Effects of Rhizopus-arrhizus-31-Assisted Pretreatment on the Extraction and Bioactivity of Total Flavonoids from Hibiscus manihot L.
Abstract
:1. Introduction
2. Results and Discussion
2.1. Changes in the Fiber Structure of HML
2.2. Effects of Extraction Factors on Total Flavonoid Production
2.2.1. Determination of Solid–Liquid Ratio
2.2.2. Determination of Extraction Time
2.2.3. Determination of Ethanol Concentration
2.2.4. Determination of Extraction Temperature
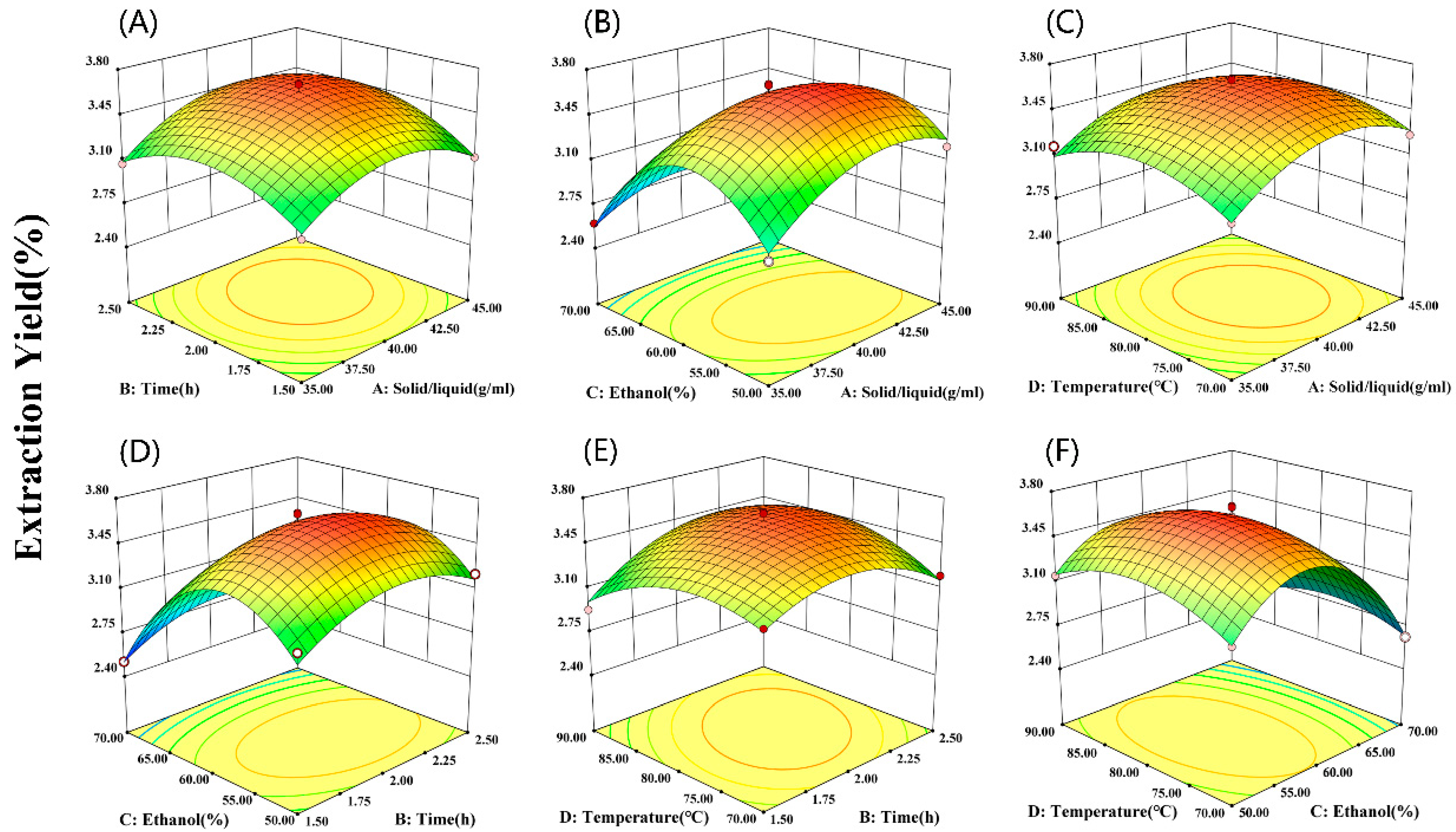
2.3. Response Surface Optimization Analysis
2.3.1. Box–Behnken Response Surface Optimization Experiment Results
2.3.2. Parameter Optimization for RFF Extraction
0.1075 BD − 0.0475 CD − 0.2521 A2 − 0.2333 B2 − 0.5521 C2 − 0.2033 D2
2.3.3. Response Surface Analysis and Process Optimization
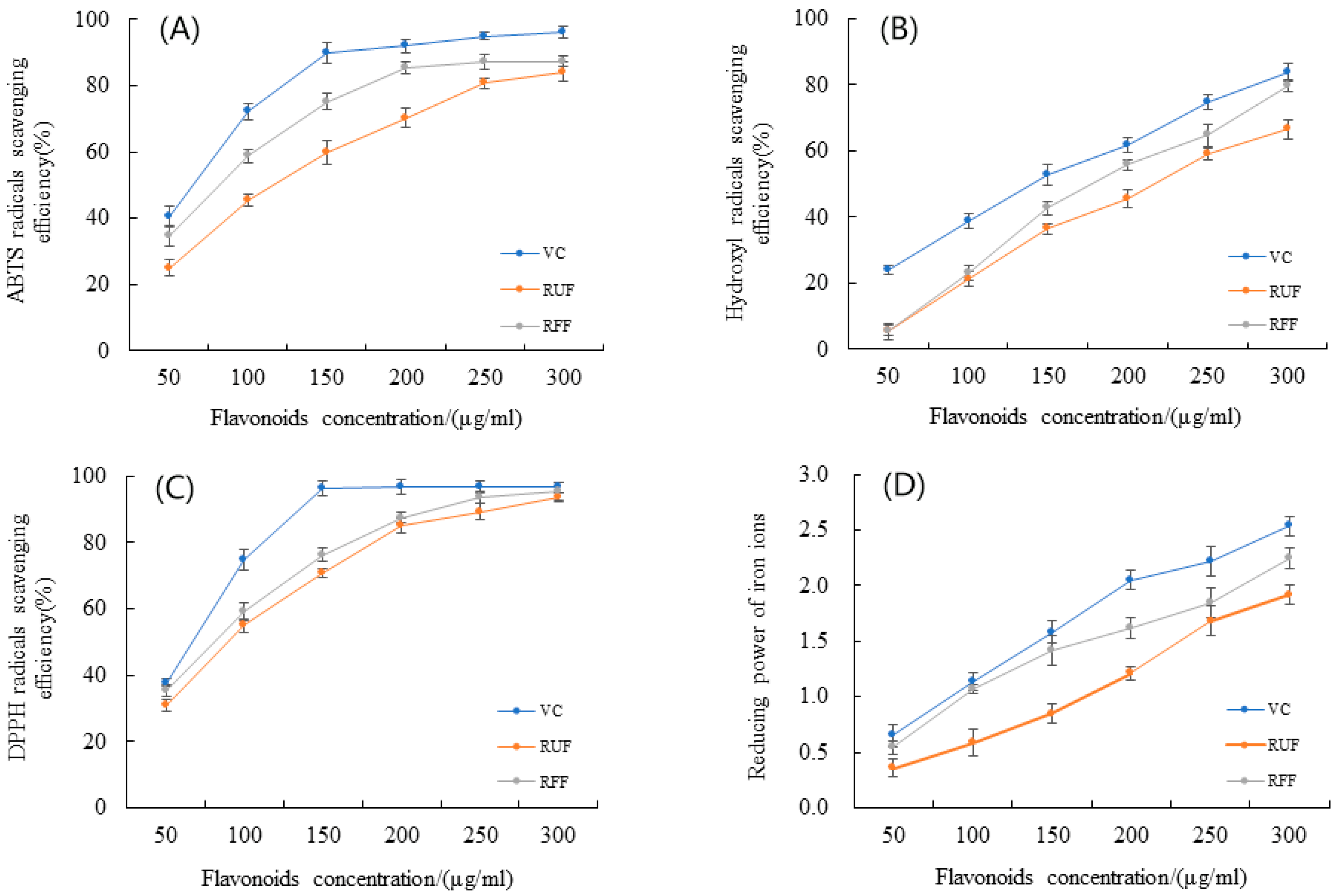
2.4. Comparison of Antioxidant Activity of RFF and RUF
2.5. UPLC-QTOF-MS Analysis of RUF and RFF
2.5.1. UPLC-QTOF-MS Analysis of RUF and RFF Chemical Composition
2.5.2. Analysis of Metabolomic and Enzymes
3. Materials and Methods
3.1. Materials
3.2. Instruments
3.3. Preparation of Spore Suspension
3.4. Process Flow of Total Flavonoid Preparation
3.5. Preparation of Fermentation Substrate of HML
3.6. Electron Microscopy Observation of HML Fibers
3.7. Determination of the Extraction Yield of Total Flavonoid Content
3.7.1. Standard Curve
3.7.2. Extraction of Total Flavonoids and Calculation of Extraction Yield
3.8. Single-Factor Experiments on the Extraction Yield of RUF and RFF
3.9. Box–Behnken Response Surface Optimization Experiment Design for RFF
3.10. Purification of RUF and RFF with Macroporous Resin
3.11. In Vitro Antioxidant of RUF and RFF
3.11.1. Determination of DPPH-Radical-Scavenging Efficiency
3.11.2. Determination of Hydroxyl-Radical-Scavenging Efficiency
3.11.3. Determination of ABTS-Radical-Scavenging Efficiency
3.11.4. Determination of Reducing Power on Iron Ions
3.12. UPLC-QTOF-MS Analysis of Changes in Flavonoid Components of RUF and RFF
3.13. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, Q.; Liu, J.Z.; Fu, Y.J. Chemical constituents from Hibiscus manihot. Chin. Tradit. Pat. Med. 2022, 44, 2187–2192. (In Chinese) [Google Scholar]
- Cui, Q.; Liu, J.Z.; Yu, L.; Gao, M.Z.; Wang, L.T.; Wang, W.; Zhao, X.H.; Fu, Y.J.; Jiang, J.C. Experimental and simulative studies on the implications of natural and green surfactant for extracting flavonoids. J. Clean. Prod. 2020, 274, 122652. [Google Scholar] [CrossRef]
- Wang, R.J.; Tian, Y.; Wu, J.W.; Wang, Y.F.; Jia, Z.H.; Bai, Z.Q.; Zhang, X.J. Analysis of medicinal components in the flower of Hibiseu manihot L. Nat. Prod. Res. Dev. 2022, 34, 588–597+721. [Google Scholar]
- Shi, H.; Li, J.; Yu, J.; Li, H.; Huang, G.; Zhang, T. Extraction, purification and antioxidant activity of polysaccharides from different parts of Hibiscus manihot L. J. Mol. Struct. 2024, 1295, 136598. [Google Scholar] [CrossRef]
- Liu, J.Z.; Zhang, C.C.; Fu, Y.J.; Cui, Q. Comparative analysis of phytochemical profile, antioxidant and anti-inflammatory activity from Hibiscus manihot L. flower. Arab. J. Chem. 2022, 15, 103503. [Google Scholar] [CrossRef]
- Xiao, M.; Zhang, T.; Cao, F.; Liang, W.; Yang, Y.; Huang, T.; Tang, Y.S.; Liu, B.; Zhao, X.; Shaw, P.C. Anti-influenza properties of tiliroside isolated from Hibiscus mutabilis L. J. Ethnopharmacol. 2023, 303, 115918. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, X.J.; Chen, J.B.; Cao, J.P.; Li, X.; Sun, C.D. Citrus flavonoids and their antioxidant evaluation. Crit. Rev. Food Sci. Nutr. 2022, 62, 3833–3854. [Google Scholar] [CrossRef] [PubMed]
- Ai, G.; Liu, Q.; Hua, W.; Huang, Z.; Wang, D. Hepatoprotective evaluation of the total flavonoids extracted from flowers of Abelmoschus manihot (L.) Medic: In vitro and in vivo studies. J. Ethnopharmacol. 2013, 146, 794–802. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Z.; Li, S.; Wang, L.; Lv, J.; Li, J.; Ma, X.; Fan, L.; Qian, F. Identification and characterization of a cytotoxic polysaccharide from the flower of Abelmoschus manihot. Int. J. Biol. Macromol. 2016, 82, 284–290. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, L.J.; Xu, Y.P.; Song, C.N.; Wang, X.Y. Optimization of the Mechanochemical Assisted Extraction of Total Flavonoids from Eleutherococcus senticosus. Lishizhen Med. Mater. Med. Res. 2007, 1, 2889–2891. [Google Scholar]
