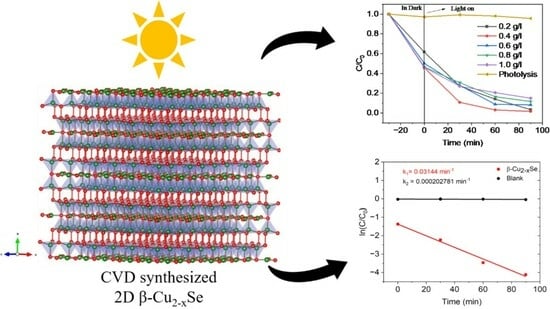
Chemical-Vapor-Deposition-Synthesized Two-Dimensional Non-Stoichiometric Copper Selenide (β-Cu2−xSe) for Ultra-Fast Tetracycline Hydrochloride Degradation under Solar Light
Abstract
:1. Introduction
2. Results and Discussion
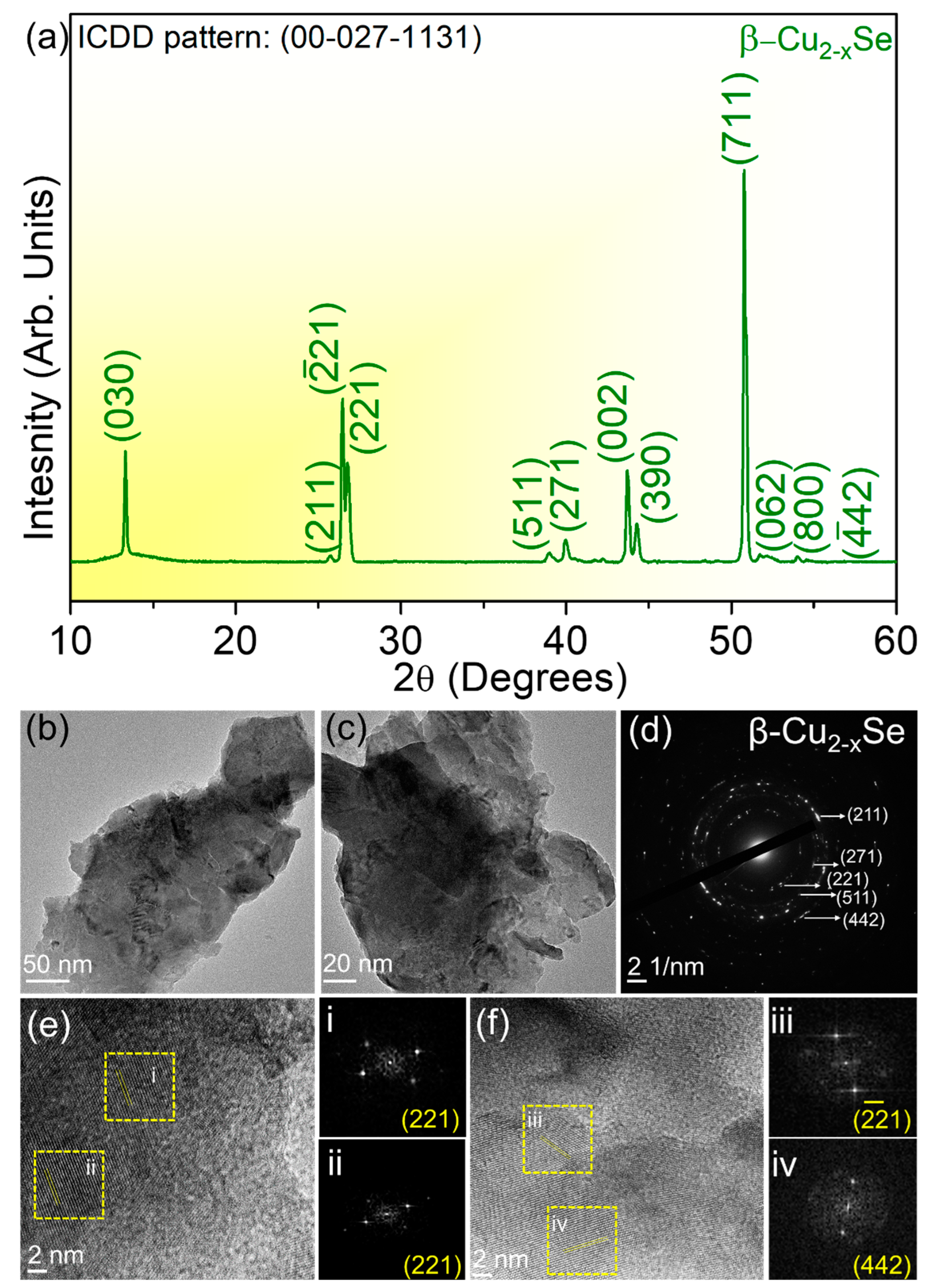
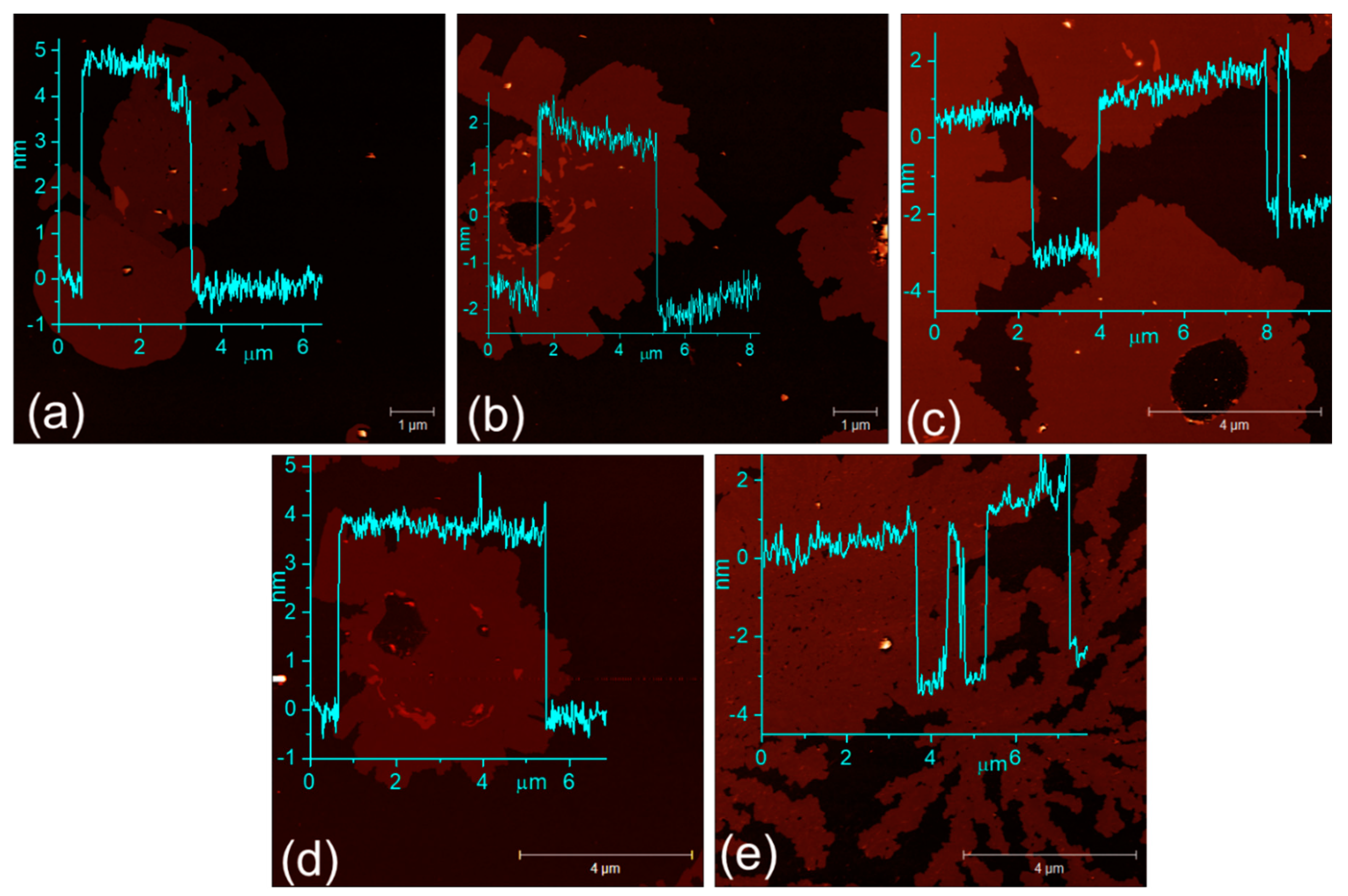
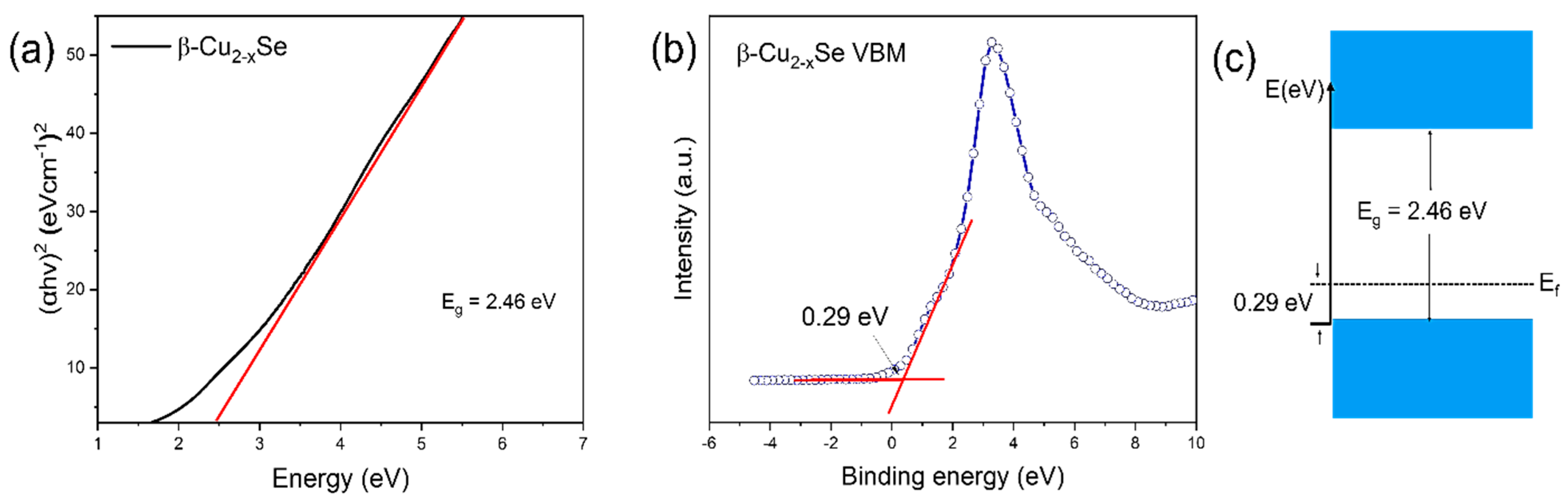
2.1. Understanding the Microstructural Growth, Phase Purity, Thickness, and Related Characterizations of β-Cu2−xSe
- (030) at 2θ~13°, (211) at 2θ~25.3°, (−221) at 2θ~26.3°, (221) at 2θ~26.4°, (511) at 2θ~38.8°, (271) at 2θ~39.8°, (002) at 2θ~43.6°, (390) at 2θ~44.2°, (711) at 2θ~50.8°, (002) at 2θ~43.6°, (062) at 2θ~51.5°, (800) at 2θ~51.8°, (−442) at 2θ~54.2°
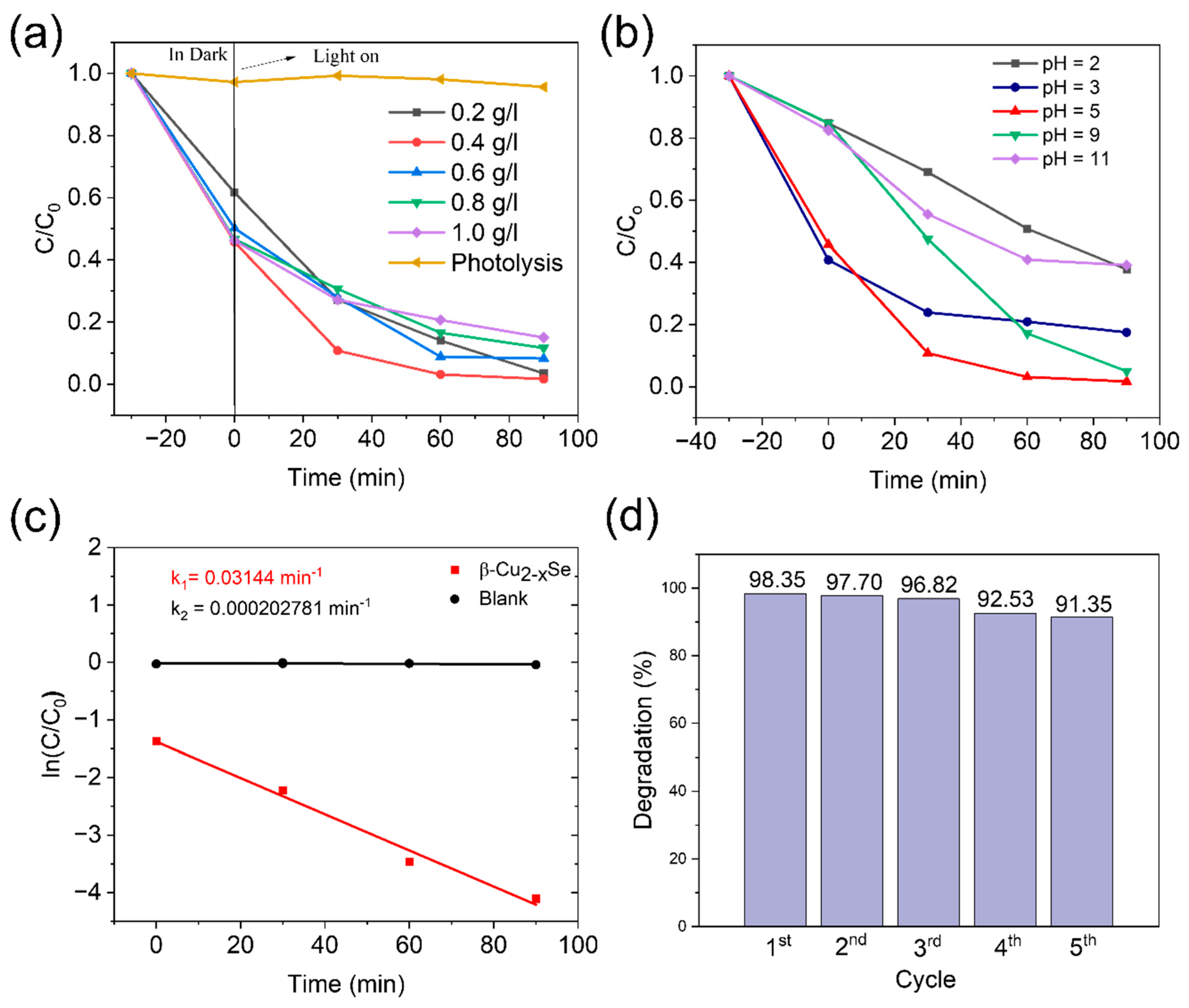
2.2. Photodegradation of Tetracycline Hydrochloride (TC-HCl)
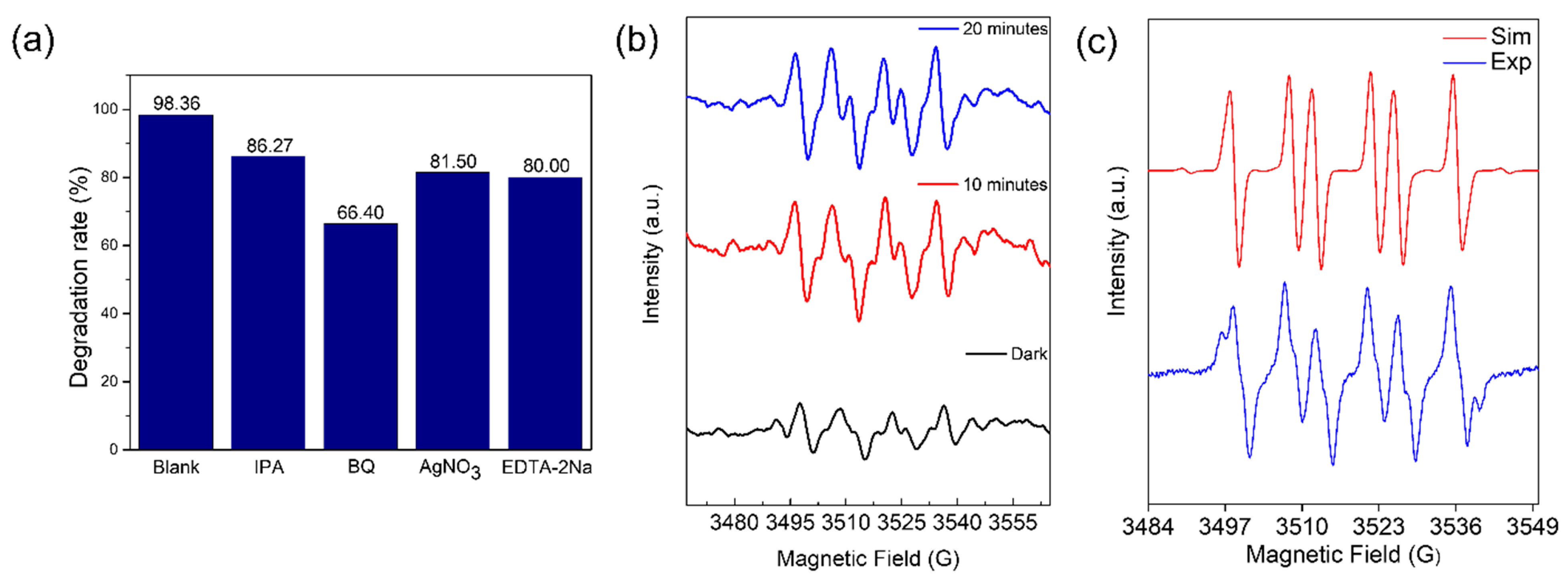
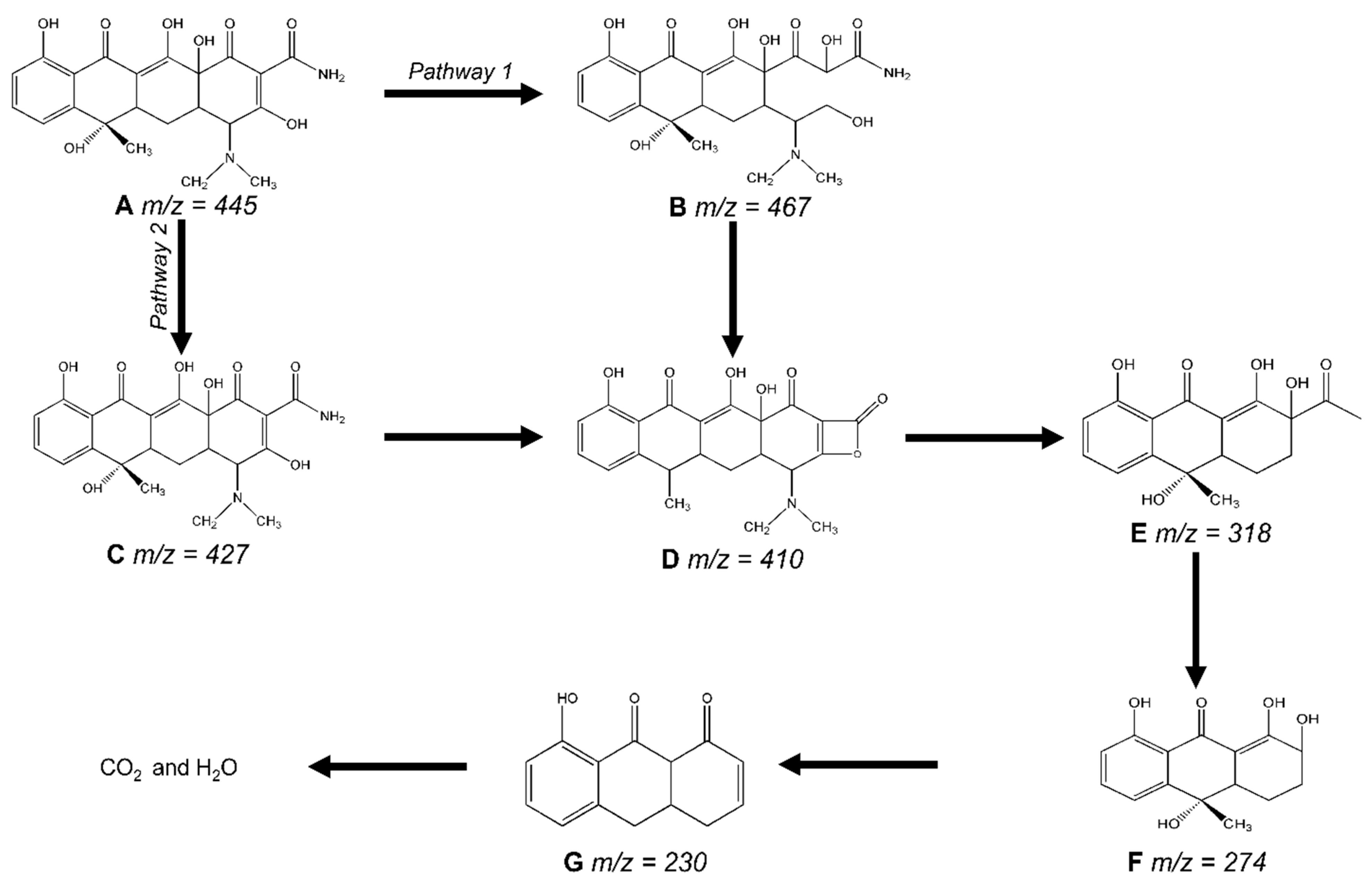
2.3. Photocatalytic Degradation Mechanism
3. Materials and Methods
3.1. CVD Synthesis of β-Cu2−xSe
3.2. Characterizations of the Bulk and Exfoliated β-Cu2−xSe
3.3. Photocatalytic Tests
3.4. Trapping Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, K.-R.; Owens, G.; Kwon, S.-I.; So, K.-H.; Lee, D.-B.; Ok, Y.S. Occurrence and Environmental Fate of Veterinary Antibiotics in the Terrestrial Environment. Water Air Soil Pollut. 2011, 214, 163–174. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Z.; Zhao, W.; Liu, C.; Qian, X.; Zhang, M.; Wei, G.; Khan, E.; Hau Ng, Y.; Sik Ok, Y. Recent Advances in Photodegradation of Antibiotic Residues in Water. Chem. Eng. J. 2021, 405, 126806. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mozaz, S.; Chamorro, S.; Marti, E.; Huerta, B.; Gros, M.; Sànchez-Melsió, A.; Borrego, C.M.; Barceló, D.; Balcázar, J.L. Occurrence of Antibiotics and Antibiotic Resistance Genes in Hospital and Urban Wastewaters and Their Impact on the Receiving River. Water Res. 2015, 69, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lv, K.; Deng, C.; Yu, Z.; Shi, J.; Johnson, A.C. Persistence and Migration of Tetracycline, Sulfonamide, Fluoroquinolone, and Macrolide Antibiotics in Streams Using a Simulated Hydrodynamic System. Environ. Pollut. 2019, 252, 1532–1538. [Google Scholar] [CrossRef] [PubMed]
- Pinkston, K.E. The Fate of Pharmaceutically Active Compounds in Water Reuse Systems; University of California: Berkeley, CA, USA, 2004. [Google Scholar]
- Mueses, M.A.; Colina-Márquez, J.; Machuca-Martínez, F.; Li Puma, G. Recent Advances on Modeling of Solar Heterogeneous Photocatalytic Reactors Applied for Degradation of Pharmaceuticals and Emerging Organic Contaminants in Water. Curr. Opin. Green Sustain. Chem. 2021, 30, 100486. [Google Scholar] [CrossRef]
- Hong, J.; Hwang, D.; Selvaraj, R. Facile Synthesis of Br-Doped g-C3N4 Nanosheets via One-Step Exfoliation Using Ammonium Bromide for Photodegradation of Oxytetracycline Antibiotics. J. Ind. Eng. Chem. 2019, 79, 473–481. [Google Scholar] [CrossRef]
