Synergistic Enhancement of Carrier Migration by SnO2/ZnO@GO Heterojunction for Rapid Degradation of RhB
Abstract
:1. Introduction
2. Results and Discussion
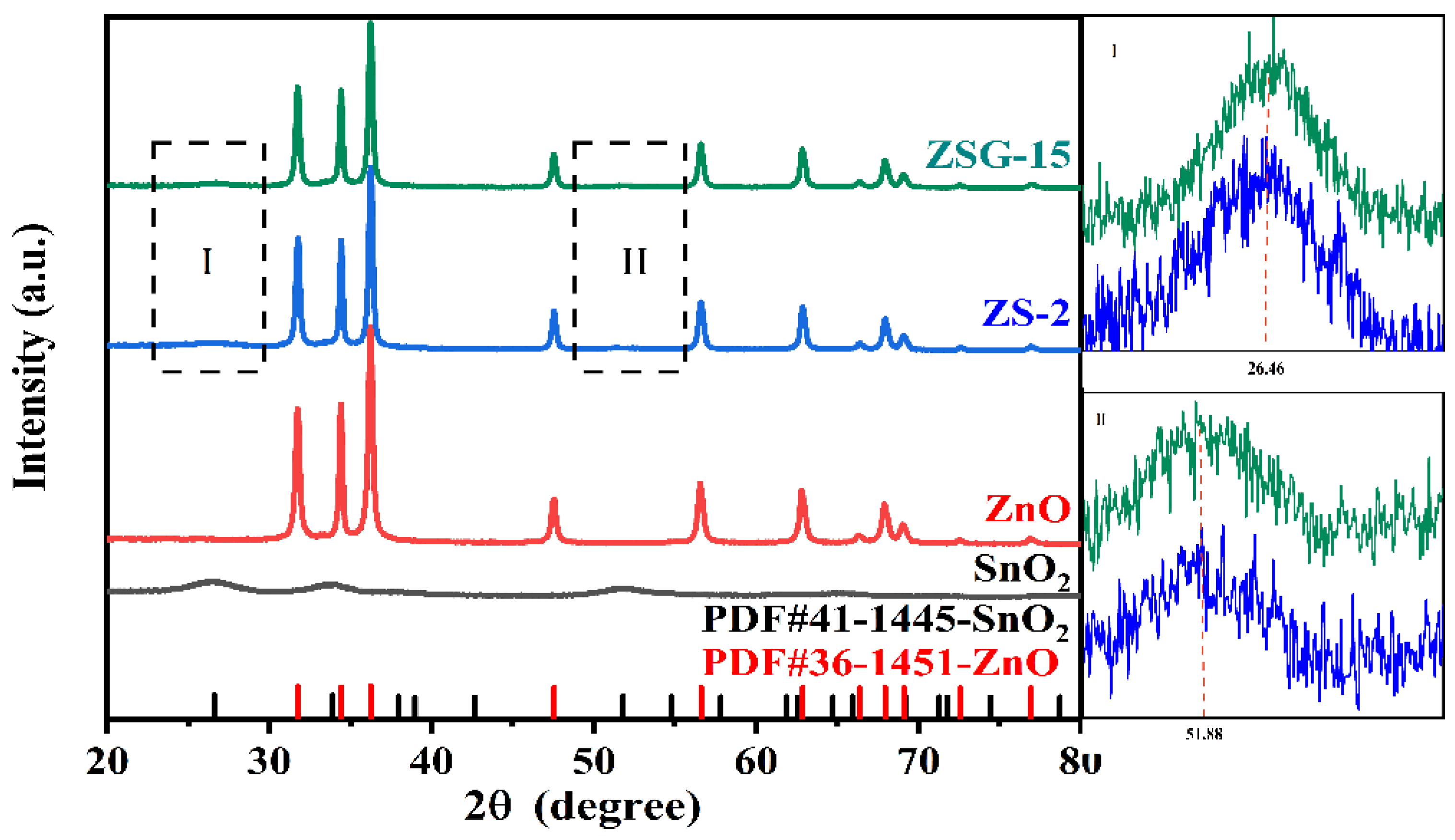
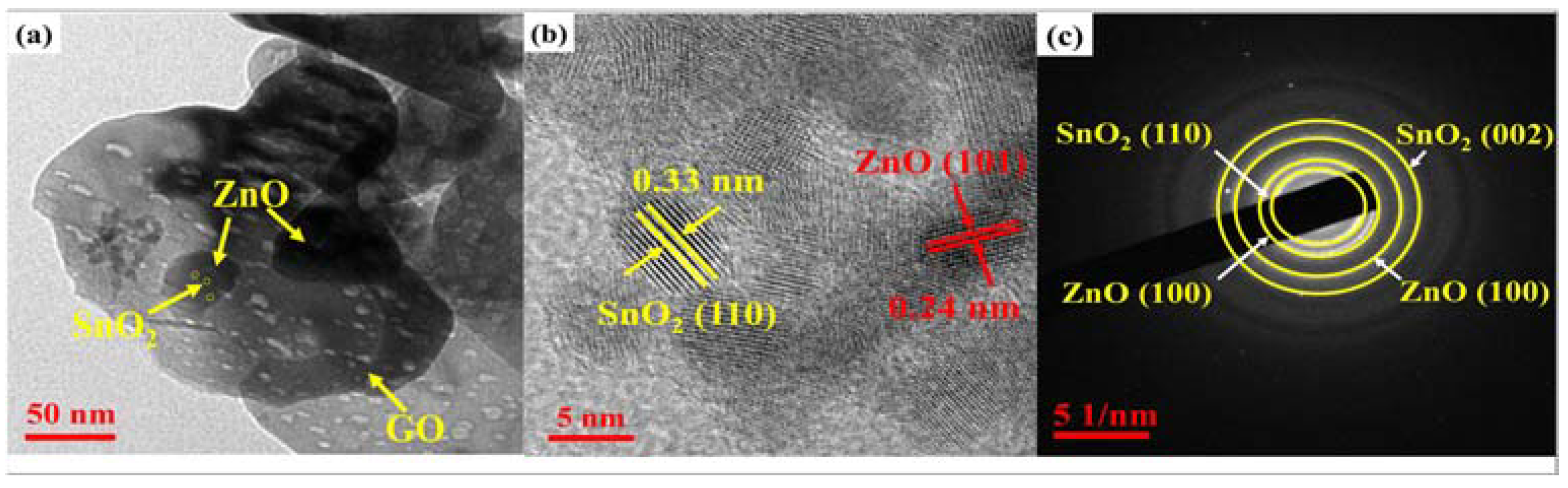
2.1. Crystal Structure and Morphology Analysis
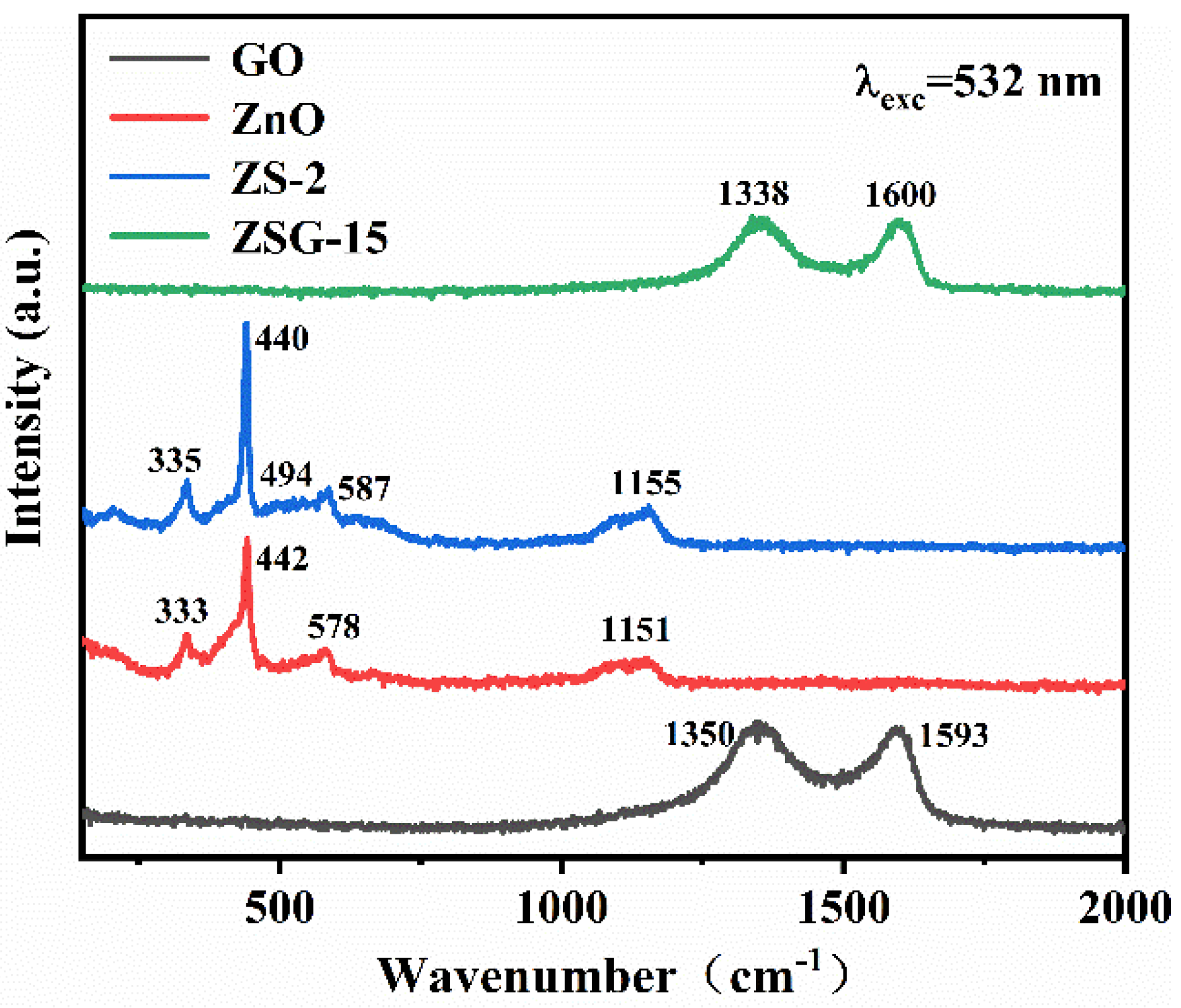
2.2. Element and Valence Analysis
2.3. Optical Properties Analysis
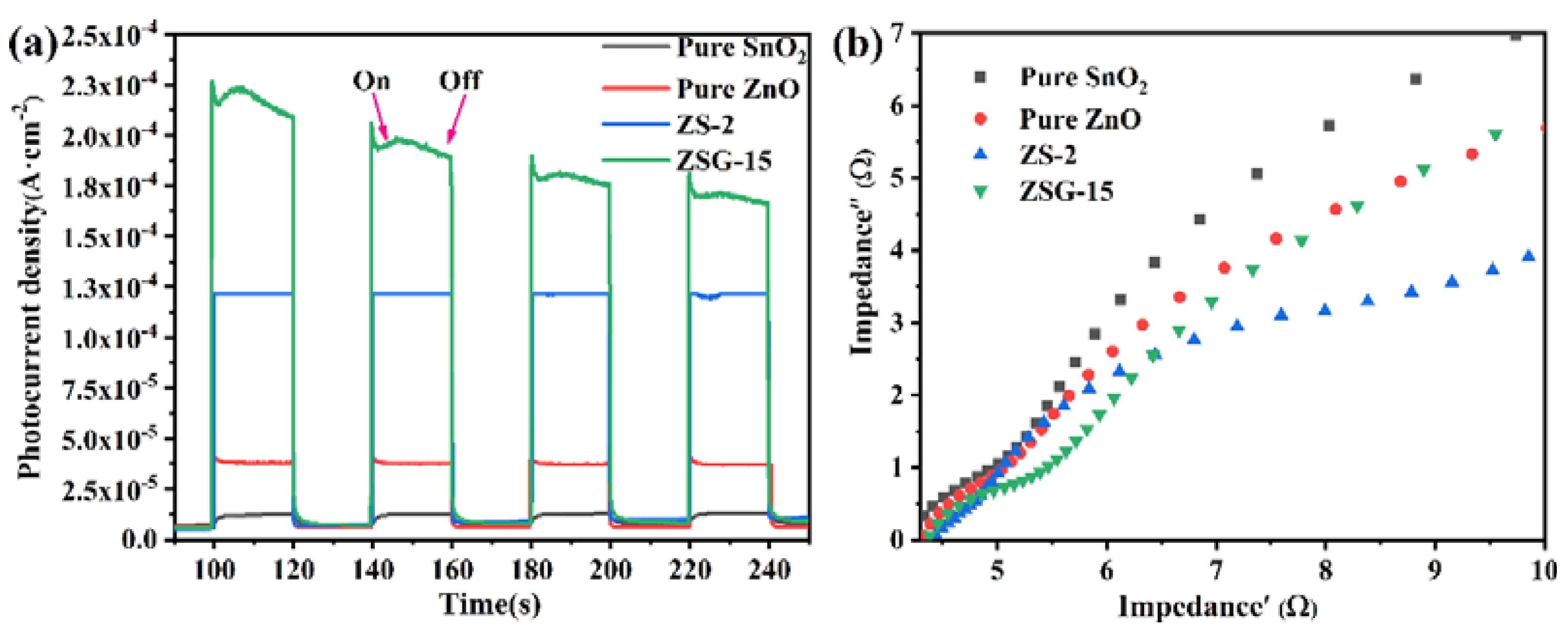
2.4. Electrochemical Properties
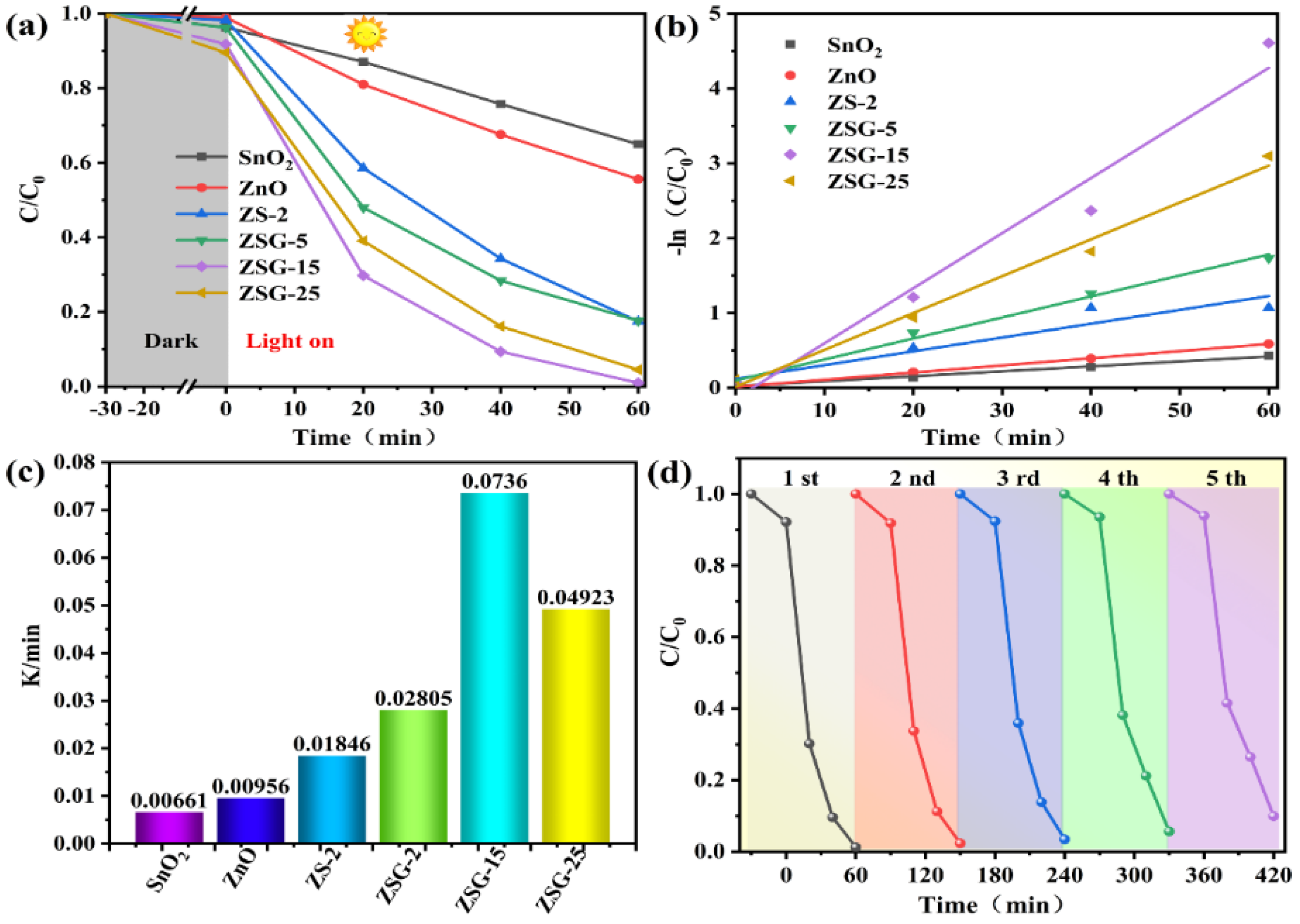
2.5. Photocatalytic Activity Evaluation
2.6. Possible Photocatalytic Mechanism Investigation
3. Materials and Methods
3.1. Materials
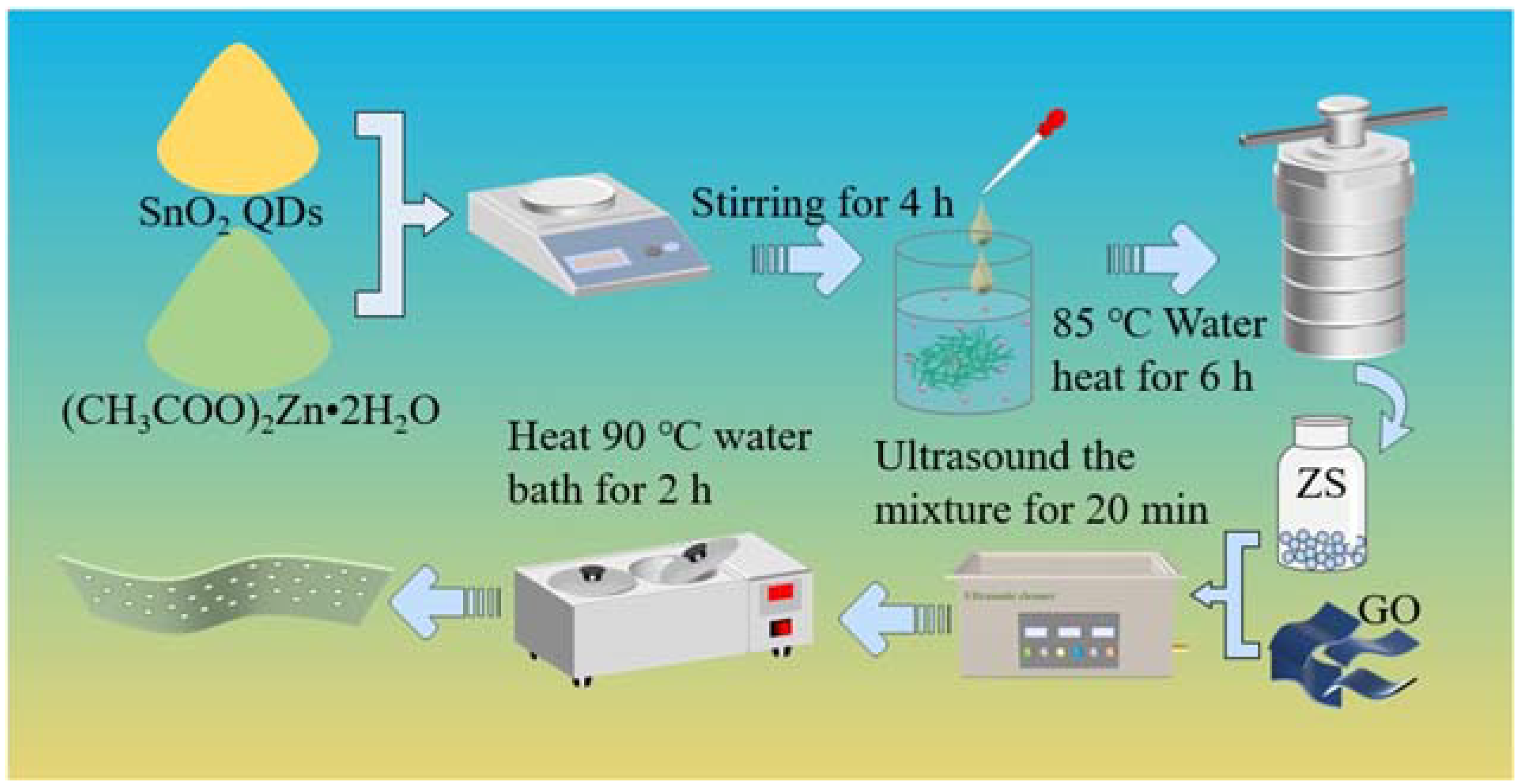
3.2. Synthesis of SnO2 Quantum Dots
3.3. Sample Preparation for GO, ZS, and ZSG
3.4. Photocatalyst Characterization
3.5. Determination of Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, J.; Li, H.; Zhang, L. New Insight into Daylight Photocatalysis of AgBr@Ag: Synergistic Effect between Semiconductor Photocatalysis and Plasmonic Photocatalysis. Chem. Eur. J. 2012, 18, 6360–6369. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, W.; Hang, T.; Wu, L.; Yang, X. Roles of MXenes in Photocatalysis. Sol. RRL 2024, 8, 2300691. [Google Scholar] [CrossRef]
- Xie, S.; Gu, A.Z.; Cen, T.; Li, D.; Chen, J. The effect and mechanism of urban fine particulate matter (PM2.5) on horizontal transfer of plasmid-mediated antimicrobial resistance genes. Sci. Total Environ. 2019, 683, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.-Y.; Ma, Y.-L.; Zhang, J.-T.; Fan, N.-S.; Huang, B.-C.; Jin, R.-C. A critical review of antibiotic removal strategies: Performance and mechanisms. J. Water Process. Eng. 2020, 38, 101681. [Google Scholar] [CrossRef]
- Kumar, S.; Kaushik, R.D.; Purohit, L.P. RGO supported ZnO/SnO2 Z-scheme heterojunctions with enriched ROS production towards enhanced photocatalytic mineralization of phenolic compounds and antibiotics at low temperature. J. Colloid Interface Sci. 2023, 632, 196–215. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Chen, C.; Cao, S.; Qian, G.; Nie, X.; Yu, W. Enhanced sunlight-driven photocatalytic activity of graphene oxide/Bi2WO6 nanoplates by silicon modification. Ceram. Int. 2015, 41, 10087–10094. [Google Scholar] [CrossRef]
