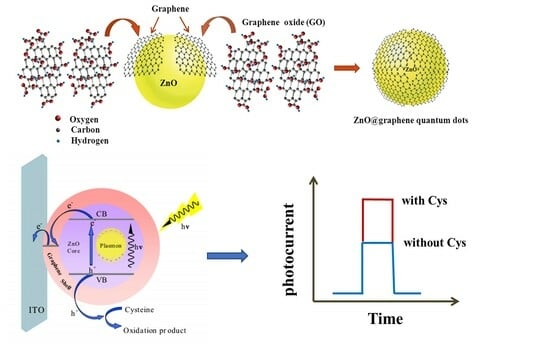
Surface Plasmon Resonance Enhanced Photoelectrochemical Sensing of Cysteine Based on Au Nanoparticle-Decorated ZnO@graphene Quantum Dots
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Prepared Nanomaterials
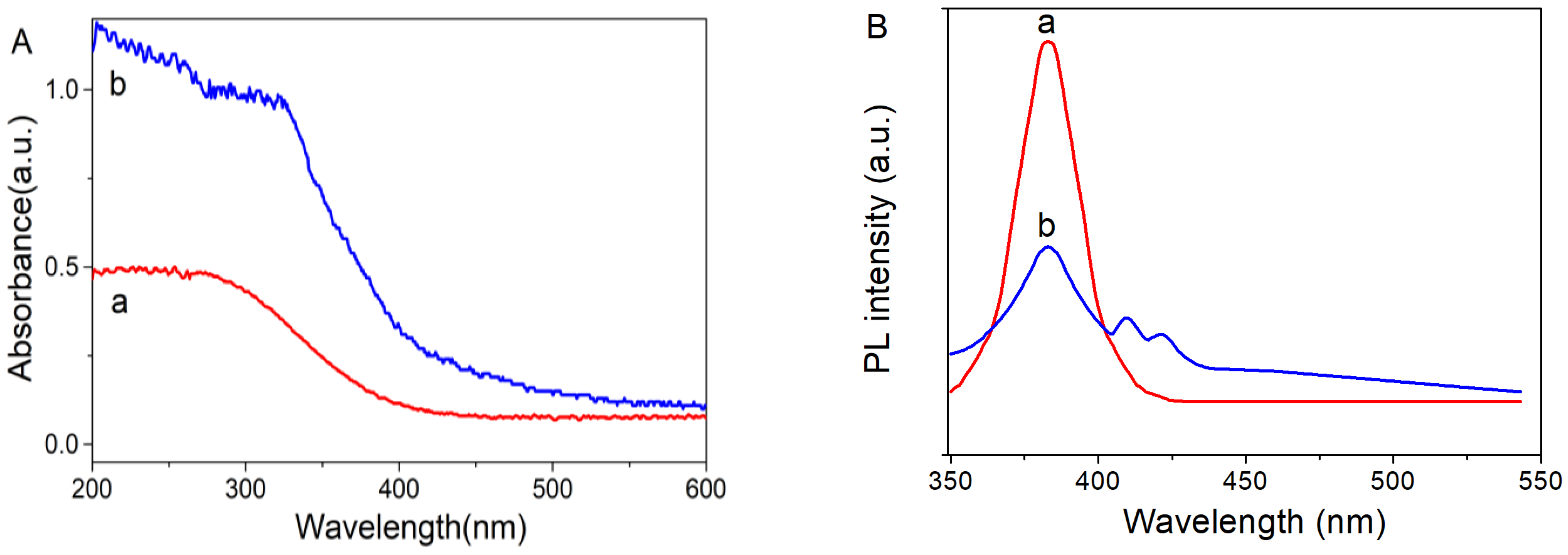
2.2. Optical Property of the Prepared Nanomaterials
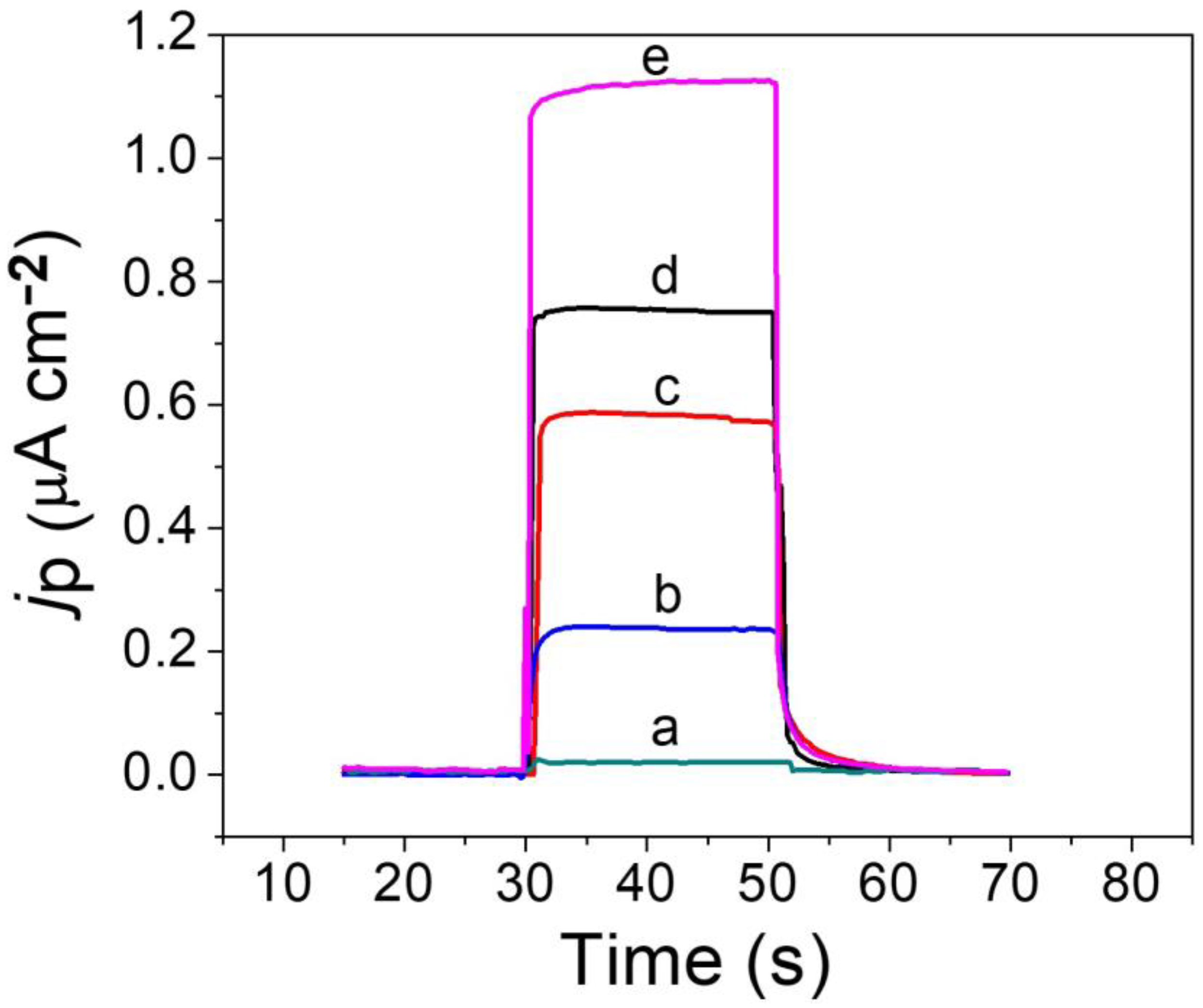
2.3. PEC Performance of the Sensor
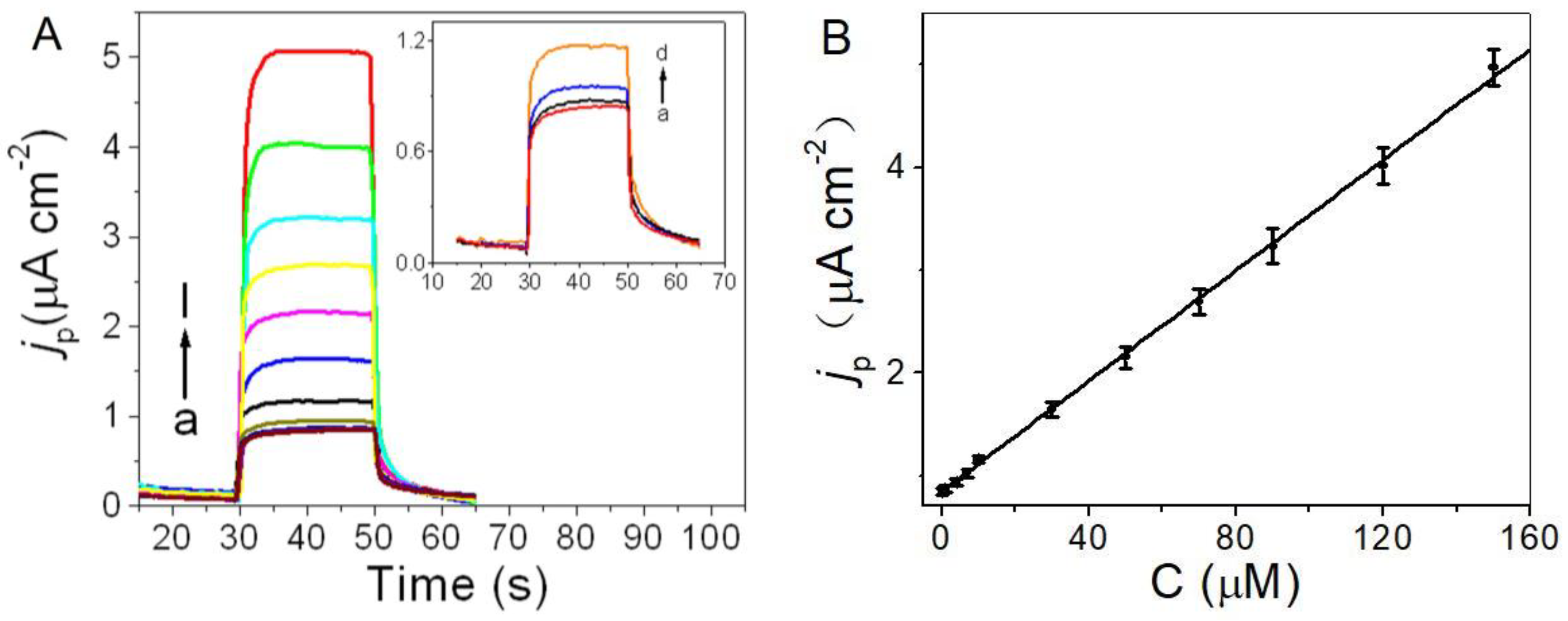
2.4. Photoelectrochemical Sensing of Cysteine
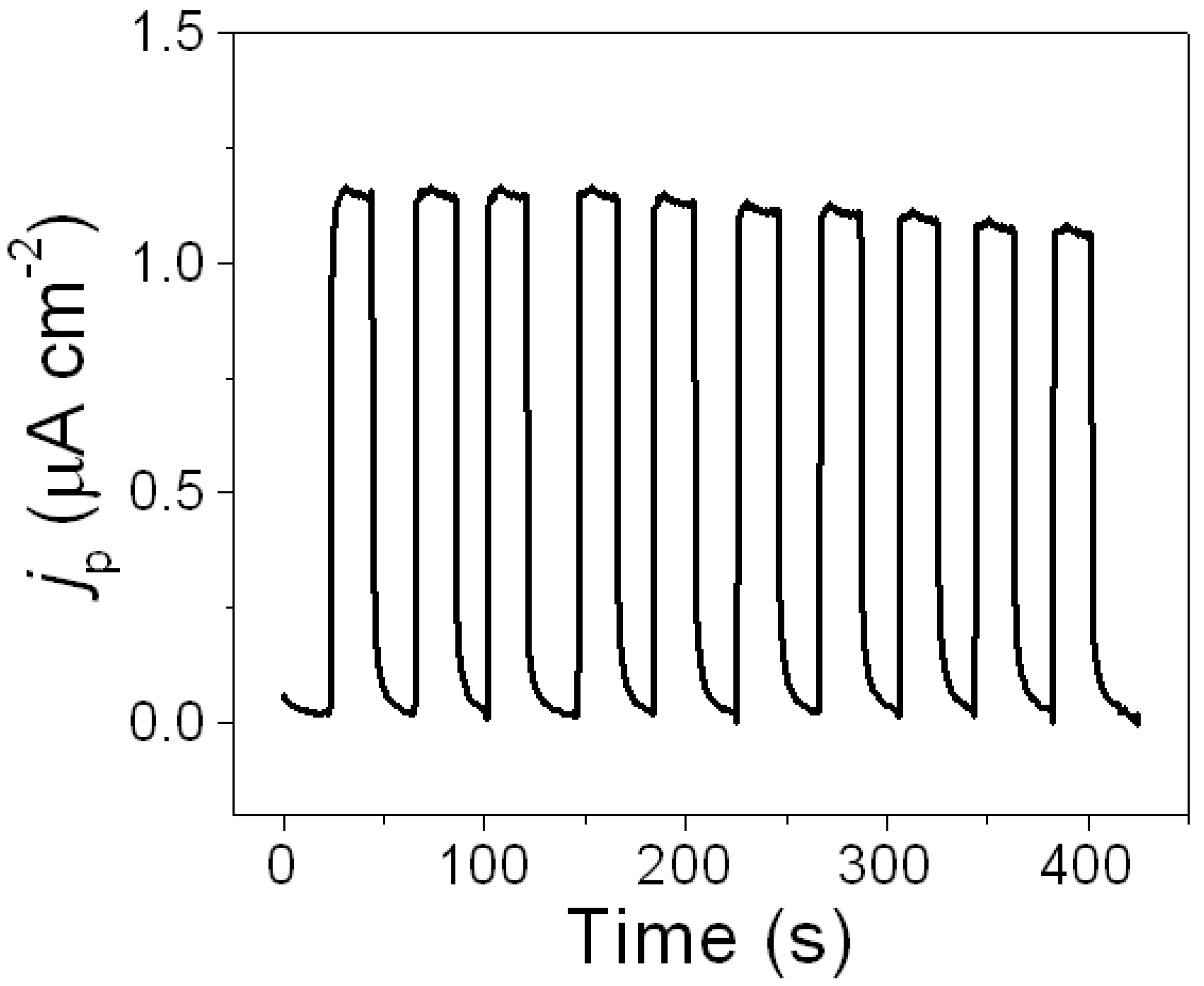
2.5. Reproducibility, Stability, and Selectivity
2.6. Application in Real Sample Analysis
3. Materials and Methods
3.1. Chemicals
3.2. Apparatus
3.3. Preparation of ZnO@graphene Quantum Dots
3.4. Construction of PEC Sensor
3.5. Photoelectrochemical Detection of Cysteine
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bertoldo, J.B.; Terenzi, H.S.; Huttelmaier, G.; Bernardes, J.L. Posttranslational chemical mutagenesis: To reveal the role of noncatalytic cysteine residues in pathogenic bacterial phosphatases. Biochemistry 2018, 57, 6144–6152. [Google Scholar] [CrossRef]
- Kurniawan, A.F.; Kurniawan, F.; Gunawan, S.H.; Chou, M.; Wang, J. Disposable electrochemical sensor based on copper-electro deposited screen-printed gold electrode and its application in sensing L-cysteine. Electrochim. Acta 2019, 293, 318–327. [Google Scholar] [CrossRef]
- Gazit, V.; Ben-Abraham, R.; Coleman, R. Cysteine-induced hypoglycemic brain damage: An alternative mechanism to excitotoxicity. Amino Acids 2004, 26, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.; Deng, J.J.; Wang, D.L.; Yang, L.F.; Yu, P.; Mao, L.Q. Aspartic acid-promoted highly selective and sensitive colorimetric sensing of cysteine in rat brain. Anal. Chem. 2012, 84, 9579–9584. [Google Scholar] [CrossRef]
