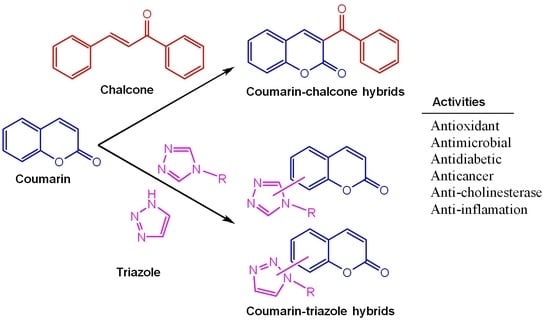
Recent Trends in the Synthesis and Bioactivity of Coumarin, Coumarin–Chalcone, and Coumarin–Triazole Molecular Hybrids
Abstract
1. Introduction
2. Synthesis of Coumarin, Coumarin–Chalcone Hybrids, and Coumarin–Triazole Hybrids
2.1. Synthesis of Coumarin Derivatives
2.2. Synthesis of Coumarin–Chalcone Molecular Hybrids
2.3. Synthesis of Coumarin–Triazole Molecular Hybrids
3. Bioactivity of Coumarins, Coumarin–Chalcones, and Coumarin–Triazoles
3.1. Antioxidant Activity
3.2. Antimicrobial Activity
3.3. Anticancer Activity
3.4. Antidiabetic Activity
3.5. Anti-Cholinesterase Activity
3.6. Anti-Inflammatory Activity
3.7. Miscellaneous Activity
4. Conclusions and Prospective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fiorot, R.G.; Westphal, R.; Lemos, B.C.; Romagna, R.A.; Gonçalves, P.R.; Fernandes, M.R.N.; Ferreira, C.V.; Taranto, A.G.; Greco, S.J. Synthesis, Molecular Modelling and Anticancer Activities of New Molecular Hybrids Containing 1,4-Naphthoquinone, 7-Chloroquinoline, 1,3,5-Triazine and Morpholine Cores as PI3K and AMPK Inhibitors in the Metastatic Melanoma Cells. J. Braz. Chem. Soc. 2019, 30, 1860–1873. [Google Scholar] [CrossRef]
- Muregi, F.W.; Ishih, A. Next-generation antimalarial drugs: Hybrid molecules as a new strategy in drug design. Drug Dev. Res. 2009, 71, 20–32. [Google Scholar] [CrossRef]
- Nepali, K.; Sharma, S.; Sharma, M.; Bedi, P.M.S.; Dhar, K.L. Rational approaches, design strategies, structure activity relationship and mechanistic insights for anticancer hybrids. Eur. J. Med. Chem. 2014, 77, 422–487. [Google Scholar] [CrossRef]
- Burch, J.D.; Farand, J.; Colucci, J.; Sturino, C.; Ducharme, Y.; Friesen, R.W.; Lévesque, J.-F.; Gagné, S.; Wrona, M.; Therien, A.G.; et al. Naphthalene/quinoline amides and sulfonylureas as potent and selective antagonists of the EP4 receptor. Bioorg. Med. Chem. Lett. 2011, 21, 1041–1046. [Google Scholar] [CrossRef]
- Viegas-Junior, C.; Danuello, A.; da Silva Bolzani, V.; Barreiro, E.J.; Fraga, C.A.M. Molecular Hybridization: A Useful Tool in the Design of New Drug Prototypes. Curr. Med. Chem. 2007, 14, 1829–1852. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Cruz-Martins, N.; López-Jornet, P.; Lopez, E.P.-F.; Harun, N.; Yeskaliyeva, B.; Beyatli, A.; Sytar, O.; Shaheen, S.; Sharopov, F.; et al. Natural Coumarins: Exploring the Pharmacological Complexity and Underlying Molecular Mechanisms. Oxidative Med. Cell. Longev. 2021, 2021, 6492346. [Google Scholar] [CrossRef]
- Adimule, V.M.; Nandi, S.S.; Kerur, S.S.; Khadapure, S.A.; Chinnam, S. Recent Advances in the One-Pot Synthesis of Coumarin Derivatives from Different Starting Materials Using Nanoparticles: A Review. Top. Catal. 2022, 1–31. [Google Scholar] [CrossRef]
- Jung, J.-C.; Jung, Y.-J.; Park, O.-S. A Convenient One-Pot Synthesis of 4-Hydroxycoumarin, 4-Hydroxythiocoumarin, and 4-Hydroxyquinolin-2(1H)-One. Synth. Commun. 2001, 31, 1195–1200. [Google Scholar] [CrossRef]
- Kostova, I.; Momekov, G.; Tzanova, T.; Karaivanova, M. Synthesis, characterization, and cytotoxic activity of new lanthanum(III) complexes of bis-coumarins. Bioinorg. Chem. Appl. 2006, 2006, 25651. [Google Scholar] [CrossRef] [PubMed]
- Turkekul, K.; Colpan, R.D.; Baykul, T.; Ozdemir, M.D.; Erdogan, S. Esculetin Inhibits the Survival of Human Prostate Cancer Cells by Inducing Apoptosis and Arresting the Cell Cycle. J. Cancer Prev. 2018, 23, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Fan, J.; Liu, L.; Liu, X.; Gao, F. Coumarin derivatives with anticancer activities: An update. Arch. Pharm. 2020, 353, e2000025. [Google Scholar] [CrossRef]
- Thomas, V.; Giles, D.; Basavarajaswamy, G.; Das, A.; Patel, A. Coumarin Derivatives as Anti-inflammatory and Anticancer Agents. Anti-Cancer Agents Med. Chem. 2017, 17, 415–423. [Google Scholar] [CrossRef]