- Chen, C.; Yu, W.; Liu, T.; Cao, S.; Tsang, Y. Graphene oxide/WS2/Mg-doped ZnO nanocomposites for solar-light catalytic and anti-bacterial applications. Sol. Energy Mater Sol. Cells 2017, 160, 43–53. [Google Scholar] [CrossRef]
- Long, H.; Huang, R.; Liu, X.; Chen, C. Preparation and photocatalytic performance of ZnO/CuO/GO heterojunction under visible light. Inorg. Chem. Commun. 2022, 136, 109097. [Google Scholar] [CrossRef]
- Hernández-Carrillo, M.A.; Torres-Ricárdez, R.; García-Mendoza, M.F.; Ramírez-Morales, E.; Rojas-Blanco, L.; Díaz-Flores, L.L.; Sepúlveda-Palacios, G.E.; Paraguay-Delgado, F.; Pérez-Hernández, G. Eu-modified ZnO nanoparticles for applications in photocatalysis. Catal. Today 2020, 349, 191–197. [Google Scholar] [CrossRef]
- Wu, L.; Fu, C.; Huang, W. Surface chemistry of TiO2 connecting thermal catalysis and photocatalysis. Phys. Chem. Chem. Phys. 2020, 22, 9875–9909. [Google Scholar] [CrossRef]
- Kandasamy, M.; Seetharaman, A.; Sivasubramanian, D.; Nithya, A.; Jothivenkatachalam, K.; Maheswari, N.; Gopalan, M.; Dillibabu, S.; Eftekhari, A. Ni-Doped SnO2 Nanoparticles for Sensing and Photocatalysis. ACS Appl. Nano Mater. 2018, 1, 5823–5836. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, K.; Sun, J.; Luo, R.; Li, D.; Chen, A. Surface decoration of WO3 architectures with Fe2O3 nanoparticles for visible-light-driven photocatalysis. CrystEngComm 2014, 16, 3289. [Google Scholar] [CrossRef]
- Pandith, A.; Jayaprakash, G.K.; ALOthman, Z.A. Surface-modified CuO nanoparticles for photocatalysis and highly efficient energy storage devices. Environ. Sci. Pollut. Res. 2023, 30, 43320–43330. [Google Scholar] [CrossRef] [PubMed]
- Hou, G.; Cheng, Z.; Kang, L.; Xu, X.; Zhang, F.; Yang, H. Controllable synthesis of CuS decorated TiO2 nanofibers for enhanced photocatalysis. CrystEngComm 2015, 17, 5496–5501. [Google Scholar] [CrossRef]
- Upadhaya, D.; Dhar Purkayastha, D. Self-cleaning activity of CuO/ZnO heterostructure: A synergy of photocatalysis and hydrophilicity. J. Taiwan Inst. Chem. Eng. 2022, 132, 104216. [Google Scholar] [CrossRef]