- Panneri, S.; Ganguly, P.; Nair, B.; Mohamed, A.; Warrier, K.; Hareesh, U.S. Role of Precursors on the Photophysical Properties of Carbon Nitride and Its Application for Antibiotic Degradation. Environ. Sci. Pollut. Res. 2017, 24, 8609–8618. [Google Scholar] [CrossRef]
- Luo, B.; Liu, G.; Wang, L. Recent Advances in 2D Materials for Photocatalysis. Nanoscale 2016, 8, 6904–6920. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, X.; Xie, Y. Advances and Challenges in Chemistry of Two-Dimensional Nanosheets. Nano Today 2016, 11, 793–816. [Google Scholar] [CrossRef]
- Tan, C.; Cao, X.; Wu, X.-J.; He, Q.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.-H.; et al. Recent Advances in Ultrathin Two-Dimensional Nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef]
- Zaki, T. Catalytic Dehydration of Ethanol Using Transition Metal Oxide Catalysts. J. Colloid Interface Sci. 2005, 284, 606–613. [Google Scholar] [CrossRef]
- Yun, Q.; Li, L.; Hu, Z.; Lu, Q.; Chen, B.; Zhang, H. Layered Transition Metal Dichalcogenide-Based Nanomaterials for Electrochemical Energy Storage. Adv. Mater. 2020, 32, 1903826. [Google Scholar] [CrossRef]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.-J.; Loh, K.P.; Zhang, H. The Chemistry of Two-Dimensional Layered Transition Metal Dichalcogenide Nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef]
- Singh, V.K.; Jain, P.; Panda, S.; Kuila, B.K.; Pitchaimuthu, S.; Das, S. Sulfonic Acid/Sulfur Trioxide (SO3H/SO3) Functionalized Two-Dimensional MoS2 Nanosheets for High-Performance Photocatalysis of Organic Pollutants. New J. Chem. 2022, 46, 13636–13642. [Google Scholar] [CrossRef]
- Gao, H.; Hu, Y.; Xuan, Y.; Li, J.; Yang, Y.; Martinez, R.V.; Li, C.; Luo, J.; Qi, M.; Cheng, G.J. Large-Scale Nanoshaping of Ultrasmooth 3D Crystalline Metallic Structures. Science 2014, 346, 1352–1356. [Google Scholar] [CrossRef]
- Wang, L.; Han, Y.; Feng, X.; Zhou, J.; Qi, P.; Wang, B. Metal–Organic Frameworks for Energy Storage: Batteries and Supercapacitors. Coord. Chem. Rev. 2016, 307, 361–381. [Google Scholar] [CrossRef]
- Lu, X.F.; Fang, Y.; Luan, D.; Lou, X.W.D. Metal–Organic Frameworks Derived Functional Materials for Electrochemical Energy Storage and Conversion: A Mini Review. Nano Lett. 2021, 21, 1555–1565. [Google Scholar] [CrossRef]
- Wu, S.; Hui, K.S.; Hui, K.N. 2D Black Phosphorus: From Preparation to Applications for Electrochemical Energy Storage. Adv. Sci. 2018, 5, 1700491. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.R.; Rao, G.S.; Luo, W.; Ahuja, R.; Hussain, T. Hexagonal Boron Nitride (h-BN) Sheets Decorated with OLi, ONa, and Li2F Molecules for Enhanced Energy Storage. ChemPhysChem 2017, 18, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Zhang, K.; Wang, Z.; Li, C.; Zhu, K.; Yao, Y.; Hong, G. Synthesis and Modification of Boron Nitride Nanomaterials for Electrochemical Energy Storage: From Theory to Application. Adv. Funct. Mater. 2021, 31, 2106315. [Google Scholar] [CrossRef]