- Gao, F.; Xiao, J.; Huang, G. Current scenario of tetrazole hybrids for antibacterial activity. Eur. J. Med. Chem. 2019, 184, 111744. [Google Scholar] [CrossRef]
- Hu, X.-L.; Xu, Z.; Liu, M.-L.; Feng, L.-S.; Zhang, G.-D. Recent Developments of Coumarin Hybrids as Anti-fungal Agents. Curr. Top. Med. Chem. 2018, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, Y.F.; Mohammed, E.T.; Khalil, R.R. Antioxidant and antitumor activities of methanolic extracts obtained from Red delicious and Granny Smith apples’ seeds. Syst. Rev. Pharm. 2020, 11, 570–576. [Google Scholar] [CrossRef]
- Kostova, I. Synthetic and Natural Coumarins as Cytotoxic Agents. Curr. Med. Chem. Anti-Cancer Agents 2005, 5, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, M. Synthesis and structure–activity relationships of novel warfarin derivatives. Bioorg. Med. Chem. 2007, 15, 2414–2420. [Google Scholar] [CrossRef] [PubMed]
- Jasim, H.A.; Nahar, L.; Jasim, M.A.; Moore, S.A.; Ritchie, K.J.; Sarker, S.D. Chalcones: Synthetic chemistry follows where nature leads. Biomolecules 2021, 11, 1203. [Google Scholar] [CrossRef] [PubMed]
- Sebti, S.; Solhy, A.; Smahi, A.; Kossir, A.; Oumimoun, H. Dramatic activity enhancement of natural phosphate catalyst by lithium nitrate. An efficient synthesis of chalcones. Catal. Commun. 2002, 3, 335–339. [Google Scholar] [CrossRef]
- Sahu, N.K.; Balbhadra, S.S.; Choudhary, J.; Kohli, D.V. Exploring Pharmacological Significance of Chalcone Scaffold: A Review. Curr. Med. Chem. 2012, 19, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef] [PubMed]
- Nowakowska, Z. A review of anti-infective and anti-inflammatory chalcones. Eur. J. Med. Chem. 2007, 42, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Vogel, S.; Barbic, M.; Jürgenliemk, G.; Heilmann, J. Synthesis, cytotoxicity, anti-oxidative and anti-inflammatory activity of chalcones and influence of A-ring modifications on the pharmacological effect. Eur. J. Med. Chem. 2010, 45, 2206–2213. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, J.; Cai, Y.; Pan, Y.; Ye, F.; Zhang, Y.; Zhao, Y.; Yang, S.; Li, X.; Liang, G. Evaluation and Discovery of Novel Synthetic Chalcone Derivatives as Anti-Inflammatory Agents. J. Med. Chem. 2011, 54, 8110–8123. [Google Scholar] [CrossRef]
- Xu, M.; Wu, P.; Shen, F.; Ji, J.; Rakesh, K. Chalcone derivatives and their antibacterial activities: Current development. Bioorg. Chem. 2019, 91, 103133. [Google Scholar] [CrossRef]
- Acharjee, S.; Maity, T.K.; Samanta, S.; Mana, S.; Chakraborty, T.; Singha, T.; Mondal, A. Antihyperglycemic activity of chalcone based novel 1-{3-[3-(substituted phenyl) prop-2-enoyl] phenyl} thioureas. Synth. Commun. 2018, 48, 3015–3024. [Google Scholar] [CrossRef]
- Mahapatra, D.K.; Bharti, S.K.; Asati, V. Anti-cancer chalcones: Structural and molecular target perspectives. Eur. J. Med. Chem. 2015, 98, 69–114. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Johnson, T.E.; Lad, R.; Xing, C. Structure−Activity Relationship Studies of Chalcone Leading to 3-Hydroxy-4,3′,4′,5′-tetramethoxychalcone and Its Analogues as Potent Nuclear Factor κB Inhibitors and Their Anticancer Activities. J. Med. Chem. 2009, 52, 7228–7235. [Google Scholar] [CrossRef]
- Park, J.-Y.; Ko, J.-A.; Kim, D.W.; Kim, Y.M.; Kwon, H.-J.; Jeong, H.J.; Kim, C.Y.; Park, K.H.; Lee, W.S.; Ryu, Y.B. Chalcones isolated from Angelica keiskei inhibit cysteine proteases of SARS-CoV. J. Enzyme Inhib. Med. Chem. 2015, 31, 23–30. [Google Scholar] [CrossRef]
- Franceschelli, S.; Pesce, M.; Vinciguerra, I.; Ferrone, A.; Riccioni, G.; Antonia, P.; Grilli, A.; Felaco, M.; Speranza, L. Licocalchone-C Extracted from Glycyrrhiza Glabra Inhibits Lipopolysaccharide-Interferon-γ Inflammation by Improving Antioxidant Conditions and Regulating Inducible Nitric Oxide Synthase Expression. Molecules 2011, 16, 5720–5734. [Google Scholar] [CrossRef]