- Wei, L.; Lu, X.; Kang, X.; Song, Y. Determination of glutathione and cysteine in human breast milk by high-performance liquid chromatography with chemiluminescence detection for evaluating the oxidative stress and exposure to heavy metals of lactating women. Anal. Lett. 2020, 53, 2607–2618. [Google Scholar] [CrossRef]
- Ummadi, M.; Weimer, B.C. Use of capillary electrophoresis and laser-induced fluorescence for attomole detection of amino acids. J. Chromatogr. A 2002, 964, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Li, Z.; Fu, X. Carbon dots-based fluorescence and UV–vis absorption dual-modal sensors for Ag+ and L-cysteine detection. Dyes Pigm. 2021, 187, 109126. [Google Scholar] [CrossRef]
- Li, H.; Ye, L.; Wang, Y. A glassy carbon electrode modified with hollow cubic cuprous oxide for voltammetric sensing of L-cysteine. Microchim. Acta 2017, 185, 5. [Google Scholar] [CrossRef]
- Zare, H.R.; Jahangiri-Dehaghani, F.; Shekari, Z.; Benvidi, A. Electrocatalytic simultaneous determination of ascorbic acid, uric acid and L-cysteine in real samples using quercetin silver nanoparticles graphene nanosheets modified glassy carbon electrode. Appl. Surf. Sci. 2016, 375, 169–178. [Google Scholar] [CrossRef]
- Long, Y.T.; Kong, C.; Li, D.W. Ultrasensitive determination of cysteine based on the photocurrent of nafion-functionalized CdS-MV quantum dots on an ITO electrode. Small 2011, 7, 1624–1628. [Google Scholar] [CrossRef]