- Manzeli, S.; Ovchinnikov, D.; Pasquier, D.; Yazyev, O.V.; Kis, A. 2D Transition Metal Dichalcogenides. Nat. Rev. Mater. 2017, 2, 17033. [Google Scholar] [CrossRef]
- Xu, X.; Yao, W.; Xiao, D.; Heinz, T.F. Spin and Pseudospins in Layered Transition Metal Dichalcogenides. Nat. Phys. 2014, 10, 343–350. [Google Scholar] [CrossRef]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and Optoelectronics of Two-Dimensional Transition Metal Dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef]
- Mak, K.F.; Shan, J. Photonics and Optoelectronics of 2D Semiconductor Transition Metal Dichalcogenides. Nat. Photonics 2016, 10, 216–226. [Google Scholar] [CrossRef]
- Telkhozhayeva, M.; Konar, R.; Lavi, R.; Teblum, E.; Malik, B.; Ruthstein, S.; Moretti, E.; Nessim, G.D. Phase-Dependent Photocatalytic Activity of Bulk and Exfoliated Defect-Controlled Flakes of Layered Copper Sulfides under Simulated Solar Light. ACS Sustain. Chem. Eng. 2021, 9, 16103–16114. [Google Scholar] [CrossRef]
- Xu, W.; Zhu, S.; Liang, Y.; Li, Z.; Cui, Z.; Yang, X.; Inoue, A. Nanoporous CuS with Excellent Photocatalytic Property. Sci. Rep. 2015, 5, 18125. [Google Scholar] [CrossRef]
- Coughlan, C.; Ibáñez, M.; Dobrozhan, O.; Singh, A.; Cabot, A.; Ryan, K.M. Compound Copper Chalcogenide Nanocrystals. Chem. Rev. 2017, 117, 5865–6109. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, X.; Yang, Z.; Xu, S.; Zhang, W.; Su, Y.; Hu, N.; Lu, W.; Feng, J.; Zhang, Y. Morphology Control and Photocatalysis Enhancement by in Situ Hybridization of Cuprous Oxide with Nitrogen-Doped Carbon Quantum Dots. Langmuir 2016, 32, 9418–9427. [Google Scholar] [CrossRef] [PubMed]
- Fuhr, O.; Dehnen, S.; Fenske, D. Chalcogenide Clusters of Copper and Silver from Silylated Chalcogenide Sources. Chem. Soc. Rev. 2013, 42, 1871–1906. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, J.; Wu, T.; Li, L.; Luo, W.; Jiang, W.; Wang, L. Nanostructured Binary Copper Chalcogenides: Synthesis Strategies and Common Applications. Nanoscale 2018, 10, 15130–15163. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shen, T.; Bai, H.; Li, B.; Tian, J. Cu3Se2 Nanostructure as a Counter Electrode for High Efficiency Quantum Dot-Sensitized Solar Cells. J. Mater. Chem. C 2016, 4, 8020–8026. [Google Scholar] [CrossRef]
- Meng, K.; Chen, G.; Thampi, K.R. Metal Chalcogenides as Counter Electrode Materials in Quantum Dot Sensitized Solar Cells: A Perspective. J. Mater. Chem. A 2015, 3, 23074–23089. [Google Scholar] [CrossRef]
- Eskandari, M.; Ahmadi, V. Copper Selenide as a New Counter Electrode for Zinc Oxide Nanorod Based Quantum Dot Solar Cells. Mater. Lett. 2015, 142, 308–311. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, Z.; Wang, X.; Guo, X.; Guo, L. Noble-Metal-Free Cu2S-Modified Photocatalysts for Enhanced Photocatalytic Hydrogen Production by Forming Nanoscale p–n Junction Structure. RSC Adv. 2015, 5, 18159–18166. [Google Scholar] [CrossRef]