- Egbune, E.O.; Ezedom, T.; Orororo, O.C.; Egbune, O.U.; Avwioroko, O.J.; Aganbi, E.; Anigboro, A.A.; Tonukari, N.J. Solid-state fermentation of cassava (Manihot esculenta Crantz): A review. World J. Microbiol. Biotechnol. 2023, 39, 259. [Google Scholar] [CrossRef] [PubMed]
- García, I.L.; López, J.A.; Dorado, M.P.; Kopsahelis, N.; Alexandri, M.; Papanikolaou, S.; Villar, M.A.; Koutinas, A.A. Evaluation of by-products from the biodiesel industry as fermentation feedstock for poly(3-hydroxybutyrate-co-3-hydroxyvalerate) production by Cupriavidus necator. Bioresour. Technol. 2013, 130, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Lomascolo, A.; Uzan-Boukhris, E.; Sigoillot, J.C.; Fine, F. Rapeseed and sunflower meal: A review on biotechnology status and challenges. Appl. Microbiol. Biotechnol. 2012, 95, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Reddy, C.K.; Ryu, J.J.; Kyung, S.; Lim, Y.; Park, M.S.; Kang, S.; Lee, C.H. Solid-State Fermentation With Aspergillus cristatus Enhances the Protopanaxadiol- and Protopanaxatriol-Associated Skin Anti-aging Activity of Panax notoginseng. Front. Microbiol. 2022, 14, 602135. [Google Scholar] [CrossRef]
- Juan, M.Y.; Chou, C.C. Enhancement of antioxidant activity, total phenolic and flavonoid content of black soybeans by solid state fermentation with Bacillus subtilis BCRC 14715. Food Microbiol. 2010, 27, 586–591. [Google Scholar] [CrossRef]
- Yin, Z.N.; Wu, W.J.; Sun, C.Z.; Lei, Z.G.; Chen, H.M.; Liu, H.F.; Chen, W.B.; Ma, J.J.; Min, T.; Zhang, M.M.; et al. Comparison of releasing bound phenolic acids from wheat bran by fermentation of three Aspergillus species. Int. J. Food Sci. Technol. 2018, 53, 1120–1130. [Google Scholar] [CrossRef]
- Liu, N.; Song, M.; Wang, N.F.; Wang, Y.; Wang, R.F.; An, X.P.; Qi, J.W. The effects of solid-state fermentation on the content, composition and in vitro antioxidant activity of flavonoids from dandelion. PLoS ONE 2020, 15, e0239076. [Google Scholar] [CrossRef]
- Corzo-León, D.E.; Uehling, J.K.; Ballou, E.R. Rhizopus arrhizus. Trends Microbiol. 2023, 31, 985–987. [Google Scholar] [CrossRef]
- Razak, A.L.D.; Rashid, A.Y.N.; Jamaluddin, A.; Sharifudin, A.S.; Kahar, A.A.; Long, K. Cosmeceutical potentials and bioactive compounds of rice bran fermented with single and mix culture of Aspergillus oryzae and Rhizopus oryzae. J. Saudi Soc. Agric. Sci. 2017, 16, 127–134. [Google Scholar]
- Zhang, Y.R.; Chang, C.X.; Liang, H.Q.; Yang, H.; Zhou, H.Z. Investigation on fermentation Conditions of a Rhizopus arrhizu producing fibrinolytic enzyme. Chin. J. Bioprocess. Eng. 2007, 5, 43–47. [Google Scholar]
- Long, K.; Zhao, Z.K.; Ma, Y.Y.; Yang, J.G. Progresses of Researches on Rhizopus for Liquor-makin. Mod. Food Sci. Technol. 2013, 29, 443–447. [Google Scholar]
- Liu, H.; Zhang, S.; Yu, N.; Dou, L.; Deng, L.; Wang, F.; Tan, T. Direct Utilization of Non-pretreated Hydrolytic Liquid of Dried Distiller’s Grains with Solubles for Bio-Ethanol by Rhizopus arrhizus RH 7-13-9#. Appl. Biochem. Biotechnol. 2018, 186, 590–596. [Google Scholar] [PubMed]
- Lei, Y.P.; Di, S.; Zhang, F.L.; Zhang, L. Study on Extraction of Flavonoids from Fruit of Rosa Davurica Pall by Different Methods and its Antioxidant Activity. In Proceedings of the 2018 International Conference on New Materials and Intelligent Manufacturing, Jilin City, China, 23–24 June 2018; pp. 428–431. [Google Scholar]
- Xu, J.; Cao, C.B.; Li, D.H.; Liu, Y.F.; Liang, Z.Y.; Yang, X.B. Study on the Optimization of Extraction Technology of Flavonoids in Clerodendrum inerme (L.) Gaertn. Roots. Adv. Mater. Res. 2012, 560–561, 349–354. [Google Scholar] [CrossRef]
- Chen, F.Z.; Wang, B.; Zhao, G.H.; Liang, X.P.; Liu, S.H.; Liu, J. Optimization extraction of flavonoids from peony pods by response surface methodology, antioxidant activity and bioaccessibility in vitro. J. Food Meas. Charact. 2023, 17, 460–471. [Google Scholar] [CrossRef]
- Huang, Q.P.; Huang, Q.C.; Zheng, Y.F.; Zen, Z.F.; Huang, L.B.; Wei, Y.H. Extraction and Antioxidant Activities of Total Flavonoids from Ficus Pumila Fruit. Chem. Reagents. 2021, 43, 1262–1267. [Google Scholar]