- Kulkarni, R.R.; Tupe, S.G.; Gample, S.P.; Chandgude, M.G.; Sarkar, D.; Deshpande, M.V.; Joshi, S.P. Antifungal dimeric chalcone derivative kamalachalcone E from Mallotus philippinensis. Nat. Prod. Res. 2012, 28, 245–250. [Google Scholar] [CrossRef]
- Enoki, T.; Ohnogi, H.; Nagamine, K.; Kudo, Y.; Sugiyama, K.; Tanabe, M.; Kobayashi, E.; Sagawa, H.; Kato, I. Antidiabetic Activities of Chalcones Isolated from a Japanese Herb, Angelica keiskei. J. Agric. Food Chem. 2007, 55, 6013–6017. [Google Scholar] [CrossRef]
- Cui, Y.; Ao, M.; Hu, J.; Yu, L. Anti-Inflammatory Activity of Licochalcone A Isolated from Glycyrrhiza inflata. Z. Naturforsch. C J. Biosci. 2008, 63, 5–6. [Google Scholar] [CrossRef]
- Takahashi, M.; Maeda, S.; Ogura, K.; Terano, A.; Omata, M. The Possible Role of Vascular Endothelial Growth Factor (VEGF) in Gastric Ulcer Healing: Effect of Sofalcone on VEGF Release In Vitro. J. Clin. Gastroenterol. 1998, 27, S178–S182. [Google Scholar] [CrossRef]
- Fu, Y.; Hsieh, T.-C.; Guo, J.; Kunicki, J.; Lee, M.Y.; Darzynkiewicz, Z.; Wu, J.M. Licochalcone-A, a novel flavonoid isolated from licorice root (Glycyrrhiza glabra), causes G2 and late-G1 arrests in androgen-independent PC-3 prostate cancer cells. Biochem. Biophys. Res. Commun. 2004, 322, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Tsukiyama, R.-I.; Katsura, H.; Tokuriki, N.; Kobayashi, M. Antibacterial activity of licochalcone A against spore-forming bacteria. Antimicrob. Agents Chemother. 2002, 46, 1226–1230. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, Z.; Meng, R.; Shi, C.; Guo, N. Antioxidative and anticancer properties of Licochalcone A from licorice. J. Ethnopharmacol. 2017, 198, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B. Diverse Molecular Targets for Chalcones with Varied Bioactivities. Med. Chem. 2015, 5, 388–404. [Google Scholar] [CrossRef] [PubMed]
- Batovska, D.I.; Todorova, I.T. Trends in Utilization of the Pharmacological Potential of Chalcones. Curr. Clin. Pharmacol. 2010, 5, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, T.; Lungu, C.N. Anticancer Activity of Natural and Synthetic Chalcones. Int. J. Mol. Sci. 2021, 22, 11306. [Google Scholar] [CrossRef] [PubMed]
- Kilpin, K.J.; Crot, S.; Riedel, T.; Kitchen, J.A.; Dyson, P.J. Ruthenium(ii) and osmium(ii) 1,2,3-triazolylidene organometallics: A preliminary investigation into the biological activity of ‘click’ carbene complexes. Dalton Trans. 2014, 43, 1443–1448. [Google Scholar] [CrossRef]
- Schulze, B.; Schubert, U.S. Beyond click chemistry—Supramolecular interactions of 1,2,3-triazoles. Chem. Soc. Rev. 2014, 43, 2522. [Google Scholar] [CrossRef]
- Benkhellat, Z.; Allali, M.; Beley, M.; Wenger, E.; Bernard, M.; Parizel, N.; Selmeczi, K.; Joly, J.-P. Click synthesis of symmetric bis-triazol ligands and full characterisation of their copper(II)-complexes. New J. Chem. 2013, 38, 419–429. [Google Scholar] [CrossRef]
- Hou, Y.-P.; Sun, J.; Pang, Z.-H.; Lv, P.-C.; Li, D.-D.; Yan, L.; Zhang, H.-J.; Zheng, E.X.; Zhao, J.; Zhu, H.-L. Synthesis and antitumor activity of 1,2,4-triazoles having 1,4-benzodioxan fragment as a novel class of potent methionine aminopeptidase type II inhibitors. Bioorg. Med. Chem. 2011, 19, 5948–5954. [Google Scholar] [CrossRef] [PubMed]
- Chai, X.; Zhang, J.; Yu, S.; Hu, H.; Zou, Y.; Zhao, Q.; Dan, Z.; Zhang, D.; Wu, Q. Design, synthesis, and biological evaluation of novel 1-(1H-1,2,4-triazole-1-yl)-2-(2,4-difluorophenyl)-3-substituted benzylamino-2-propanols. Bioorg. Med. Chem. Lett. 2009, 19, 1811–1814. [Google Scholar] [CrossRef]