- Shen, Q.M.; Jiang, J.Y.; Liu, S.L. Facile synthesis of Au–SnO2 hybrid nanospheres with enhanced photoelectrochemical biosensing performance. Nanoscale 2014, 6, 6315–6321. [Google Scholar] [CrossRef]
- Wang, Y.; Bian, F.; Qin, X.F. Visible light photoelectrochemical aptasensor for chloramphenicol by using a TiO2 nanorod array sensitized with Eu (III)-doped CdS quantum dots. Microchim. Acta 2018, 185, 161. [Google Scholar] [CrossRef]
- Qin, X.F.; Geng, L.P.; Wang, Q.Q.; Wang, Y. Photoelectrochemical aptasensing of ofloxacin based on the use of a TiO2 nanotube array co-sensitized with a nanocomposite prepared from polydopamine and Ag2S nanoparticles. Microchim. Acta 2019, 186, 430. [Google Scholar] [CrossRef]
- Son, D.I.; Kim, T.W.; Shim, J.H. Flexible organic bistable devices based on graphene embedded in an insulating poly (methyl methacrylate) polymer layer. Nano Lett. 2010, 10, 2441–2447. [Google Scholar] [CrossRef]
- Qin, X.F.; Geng, L.P.; Wang, Q.Q.; Shu, X.L.; Wang, Y. A “signal-on” photoelectrochemical aptasensor based on graphene quantum dots-sensitized TiO2 nanotube arrays for sensitive detection of chloramphenicol. Talanta 2019, 197, 28–35. [Google Scholar] [CrossRef]
- Graeme, W.; Brian, S.; Kamat, P.V. TiO2-graphene nanocomposites. UV-assisted photocatalytic reduction of graphene oxide. ACS Nano 2008, 2, 1487–1491. [Google Scholar]
- Zhang, X.Y.; Li, H.P.; Cui, X.L. Graphene/TiO2 nanocomposites: Synthesis, characterization and application in hydrogen evolution from water photocatalytic splitting. J. Mater. Chem. 2010, 20, 280–2806. [Google Scholar] [CrossRef]