- Tham, M.W.; Liew, K.C. Influence of different extraction temperatures and methanol solvent percentages on the total phenols and total flavonoids from the heartwood and bark of Acacia auriculiformis. Eur. J. Wood Wood Prod. 2014, 72, 67–72. [Google Scholar] [CrossRef]
- You, J.; Song, F.F.; Wu, W.B. Optimization of fermentation technology of pine needle chestnut black rice wine by response surface method. Food Ferment. Ind. 2023, 50, 1–10. [Google Scholar]
- Marcelo, K.L.; Means, A.R.; York, B. The Ca(2+)/Calmodulin/CaMKK2 Axis: Nature’s Metabolic CaMshaft. Trends Endocrinol. Metab. 2016, 27, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, G.R.; Kemp, B.E. AMPK in Health and Disease. Physiol. Rev. 2009, 89, 1025–1078. [Google Scholar] [CrossRef]
- Adams, M.; Jia, Z. Structural and biochemical analysis reveal pirins to possess quercetinase activity. J. Biol. Chem. 2005, 280, 28675–28682. [Google Scholar] [CrossRef]
- Zha, J.; Wu, X.; Gong, G.; Koffas, M.A.G. Pathway enzyme engineering for flavonoid production in recombinant microbes. Metab. Eng. Commun. 2019, 9, e00104. [Google Scholar] [CrossRef] [PubMed]
- Robotham, S.A.; Brodbelt, J.S. Regioselectivity of human UDP-glucuronosyltransferase isozymes in flavonoid biotransformation by metal complexation and tandem mass spectrometry. Biochem. Pharmacol. 2011, 82, 1764–1770. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Shin, I. Profiling of glycosidase activities using coumarin-conjugated glycoside cocktails. Org. Lett. 2007, 9, 619–622. [Google Scholar] [CrossRef] [PubMed]
- Ayele, D.T.; Akele, M.L.; Melese, A.T. Analysis of total phenolic contents, flavonoids, antioxidant and antibacterial activities of Croton macrostachyus root extracts. BMC Chem. 2022, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.T.; Duan, W.; Huang, G.L.; Huang, H.L. Ultrasonic-assisted extraction, analysis and properties of mung bean peel polysaccharide. Ultrason. Sonochem. 2023, 98, 106487. [Google Scholar] [CrossRef]
- Tao, A.L.; Feng, X.H.; Sheng, Y.J.; Song, Z.R. Optimization of the Artemisia Polysaccharide Fermentation Process by Aspergillus niger. Front. Nutr. 2022, 9, 842766. [Google Scholar] [CrossRef]
- Raza, A.; Li, F.; Xu, X.Q.; Tang, J. Optimization of ultrasonic-assisted extraction of antioxidant polysaccharides from the stem of Trapa quadrispinosa using response surface methodology. Int. J. Biol. Macromol. 2017, 94, 335–344. [Google Scholar] [CrossRef]
- Ye, H.; Yuan, S.; Cong, X.D. Biotransformation of puerarin into 3’-hydroxypuerarin by Trichoderma harzianum NJ01. Enzyme Microb. Technol. 2007, 40, 594–597. [Google Scholar] [CrossRef]
- Lee, Y.; Oh, J.; Jeong, Y.S. Lactobacillus plantarum-mediated onversion of flavonoid glycosides into flavonols, quercetin, and kaempferol in Cudrania tricuspidata leaves. Food Sci. Biotechnol. 2015, 24, 1817–1821. [Google Scholar] [CrossRef]




| Run | A Material–Liquid Ratio (g/mL) | B Extraction Time (h) | C Ethanol Concentration (%) | D Extraction Temperature (°C) | Y Total Flavonoid Extraction Yield (%) |
|---|---|---|---|---|---|
| 1 | −1 | −1 | 0 | 0 | 2.99 |
| 2 | 1 | −1 | 0 | 0 | 3.11 |
| 3 | −1 | 1 | 0 | 0 | 3.07 |
| 4 | 1 | 1 | 0 | 0 | 3.34 |
| 5 | 0 | 0 | −1 | −1 | 3.10 |
| 6 | 0 | 0 | 1 | −1 | 2.66 |
| 7 | 0 | 0 | −1 | 1 | 3.15 |
| 8 | 0 | 0 | 1 | 1 | 2.52 |
| 9 | −1 | 0 | 0 | −1 | 3.08 |
| 10 | 1 | 0 | 0 | −1 | 3.26 |
| 11 | −1 | 0 | 0 | 1 | 3.17 |
| 12 | 1 | 0 | 0 | 1 | 3.25 |
| 13 | 0 | −1 | −1 | 0 | 3.11 |
| 14 | 0 | 1 | −1 | 0 | 3.22 |
| 15 | 0 | −1 | 1 | 0 | 2.52 |
| 16 | 0 | 1 | 1 | 0 | 2.59 |
| 17 | −1 | 0 | −1 | 0 | 2.85 |
| 18 | 1 | 0 | −1 | 0 | 3.21 |
| 19 | −1 | 0 | 1 | 0 | 2.60 |
| 20 | 1 | 0 | 1 | 0 | 2.56 |
| 21 | 0 | −1 | 0 | −1 | 3.27 |
| 22 | 0 | 1 | 0 | −1 | 3.20 |
| 23 | 0 | −1 | 0 | 1 | 2.93 |
| 24 | 0 | 1 | 0 | 1 | 3.29 |
| 25 | 0 | 0 | 0 | 0 | 3.80 |
| 26 | 0 | 0 | 0 | 0 | 3.51 |
| 27 | 0 | 0 | 0 | 0 | 3.61 |
| 28 | 0 | 0 | 0 | 0 | 3.69 |
| 29 | 0 | 0 | 0 | 0 | 3.68 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value Prop > F | Salience |
|---|---|---|---|---|---|---|
| Model | 3.04 | 14 | 0.2171 | 48.64 | <0.0001 | ** |
| A | 0.0784 | 1 | 0.0784 | 17.56 | 0.0011 | ** |
| B | 0.0507 | 1 | 0.0507 | 11.36 | 0.0050 | ** |
| C | 0.848 | 1 | 0.8480 | 189.96 | <0.0001 | ** |
| D | 5.6 × 10−3 | 1 | 5.6 × 10−3 | 1.26 | 0.2816 | |