- Navidpour, L.; Shafaroodi, H.; Abdi, K.; Amini, M.; Ghahremani, M.H.; Dehpour, A.R.; Shafiee, A. Design, synthesis, and biological evaluation of substituted 3-alkylthio-4,5-diaryl-4H-1,2,4-triazoles as selective COX-2 inhibitors. Bioorg. Med. Chem. 2006, 14, 2507–2517. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.-M.; Wang, C.; Wang, W.-L.; Liang, L.-L.; Liu, W.; Gong, K.-K.; Sun, K.-L. Triazole derivatives and their antiplasmodial and antimalarial activities. Eur. J. Med. Chem. 2019, 166, 206–223. [Google Scholar] [CrossRef] [PubMed]
- Manohar, S.; Khan, S.I.; Rawat, D.S. Synthesis of 4-aminoquinoline-1,2,3-triazole and 4-aminoquinoline-1,2,3-triazole-1,3,5-triazine Hybrids as Potential Antimalarial Agents. Chem. Biol. Drug Des. 2011, 78, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Labanauskas, L.; Udrenaite, E.; Gaidelis, P.; Brukštus, A. Synthesis of 5-(2-,3- and 4-methoxyphenyl)-4H-1,2,4-triazole-3-thiol derivatives exhibiting anti-inflammatory activity. Il Farm. 2004, 59, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Boechat, N.; Ferreira, V.F.; Ferreira, S.B.; Ferreira, M.d.L.G.; Silva, F.d.C.d.; Bastos, M.M.; Costa, M.d.S.; Lourenço, M.C.S.; Pinto, A.C.; Krettli, A.U.; et al. Novel 1,2,3-Triazole Derivatives for Use against Mycobacterium tuberculosis H37Rv (ATCC 27294) Strain. J. Med. Chem. 2011, 54, 5988–5999. [Google Scholar] [CrossRef]
- De La Rosa, M.; Kim, H.W.; Gunic, E.; Jenket, C.; Boyle, U.; Koh, Y.-H.; Korboukh, I.; Allan, M.; Zhang, W.; Chen, H.; et al. Tri-substituted triazoles as potent non-nucleoside inhibitors of the HIV-1 reverse transcriptase. Bioorg. Med. Chem. Lett. 2006, 16, 4444–4449. [Google Scholar] [CrossRef] [PubMed]
- Zoumpoulakis, P.; Camoutsis, C.; Pairas, G.; Soković, M.; Glamočlija, J.; Potamitis, C.; Pitsas, A. Synthesis of novel sulfonamide-1,2,4-triazoles, 1,3,4-thiadiazoles and 1,3,4-oxadiazoles, as potential antibacterial and antifungal agents. Biological evaluation and conformational analysis studies. Bioorg. Med. Chem. 2012, 20, 1569–1583. [Google Scholar] [CrossRef] [PubMed]
- Sanders, W.E.; Sanders, C.C. Piperacillin/Tazobactam: A Critical Review of the Evolving Clinical Literature. Clin. Infect. Dis. 1996, 22, 107–123. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Perry, C.M.; Markham, A.; Fass, R.J.; Wilson, S.E. Piperacillin/Tazobactam An Updated Review of its Use in the Treatment of Bacterial Infections. Pharmacoeconomics 2001, 19, 1135–1175. [Google Scholar] [CrossRef]
- Khan, F.Y.; Elhiday, A.; Khudair, I.F.; Yousef, H.; Omran, A.H.; Alsamman, S.H.; Elhamid, M. Evaluation of the use of piperacillin/tazobactam (Tazocin) at Hamad General Hospital, Qatar: Are there unjustified prescriptions? Infect. Drug Resist. 2012, 5, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Yan, H.; Wang, J.; Liu, K.; Liu, B.-S.; Shi, Y.-M. 1,2,3-Triazole-containing hybrids with potential antibacterial activity against ESKAPE pathogens. Eur. J. Med. Chem. 2022, 244, 114888. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Karthikeyan, C.; Moorthy, N.S.H.N.; Deora, G.S.; Solomon, V.R.; Lee, H.; Trivedi, P. Design, synthesis and biological evaluation of some novel 3-cinnamoyl-4-hydroxy-2H-chromen-2-ones as antimalarial agents. Med. Chem. Res. 2011, 21, 1780–1784. [Google Scholar] [CrossRef]
- Sashidhara, K.V.; Kumar, A.; Kumar, M.; Sarkar, J.; Sinha, S. Synthesis and in vitro evaluation of novel coumarin–chalcone hybrids as potential anticancer agents. Bioorg. Med. Chem. Lett. 2010, 20, 7205–7211. [Google Scholar] [CrossRef]
- Sashidhara, K.V.; Kumar, M.; Modukuri, R.K.; Sonkar, R.; Bhatia, G.; Khanna, A.; Rai, S.; Shukla, R. Synthesis and anti-inflammatory activity of novel biscoumarin–chalcone hybrids. Bioorg. Med. Chem. Lett. 2011, 21, 4480–4484. [Google Scholar] [CrossRef]
