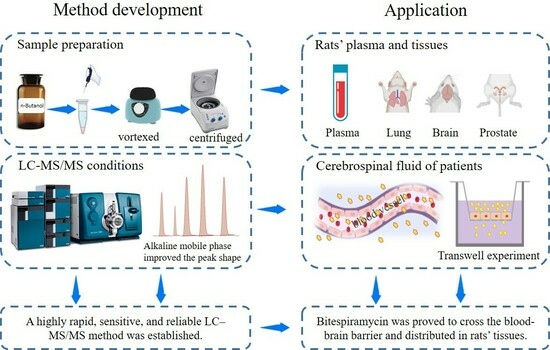
Study of Bitespiramycin Distribution in Rats and Cerebrospinal Fluid of Patients by a Sensitive LC-MS/MS Method with Rapid Sample Preparation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Method Development
2.2. Method Validation
2.2.1. Specificity and Selectivity
2.2.2. Linearity and LLOQ
2.2.3. Accuracy and Precision
2.2.4. Extraction Recovery Rate and Matrix Effect
2.2.5. Stability
2.3. Distribution of Bitespiramycin in Rats
2.3.1. Distribution in Rat Plasma
2.3.2. Distribution in Rat Tissues
2.4. Distribution in Cerebrospinal Fluid of Patients
3. Material and Methods
3.1. Reagents
3.2. Animals
3.3. Patient Cerebrospinal Fluid Collection
3.4. Standards and Sample Preparation for UHPLC–MS/MS
3.5. UHPLC–MS/MS
3.6. Method Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, H.Y.; Sun, J.; Wang, K.; Wang, H.; Wu, S.; Bao, L.; He, W.; Wang, D.; Zhu, A.; Zhang, T.; et al. Repurposing CFDA-approved drug carrimycin as an antiviral agent against human coronaviruses, including the currently pandemic SARS-CoV-2. Acta Pharm. Sin. B 2021, 11, 2850–2858. [Google Scholar] [CrossRef] [PubMed]
- U. S. National Library of Medicine. Study to Evaluate Safety and Efficacy of Carrimycin for Treatment of Severe COVID-19 in Hospitalized Patients. Available online: https://clinicaltrials.gov/study/NCT04672564 (accessed on 19 February 2024).
- Cui, J.; Zhou, J.; He, W.; Ye, J.; Westlake, T.; Medina, R.; Wang, H.; Thakur, B.L.; Liu, J.; Xia, M.; et al. Targeting selenoprotein H in the nucleolus suppresses tumors and metastases by Isovalerylspiramycin I. J. Exp. Clin. Cancer Res. 2022, 41, 126. [Google Scholar] [CrossRef]
- Wang, M.J.; Xue, J.; Zou, W.-B.; Wang, Y.; Hu, C.-Q.; Hoogmartens, J.; Adams, E. Identification of multi components in bitespiramycin by liquid chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2012, 66, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Yang, J.N.; Hu, M.; Hu, C.Q. Analysis of components of bitespiramycin by HPLC. Acta Pharm. Sin. B 2009, 44, 1183–1186. [Google Scholar] [CrossRef]
- Shi, X.G.; Fawcett, J.P.; Chen, X.Y.; Zhong, D.F. Structural identification of bitespiramycin metabolites in rat: A single oral dose study. Xenobiotica 2005, 35, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.G.; Zhong, D.F.; Sun, Y.M.; Zhang, Y.F. Metabolites of A Novel Antibiotic Bitespiramycin in Rat Urine and Bile. Chin. Chem. Lett. 2004, 4, 431–434. [Google Scholar]
- Zhong, D.; Shi, X.; Sun, L.; Chen, X. Determination of three major components of bitespiramycin and their major active metabolites in rat plasma by liquid chromatography-ion trap mass spectrometry. J. Chromatogr. B 2003, 791, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.G.; Sun, Y.M.; Zhang, Y.F.; Zhong, D.F. Tissue distribution of bitespiramycin and spiramycin in rats. Acta Pharmacol. Sin. 2004, 25, 1396–1401. [Google Scholar] [PubMed]
- Gao, H.L.; Liu, Q.; Liu, H.Z.; He, Z.G. Pharmacokinetics of kelimycin tablets in beagle dogs. J. Shenyang Pharma Univ. 2019, 36, 973–980. [Google Scholar] [CrossRef]





| Tissues/Body Fluid | Linear Ranges | |
|---|---|---|
| ISV-SPM I, SPM I, II, III | ISV-SPM II, III | |
| Brain | 1.2–72.8 ng/g | 0.6–38.4 ng/g |
| Testis | 0.6–800 ng/g | 0.3–400 ng/g |
| Prostate | 1.2–8000 ng/g | 0.6–4000 ng/g |
| Uterus and ovary | 1.8–12,000 ng/g | 0.9–6000 ng/g |