- Nandi, P.; Das, D. ZnO/CdS/CuS heterostructure: A suitable candidate for applications in visible-light photocatalysis. J. Phys. Chem. Solids 2022, 160, 110344. [Google Scholar] [CrossRef]
- Aziz, N.A.B.; Sheng, C.K. Impact of CuO loading for enhanced photocatalytic performance of CdS/CuO photocatalyst on rhodamine 6G dye decomposition under ultraviolet irradiation. Mater. Lett. 2023, 347, 134589. [Google Scholar] [CrossRef]
- Pan, J.; Li, J.; Yan, Z.; Zhou, B.; Wu, H.; Xiong, X. SnO2@CdS nanowire-quantum dots heterostructures: Tailoring optical properties of SnO2 for enhanced photodetection and photocatalysis. Nanoscale 2013, 5, 3022. [Google Scholar] [CrossRef] [PubMed]
- Zalfani, M.; Van Der Schueren, B.; Mahdouani, M.; Bourguiga, R.; Yu, W.-B.; Wu, M.; Deparis, O.; Li, Y.; Su, B.-L. ZnO quantum dots decorated 3DOM TiO2 nanocomposites: Symbiose of quantum size effects and photonic structure for highly enhanced photocatalytic degradation of organic pollutants. Appl. Catal. B 2016, 199, 187–198. [Google Scholar] [CrossRef]
- Mohamed, I.M.A.; Dao, V.-D.; Yasin, A.S.; Choi, H.-S.; Khalil, K.; Barakat, N.A.M. Facile synthesis of GO@SnO2/TiO2 nanofibers and their behavior in photovoltaics. J. Colloid Interface Sci. 2017, 490, 303–313. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, J.; Chen, T.; Xiang, C.; Zhao, Y.; Zhang, N. Hydrothermal synthesis of Pd-doped rGO/ZnO-SnO2 nanocomposites for efficient hydrogen detection. Ionics 2022, 28, 3013–3021. [Google Scholar] [CrossRef]
- Sen, S.; Kundu, S. Reduced graphene oxide (rGO) decorated ZnO-SnO2: A ternary nanocomposite towards improved low concentration VOC sensing performance. J. Alloys Compd. 2021, 881, 160406. [Google Scholar] [CrossRef]
- Yang, L.; Xu, C.; Wan, F.; He, H.; Gu, H.; Xiong, J. Synthesis of RGO/BiOI/ZnO composites with efficient photocatalytic reduction of aqueous Cr(VI) under visible-light irradiation. Mater. Res. Bull. 2019, 112, 154–158. [Google Scholar] [CrossRef]
- Chen, D.; Huang, S.; Huang, R.; Zhang, Q.; Le, T.-T.; Cheng, E.; Yue, R.; Hu, Z.; Chen, Z. Construction of Ni-doped SnO2-SnS2 heterojunctions with synergistic effect for enhanced photodegradation activity. J. Hazard. Mater. 2019, 368, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Lipson, H. Elements of X-ray diffraction. Contemp. Phys. 1979, 20, 87–88. [Google Scholar]