- Pérez-Cruz, F.; Vazquez-Rodriguez, S.; Matos, M.J.; Herrera-Morales, A.; Villamena, F.A.; Das, A.; Gopalakrishnan, B.; Olea-Azar, C.; Santana, L.; Uriarte, E. Synthesis and Electrochemical and Biological Studies of Novel Coumarin–Chalcone Hybrid Compounds. J. Med. Chem. 2013, 56, 6136–6145. [Google Scholar] [CrossRef] [PubMed]
- Wanare, G.; Aher, R.; Kawathekar, N.; Ranjan, R.; Kaushik, N.K.; Sahal, D. Synthesis of novel α-pyranochalcones and pyrazoline derivatives as Plasmodium falciparum growth inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 4675–4678. [Google Scholar] [CrossRef]
- Stefani, H.A.; Gueogjan, K.; Manarin, F.; Farsky, S.H.; Zukerman-Schpector, J.; Caracelli, I.; Rodrigues, S.R.P.; Muscará, M.N.; Teixeira, S.A.; Santin, J.R.; et al. Synthesis, biological evaluation and molecular docking studies of 3-(triazolyl)-coumarin derivatives: Effect on inducible nitric oxide synthase. Eur. J. Med. Chem. 2012, 58, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Ouellet, S.G.; Gauvreau, D.; Cameron, M.; Dolman, S.; Campeau, L.-C.; Hughes, G.; O’shea, P.D.; Davies, I.W. Convergent, Fit-For-Purpose, Kilogram-Scale Synthesis of a 5-Lipoxygenase Inhibitor. Org. Process. Res. Dev. 2012, 16, 214–219. [Google Scholar] [CrossRef]
- Kushwaha, K.; Kaushik, N.; Lata; Jain, S.C. Design and synthesis of novel 2H-chromen-2-one derivatives bearing 1,2,3-triazole moiety as lead antimicrobials. Bioorg. Med. Chem. Lett. 2014, 24, 1795–1801. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, Z.; Zhou, M.; Wu, F.; Hou, X.; Luo, H.; Liu, H.; Han, X.; Yan, G.; Ding, Z.; et al. Synthesis and biological evaluation of 4-(1,2,3-triazol-1-yl)coumarin derivatives as potential antitumor agents. Bioorg. Med. Chem. Lett. 2013, 24, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Mukusheva, G.K.; Lipeeva, A.V.; Zhanymkhanova, P.Z.; Shults, E.E.; Gatilov, Y.V.; Shakirov, M.M.; Adekenov, S.M. The flavanone pinostrobin in the synthesis of coumarin-chalcone hybrids with a triazole linker. Chem. Heterocycl. Compd. 2015, 51, 146–152. [Google Scholar] [CrossRef]
- Annunziata, F.; Pinna, C.; Dallavalle, S.; Tamborini, L.; Pinto, A. An Overview of Coumarin as a Versatile and Readily Accessible Scaffold with Broad-Ranging Biological Activities. Int. J. Mol. Sci. 2020, 21, 4618. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lan, Y.; Wang, S.; Zhang, H.; Xu, X.; Liu, X.; Yu, M.; Liu, B.-F.; Zhang, G. Synthesis and evaluation of new coumarin derivatives as potential atypical antipsychotics. Eur. J. Med. Chem. 2014, 74, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Elbastawesy, M.A.I.; Youssif, B.G.M.; Abdelrahman, M.H.; Hayallah, A.M. Synthesis and biological evaluation of some new coumarin derivatives as potential antimicrobial, analgesic and anti-inflammatory agents. Pharma Chem. 2015, 7, 337–349. [Google Scholar]
- Rasool, S.; Rehman, A.U.; Abbasi, M.A.; Siddiqui, S.Z.; Shah, S.A.A.; Hassan, S.; Ahmad, I. Synthesis, Structural Elucidation, and Antibacterial Evaluation of Some New Molecules Derived from Coumarin, 1,3,4-Oxadiazole, and Acetamide. Org. Chem. Int. 2016, 2016, 8696817. [Google Scholar] [CrossRef]
- Weng, K.-G.; Yuan, Y.-L. Synthesis and evaluation of coumarin derivatives against human lung cancer cell lines. Braz. J. Med. Biol. Res. 2017, 50, e6455. [Google Scholar] [CrossRef]
- Fayed, E.A.; Sabour, R.; Harras, M.F.; Mehany, A.B.M. Design, synthesis, biological evaluation and molecular modeling of new coumarin derivatives as potent anticancer agents. Med. Chem. Res. 2019, 28, 1284–1297. [Google Scholar] [CrossRef]
- Naik, C.G.; Malik, G.M.; Parekh, H.M. Novel Coumarin Derivatives: Synthesis, Characterization and Antimicrobial Activity. S. Afr. J. Chem. 2019, 72, 248–252. [Google Scholar] [CrossRef]