- Chang, H.; Sun, Z.; Ho, K.Y.F.; Tao, X.; Yan, F.; Kwok, W.M.; Zheng, Z. A highly sensitive ultraviolet sensor based on a facile in situ solution-grown ZnO nanorod/graphene heterostructure. Nanoscale 2011, 3, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Huo, P.W.; Zhou, M.J.; Tang, Y.F. Incorporation of N-ZnO/CdS/Graphene oxide composite photocatalyst for enhanced photocatalytic activity under visible light. J. Alloys Compd. 2016, 247, 198–209. [Google Scholar] [CrossRef]
- Zhao, X.M.; Zhou, S.W.; Shen, Q.M. Fabrication of glutathione photoelectrochemical biosensor using graphene-CdS nanocomposites. Analyst 2012, 137, 3697–3703. [Google Scholar] [CrossRef]
- Fu, Y.S.; Wang, X. Magnetically separable ZnFe2O4-Graphene catalyst and its high photocatalytic performance under visible light irradiation. Ind. Eng. Chem. Res. 2011, 50, 7210–7218. [Google Scholar] [CrossRef]
- Nipane, S.V.; Korake, P.V.; Gokavi, G.S. Graphene-zinc oxide nanorod nanocomposite as photocatalyst for enhanced degradation of dyes under UV light. Ceram. Int. 2015, 41, 4549–4557. [Google Scholar] [CrossRef]
- Zhang, L.Z.; Zhang, J.N.; Huang, Y.H. Hexagonal ZnO nanoplates/graphene composites with excellent sensing performance to NO2 at room temperature. Appl. Surf. Sci. 2021, 537, 147785. [Google Scholar] [CrossRef]
- Niavol, S.S.; Khatibani, A.B.; Chauhanc, D.; Moghaddam, H.M.; Gao, G.H. ZnO quantum dots decorated on graphene oxide and graphene nanoplatelets: Comparison the structure and sensing properties. Inorg. Chem. Commun. 2024, 160, 111957. [Google Scholar] [CrossRef]
- Low, S.S.; Michelle, T.T.; Loh, H.S.; Khiew, P.S.; Chiu, W.S. Facile hydrothermal growth graphene/ZnO nanocomposite for development of enhanced biosensor. Anal. Chim. Acta 2016, 903, 131–141. [Google Scholar] [CrossRef]
