood 2006, 108, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Grisouard, J.; Shimizu, T.; Duek, A.; Kubovcakova, L.; Hao-Shen, H.; Dirnhofer, S.; Skoda, R.C. Deletion of Stat3 in hematopoietic cells enhances thrombocytosis and shortens survival in a JAK2-V617F mouse model of MPN. Blood 2015, 125, 2131–2140. [Google Scholar] [CrossRef]
- Xing, S.; Shen, X.; Yang, J.K.; Wang, X.R.; Ou, H.L.; Zhang, X.W.; Xiong, G.L.; Shan, Y.J.; Cong, Y.W.; Luo, Q.L. Single-Dose Administration of Recombinant Human Thrombopoietin Mitigates Total Body Irradiation-Induced Hematopoietic System Injury in Mice and Nonhuman Primates. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 1357–1367. [Google Scholar] [CrossRef]
- Bai, F.; Wang, L.M.; Li, Z.T.; Yi, L.R.; Zuo, Z.Z.; Zhang, C.; Shan, Y.J.; Yang, C.; Peng, R.J.; Yang, R.F. 17α-Ethinyl-androst-5-ene-3β, 17β-diol, a Novel Potent Oral Radioprotective Agent, Confers Radioprotection of Hematopoietic Stem and Progenitor Cells in a Granulocyte Colony-Stimulating Factor-Independent Manner. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 217–228. [Google Scholar] [CrossRef]
- Shen, X.; Xiong, G.L.; Jing, Y.; Xiao, H.; Cui, Y.; Zhang, Y.F.; Shan, Y.J.; Xing, S.; Yang, M.; Liu, X.L. The protein kinase C agonist prostratin induces differentiation of human myeloid leukemia cells and enhances cellular differentiation by chemotherapeutic agents. Cancer Lett. 2015, 356, 686–696. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Qiao, Z.; Guan, B.; Wang, F.; Shen, X.; Shu, H.; Shan, Y.; Cong, Y.; Xing, S.; Yu, Z. Fluacrypyrim Protects Hematopoietic Stem and Progenitor Cells against Irradiation via Apoptosis Prevention. Molecules 2024, 29, 816. https://doi.org/10.3390/molecules29040816
Zhang X, Qiao Z, Guan B, Wang F, Shen X, Shu H, Shan Y, Cong Y, Xing S, Yu Z. Fluacrypyrim Protects Hematopoietic Stem and Progenitor Cells against Irradiation via Apoptosis Prevention. Molecules. 2024; 29(4):816. https://doi.org/10.3390/molecules29040816
Chicago/Turabian StyleZhang, Xuewen, Zizhi Qiao, Bo Guan, Fangming Wang, Xing Shen, Hui Shu, Yajun Shan, Yuwen Cong, Shuang Xing, and Zuyin Yu. 2024. "Fluacrypyrim Protects Hematopoietic Stem and Progenitor Cells against Irradiation via Apoptosis Prevention" Molecules 29, no. 4: 816. https://doi.org/10.3390/molecules29040816