- Xu, X.-T.; Deng, X.-Y.; Chen, J.; Liang, Q.-M.; Zhang, K.; Li, D.-L.; Wu, P.-P.; Zheng, X.; Zhou, R.-P.; Jiang, Z.-Y.; et al. Synthesis and biological evaluation of coumarin derivatives as α-glucosidase inhibitors. Eur. J. Med. Chem. 2020, 189, 112013. [Google Scholar] [CrossRef]
- Alshibl, H.M.; Al-Abdullah, E.S.; Haiba, M.E.; Alkahtani, H.M.; Awad, G.E.; Mahmoud, A.H.; Ibrahim, B.M.; Bari, A.; Villinger, A. Synthesis and Evaluation of New Coumarin Derivatives as Antioxidant, Antimicrobial, and Anti-Inflammatory Agents. Molecules 2020, 25, 3251. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Peng, T.; Wen, X.; Wang, G.; Liu, S.; Sun, Y.; Zhang, S.; Wang, L. Design, Synthesis and Evaluation of 3-Substituted Coumarin Derivatives as Anti-inflammatory Agents. Chem. Pharm. Bull. 2020, 68, 443–446. [Google Scholar] [CrossRef]
- Abduljabbar, T.T.; Hadi, M.K. Synthesis, Characterization and Antibacterial Evaluation of Some Coumarin Derivatives. Iraqi J. Pharm. Sci. 2021, 30, 249–257. [Google Scholar] [CrossRef]
- Mzezewa, S.C.; Omoruyi, S.I.; Zondagh, L.S.; Malan, S.F.; Ekpo, O.E.; Joubert, J. Design, synthesis, and evaluation of 3,7-substituted coumarin derivatives as multifunctional Alzheimer’s disease agents. J. Enzyme Inhib. Med. Chem. 2021, 36, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Kokat, J.; Jadhav, B. Synthesis And Biological Evaluation Of Newer Coumarin Derivatives As Antimicrobial Agents. Int. J. Pharm. Sci. Res. 2022, 13, 3249. [Google Scholar] [CrossRef]
- Yadav, S.; Kumar, N.; Bhalla, V. Synthesis and evaluation of novel 4-anilinocoumarin derivatives as potential antimicrobial agents. J. Appl. Pharm. Sci. 2022, 12, 196–204. [Google Scholar] [CrossRef]
- Zhou, R.; Yu, Y.H.; Kim, H.; Ha, H.-H. Synthesis of coumarin derivatives and investigation of their inhibitory effects on lung cancer cell motility. Sci. Rep. 2022, 12, 21635. [Google Scholar] [CrossRef]
- Ghouse, S.M.; Bahatam, K.; Angeli, A.; Pawar, G.; Chinchilli, K.K.; Yaddanapudi, V.M.; Mohammed, A.; Supuran, C.T.; Nanduri, S. Synthesis and biological evaluation of new 3-substituted coumarin derivatives as selective inhibitors of human carbonic anhydrase IX and XII. J. Enzyme Inhib. Med. Chem. 2023, 38, 2185760. [Google Scholar] [CrossRef]
- Prathap, K.N.C.; Lokanath, N.K. Synthesis, characterization, crystal structure and quantum chemical investigations of three novel coumarin-benzenesulfonohydrazide derivatives. J. Mol. Struct. 2018, 1158, 26–38. [Google Scholar] [CrossRef]
- Amin, K.M.; Eissa, A.A.M.; Abou-Seri, S.M.; Awadallah, F.M.; Hassan, G.S. Synthesis and biological evaluation of novel coumarin–pyrazoline hybrids endowed with phenylsulfonyl moiety as antitumor agents. Eur. J. Med. Chem. 2013, 60, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Rodriguez, S.; Figueroa-Guíñez, R.; Matos, M.J.; Santana, L.; Uriarte, E.; Lapier, M.; Maya, J.D.; Olea-Azar, C. Synthesis of coumarin–chalcone hybrids and evaluation of their antioxidant and trypanocidal properties. MedChemComm 2013, 4, 993–1000. [Google Scholar] [CrossRef]
- Rodriguez, S.-V. Synthesis and Trypanocidal Properties of New Coumarin-Chalcone Derivatives. Med. Chem. 2015, 5, 173–177. [Google Scholar] [CrossRef]
- Vazquez-Rodriguez, S.; López, R.L.; Matos, M.J.; Armesto-Quintas, G.; Serra, S.; Uriarte, E.; Santana, L.; Borges, F.; Crego, A.M.; Santos, Y. Design, synthesis and antibacterial study of new potent and selective coumarin-chalcone derivatives for the treatment of tenacibaculosis. Bioorg. Med. Chem. 2015, 23, 7045–7052. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Rajput, S.; Hospete, S.; Irkal, S. Synthesis of coumarin-Chalcone Derivatives. IOSR J. Appl. Chem. (IOSR-JAC) 2019, 12, 46–51. [Google Scholar] [CrossRef]