- Hwa, K.Y.; Subramani, B. Synthesis of zinc oxide nanoparticles on graphene–carbon nanotube hybrid for glucose biosensor applications. Biosens. Bioelectron. 2014, 62, 127–133. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.C.; Ma, L.X. One-pot facile fabrication of graphene-zinc oxide composite and its enhanced sensitivity for simultaneous electrochemical detection of ascorbic acid, dopamine and uric acid. Sens. Actuators B Chem. 2016, 227, 488–496. [Google Scholar] [CrossRef]
- Yukird, J.; Kongsittikul, P.; Rodthongkum, N. ZnO@ graphene nanocomposite modified electrode for sensitive and simultaneous detection of Cd (II) and Pb (II). Synthetic Met. 2018, 245, 251–259. [Google Scholar] [CrossRef]
- Shen, Y.L.; Yang, J.; Wan, Q.J. Fabrication of a branch-like Ni-doped ZnO/graphene nanoplatelet composite for enhanced electrochemical determination of 17β-estradiol and acetaminophen. J. Electron. Mater. 2022, 51, 5310–5321. [Google Scholar] [CrossRef]
- Li, L.; Zhai, T.Y.; Bando, Y. Recent progress of one-dimensional ZnO nanostructured solar cells. Nano Energy 2012, 1, 91–106. [Google Scholar] [CrossRef]
- Mao, Y.C.; Yang, H.; Chen, J.X. Significant performance enhancement of ZnO photoanodes from Ni (OH)2 electrocatalyst nanosheets overcoating. Nano Energy 2014, 6, 10–18. [Google Scholar] [CrossRef]
- Peng, Y.; Ji, Y.J.; Chen, D. Ultrasound assisted synthesis of ZnO/reduced graphene oxide composites with enhanced photocata lytic activity and anti-photocorrosion. Appl. Surf. Sci. 2015, 356, 762–768. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Y.; Yu, J.H.; Song, X.R. Application of ZnO/graphene and S6 aptamers for sensitive photoelectrochemical detection of SK-BR-3 breast cancer cells based on a disposable indium tin oxide device. Biosens. Bioelectron. 2014, 51, 413–420. [Google Scholar] [CrossRef]