- Miao, X.; Shen, X.; Wu, J.; Ji, Z.; Wang, J.; Kong, L.; Liu, M.; Song, C. Fabrication of an all solid Z-scheme photocatalyst g-C3N4/GO/AgBr with enhanced visible light photocatalytic activity. Appl. Catal. A-Gen. 2017, 539, 104–113. [Google Scholar] [CrossRef]
- Mohd Raub, A.A.; Yunas, J.; Mohamed, M.A.; Bais, B.; Hamzah, A.A.; Ridwan, J.; Kazmi, J.; Hassan, M.A. Synthesis and characterization of ZnO NRs with spray coated GO for enhanced photocatalytic activity. Ceram. Int. 2022, 48, 18238–18245. [Google Scholar] [CrossRef]
- Moussa, H.; Girot, E.; Mozet, K.; Alem, H.; Medjahdi, G.; Schneider, R. ZnO rods/reduced graphene oxide composites prepared via a solvothermal reaction for efficient sunlight-driven photocatalysis. Appl. Catal. B 2016, 185, 11–21. [Google Scholar] [CrossRef]
- Huang, C.; Li, J. A synergistic effect between ZnO/CdS S-scheme heterojunction and GO cocatalyst for boosting photocatalytic performance. Opt. Mater. 2023, 139, 113726. [Google Scholar] [CrossRef]
- Naik, A.; Parkin, I.; Binions, R. Gas Sensing Studies of an n-n Hetero-Junction Array Based on SnO2 and ZnO Composites. Chemosensors 2016, 4, 3. [Google Scholar] [CrossRef]
- Prabakaran, S.; Nisha, K.D.; Harish, S.; Archana, J.; Navaneethan, M.; Ponnusamy, S.; Muthamizhchelvan, C.; Ikeda, H.; Hayakawa, Y. Synergistic effect and enhanced electrical properties of TiO2/SnO2/ZnO nanostructures as electron extraction layer for solar cell application. Appl. Surf. Sci. 2019, 498, 143702. [Google Scholar] [CrossRef]
- Hu, K.; Wang, F.; Yan, Y.; Liu, H.; Shen, Z. One step from nanofiber to functional hybrid structure: Pd doped ZnO/SnO2 heterojunction nanofibers with hexagonal ZnO columns for enhanced low-temperature hydrogen gas sensing. Ceram. Int. 2021, 47, 15228–15236. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Radiman, S.; Daud, A.R.; Tabet, N.; Al-Douri, Y. XPS and optical studies of different morphologies of ZnO nanostructures prepared by microwave methods. Ceram. Int. 2013, 39, 2283–2292. [Google Scholar] [CrossRef]