- Nouri, M.; Azimi, H.R.; Saray, A.M.; Yousefi, R. S-Doping Effects on Optical Properties and Highly Enhanced Photocatalytic Performance of Cu3Se2 Nanoparticles under Solar-Light Irradiation. Ceram. Int. 2017, 43, 14983–14988. [Google Scholar] [CrossRef]
- Baghchesara, M.A.; Azimi, H.R.; Ghorban Shiravizadeh, A.; Asri Mat Teridi, M.; Yousefi, R. Improving the Intrinsic Properties of RGO Sheets by S-Doping and the Effects of RGO Improvements on the Photocatalytic Performance of Cu3Se2/RGO Nanocomposites. Appl. Surf. Sci. 2019, 466, 401–410. [Google Scholar] [CrossRef]
- Zhao, L.; Fei, F.Y.; Wang, J.; Wang, F.; Wang, C.; Li, J.; Wang, J.; Cheng, Z.; Dou, S.; Wang, X. Improvement of Thermoelectric Properties and Their Correlations with Electron Effective Mass in Cu1.98SxSe1−x. Sci. Rep. 2017, 7, 40436. [Google Scholar] [CrossRef]
- Ni, S.; Su, Y.; Ai, Z.; Hu, J.; Yang, Z.; Kong, E.; Zhang, Y. Bandgap Tuning and Photocatalytic Activities of CuSe1–XSx Nanoflakes. Ceram. Int. 2016, 42, 211–219. [Google Scholar] [CrossRef]
- Sonia, S.; Kumar, P.S.; Mangalaraj, D.; Ponpandian, N.; Viswanathan, C. Influence of Growth and Photocatalytic Properties of Copper Selenide (CuSe) Nanoparticles Using Reflux Condensation Method. Appl. Surf. Sci. 2013, 283, 802–807. [Google Scholar] [CrossRef]
- Ren, X.; Wang, H.; Chen, J.; Xu, W.; He, Q.; Wang, H.; Zhan, F.; Chen, S.; Chen, L. Emerging 2D Copper-Based Materials for Energy Storage and Conversion: A Review and Perspective. Small 2023, 19, 2204121. [Google Scholar] [CrossRef]
- Masud, J.; Liyanage, W.P.R.; Cao, X.; Saxena, A.; Nath, M. Copper Selenides as High-Efficiency Electrocatalysts for Oxygen Evolution Reaction. ACS Appl. Energy Mater. 2018, 1, 4075–4083. [Google Scholar] [CrossRef]
- Hu, Y.; Afzaal, M.; Malik, M.A.; O’Brien, P. Deposition of Copper Selenide Thin Films and Nanoparticles. J. Cryst. Growth 2006, 297, 61–65. [Google Scholar] [CrossRef]
- Gorelenok, A.T.; Mdivani, V.N.; Moskvin, P.P.; Sorokin, V.S.; Usikov, A.S. Phase Equilibria in the In-Ga-As-P System. J. Cryst. Growth 1982, 60, 355–362. [Google Scholar] [CrossRef]
- Konar, R.; Rajeswaran, B.; Paul, A.; Teblum, E.; Aviv, H.; Perelshtein, I.; Grinberg, I.; Tischler, Y.R.; Nessim, G.D. CVD-Assisted Synthesis of 2D Layered MoSe2 on Mo Foil and Low Frequency Raman Scattering of Its Exfoliated Few-Layer Nanosheets on CaF2 Substrates. ACS Omega 2022, 7, 4121–4134. [Google Scholar] [CrossRef]
- Konar, R.; Tamari, R.; Teblum, E.; Nessim, G.D.; Meshi, L. In-Depth Characterization of Stacking Faults Forming during the Growth of Transition-Metal Di-Chalcogenides (TMDCs) by Ambient Pressure-CVD. Mater. Charact. 2022, 184, 111666. [Google Scholar] [CrossRef]