| AB | 5.6 × 10−3 | 1 | 5.6 × 10−3 | 1.26 | 0.2819 | |
| AC | 0.04 | 1 | 0.04 | 8.96 | 0.0104 | * |
| AD | 2.5 × 10−3 | 1 | 2.5 × 10−3 | 0.56 | 0.4676 | |
| BC | 4 × 10−4 | 1 | 4 × 10−4 | 0.0896 | 0.7694 | |
| BD | 0.0462 | 1 | 0.0462 | 10.35 | 0.0067 | ** |
| CD | 9 × 10−3 | 1 | 9 × 10−3 | 2.02 | 0.1786 | |
| A2 | 0.3813 | 1 | 0.3813 | 85.41 | <0.0001 | ** |
| B2 | 0.3267 | 1 | 0.3267 | 73.18 | <0.0001 | ** |
| C2 | 1.83 | 1 | 1.83 | 409.66 | <0.0001 | ** |
| D2 | 0.2481 | 1 | 0.2481 | 55.57 | <0.0001 | ** |
| Residual | 0.0580 | 13 | 4.5 × 10−3 | |||
| Lack of fit | 0.0374 | 10 | 3.7 × 10−3 | 0.5421 | 0.7972 | |
| Pure error | 0.0207 | 3 | 6.9 × 10−4 | |||
| Cor total | 3.10 | 27 | ||||
| R2 | 0.9813 | |||||
| Adj-R2 | 0.9611 |
| No. | RT/min | Precursor Ion | Meas.m/z | m/z | Error/ppm | Formula | Fragments | Identification | Response |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.667 | [M−H]− | 266.0891 | 266.0887 | 1.5 | C10H13N5O4 | 217, 215, 179, 101 | Adenosine | 4336 |
| 2 | 1.232 | [M−H]− | 610.1539 | 610.1524 | 2.5 | C27H31O16 | 471, 470, 443, 308, 279, 133, 128 | Enocyanin | 124 |
| 3 | 1.769 | [M−H]− | 492.136 | 492.1354 | 1.2 | C23H28Cl-N3O5S | 424, 358, 334, 213 | Glyburide | 239 |
| 4 | 3.553 | [M−H]− | 153.0187 | 153.0186 | 0.7 | C7H6O4 | — | Protocatechuic acid | 8653 |
| 5 | 3.692 | [M−H]− | 369.0821 | 369.0816 | 1.4 | C16H18O10 | 247, 232, 216, 201, 191 | Fraxin | 893 |
| 6 | 3.718 | [M−H]− | 677.1536 | 677.1497 | 5.8 | C34H30O15 | 611, 477, 305, 189, 146, 144 | 3,4,5-Tricaffeoylquinic acid | 211 |
| 7 | 3.740 | [M−H]− | 299.0765 | 299.0752 | 4.3 | C13H16O8 | 196, 146, 93 | Salicylic acid-β-d-glucoside | 36,072 |
| 8 | 3.795 | [M−H]− | 479.0794 | 479.0819 | −5.2 | C21H20O13 | 447, 425, 333, 300, 175 | Myricetin-3-O-β-d-glucoside | 1,029,648 |
| 9 | 3.796 | [M−H]− | 595.1263 | 595.129 | −4.5 | C26H28O16 | 495, 479, 447, 425, 333, 300, 271 | Quercetin-3-arabinoside-7-glucoside | 273,676 |
| 10 | 3.824 | [M−H]− | 353.0856 | 353.0867 | −3.1 | C16H18O9 | 333, 316, 271, 191, 175 | Chlorogenic acid | 7520 |
| 11 | 3.876 | [M−H]− | 325.0911 | 325.0918 | −2.2 | C15H18O8 | 318, 305, 181, 146, 135 | 4-O-β-d-glucosy-l-4-coumarate | 6416 |
| 12 | 3.932 | [M−H]− | 623.1583 | 623.1602 | −3.0 | C28H32O16 | 595, 480, 479, 463, 461, 301, 300 | Isorhamnetin-3-O-neohespeidoside | 3704 |
| 13 | 3.982 | [M−H]− | 495.0743 | 495.0768 | −5.0 | C21H20O14 | 387, 334, 333, 316, 305, 179, 137 | Hibiscetin-3-O-glucoside | 195,097 |
| 14 | 3.987 | [M−H]− | 741.1829 | 741.1866 | −5.0 | C32H38O20 | 611, 495, 387, 333, 300 | Quercetin-3-glucosyl-(1->4)-xylosyl-(1->4)-Rhamnoside | 13,845 |
| 15 | 3.993 | [M−H]− | 137.0242 | 137.0237 | 3.6 | C7H6O3 | — | Salicylic acid | 1120 |
| 16 | 4.026 | [M−H]− | 479.0792 | 479.0819 | −5.6 | C21H20O13 | 447, 417, 223, 179, 61 | Cannabiscitrin | 759,151 |
| 17 | 4.067 | [M−H]− | 625.1361 | 625.1395 | −5.4 | C27H30O17 | 521, 480, 479, 463, 337, 300, 271 | Myricetin-3-neohesperidoside | 197,243 |
| 18 | 4.080 | [M−H]− | 289.0679 | 289.0708 | −10.0 | C15H14O6 | 271, 255, 210, 151 | Cianidanol | 855 |
| 19 | 4.101 | [M−H]− | 609.1406 | 609.1446 | −6.6 | C27H30O16 | 542, 480, 479, 463, 385, 300, 210 | Rutin | 614,274 |
| 4.336 | [M+H]+ | 611.1582 | 611.1602 | −3.3 | C27H30O16 | 531, 450, 447, 445, 439, 229 | Rutin | 959 | |
| 20 | 4.120 | [M−H]− | 179.0348 | 179.0342 | 3.4 | C9H8O4 | 167 | Caffeic acid | 181 |
| 21 | 4.233 | [M−H]− | 463.0839 | 463.087 | −6.7 | C21H20O12 | 381, 273, 315, 151 | Hyperoside | 2,443,396 |
| 22 | 4.503 | [M−H]− | 463.0839 | 463.087 | −6.7 | C21H20O12 | 461, 455, 301, 151 | Isoquercetin | 1,713,071 |
| 23 | 4.662 | [M−H]− | 163.0391 | 163.0393 | −1.2 | C9H8O3 | 139 | Trans-p-coumaric acid | 1168 |
| 24 | 4.679 | [M−H]− | 165.0546 | 165.0549 | −1.8 | C9H10O3 | 139 | Ethylparaben | 1965 |
| 25 | 4.854 | [M−H]− | 447.0907 | 447.0921 | −3.1 | C21H20O11 | 326, 318, 317, 195, 139 | Astragalin | 1953 |
| 26 | 4.893 | [M−H]− | 477.0999 | 477.1026 | −5.7 | C22H22O12 | 445, 429, 300, 271, 243, 242, 231 | Isorhamnetin-3-glucoside | 6568 |
| 27 | 4.928 | [M−H]− | 447.0913 | 447.0921 | −1.8 | C21H20O11 | 301, 178, 151 | Isoorientin | 303 |
| 28 | 5.114 | [M−H]− | 493.0577 | 493.0614 | −7.5 | C21H18O14 | 385, 339, 318, 243, 139 | Hibifolin | 3,417,545 |