- Cheng, Y.; Shao, T.; Dong, J.; Kou, H.; Zhang, F.; Guo, J.; Liu, X. MOF-derived SnO2@ZnO ethanol sensors with enhanced gas sensing properties. Vacuum 2023, 216, 112440. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, H. Cu2ZnSnS4 synthesized through a green and economic process. J. Alloys Compd. 2011, 509, 9627–9630. [Google Scholar] [CrossRef]
- Avendaño, C.A.M.; Mathews, N.R.; Pal, M.; Delgado, F.P.; Mathew, X. Structural Evolution of Multilayer SnS/Cu/ZnS Stack to Phase-Pure Cu2ZnSnS4 Thin Films by Thermal Processing. ECS J. Solid State Sci. Technol. 2015, 4, P91–P96. [Google Scholar] [CrossRef]
- Thiyagarajan, K.; Sivakumar, K. Oxygen vacancy-induced room temperature ferromagnetism in graphene–SnO2 nanocomposites. J. Mater. Sci. 2017, 52, 8084–8096. [Google Scholar] [CrossRef]
- Choi, S.-J.; Jang, B.-H.; Lee, S.-J.; Min, B.K.; Rothschild, A.; Kim, I.-D. Selective Detection of Acetone and Hydrogen Sulfide for the Diagnosis of Diabetes and Halitosis Using SnO2 Nanofibers Functionalized with Reduced Graphene Oxide Nanosheets. ACS Appl. Mater. Interfaces 2014, 6, 2588–2597. [Google Scholar] [CrossRef]
- Beura, R.; Thangadurai, P. Effect of Sn doping in ZnO on the photocatalytic activity of ZnO-Graphene nanocomposite with improved activity. J. Environ. Chem. Eng. 2018, 6, 5087–5100. [Google Scholar] [CrossRef]
- Zhu, Y.-P.; Li, M.; Liu, Y.-L.; Ren, T.-Z.; Yuan, Z.-Y. Carbon-Doped ZnO Hybridized Homogeneously with Graphitic Carbon Nitride Nanocomposites for Photocatalysis. J. Phys. Chem. C. 2014, 118, 10963–10971. [Google Scholar] [CrossRef]
- Liu, S.; Sun, H.; Suvorova, A.; Wang, S. One-pot hydrothermal synthesis of ZnO-reduced graphene oxide composites using Zn powders for enhanced photocatalysis. Chem. Eng. J. 2013, 229, 533–539. [Google Scholar] [CrossRef]
- Xie, Y.P.; Yang, Y.; Wang, G.; Liu, G. Oxygen vacancies promoted interfacial charge carrier transfer of CdS/ZnO heterostructure for photocatalytic hydrogen generation. J. Colloid Interface Sci. 2017, 503, 198–204. [Google Scholar] [CrossRef]