- Konar, R.; Rosy; Perelshtein, I.; Teblum, E.; Telkhozhayeva, M.; Tkachev, M.; Richter, J.J.; Cattaruzza, E.; Pietropolli Charmet, A.; Stoppa, P.; et al. Scalable Synthesis of Few-Layered 2D Tungsten Diselenide (2H-WSe2) Nanosheets Directly Grown on Tungsten (W) Foil Using Ambient-Pressure Chemical Vapor Deposition for Reversible Li-Ion Storage. ACS Omega 2020, 5, 19409–19421. [Google Scholar] [CrossRef]
- Dodd, L.E. The Vapor Pressure Curves of Solid and Liquid Selenium Near the Melting Point. J. Am. Chem. Soc. 1920, 42, 1579–1594. [Google Scholar] [CrossRef]
- Baker, E.H. The Vapour Pressure and Resistivity of Selenium at High Temperatures. J. Chem. Soc. A Inorg. Phys. Theor. 1968, 1089–1092. [Google Scholar] [CrossRef]
- Moumen, A.; Konar, R.; Zappa, D.; Teblum, E.; Perelshtein, I.; Lavi, R.; Ruthstein, S.; Nessim, G.D.; Comini, E. Robust Room-Temperature NO2 Sensors from Exfoliated 2D Few-Layered CVD-Grown Bulk Tungsten Di-Selenide (2H-WSe2). ACS Appl. Mater. Interfaces 2021, 13, 4316–4329. [Google Scholar] [CrossRef]
- Konar, R.; Das, S.; Teblum, E.; Modak, A.; Perelshtein, I.; Richter, J.J.; Schechter, A.; Nessim, G.D. Facile and Scalable Ambient Pressure Chemical Vapor Deposition-Assisted Synthesis of Layered Silver Selenide (β-Ag2Se) on Ag Foil as a Possible Oxygen Reduction Catalyst in Alkaline Medium. Electrochim. Acta 2021, 370, 137709. [Google Scholar] [CrossRef]
- Rajeswaran, B.; Konar, R.; Guddala, S.; Sharabani, T.; Teblum, E.; Tischler, Y.R.; Nessim, G.D. Nanostructure-Free Metal–Dielectric Stacks for Raman Scattering Enhancement and Defect Identification in CVD-Grown Tungsten Disulfide (2H-WS2) Nanosheets. J. Phys. Chem. C 2022, 126, 20511–20523. [Google Scholar] [CrossRef]
- Maiti, S.; Konar, R.; Sclar, H.; Grinblat, J.; Talianker, M.; Tkachev, M.; Wu, X.; Kondrakov, A.; Nessim, G.D.; Aurbach, D. Stabilizing High-Voltage Lithium-Ion Battery Cathodes Using Functional Coatings of 2D Tungsten Diselenide. ACS Energy Lett. 2022, 7, 1383–1391. [Google Scholar] [CrossRef]
- Roth, N.; Iversen, B.B. Solving the Disordered Structure of β-Cu 2 x Se Using the Three-Dimensional Difference Pair Distribution Function. Acta Crystallogr. Sect. A Found. Adv. 2019, 75, 465–473. [Google Scholar] [CrossRef]
- Eikeland, E.; Blichfeld, A.B.; Borup, K.A.; Zhao, K.; Overgaard, J.; Shi, X.; Chen, L.; Iversen, B.B. Crystal Structure across the β to α Phase Transition in Thermoelectric Cu 2-x Se. IUCrJ 2017, 4, 476–485. [Google Scholar] [CrossRef]
- Liu, H.; Yuan, X.; Lu, P.; Shi, X.; Xu, F.; He, Y.; Tang, Y.; Bai, S.; Zhang, W.; Chen, L.; et al. Ultrahigh Thermoelectric Performance by Electron and Phonon Critical Scattering in Cu2Se1-XIx. Adv. Mater. 2013, 25, 6607–6612. [Google Scholar] [CrossRef]
- Lu, P.; Liu, H.; Yuan, X.; Xu, F.; Shi, X.; Zhao, K.; Qiu, W.; Zhang, W.; Chen, L. Multiformity and Fluctuation of Cu Ordering in Cu2Se Thermoelectric Materials. J. Mater. Chem. A 2015, 3, 6901–6908. [Google Scholar] [CrossRef]