| 29 | 5.171 | [M−H]− | 463.0845 | 463.087 | −5.4 | C21H20O12 | 461, 439, 319, 301, 243, 165 | Quercetin 3′-O-β-d-glucoside | 1,144,905 |
| 30 | 5.248 | [M−H]− | 447.0919 | 447.0921 | −0.4 | C21H20O11 | 385, 339, 323, 319, 300, 271, 137 | Quercitrin | 118 |
| 31 | 5.307 | [M−H]− | 187.0962 | 187.0966 | −2.1 | C9H16O4 | — | Nonanedioic acid | 985 |
| 32 | 5.396 | [M−H]− | 505.0951 | 505.0975 | −4.8 | C23H22O13 | 445, 339, 317, 316, 315, 146, 137 | Quercetin-3-O-(6″-acetyl-glucoside) | 60,696 |
| 33 | 5.468 | [M−H]− | 317.0271 | 317.0294 | −7.3 | C15H10O8 | 315, 178, 151, 109 | Myricetin | 1,193,716 |
| 34 | 5.672 | [M−H]− | 505.0934 | 505.0975 | −8.1 | C23H22O13 | 445, 323, 301, 300, 202, 121 | Quercetin-3-(2″-acetyl-galactoside) | 52,592 |
| 35 | 6.028 | [M−H]− | 489.105 | 489.1026 | 4.9 | C23H22O12 | 409, 307 | 3-O-beta-d-Glucuronoside,etester-3,4′,5,7-tetrahydroxyflavone | 68 |
| 36 | 6.382 | [M−H]− | 465.1732 | 465.1752 | −4.3 | C23H30O10 | 303, 259, 215 | Ilexin II | 1658 |
| 37 | 6.451 | [M−H]− | 201.1123 | 201.1122 | 0.5 | C10H18O4 | 179 | Sebacic acid | 68 |
| 38 | 6.846 | [M−H]− | 593.1281 | 593.1287 | −1.0 | C30H26O13 | 545, 401, 313, 285, 129 | Tribuloside | 219 |
| 39 | 6.949 | [M−H]− | 301.0324 | 301.0345 | −7.0 | C15H10O7 | 249, 179, 151 | Quercetin | 1,500,688 |
| 40 | 7.794 | [M−H]− | 395.2022 | 395.2061 | −9.9 | C21H32O7 | 327, 215 | Furonewguinone B | 792 |
| 41 | 8.101 | [M−H]− | 269.0493 | 269.0447 | 17.1 | C15H10O5 | — | Genistein | 38 |
| 42 | 8.435 | [M−H]− | 285.0388 | 285.0396 | −2.8 | C15H10O6 | — | Kaempferol | 2541 |
| 43 | 8.565 | [M−H]− | 315.049 | 315.0501 | −3.5 | C16H12O7 | 300, 151 | Isorhamnetin | 6714 |
| 44 | 8.751 | [M−H]− | 289.1433 | 289.1434 | −0.3 | C17H22O4 | 171, 130 | 6-Isobutyryl-5,7-dimethoxy-2,2-dimethyl benzopyran | 378 |
| 45 | 8.831 | [M−H]− | 491.118 | 491.1182 | −0.4 | C23H24O12 | 401, 357, 313, 121 | Yixingensin | 357 |
| 46 | 9.363 | [M−H]− | 287.0933 | 287.0915 | 6.3 | C16H16O5 | — | Alkannin | 112 |
| 47 | 12.557 | [M−H]− | 593.1258 | 593.1287 | −4.9 | C30H26O13 | 474, 397, 277, 239, 129, 112 | Kaempferol-3-glucoside-2″-p-coumaroyl | 5171 |
| 48 | 13.537 | [M−H]− | 255.2316 | 255.2316 | 0.0 | C16H32O2 | 145 | Palmitic acid | 1147 |
| 49 | 13.880 | [M−H]− | 361.1978 | 361.2007 | −8.0 | C21H30O5 | 293, 255, 130 | Enaimeone B | 1448 |
| 50 | 14.022 | [M−H]− | 277.144 | 277.1434 | 2.2 | C16H22O4 | 255, 179 | Diisobutyl phthalate | 539 |
| 51 | 14.713 | [M−H]− | 297.1505 | 297.1485 | 6.7 | C19H22O3 | 255, 183, 149 | Aurapten | 7267 |
| 52 | 14.798 | [M−H]− | 225.2195 | 225.2211 | −7.1 | C15H30O | 101 | 2-Pentadecanone | 61 |
| 53 | 15.103 | [M−H]− | 279.2301 | 279.2316 | −5.4 | C18H32O2 | 208, 150, 100 | 9,12-Octadecadienoic acid | 4641 |
| 54 | 16.199 | [M−H]− | 389.2709 | 389.2682 | 6.9 | C24H38O4 | 313, 267 | Diisooctyl phthalate | 27 |
| 55 | 3.961 | [M+H]+ | 525.229 | 525.2325 | −6.7 | C26H36O11 | 520, 213 | Neocaesalpin L | 229 |
| 56 | 4.370 | [M+H]+ | 582.1758 | 582.1813 | −9.4 | C26H28O14 | 428, 425, 423, 417, 229 | 3,4,5-Trihydroxy-6-[7-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-6,8-dimethoxy-4-oxochromen-5-yl]oxyoxan-2-yl]methylacetate | 119 |
| 57 | 4.609 | [M+H]+ | 563.236 | 563.2348 | 2.1 | C27H34N5O7 | 495, 494, 492, 489, 483, 481 | 1-[2-(2-acetylammonium)-3-phenyl propionyl-pyrrolidine-2-carboxylic acid | 22 |
| 58 | 4.668 | [M+H]+ | 505.2594 | 505.2637 | −8.5 | C24H40O11 | 479, 477, 470, 271, 163 | leeaoside | 323 |
| 59 | 8.178 | [M+H]+ | 277.2848 | 277.2862 | −5.0 | C18H38 | 266, 261, 244, 213 | n-Octadecane | 207 |
| 60 | 12.800 | [M+H]+ | 522.114 | 522.1183 | −8.2 | C30H16O8 | 460, 454, 453, 437, 415, 299, 119 | Hypericin | 172 |
| 61 | 12.801 | [M+H]+ | 415.2805 | 415.2838 | −7.9 | C26H38O4 | 303, 299, 247, 135, 119 | Hyperpapuanone | 20,517 |
| 62 | 15.393 | [M+H]+ | 457.3708 | 457.3669 | 8.5 | C30H48O3 | 413, 371, 369, 355 | Ursolic acid | 1389 |
| 63 | 16.636 | [M+H]+ | 311.0865 | 311.0891 | −8.4 | C16H16O5 | 170, 140 | (2S)-5,3′4′-trihydroxy-7-methoxyflavane | 211 |
| NO. | RT/min | Precursor Ion | Meas.m/z | m/z | Error/ppm | Formula | Fragments | Identification | Response |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.614 | [M−H]− | 289.0685 | 289.0708 | −8.0 | C15H14O6 | 191, 133, 128 | Cianidanol | 583 |