| Bladder | 2.4–16,000 ng/g | 1.2–8000 ng/g |
| Lung | 3.6–24,000 ng/g | 1.8–12,000 ng/g |
| Plasma | 0.6–4000 ng/mL | 0.3–2000 ng/mL |
| Cerebrospinal fluid | 0.6–38.4 ng/mL | 0.3–19.2 ng/mL |
| ISV-SPM I | ISV-SPM I | ISV-SPM I | SPM I | SPM II | SPM III | |
|---|---|---|---|---|---|---|
| LLOQ reported in [9] | 4 | 12 | 18 | 4 | 12 | 18 |
| LLOQ reported in [10] | 1 | 1 | 1 | 1 | 1 | 1 |
| LLOQ of this study | 0.3 | 0.6 | 0.6 | 0.6 | 0.3 | 0.3 |
| Brain | Lung | Prostate | Testis | Womb | Ovary | Bladder | |
|---|---|---|---|---|---|---|---|
| ISV-SPM I | / | / | / | / | / | / | / |
| ISV-SPM II | 0.31 | 83.10 | 78.81 | 2.70 | 30.66 | 26.28 | 12.35 |
| ISV-SPM III | 1.34 | 221.04 | 182.44 | 5.82 | 60.86 | 57.28 | 37.17 |
| SPM I | 0.22 | 89.29 | 53.50 | 5.60 | 62.18 | 35.75 | 31.27 |
| SPM II | 0.40 | 61.84 | 61.11 | 8.34 | 49.47 | 17.06 | 19.91 |
| SPM III | 0.26 | 69.01 | 34.46 | 1.95 | 29.59 | 14.19 | 9.87 |
| No. | Sex | Age | Sampling Time * | Concentration (ng/mL) | |||||
|---|---|---|---|---|---|---|---|---|---|
| ISV- SPM I | ISV- SPM II | ISV- SPM III | SPM I | SPM II | SPM III | ||||
| 1 | Male | 77 | 1 | ND | 0.47 | 0.60 | ND | ND | ND |
| 5 | ND | 1.54 | 1.76 | ND | ND | 1.27 | |||
| 2 | Male | 44 | 1 | ND | ND | ND | ND | ND | ND |
| 5 | ND | 0.31 | 0.42 | ND | ND | 0.75 | |||
| 3 | Male | 57 | 1 | ND | ND | ND | ND | ND | ND |
| 5 | ND | ND | ND | ND | 0.99 | 0.67 | |||
| 4 | Female | 53 | 1 | ND | ND | 0.33 | ND | 1.36 | 0.83 |
| 5 | ND | 1.73 | 1.83 | ND | 2.77 | 3.40 | |||
| 5 | Male | 43 | 1 | ND | ND | ND | ND | 0.74 | ND |
| 5 | ND | ND | ND | ND | 1.13 | 0.86 | |||
| 6 | Male | 72 | 19 | 0.72 | 5.21 | 9.14 | ND | 4.69 | 13.00 |
| 20 | 1.05 | 6.55 | 9.82 | ND | 5.61 | 11.90 | |||
| Component | m/z | CE/V | DP/V |
|---|---|---|---|
| SPM I | 843.7 → 174.2 | 43 | 80 |
| SPM II | 885.7 → 174.3 | 44 | 80 |
| SPM III | 899.7 → 174.4 | 46 | 80 |
| ISV-SPM I | 927.7 → 174.0 | 51 | 80 |
| ISV-SPM II | 969.8 → 174.1 | 48 | 80 |
| ISV-SPM III | 983.8 → 174.1 | 48 | 80 |
| Azithromycin | 749.7 → 591.6 | 42 | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Cao, J.; Su, J.; He, T.; Wang, Q.; Wei, F.; Guo, X.; Mei, Q.; Zeng, J. Study of Bitespiramycin Distribution in Rats and Cerebrospinal Fluid of Patients by a Sensitive LC-MS/MS Method with Rapid Sample Preparation. Molecules 2024, 29, 1037. https://doi.org/10.3390/molecules29051037
Zhang Y, Cao J, Su J, He T, Wang Q, Wei F, Guo X, Mei Q, Zeng J. Study of Bitespiramycin Distribution in Rats and Cerebrospinal Fluid of Patients by a Sensitive LC-MS/MS Method with Rapid Sample Preparation. Molecules. 2024; 29(5):1037. https://doi.org/10.3390/molecules29051037
Chicago/Turabian StyleZhang, Yujie, Jingjie Cao, Jiahan Su, Tingting He, Qianru Wang, Feng Wei, Xin Guo, Qibing Mei, and Jing Zeng. 2024. "Study of Bitespiramycin Distribution in Rats and Cerebrospinal Fluid of Patients by a Sensitive LC-MS/MS Method with Rapid Sample Preparation" Molecules 29, no. 5: 1037. https://doi.org/10.3390/molecules29051037
APA StyleZhang, Y., Cao, J., Su, J., He, T., Wang, Q., Wei, F., Guo, X., Mei, Q., & Zeng, J. (2024). Study of Bitespiramycin Distribution in Rats and Cerebrospinal Fluid of Patients by a Sensitive LC-MS/MS Method with Rapid Sample Preparation. Molecules, 29(5), 1037. https://doi.org/10.3390/molecules29051037