- Kurt, B.Z.; Kandas, N.O.; Dag, A.; Sonmez, F.; Kucukislamoglu, M. Synthesis and biological evaluation of novel coumarin-chalcone derivatives containing urea moiety as potential anticancer agents. Arab. J. Chem. 2020, 13, 1120–1129. [Google Scholar] [CrossRef]
- Emam, S.H.; Sonousi, A.; Osman, E.O.; Hwang, D.; Kim, G.-D.; Hassan, R.A. Design and synthesis of methoxyphenyl- and coumarin-based chalcone derivatives as anti-inflammatory agents by inhibition of NO production and down-regulation of NF-κB in LPS-induced RAW264.7 macrophage cells. Bioorg. Chem. 2021, 107, 104630. [Google Scholar] [CrossRef] [PubMed]
- Konidala, S.K.; Kotra, V.; Danduga, R.C.S.R.; Kola, P.K.; Bhandare, R.R.; Shaik, A.B. Design, multistep synthesis and in-vitro antimicrobial and antioxidant screening of coumarin clubbed chalcone hybrids through molecular hybridization approach. Arab. J. Chem. 2021, 14, 103154. [Google Scholar] [CrossRef]
- Hu, C.-M.; Luo, Y.-X.; Wang, W.-J.; Li, J.-P.; Li, M.-Y.; Zhang, Y.-F.; Xiao, D.; Lu, L.; Xiong, Z.; Feng, N.; et al. Synthesis and Evaluation of Coumarin-Chalcone Derivatives as α-Glucosidase Inhibitors. Front. Chem. 2022, 10, 926543. [Google Scholar] [CrossRef]
- Pavić, K.; Beus, M.; Poje, G.; Uzelac, L.; Kralj, M.; Rajić, Z. Synthesis and biological evaluation of harmirins, novel harmine–coumarin hybrids as potential anticancer agents. Molecules 2021, 26, 6490. [Google Scholar] [CrossRef]
- Shaikh, M.H.; Subhedar, D.D.; Khan, F.A.K.; Sangshetti, J.N.; Shingate, B.B. 1,2,3-Triazole incorporated coumarin derivatives as potential antifungal and antioxidant agents. Chin. Chem. Lett. 2016, 27, 295–301. [Google Scholar] [CrossRef]
- Shaikh, M.H.; Subhedar, D.D.; Shingate, B.B.; Khan, F.A.K.; Sangshetti, J.N.; Khedkar, V.M.; Nawale, L.; Sarkar, D.; Navale, G.R.; Shinde, S.S. Synthesis, biological evaluation and molecular docking of novel coumarin incorporated triazoles as antitubercular, antioxidant and antimicrobial agents. Med. Chem. Res. 2016, 25, 790–804. [Google Scholar] [CrossRef]
- Sinha, S.; Kumaran, A.P.; Mishra, D.; Paira, P. Synthesis and cytotoxicity study of novel 3-(triazolyl)coumarins based fluorescent scaffolds. Bioorg. Med. Chem. Lett. 2016, 26, 5557–5561. [Google Scholar] [CrossRef]
- Al-Wahaibi, L.H.; Abu-Melha, H.M.; Ibrahim, D.A. Synthesis of novel 1,2,4-triazolyl coumarin derivatives as potential anticancer agents. J. Chem. 2018, 2018, 5201374. [Google Scholar] [CrossRef]
- Kumar K., A.; Kalluraya, B.; Kumar, S.M. Synthesis and in-vitro antioxidant activities of some coumarin derivatives containing 1,2,3-triazole ring. Phosphorus Sulfur Silicon Relat. Elem. 2018, 193, 294–299. [Google Scholar] [CrossRef]
- Nouraie, P.; Dehaghi, S.M.; Foroumadi, A. Coumarin-1,2,3-triazole hybrid derivatives: Green synthesis and DFT calculations. Synth. Commun. 2019, 49, 386–394. [Google Scholar] [CrossRef]
- Krishna, B.R.; Thummuri, D.; Naidu, V.; Ramakrishna, S.; Mallavadhani, U.V. Synthesis of some novel orcinol based coumarin triazole hybrids with capabilities to inhibit RANKL-induced osteoclastogenesis through NF-κB signaling pathway. Bioorg. Chem. 2018, 78, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Agarwal, D.; Kumar, S.; Dixit, A.; Gupta, R.D.; Awasthi, S.K. In vitro antiplasmodial efficacy of synthetic coumarin-triazole analogs. Eur. J. Med. Chem. 2018, 145, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, F.; Faiz, Ö. Microwave-assisted synthesis and biological evaluation of some coumarin hydrazides. J. Turk. Chem. Soc. Sect. A Chem. 2018, 5, 551–568. [Google Scholar] [CrossRef]
- Özdemir, M.; Abliatipova, A.; Benian, S.; Yalçın, B.; Salan, Ü.; Durmuş, M.; Bulut, M. 1,2,3-Triazole incorporated coumarin carrying metal-free, Zn(II), Mg(II) phthalocyanines: Synthesis, characterization, theoretical studies, photophysical and photochemical properties. J. Photochem. Photobiol. A Chem. 2020, 403, 112845. [Google Scholar] [CrossRef]