| 2 | 0.658 | [M−H]− | 503.1603 | 503.1602 | 0.2 | C18H32O16 | 379, 377, 321, 215, 195, 161, 133 | Gentianose | 506 |
| 13.215 | [M+H]+ | 522.2048 | 522.2048 | 0.0 | C18H32O16 | 500, 478, 457, 384, 313, 188, 122 | Gentianose | 418 | |
| 3 | 0.668 | [M−H]− | 266.0871 | 266.0887 | −6.0 | C10H13N5O4 | 164, 150 | Adenosine | 6287 |
| 4 | 1.493 | [M−H]− | 285.0588 | 285.0606 | −6.3 | C12H14O8 | 195 | Uralenneoside | 110 |
| 5 | 2.697 | [M−H]− | 303.0843 | 303.0864 | −6.9 | C16H16O6 | 253, 249, 232, 164, 147, 94 | 3-Methoxypyrocatechol | 152 |
| 6 | 3.548 | [M−H]− | 153.0178 | 153.0186 | −5.2 | C7H6O4 | — | Protocatechuic acid | 62,326 |
| 7 | 3.549 | [M−H]− | 109.0290 | 109.0288 | 1.8 | C6H6O2 | — | Hydroquinone | 997 |
| 8 | 3.686 | [M−H]− | 369.0851 | 369.0816 | 9.5 | C16H18O10 | 316, 291, 287, 203, 153 | Fraxin | 43 |
| 9 | 3.795 | [M−H]− | 479.0791 | 479.0819 | −5.8 | C21H20O13 | 463, 339, 301, 271, 269, 190 | Myricetin-3-O-β-d-glucoside | 214,470 |
| 10 | 3.797 | [M−H]− | 595.1255 | 595.1290 | −5.9 | C26H28O16 | 463, 439, 301, 255, 243 | Quercetin-3-arabinoside-7-glucoside | 308,061 |
| 16.912 | [M+H]+ | 597.1513 | 597.1556 | −7.2 | C26H28O16 | 539, 496, 393, 355, 323, 263 | Quercetin-3-arabinoside-7-glucoside | 849 | |
| 11 | 3.821 | [M−H]− | 353.0850 | 353.0867 | −4.8 | C16H18O9 | 290, 200, 128, 161 | Chlorogenic acid | 6601 |
| 12 | 3.931 | [M−H]− | 623.1574 | 623.1602 | −4.5 | C28H32O16 | 595, 479, 314, 281, 271, 255 | Isorhamnetin-3-O-neohespeidoside | 9866 |
| 13 | 3.932 | [M−H]− | 179.0346 | 179.0342 | 2.2 | C9H8O4 | 147 | Caffeic acid | 2735 |
| 14 | 3.982 | [M−H]− | 495.0747 | 495.0768 | −4.2 | C21H20O14 | 464, 463, 333, 303, 179 | Hibiscetin-3-O-glucoside | 10,639 |
| 15 | 3.984 | [M−H]− | 741.1816 | 741.1866 | −6.7 | C32H38O20 | 612, 611, 495, 461, 323, 316, 300, 287, 151, 137 | Quercetin-3-glucosyl-(1->4)-xylosyl-(1->4)-rhamnoside | 15,593 |
| 16 | 3.988 | [M−H]− | 137.0233 | 137.0237 | −2.9 | C7H6O3 | 124 | Salicylic acid | 5443 |
| 17 | 4.026 | [M−H]− | 479.0780 | 479.0819 | −8.1 | C21H20O13 | 477, 318, 316, 291, 287, 179, 201 | Cannabiscitrin | 354,843 |
| 18 | 4.110 | [M−H]− | 609.1393 | 609.1446 | −8.7 | C27H30O16 | 441, 300, 255, 243, 151 | Rutin | 1,226,910 |
| 5.367 | [M+H]+ | 628.1877 | 628.1867 | 1.6 | C27H30O16 | 551, 487, 481, 367, 352, 319, 254 | Rutin | 763 | |
| 19 | 4.231 | [M−H]− | 463.0832 | 463.0870 | −8.2 | C21H20O12 | 461, 303, 179 | Hyperoside | 776,080 |
| 20 | 4.307 | [M−H]− | 625.1360 | 625.1395 | −5.6 | C27H30O17 | 565, 521, 477, 466, 319, 316, 303 | Myricetin-3-neohesperidoside | 153,458 |
| 12.994 | [M+H]+ | 649.1419 | 649.1371 | 7.4 | C27H30O17 | 640, 556, 494, 337, 188, 184, 104 | Myricetin-3-neohesperidoside | 1343 | |
| 21 | 4.498 | [M−H]− | 463.0828 | 463.0870 | −9.1 | C21H20O12 | 455, 301, 151 | Isoquercetin | 2,318,366 |
| 1.090 | [M+H]+ | 482.1275 | 482.1275 | 0.0 | C21H20O12 | 423, 330, 314, 296, 274, 203, 130 | Isoquercetin | 523 | |
| 22 | 4.561 | [M−H]− | 593.1506 | 593.1497 | 1.5 | C27H30O15 | 493, 479, 318, 317, 139 | Aempferol-3-O-rutinoside | 179 |
| 23 | 4.673 | [M−H]− | 165.0541 | 165.0549 | −4.8 | C9H10O3 | 139 | Ethylparaben | 7244 |
| 24 | 4.691 | [M−H]− | 167.0345 | 167.0342 | 1.8 | C8H8O4 | 165 | Vanillic acid | 846 |
| 25 | 4.888 | [M−H]− | 477.0996 | 477.1026 | −6.3 | C22H22O12 | 345, 323, 300, 271, 245, 243, 164, 231, 169 | Isorhamnetin-3-glucoside | 4277 |
| 26 | 4.931 | [M−H]− | 447.0938 | 447.0921 | 3.8 | C21H20O11 | 315, 301, 240, 151 | Isoorientin | 271 |
| 27 | 5.114 | [M−H]− | 493.0565 | 493.0614 | −9.9 | C21H18O14 | 343, 243, 380, 339, 193 | Hibifolin | 5,596,770 |
| 1.624 | [M+H]+ | 517.0566 | 517.0588 | −4.3 | C21H18O14 | 365, 266, 210, 203, 140, 136 | Hibifolin | 89 | |
| 28 | 5.175 | [M−H]− | 463.0826 | 463.0870 | −9.5 | C21H20O12 | 323, 301, 178, 151 | Quercetin 3′-O-β-d-glucoside | 530,689 |
| 29 | 5.242 | [M−H]− | 447.0896 | 447.0921 | −5.6 | C21H20O11 | 407, 385, 317, 285, 249, 150, 137 | Quercitrin | 64 |
| 30 | 5.307 | [M−H]− | 187.0962 | 187.0966 | −2.1 | C9H16O4 | 179, 151 | Nonanedioicacid | 1519 |
| 31 | 5.396 | [M−H]− | 505.0950 | 505.0975 | −4.9 | C23H22O13 | 406, 300, 137, 61 | Quercetin-3-O-(6″-acetyl-glucoside) | 34,203 |