- Gáborová, K.; Achimovičová, M.; Hegedüs, M.; Girman, V.; Kaňuchová, M.; Dutková, E. Advantageous Mechanochemical Synthesis of Copper(I) Selenide Semiconductor, Characterization, and Properties. Front. Chem. Sci. Eng. 2022, 16, 433–442. [Google Scholar] [CrossRef]
- Nelson, P.I.; Kannan, R.R.; Mohan, A.; Rajesh, S.; Vidhya, B. Impact of Sequential Annealing on the Characteristics of Thermally Evaporated Semiconductor Cu2Se/ZnSe/Cu2Se Sandwich Structure. J. Mater. Sci. Mater. Electron. 2018, 29, 7393–7401. [Google Scholar] [CrossRef]
- Zheng, J.; Fan, C.; Li, X.; Yang, Q.; Wang, D.; Duan, A.; Ding, J.; Rong, S.; Chen, Z.; Luo, J.; et al. Enhanced Photodegradation of Tetracycline Hydrochloride by Hexameric AgBr/Zn-Al MMO S-Scheme Heterojunction Photocatalysts: Low Metal Leaching, Degradation Mechanism and Intermediates. Chem. Eng. J. 2022, 446, 137371. [Google Scholar] [CrossRef]
- Babu, B.; Koutavarapu, R.; Shim, J.; Yoo, K. Enhanced Solar Light–Driven Photocatalytic Degradation of Tetracycline and Organic Pollutants by Novel One–Dimensional ZnWO4 Nanorod–Decorated Two–Dimensional Bi2WO6 Nanoflakes. J. Taiwan Inst. Chem. Eng. 2020, 110, 58–70. [Google Scholar] [CrossRef]
- Pan, Y.; Yuan, X.; Jiang, L.; Wang, H.; Yu, H.; Zhang, J. Stable Self-Assembly AgI/UiO-66(NH2) Heterojunction as Efficient Visible-Light Responsive Photocatalyst for Tetracycline Degradation and Mechanism Insight. Chem. Eng. J. 2020, 384, 123310. [Google Scholar] [CrossRef]
- Deng, F.; Zhao, L.; Luo, X.; Luo, S.; Dionysiou, D.D. Highly Efficient Visible-Light Photocatalytic Performance of Ag/AgIn5S8 for Degradation of Tetracycline Hydrochloride and Treatment of Real Pharmaceutical Industry Wastewater. Chem. Eng. J. 2018, 333, 423–433. [Google Scholar] [CrossRef]
- Tang, T.; Yin, Z.; Chen, J.; Zhang, S.; Sheng, W.; Wei, W.; Xiao, Y.; Shi, Q.; Cao, S. Novel P-n Heterojunction Bi2O3/Ti3+-TiO2 Photocatalyst Enables the Complete Removal of Tetracyclines under Visible Light. Chem. Eng. J. 2021, 417, 128058. [Google Scholar] [CrossRef]
- Chen, M.; Guo, C.; Hou, S.; Lv, J.; Zhang, Y.; Zhang, H.; Xu, J. A Novel Z-Scheme AgBr/P-g-C3N4 Heterojunction Photocatalyst: Excellent Photocatalytic Performance and Photocatalytic Mechanism for Ephedrine Degradation. Appl. Catal. B Environ. 2020, 266, 118614. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, K.; Qiu, P.; Shi, X.; Chen, L. Thermoelectric Properties of Copper-Deficient Cu2-XSe (0.05 ≤ x ≤ 0.25) Binary Compounds. Ceram. Int. 2017, 43, 11142–11148. [Google Scholar] [CrossRef]
- Ding, C.; Zhu, Q.; Yang, B.; Petropoulos, E.; Xue, L.; Feng, Y.; He, S.; Yang, L. Efficient Photocatalysis of Tetracycline Hydrochloride (TC-HCl) from Pharmaceutical Wastewater Using AgCl/ZnO/g-C3N4 Composite under Visible Light: Process and Mechanisms. J. Environ. Sci. 2023, 126, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Jiang, L.; Wang, J.; Pu, Y.; Zhu, Y.; Wang, Y.; Xiao, B. Efficient and Stable Photocatalytic Degradation of Tetracycline Wastewater by 3D Polyaniline/Perylene Diimide Organic Heterojunction under Visible Light Irradiation. Chem. Eng. J. 2020, 397, 125476. [Google Scholar] [CrossRef]