- Channabasappa, V.; Kumara, K.; Kariyappa, A.K. Design, synthesis of coumarin tethered 1,2,3-triazoles analogues, evaluation of their antimicrobial and a-amylase inhibition activities. J. Chem. Sci. 2021, 133, 1–8. [Google Scholar] [CrossRef]
- de Sousa, B.L.; Leite, J.; Mendes, T.; Varejão, E.; Chaves, A.; da Silva, J.; Agrizzi, A.; Ferreira, P.; Pilau, E.J.; Silva, E.; et al. Inhibition of acetylcholinesterase by coumarin-linked amino acids synthetized via triazole associated with molecule partition coefficient. J. Braz. Chem. Soc. 2021, 32, 652–664. [Google Scholar] [CrossRef]
- Vagish, C.B.; Kumara, K.; Vivek, H.K.; Bharath, S.; Lokanath, N.K.; Kumar, K.A. Coumarin-triazole hybrids: Design, microwave-assisted synthesis, crystal and molecular structure, theoretical and computational studies and screening for their anticancer potentials against PC-3 and DU-145. J. Mol. Struct. 2021, 1230, 129899. [Google Scholar] [CrossRef]
- Basappa, V.C.; Kameshwar, V.H.; Kumara, K.; Achutha, D.K.; Krishnappagowda, L.N.; Kariyappa, A.K. Design and synthesis of coumarin-triazole hybrids: Biocompatible anti-diabetic agents, in silico molecular docking and ADME screening. Heliyon 2020, 6, e05290. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Al-Shuaeeb, A. Synthesis of new coumarin derivatives containing aminobenzotriazole, triazole moieties and their antimicrobial activities. Al-Kitab J. Med. Sci. 2023, 1, 47–57. Available online: https://www.isnra.net/index.php/kjms/article/view/861 (accessed on 25 October 2023).
- Adam, R.W.; Zimam, E.H. Design, Synthesis, Anticancer Activity and Molecular Docking of New 1,2,3-Triazole combined Glucosides with coumarin. J. Popul. Ther. Clin. Pharmacol. 2023, 30, E345–E356. [Google Scholar] [CrossRef]
- Omar, R.A.; Koparir, P.; Sarac, K.; Koparir, M.; A Safin, D. A novel coumarin-triazole-thiophene hybrid: Synthesis, characterization, ADMET prediction, molecular docking and molecular dynamics studies with a series of SARS-CoV-2 proteins. J. Chem. Sci. 2023, 135, 1–15. [Google Scholar] [CrossRef]
- Sharma, A.; Bharate, S.B. Synthesis and Biological Evaluation of Coumarin Triazoles as Dual Inhibitors of Cholinesterases and β-Secretase. ACS Omega 2023, 8, 11161–11176. [Google Scholar] [CrossRef] [PubMed]
- Pingaew, R.; Saekee, A.; Mandi, P.; Nantasenamat, C.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Synthesis, biological evaluation and molecular docking of novel chalcone-coumarin hybrids as anticancer and antimalarial agents. Eur. J. Med. Chem. 2014, 85, 65–76. [Google Scholar] [CrossRef] [PubMed]
























































Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rohman, N.; Ardiansah, B.; Wukirsari, T.; Judeh, Z. Recent Trends in the Synthesis and Bioactivity of Coumarin, Coumarin–Chalcone, and Coumarin–Triazole Molecular Hybrids. Molecules 2024, 29, 1026. https://doi.org/10.3390/molecules29051026
Rohman N, Ardiansah B, Wukirsari T, Judeh Z. Recent Trends in the Synthesis and Bioactivity of Coumarin, Coumarin–Chalcone, and Coumarin–Triazole Molecular Hybrids. Molecules. 2024; 29(5):1026. https://doi.org/10.3390/molecules29051026
Chicago/Turabian StyleRohman, Nur, Bayu Ardiansah, Tuti Wukirsari, and Zaher Judeh. 2024. "Recent Trends in the Synthesis and Bioactivity of Coumarin, Coumarin–Chalcone, and Coumarin–Triazole Molecular Hybrids" Molecules 29, no. 5: 1026. https://doi.org/10.3390/molecules29051026
APA StyleRohman, N., Ardiansah, B., Wukirsari, T., & Judeh, Z. (2024). Recent Trends in the Synthesis and Bioactivity of Coumarin, Coumarin–Chalcone, and Coumarin–Triazole Molecular Hybrids. Molecules, 29(5), 1026. https://doi.org/10.3390/molecules29051026