- Yan, Y.; Ge, Y.; Mao, H. Annealing temperature-dependent photoelectrochemical property of zinc oxide/graphene nanocomposite and the application for fabricating a “signal-off” photoelectrochemical aptasensing for ATP. IEEE Sens. J. 2023, 23, 6489–6498. [Google Scholar] [CrossRef]
- Son, D.I.B.; Kwon, W.; Park, D.H.; Se, W.S.; Angadi, B.; Lee, C.L.; Choi, W.K. Emissive ZnO–graphene quantum dots for white-light-emitting diodes. Nat. Nano. 2012, 7, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.Y.; Chen, Z.Y.; Li, W.B.; Hou, B.R. Highly efficient photocatalytic performance of grapheme-ZnO quasi-shell-core com posite material. ACS Appl. Mater. Inter. 2013, 5, 12361–12368. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Bender, S.L.; Hranisavljevic, J. Metal nanoparticle plasmon-enhanced light-harvesting in a photosystem I thin film. Nano Lett. 2011, 11, 3091–3098. [Google Scholar] [CrossRef] [PubMed]
- Grabar, K.C.; Freeman, R.G.; Hommer, M.B. Preparation and characterization of Au colloid monolayers. Anal. Chem. 1995, 67, 735–743. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Wang, S.; Chu, W.; Wei, T.; Tao, H.; Zhang, C.; Sun, Y. Enhanced photoelectrochemical detection of L-cysteine based on the ultrathin polythiophene layer sensitized anatase TiO2 on F-doped tin oxide substrates. Sens. Actuators B Chem. 2016, 232, 448–453. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Xu, Z.W.; Yan, K.; Zhao, H.B.; Zhang, J.D. One-step synthesis of CuO-Cu2O heterojunction by flame spray pyrolysis for cathodic photoelectrochemical sensing of L-cysteine. ACS Appl. Mater. Inter. 2017, 9, 40452–40460. [Google Scholar] [CrossRef]