- Xu, F.; Chai, B.; Liu, Y.; Liu, Y.; Fan, G.; Song, G. Superior Photo-Fenton Activity toward Tetracycline Degradation by 2D α-Fe2O3 Anchored on 2D g-C3N4: S-Scheme Heterojunction Mechanism and Accelerated Fe3+/Fe2+ Cycle. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129854. [Google Scholar] [CrossRef]
- Telkhozhayeva, M.; Hirsch, B.; Konar, R.; Teblum, E.; Lavi, R.; Weitman, M.; Malik, B.; Moretti, E.; Nessim, G.D. 2D TiS2 Flakes for Tetracycline Hydrochloride Photodegradation under Solar Light. Appl. Catal. B Environ. 2022, 318, 121872. [Google Scholar] [CrossRef]
- Dong, H.; Jiang, Z.; Zhang, C.; Deng, J.; Hou, K.; Cheng, Y.; Zhang, L.; Zeng, G. Removal of Tetracycline by Fe/Ni Bimetallic Nanoparticles in Aqueous Solution. J. Colloid Interface Sci. 2018, 513, 117–125. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, K.; Ling, W.; Wei, X.; Wang, Y.; Wang, J. Investigation of the Kinetics and Reaction Mechanism for Photodegradation Tetracycline Antibiotics over Sulfur-Doped Bi2WO6-x/ZnIn2S4 Direct Z-Scheme Heterojunction. Nanomaterials 2021, 11, 2123. [Google Scholar] [CrossRef]
- Yang, P.; Chen, C.; Wang, D.; Ma, H.; Du, Y.; Cai, D.; Zhang, X.; Wu, Z. Kinetics, Reaction Pathways, and Mechanism Investigation for Improved Environmental Remediation by 0D/3D CdTe/Bi2WO6 Z-Scheme Catalyst. Appl. Catal. B Environ. 2021, 285, 119877. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, Y.; Wang, W.; Yang, Y.; Zhou, C.; Wang, L.; Lei, L.; He, D.; Luo, H.; Huang, D. Formation of Mo2C/Hollow Tubular g-C3N4 Hybrids with Favorable Charge Transfer Channels for Excellent Visible-Light-Photocatalytic Performance. Appl. Surf. Sci. 2020, 527, 146757. [Google Scholar] [CrossRef]
- Li, C.; Yu, S.; Dong, H.; Liu, C.; Wu, H.; Che, H.; Chen, G. Z-Scheme Mesoporous Photocatalyst Constructed by Modification of Sn3O4 Nanoclusters on g-C3N4 Nanosheets with Improved Photocatalytic Performance and Mechanism Insight. Appl. Catal. B Environ. 2018, 238, 284–293. [Google Scholar] [CrossRef]
- Zhu, Q.; Sun, Y.; Na, F.; Wei, J.; Xu, S.; Li, Y.; Guo, F. Fabrication of CdS/Titanium-Oxo-Cluster Nanocomposites Based on a Ti32 Framework with Enhanced Photocatalytic Activity for Tetracycline Hydrochloride Degradation under Visible Light. Appl. Catal. B Environ. 2019, 254, 541–550. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Qiu, H.-B.; Huang, G.; Yu, H.-Q. Bi24O31Br10 Nanosheets with Controllable Thickness for Visible–Light–Driven Catalytic Degradation of Tetracycline Hydrochloride. Appl. Catal. B Environ. 2017, 205, 615–623. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.-Z.; Zeng, G.; Liang, J.; Chen, X.; Yu, H.; Hou, W.; Wu, Z.; Zhang, J.; Xiong, T. In-Situ Synthesis of Direct Solid-State Dual Z-Scheme WO3/g-C3N4/Bi2O3 Photocatalyst for the Degradation of Refractory Pollutant. Appl. Catal. B Environ. 2018, 227, 376–385. [Google Scholar] [CrossRef]