- Svoboda, L.; Škuta, R.; Matějka, V.; Dvorský, R.; Matýsek, D.; Henych, J. Graphene oxide and graphitic carbon nitride nanocom posites assembled by electrostatic attraction forces: Synthesis and characterization. Mater. Chem. Phys. 2019, 228, 228–236. [Google Scholar] [CrossRef]
- Chen, C.-C.; Chang, S.-H.; Shaya, J.; Liu, F.-Y.; Lin, Y.-Y.; Wang, L.-G.; Tsai, H.-Y.; Lu, C.-S. Hydrothermal synthesis of BiOxBry/BiOmIn/GO composites with visible-light photocatalytic activity. J. Taiwan Inst. Chem. Eng. 2022, 133, 104272. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, K.; Xu, R.; Yu, D.; Wang, W.; Gao, P.; Liu, B. Fabrication of BiVO4/BiPO4/GO composite photocatalytic material for the visible light-driven degradation. J. Clean. Prod. 2020, 247, 119108. [Google Scholar] [CrossRef]
- Luo, W.; Ying, J.; Yu, S.; Yang, X.; Jia, Y.; Chen, M.; Zhang, H.; Gao, J.; Li, Y.; Mai, Y.-W.; et al. ZnS:Cu powders with strong visible-light photocatalysis and pyro-catalysis for room-temperature dye decomposition. Ceram. Int. 2020, 46, 12096–12101. [Google Scholar]
- Segovia, M.; Alegría, M.; Aliaga, J.; Celedon, S.; Ballesteros, L.; Sotomayor-Torres, C.; González, G.; Benavente, E. Heterostructured 2D ZnO hybrid nanocomposites sensitized with cubic Cu2O nanoparticles for sunlight photocatalysis. J. Mater. Sci. 2019, 54, 13523–13536. [Google Scholar] [CrossRef]
- Bayantong, A.R.B.; Shih, Y.-J.; Dong, C.-D.; Garcia-Segura, S.; De Luna, M.D.G. Nickel ferrite nanoenabled graphene oxide (NiFe2O4@GO) as photoactive nanocomposites for water treatment. Environ. Sci. Pollut. Res. 2021, 28, 5472–5481. [Google Scholar] [CrossRef] [PubMed]
- Thirugnanam, N.; Song, H.; Wu, Y. Photocatalytic degradation of Brilliant Green dye using CdSe quantum dots hybridized with graphene oxide under sunlight irradiation. Chin. J. Catal. 2017, 38, 2150–2159. [Google Scholar] [CrossRef]
- De Almeida, G.C.; Della Santina Mohallem, N.; Viana, M.M. Ag/GO/TiO2 nanocomposites: The role of the interfacial charge transfer for application in photocatalysis. Nanotechnology 2022, 33, 035710. [Google Scholar] [CrossRef]
- Tang, J.; Duan, Z.; Xv, Q.; Li, C.; Gao, G.; Luo, W.; Hou, D.; Zhu, Y. ZIF-8-Derived ZnO and SnO2 Form ZnO@SnO2 Composites for Enhanced Photocatalysis under Visible Light. ChemistrySelect 2022, 7, e202201642. [Google Scholar] [CrossRef]