- Li, F.; Zhou, B.; Zhang, W.B. A novel photoelectrochemical assay for cysteine based on the target-induced generation of photosensitizer. Int. J. Electrochem. Sci. 2018, 13, 7183–7192. [Google Scholar] [CrossRef]
- Peng, J.Y.; Huang, Q.; Liu, Y.X.; Huang, Y.Y.; Xiang, G. Photoelectrochemical detection of L-cysteine with a covalently grafted ZnTAPc-Gr-based probe. Electroanalysis 2020, 32, 1237–1242. [Google Scholar] [CrossRef]
- Li, Y.R.; Liu, G.; Ji, D.Z.; Zhang, F.N.; Liu, Q.G. Smartphone-based label-free photoelectrochemical sensing of cysteine with cadmium ion chelation. Analyst 2022, 147, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.T.; Yao, C.F.; Cui, H.; Cang, Y.G.; Zhang, Z.H.; Miao, Y.Q.; Xin, Y.M. A nano-enzymatic photoelectrochemical L-cysteine biosensor based on Bi2MoO6 modified honeycomb TiO2 nanotube arrays composite. Microchem. J. 2022, 175, 107200. [Google Scholar] [CrossRef]
- Xin, Y.M.; Wang, Z.; Yao, H.Z.; Liu, W.T.; Miao, Y.Q.; Zhang, Z.G.; Wu, D. Au-mediated Z-scheme TiO2-Au-BiOI photoelectrode for sensitive and selective photoelectrochemical detection of L-cysteine. Sens. Actuators B Chem. 2023, 393, 134285. [Google Scholar] [CrossRef]
- Chen, S.; Gao, H.L.; Shen, W.W.; Lu, C.; Yuan, Q.P. Colorimetric detection of cysteine using noncrosslinking aggregation of fluorosurfactant-capped silver nanoparticles. Sens. Actuators B Chem. 2014, 190, 673–678. [Google Scholar] [CrossRef]



| Working Electrode | Linear Range (μM) | Detection Limit (nM) | Reference |
|---|---|---|---|
| Nafion/CdS-MV/ITO | 0.2–2.8 | 100 | [10] |
| Au-SnO2/CdS/ITO | 0.4–120 | 100 | [11] |