| 1.689 | [M+H]+ | 524.1377 | 524.1396 | −3.6 | C23H22O13 | 258, 176, 182, 165, 144, 91 | Quercetin-3-O-(6″-acetyl-glucoside) | 1155 | |
| 32 | 5.462 | [M−H]− | 317.0267 | 317.0294 | −8.5 | C15H10O8 | 300, 178, 62 | Myricetin | 1,338,137 |
| 33 | 5.665 | [M−H]− | 505.0934 | 505.0975 | −8.1 | C23H22O13 | 445, 377, 301, 300, 121 | Quercetin-3-(2″-acetyl-galactoside) | 24,390 |
| 34 | 6.141 | [M−H]− | 201.1136 | 201.1122 | 7.0 | C10H18O4 | — | Sebacic acid | 202 |
| 35 | 6.763 | [M−H]− | 287.0561 | 287.0552 | 3.1 | C15H12O6 | 137 | Steppogenin | 610 |
| 36 | 6.838 | [M−H]− | 593.1276 | 593.1287 | −1.9 | C30H26O13 | 549, 505, 493, 319, 245, 167 | Tribuloside | 181 |
| 37 | 6.947 | [M−H]− | 301.0320 | 301.0345 | −8.3 | C15H10O7 | 253, 271, 179, 151 | Quercetin | 4,286,867 |
| 38 | 8.424 | [M−H]− | 285.0382 | 285.0396 | −4.9 | C15H10O6 | 255, 145 | Kaempferol | 11,321 |
| 39 | 8.555 | [M−H]− | 315.0485 | 315.0501 | −5.1 | C16H12O7 | 300, 239, 151 | Isorhamnetin | 9328 |
| 40 | 8.738 | [M−H]− | 289.1431 | 289.1434 | −1.0 | C17H22O4 | — | 6-iso butyryl-5, 7-dimethoxy-2, 2-dimethyl-benzopyran | 1277 |
| 41 | 8.823 | [M−H]− | 491.1168 | 491.1182 | −2.9 | C23H24O12 | 401, 393, 357, 313, 121 | Yixingensin | 319 |
| 42 | 12.047 | [M−H]− | 431.0931 | 431.0972 | −9.5 | C21H20O10 | 363, 295, 195 | Vitexin | 46 |
| 43 | 12.555 | [M-H]− | 593.1276 | 593.1287 | −1.9 | C30H26O13 | 474, 397, 386, 277, 196, 113 | Kaempferol-3-glucoside-2″-p-coumaroyl | 1854 |
| 44 | 13.040 | [M−H]− | 439.3537 | 439.3564 | −6.1 | C30H48O2 | 367, 313, 266, 149 | Roburic acid | 175 |
| 12.897 | [M+H]+ | 458.4014 | 458.3985 | 6.3 | C30H48O2 | 444, 424, 390, 337, 335, 132, 124 | Roburic acid | 473 | |
| 45 | 13.529 | [M−H]− | 255.2336 | 255.2316 | 7.8 | C16H32O2 | — | Palmitic acid | 188 |
| 46 | 13.883 | [M−H]− | 361.1972 | 361.2007 | −9.7 | C21H30O5 | 327, 293 | Enaimeone B | 5252 |
| 47 | 14.160 | [M−H]− | 283.2634 | 283.2628 | 2.1 | C18H36O2 | 145, 125, 117 | Ethyl palmitate | 76 |
| 2.738 | [M+H]+ | 285.2761 | 285.2784 | −8.1 | C18H36O2 | 153, 136 | Ethyl palmitate | 853 | |
| 48 | 14.405 | [M−H]− | 345.2632 | 345.2631 | 0.3 | C19H38O5 | 342, 299, 267, 150 | 1, 1, 3, 3-tetrabutoxy-2-propanone | 237 |
| 49 | 14.716 | [M−H]− | 297.1509 | 297.1485 | 8.1 | C19H22O3 | 265, 150, 100 | Aurapten | 2788 |
| 50 | 14.795 | [M−H]− | 225.2222 | 225.2211 | 4.9 | C15H30O | 183 | 2-Pentadecanone | 105 |
| 51 | 14.795 | [M−H]− | 371.1506 | 371.1488 | 4.8 | C21H24O6 | 273, 271, 265, 225, 183 | (-)-Arctigenin | 1025 |
| 3.613 | [M+H]+ | 395.1451 | 395.1464 | −3.3 | C21H24O6 | 391, 367, 305, 300, 229, 192, 156 | (-)-Arctigenin | 233 | |
| 52 | 15.101 | [M−H]− | 279.2299 | 279.2316 | −6.1 | C18H32O2 | 255, 117 | 9, 12-O-ctadecadienoic acid | 5429 |
| 6.965 | [M+H]+ | 303.2272 | 303.2292 | −6.6 | C18H32O2 | 233, 153 | 9, 12-O-ctadecadienoic-acid | 147,858 | |
| 53 | 15.201 | [M−H]− | 455.3493 | 455.3513 | −4.4 | C30H48O3 | 385, 265, 183, 150, 121 | Ursolic acid | 1416 |
| 15.397 | [M+H]+ | 457.3698 | 457.3669 | 6.3 | C30H48O3 | 413, 369, 347, 270, 130 | Ursolic acid | 2232 | |
| 54 | 16.511 | [M−H]− | 603.3875 | 603.3882 | −1.2 | C35H56O8 | 381, 356, 355, 309, 307, 119 | Ziyuglycoside II | 219 |
| 55 | 0.951 | [M+H]+ | 397.2181 | 397.2217 | −9.1 | C21H32O7 | 383, 266, 248, 203, 191 | Furonewguinone B | 2102 |
| 56 | 0.983 | [M+H]+ | 489.1688 | 489.1728 | −8.2 | C23H30O10 | 381, 365, 360, 203 | Ilexin II | 974 |
| 57 | 1.109 | [M+H]+ | 522.1223 | 522.1183 | 7.7 | C30H16O8 | 423, 330, 314, 296, 274, 256 | Hypericin | 804 |
| 58 | 2.459 | [M+H]+ | 263.2720 | 263.2706 | 5.3 | C17H36 | 120 | n-Heptadecane | 156 |
| 59 | 2.871 | [M+H]+ | 345.2080 | 345.2058 | 6.4 | C21H28O4 | 328, 323, 298, 166, 146, 132, 120 | Ialibinone B | 4759 |
| 60 | 3.507 | [M+H]+ | 311.3695 | 311.3666 | 9.3 | C22H46 | 298, 280 | Docosane | 5309 |
| 61 | 4.405 | [M+H]+ | 960.5183 | 960.5146 | 3.9 | C47H74O19 | 814, 792, 487, 397, 319, 277, 114 | Deslanoside | 5645 |
| 62 | 4.623 | [M+H]+ | 255.3029 | 255.3042 | −5.1 | C18H38 | 152, 120 | n-Octadecane | 2184 |
| 63 | 4.856 | [M+H]+ | 341.3401 | 341.3408 | −2.1 | C22H44O2 | 287, 262 | Docosanoic acid | 68 |
| 64 | 5.814 | [M+H]+ | 612.1946 | 612.1918 | 4.6 | C27H30O15 | 600, 580, 581, 579, 277, 233 | Glucosylvitexin | 2889 |
| 65 | 6.252 | [M+H]+ | 554.4688 | 554.4711 | −4.1 | C40H56 | 473, 471, 303, 287, 247 | beta-Carotene | 79 |