- Puneetham, J.; Kottam, N.; Rathna, A. Investigation of photocatalytic degradation of crystal violet and its correlation with bandgap in ZnO and ZnO/GO nanohybrid. Inorg. Chem. Commun. 2021, 125, 108460. [Google Scholar]
- Akshhayya, C.; Okla, M.K.; Thomas, A.M.; AL-ghamdi, A.A.; Abdel-Maksoud, M.A.; Almunqedhi, B.; AbdElgawad, H.; Raju, L.L.; Khan, S.S. Insights into photocatalytic mechanism for the rational design of p-n heterojunction by decorating mesoporous SnS2 over ZnFe2O4 nanocomposite for accelerated visible light photocatalysis. Mater. Chem. Phys. 2022, 277, 125464. [Google Scholar] [CrossRef]
- Palanisamy, G.; Bhuvaneswari, K.; Pazhanivel, T.; Shankar, R.; Katubi, K.M.; Alsaiari, N.S.; Ouladsmane, M. ZnS quantum dots and Bi metals embedded with two dimensional β-Bi2O4 nanosheets for efficient UV-visible light driven photocatalysis. Mater. Res. Bull. 2021, 142, 111387. [Google Scholar]
- Hezam, A.; Namratha, K.; Drmosh, Q.A.; Ponnamma, D.; Nagi Saeed, A.M.; Ganesh, V.; Neppolian, B.; Byrappa, K. Direct Z-scheme Cs2O–Bi2O3–ZnO heterostructures for photocatalytic overall water splitting. J. Mater. Chem. A 2018, 6, 21379–21388. [Google Scholar] [CrossRef]
- Uddin, M.T.; Hoque, M.E.; Chandra Bhoumick, M. Facile one-pot synthesis of heterostructure SnO2 /ZnO photocatalyst for enhanced photocatalytic degradation of organic dye. RSC Adv. 2020, 10, 23554–23565. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Schoonen, M.A.A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Mineral. 2000, 85, 543–556. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, W.; Hu, X.; Xu, L.; Chen, G.; Li, X. Hollow spherical WO3/TiO2 heterojunction for enhancing photocatalytic performance in visible-light. J. Water Process. Eng. 2021, 40, 101943. [Google Scholar] [CrossRef]
- Sinar Mashuri, S.I.; Kasim, M.F.; Mohd Kaus, N.H.; Tan, Y.H.; Islam, A.; Rashid, U.; Asikin-Mijan, N.; Andas, J.; Taufiq-Yap, Y.H.; Yaakob, M.K.; et al. Photo-response range extension of Z-scheme ZnO/CdS for LED-light-driven photo-active catalyst. Renew. Sust. Energ. Rev. 2023, 184, 113602. [Google Scholar] [CrossRef]