- Yan, Y.; Tang, X.; Ma, C.; Huang, H.; Yu, K.; Liu, Y.; Lu, Z.; Li, C.; Huo, P.; Zhu, Z. 2D Mesoporous Photocatalyst Constructed by Modification of Biochar on BiOCl Ultrathin Nanosheets for Enhancing TC-HCl Degradation Activity. New J. Chem. 2019, 44, 79–86. [Google Scholar] [CrossRef]
- Pan, T.; Dongdong, C.; Xu, W.; Fang, J.; Wu, S.; Liu, Z.; Wu, K.; Fang, Z. Anionic Polyacrylamide-Assisted Construction of Thin 2D-2D WO3/g-C3N4 Step-Scheme Heterojunction for Enhanced Tetracycline Degradation under Visible Light Irradiation. J. Hazard. Mater. 2020, 393, 122366. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, Y.; Shen, X.; Duoerkun, G.; Zhu, B.; Zhang, L.; Li, M.; Chen, Z. Fabrication of G-C3N4/BiOBr Heterojunctions on Carbon Fibers as Weaveable Photocatalyst for Degrading Tetracycline Hydrochloride under Visible Light. Chem. Eng. J. 2020, 386, 124010. [Google Scholar] [CrossRef]
- Xie, Z.; Feng, Y.; Wang, F.; Chen, D.; Zhang, Q.; Zeng, Y.; Lv, W.; Liu, G. Construction of Carbon Dots Modified MoO3/g-C3N4 Z-Scheme Photocatalyst with Enhanced Visible-Light Photocatalytic Activity for the Degradation of Tetracycline. Appl. Catal. B Environ. 2018, 229, 96–104. [Google Scholar] [CrossRef]
- Ouyang, C.; Quan, X.; Zhang, C.; Pan, Y.; Li, X.; Hong, Z.; Zhi, M. Direct Z-Scheme ZnIn2S4@MoO3 Heterojunction for Efficient Photodegradation of Tetracycline Hydrochloride under Visible Light Irradiation. Chem. Eng. J. 2021, 424, 130510. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srijith; Konar, R.; Teblum, E.; Singh, V.K.; Telkhozhayeva, M.; Paiardi, M.; Nessim, G.D. Chemical-Vapor-Deposition-Synthesized Two-Dimensional Non-Stoichiometric Copper Selenide (β-Cu2−xSe) for Ultra-Fast Tetracycline Hydrochloride Degradation under Solar Light. Molecules 2024, 29, 887. https://doi.org/10.3390/molecules29040887
Srijith, Konar R, Teblum E, Singh VK, Telkhozhayeva M, Paiardi M, Nessim GD. Chemical-Vapor-Deposition-Synthesized Two-Dimensional Non-Stoichiometric Copper Selenide (β-Cu2−xSe) for Ultra-Fast Tetracycline Hydrochloride Degradation under Solar Light. Molecules. 2024; 29(4):887. https://doi.org/10.3390/molecules29040887
Chicago/Turabian StyleSrijith, Rajashree Konar, Eti Teblum, Vivek Kumar Singh, Madina Telkhozhayeva, Michelangelo Paiardi, and Gilbert Daniel Nessim. 2024. "Chemical-Vapor-Deposition-Synthesized Two-Dimensional Non-Stoichiometric Copper Selenide (β-Cu2−xSe) for Ultra-Fast Tetracycline Hydrochloride Degradation under Solar Light" Molecules 29, no. 4: 887. https://doi.org/10.3390/molecules29040887
APA StyleSrijith, Konar, R., Teblum, E., Singh, V. K., Telkhozhayeva, M., Paiardi, M., & Nessim, G. D. (2024). Chemical-Vapor-Deposition-Synthesized Two-Dimensional Non-Stoichiometric Copper Selenide (β-Cu2−xSe) for Ultra-Fast Tetracycline Hydrochloride Degradation under Solar Light. Molecules, 29(4), 887. https://doi.org/10.3390/molecules29040887