| 66 | 6.285 | [M+H]+ | 297.3528 | 297.3510 | 6.1 | C21H44 | 262, 222 | Heneicosane | 307 |
| 67 | 7.825 | [M+H]+ | 687.6081 | 687.6036 | 6.5 | C46H80O2 | — | Alpha-Amyrin palmitate | 666 |
| 68 | 8.324 | [M+H]+ | 391.2804 | 391.2838 | −8.7 | C24H38O4 | 375, 354, 353, 348, 195 | Diisooctyl phthalate | 382 |
| 69 | 8.701 | [M+H]+ | 612.1686 | 612.1680 | 1.0 | C27H31O16 | 610, 496, 351, 325, 249, 147 | Enocyanin | 388 |
| 70 | 9.048 | [M+H]+ | 394.2550 | 394.2584 | −8.6 | C22H32O5 | 334, 277 | Enaimeone C | 242 |
| 71 | 9.101 | [M+H]+ | 553.3833 | 553.3879 | −8.3 | C35H52O5 | 427, 351, 311 | Furohyperforin | 695 |
| 72 | 11.743 | [M+H]+ | 430.4040 | 430.4036 | 0.9 | C29H48O | 421, 411, 378, 310, 307 | α-Spinasterol | 811 |
| 73 | 12.904 | [M+H]+ | 657.3733 | 657.3753 | −3.0 | C39H54O7 | 561, 554, 553, 303, 196 | 3-O-trans-p-coumaroyltormentic acid | 292 |
| 74 | 12.909 | [M+H]+ | 651.4093 | 651.4092 | 0.2 | C36H58O10 | 599, 588, 453, 344, 335, 200, 124 | Pedunculoside | 254 |
| 75 | 13.263 | [M+H]+ | 547.2141 | 547.2145 | −0.7 | C26H36O11 | 543, 542, 520, 483, 313, 184 | Neocaesalpin L | 2487 |
| 76 | 13.312 | [M+H]+ | 625.1723 | 625.1758 | −5.6 | C28H32O16 | 616, 532, 437, 393, 372, 366, 307 | Narcissoside | 9633 |
| 77 | 13.332 | [M+H]+ | 339.3947 | 339.3978 | −9.1 | C24H50 | 299, 188 | Tetracosane | 4602 |
| 78 | 13.469 | [M+H]+ | 541.2522 | 541.2528 | −1.1 | C27H34N5O7 | 463, 391, 358, 339, 342, 283, 277 | 1-[2-(2-acetyl ammonium)-3-phenylpropionate-pyrrolidine-2-carboxylic acid | 6476 |
| 79 | 13.602 | [M+H]+ | 661.1721 | 661.1734 | −2.0 | C29H34O16 | 569, 310, 309, 284, 133, 89 | Quercetin-3, 3′-dimethyl ether-7-rutinoside | 16,922 |
| 80 | 13.729 | [M+H]+ | 557.3544 | 557.3594 | −9.0 | C35H50O4 | 544, 522, 485, 480, 339, 184, 104 | Pyrano-[7, 28-b]-hyperforin | 386 |
| 81 | 13.824 | [M+H]+ | 757.2179 | 757.2178 | 0.1 | C33H40O20 | 699, 684, 640, 597, 330, 305, 118 | Kaempferol-3-sophoroside-7-rhamnoside | 361 |
| 82 | 14.208 | [M+H]+ | 527.2478 | 527.2457 | 4.0 | C24H40O11 | 395, 297, 279, 278 | leeaoside | 450,363 |
| 83 | 14.327 | [M+H]+ | 696.6235 | 696.6274 | −5.6 | C46H78O3 | 541, 349, 344, 226 | 11α, 12α-Oxidotaraxerol palMitate | 767 |
| 84 | 15.501 | [M+H]+ | 701.1445 | 701.1497 | −7.4 | C36H28O15 | 690, 589, 567, 545, 501, 283 | Theaflavin gallate | 381 |
| 85 | 15.513 | [M+H]+ | 679.1591 | 679.1653 | −9.1 | C34H30O15 | 668, 593, 567, 545, 523, 457, 413 | 3, 4, 5-Tricaffeoylquinic acid | 1207 |
| 86 | 16.462 | [M+H]+ | 575.3692 | 575.3699 | −1.2 | C35H52O5 | 559, 437, 227 | 8-Hydroxyhyperforin-8, 1-hemiacetal | 437 |
| 87 | 16.644 | [M+H]+ | 594.4730 | 594.4717 | 2.2 | C35H60O6 | 546, 487, 145 | Daucosterol | 372 |
| Factors | Coded Symbols | Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Material–liquid ratio (g/mL) | A | 1:35 | 1:40 | 1:45 |
| Extraction time (h) | B | 1.5 | 2 | 2.5 |
| Ethanol concentration (%) | C | 50 | 60 | 70 |
| Extraction temperature (°C) | D | 70 | 80 | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, X.; Chen, T.; Ding, Y.; Yu, D.; Zhang, J.; Zhang, R.; Zhang, Y.; Wang, X.; Xu, T.; Li, J. Effects of Rhizopus-arrhizus-31-Assisted Pretreatment on the Extraction and Bioactivity of Total Flavonoids from Hibiscus manihot L. Molecules 2024, 29, 1046. https://doi.org/10.3390/molecules29051046
Ju X, Chen T, Ding Y, Yu D, Zhang J, Zhang R, Zhang Y, Wang X, Xu T, Li J. Effects of Rhizopus-arrhizus-31-Assisted Pretreatment on the Extraction and Bioactivity of Total Flavonoids from Hibiscus manihot L. Molecules. 2024; 29(5):1046. https://doi.org/10.3390/molecules29051046
Chicago/Turabian StyleJu, Xiurong, Tao Chen, Yutao Ding, Dan Yu, Jingyu Zhang, Ruyuan Zhang, Yang Zhang, Xinyu Wang, Tao Xu, and Jiayou Li. 2024. "Effects of Rhizopus-arrhizus-31-Assisted Pretreatment on the Extraction and Bioactivity of Total Flavonoids from Hibiscus manihot L." Molecules 29, no. 5: 1046. https://doi.org/10.3390/molecules29051046
APA StyleJu, X., Chen, T., Ding, Y., Yu, D., Zhang, J., Zhang, R., Zhang, Y., Wang, X., Xu, T., & Li, J. (2024). Effects of Rhizopus-arrhizus-31-Assisted Pretreatment on the Extraction and Bioactivity of Total Flavonoids from Hibiscus manihot L. Molecules, 29(5), 1046. https://doi.org/10.3390/molecules29051046