| PTh/TiO2/FTO | 10–800 | 12,800 | [39] |
| CuO−Cu2O/GCE | 0.2–10 | 50 | [40] |
| CA-TiO2/FTO | 2.0–100 | 650 | [41] |
| ZnTAPc-Gr/ITO | 0.25–113 | 11.4 | [42] |
| ITO/g-C3N4/Au | 10–40 | 9200 | [43] |
| Bi2MoO6/TiO2 | 0.5–600 | 150 | [44] |
| TiO2-Au-BiOI | 0.8–200 | 70 | [45] |
| Au-ZnO@graphene QDs/ITO | 0.1–150 | 8.9 | This work |
| Sample | Original (µM) | Added (µM) | Found (µM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| Serum 1 | 6.57 | 5.00 | 11.47 | 99.1 | 3.3 |
| Serum 2 | 6.97 | 5.00 | 12.16 | 101.6 | 2.8 |
| Urine 1 | 10.61 | 10.0 | 20.34 | 98.7 | 2.0 |
| Urine 2 | 13.56 | 10.0 | 23.28 | 98.8 | 1.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Lin, F.; Wang, Y. Surface Plasmon Resonance Enhanced Photoelectrochemical Sensing of Cysteine Based on Au Nanoparticle-Decorated ZnO@graphene Quantum Dots. Molecules 2024, 29, 1002. https://doi.org/10.3390/molecules29051002
Liu J, Lin F, Wang Y. Surface Plasmon Resonance Enhanced Photoelectrochemical Sensing of Cysteine Based on Au Nanoparticle-Decorated ZnO@graphene Quantum Dots. Molecules. 2024; 29(5):1002. https://doi.org/10.3390/molecules29051002
Chicago/Turabian StyleLiu, Jiaxin, Fancheng Lin, and Yan Wang. 2024. "Surface Plasmon Resonance Enhanced Photoelectrochemical Sensing of Cysteine Based on Au Nanoparticle-Decorated ZnO@graphene Quantum Dots" Molecules 29, no. 5: 1002. https://doi.org/10.3390/molecules29051002
APA StyleLiu, J., Lin, F., & Wang, Y. (2024). Surface Plasmon Resonance Enhanced Photoelectrochemical Sensing of Cysteine Based on Au Nanoparticle-Decorated ZnO@graphene Quantum Dots. Molecules, 29(5), 1002. https://doi.org/10.3390/molecules29051002