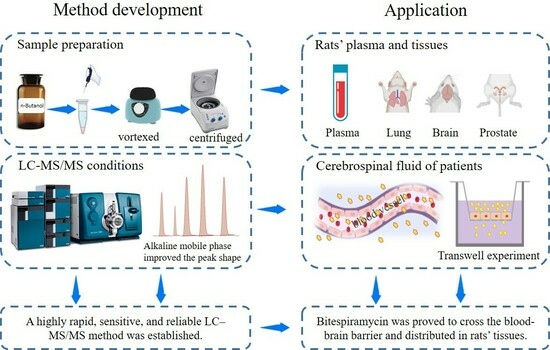
Study of Bitespiramycin Distribution in Rats and Cerebrospinal Fluid of Patients by a Sensitive LC-MS/MS Method with Rapid Sample Preparation
Abstract
Share and Cite
Zhang, Y.; Cao, J.; Su, J.; He, T.; Wang, Q.; Wei, F.; Guo, X.; Mei, Q.; Zeng, J. Study of Bitespiramycin Distribution in Rats and Cerebrospinal Fluid of Patients by a Sensitive LC-MS/MS Method with Rapid Sample Preparation. Molecules 2024, 29, 1037. https://doi.org/10.3390/molecules29051037
Zhang Y, Cao J, Su J, He T, Wang Q, Wei F, Guo X, Mei Q, Zeng J. Study of Bitespiramycin Distribution in Rats and Cerebrospinal Fluid of Patients by a Sensitive LC-MS/MS Method with Rapid Sample Preparation. Molecules. 2024; 29(5):1037. https://doi.org/10.3390/molecules29051037
Chicago/Turabian StyleZhang, Yujie, Jingjie Cao, Jiahan Su, Tingting He, Qianru Wang, Feng Wei, Xin Guo, Qibing Mei, and Jing Zeng. 2024. "Study of Bitespiramycin Distribution in Rats and Cerebrospinal Fluid of Patients by a Sensitive LC-MS/MS Method with Rapid Sample Preparation" Molecules 29, no. 5: 1037. https://doi.org/10.3390/molecules29051037
APA StyleZhang, Y., Cao, J., Su, J., He, T., Wang, Q., Wei, F., Guo, X., Mei, Q., & Zeng, J. (2024). Study of Bitespiramycin Distribution in Rats and Cerebrospinal Fluid of Patients by a Sensitive LC-MS/MS Method with Rapid Sample Preparation. Molecules, 29(5), 1037. https://doi.org/10.3390/molecules29051037