- Molahalli, V.; Bhat, V.S.; Shetty, A.; Hundekal, D.; Toghan, A.; Hegde, G. ZnO doped SnO2 nano flower decorated on graphene oxide/polypyrrole nanotubes for symmetric supercapacitor applications. J. Energy Storage 2023, 69, 107953. [Google Scholar] [CrossRef]
- Zhao, Y.-Y.; Wang, X.-B.; Xu, Q.-K.; Chakir, S.; Xu, Y.-F.; Xu, B.; Hu, Y.-H. Micro-/nanostructured ZnFe2O4 hollow sphere/GO composite for structurally enhanced photocatalysis performance. Rare Met. 2023, 42, 813–821. [Google Scholar] [CrossRef]
- Chen, S.; Liu, F.; Xu, M.; Yan, J.; Zhang, F.; Zhao, W.; Zhang, Z.; Deng, Z.; Yun, J.; Chen, R.; et al. First-principles calculations and experimental investigation on SnO2@ZnO heterojunction photocatalyst with enhanced photocatalytic performance. J. Colloid. Interface Sci. 2019, 553, 613–621. [Google Scholar] [CrossRef] [PubMed]

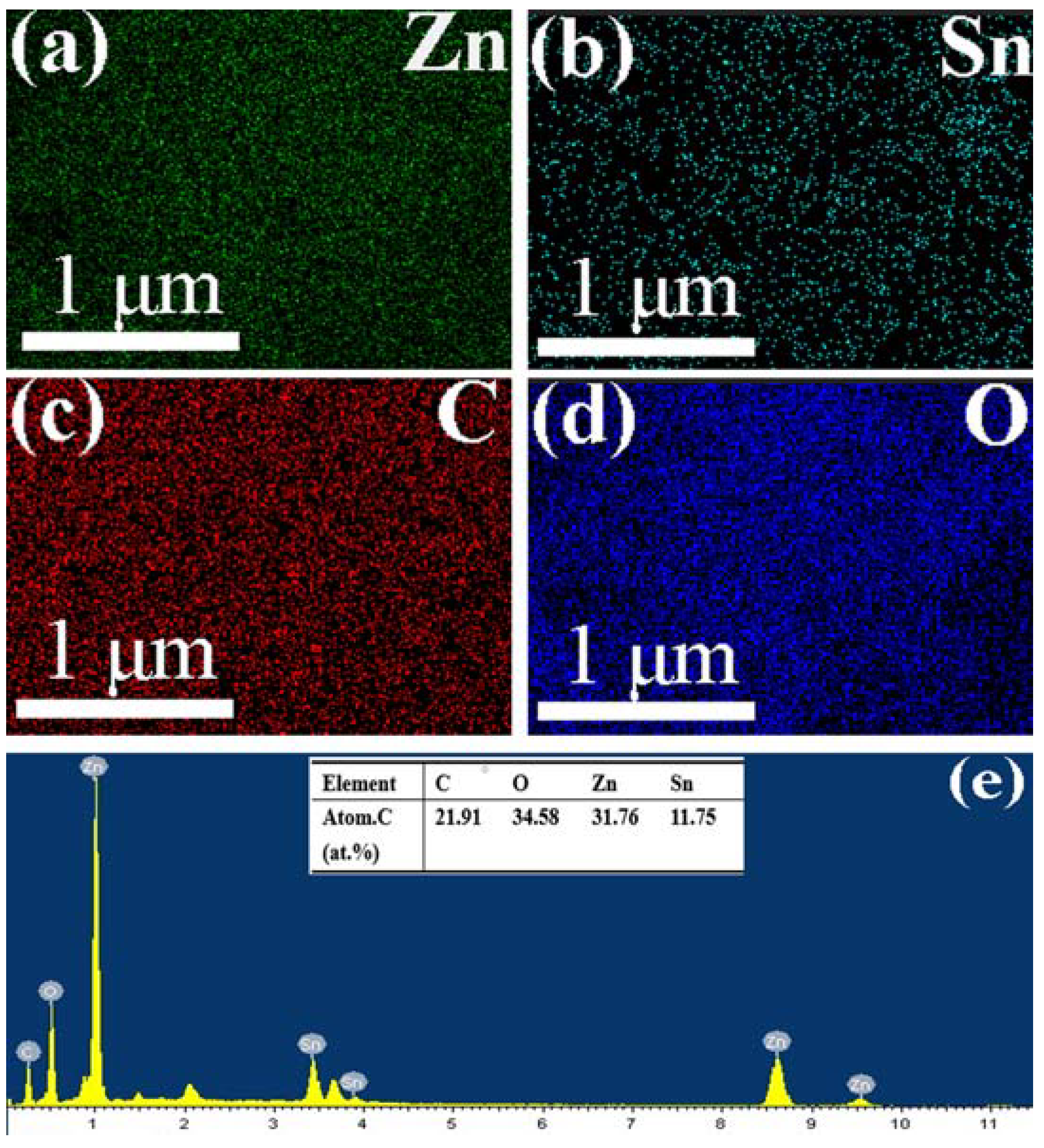
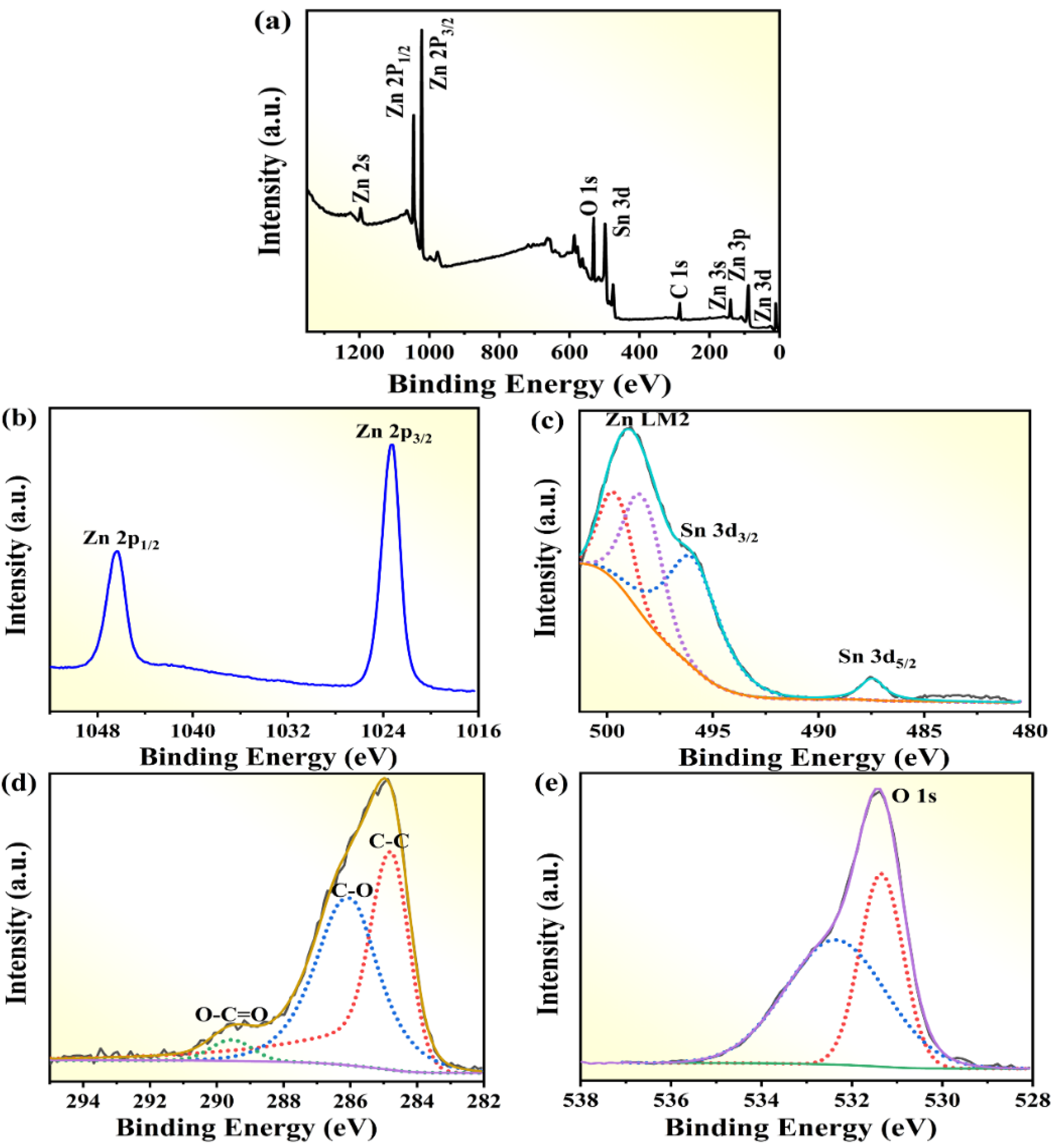
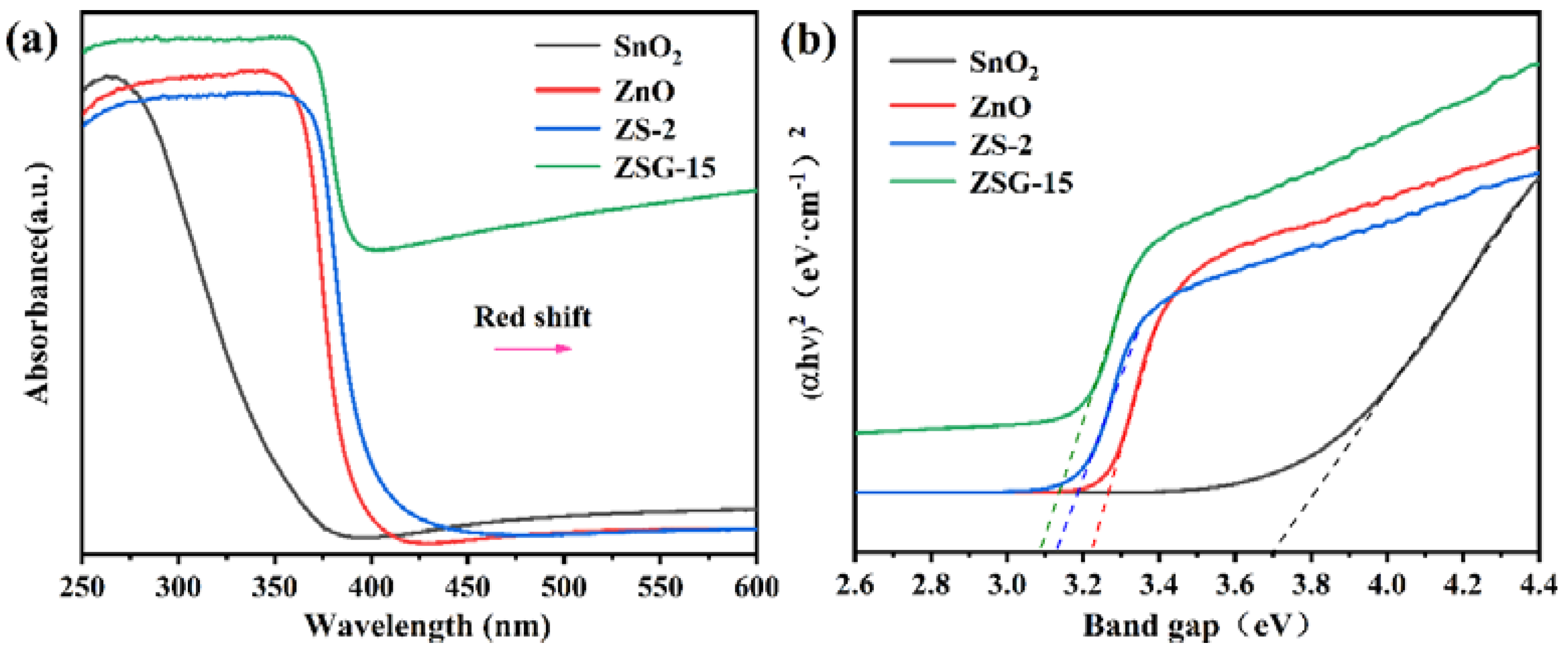
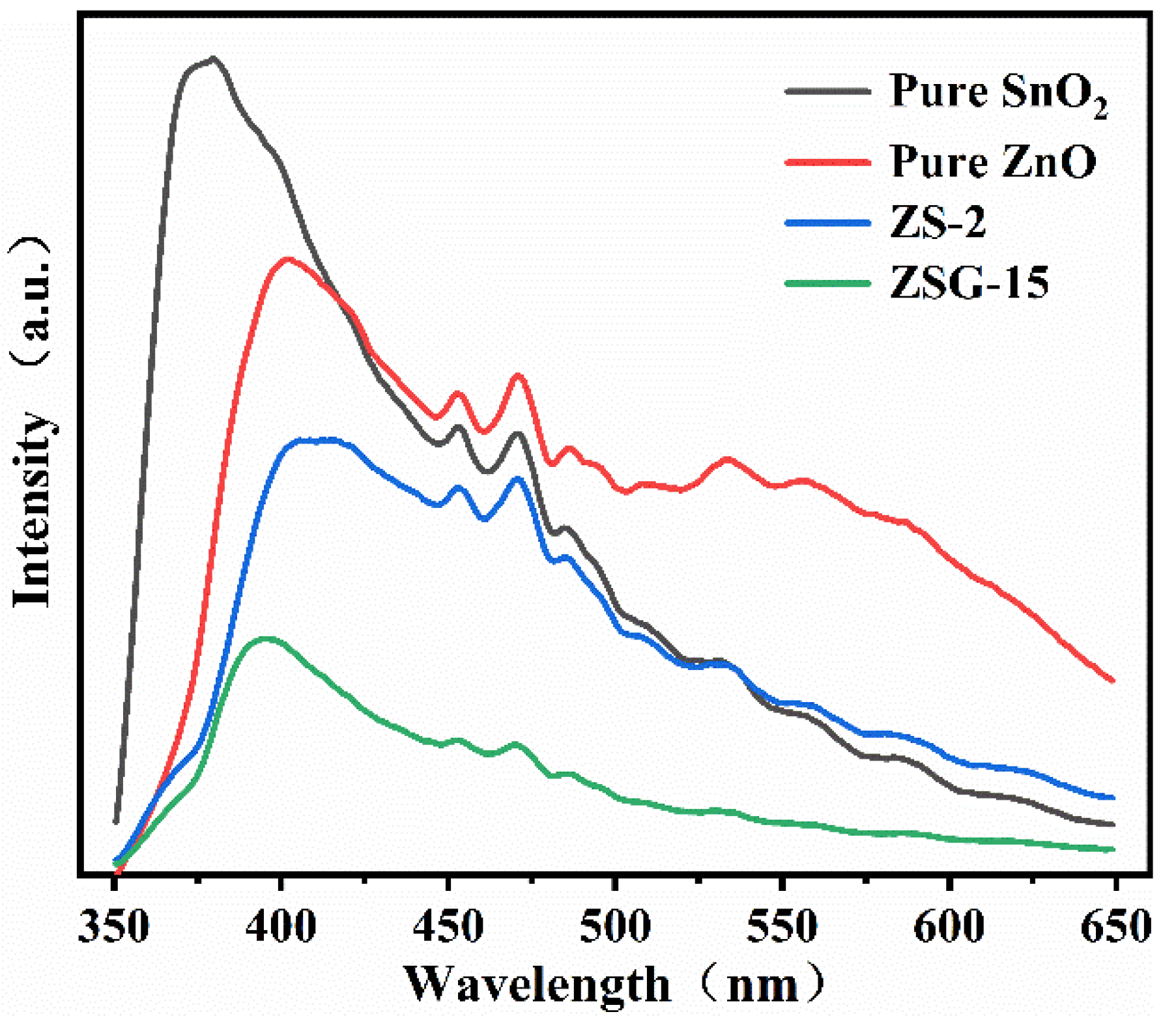


| S.NO | Photocatalyst | Preparation Method | Pollutant | Degradation Efficiency | Ref. |
|---|---|---|---|---|---|
| 1 | NiFe2O4@GO | Hydrothermal | MB | 100% in 120 min | [48] |
| 2 | CdSe/GO | Aqueous phase | BG | 95% in 90 min | [49] |
| 3 | Ag/GO/TiO2 | Aqueous phase | MB | 97% in 180 min | [50] |
| 4 | ZnO@SnO2 | Hydrothermal | TC | 91.2% in 90 min | [51] |
| 5 | ZnO/GO | Solid phase | CV | 99% in 240 min | [52] |
| 6 | SnO2/ZnO@GO | Aqueous phase | RhB | 98.9% in 60 min | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.; Li, J.; Wang, J.; Deng, L. Synergistic Enhancement of Carrier Migration by SnO2/ZnO@GO Heterojunction for Rapid Degradation of RhB. Molecules 2024, 29, 854. https://doi.org/10.3390/molecules29040854
Chen P, Li J, Wang J, Deng L. Synergistic Enhancement of Carrier Migration by SnO2/ZnO@GO Heterojunction for Rapid Degradation of RhB. Molecules. 2024; 29(4):854. https://doi.org/10.3390/molecules29040854
Chicago/Turabian StyleChen, Pengfei, Jin Li, Jianing Wang, and Lihan Deng. 2024. "Synergistic Enhancement of Carrier Migration by SnO2/ZnO@GO Heterojunction for Rapid Degradation of RhB" Molecules 29, no. 4: 854. https://doi.org/10.3390/molecules29040854





