Treatment of Coking Wastewater Using Hydrodynamic Cavitation Coupled with Fenton Oxidation Process
Abstract
:1. Introduction
2. Results
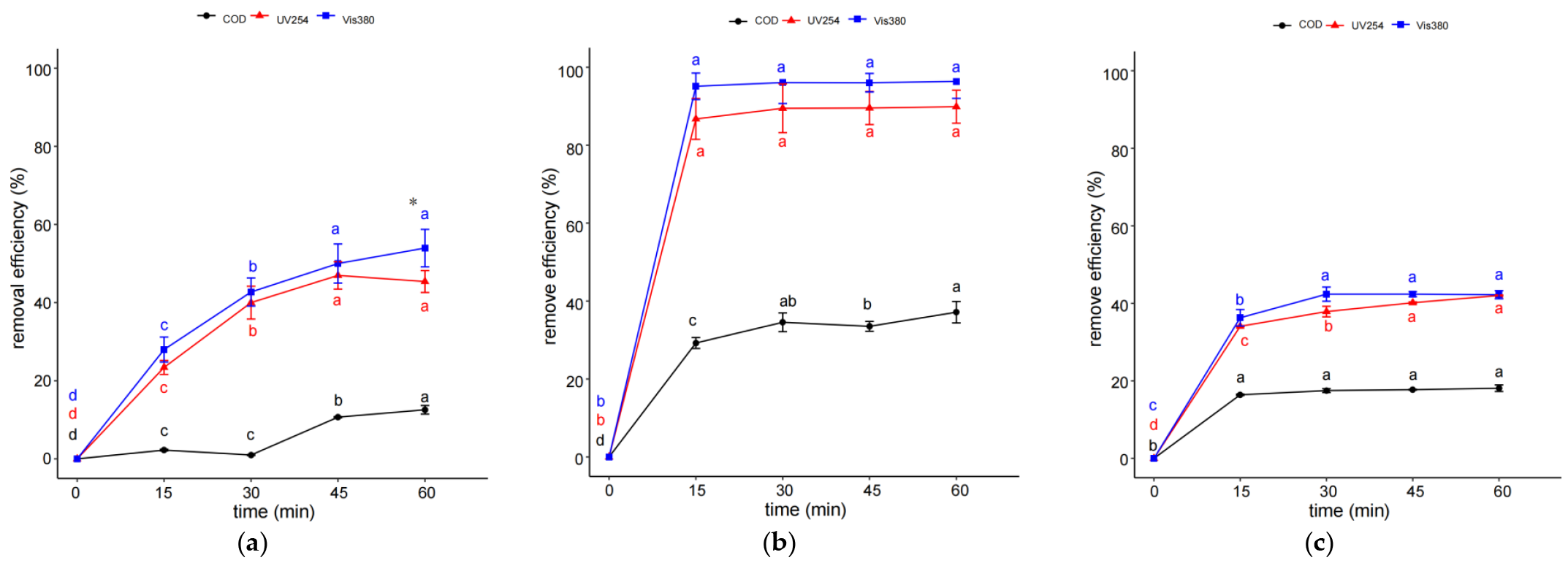
2.1. Effect of Treating Time
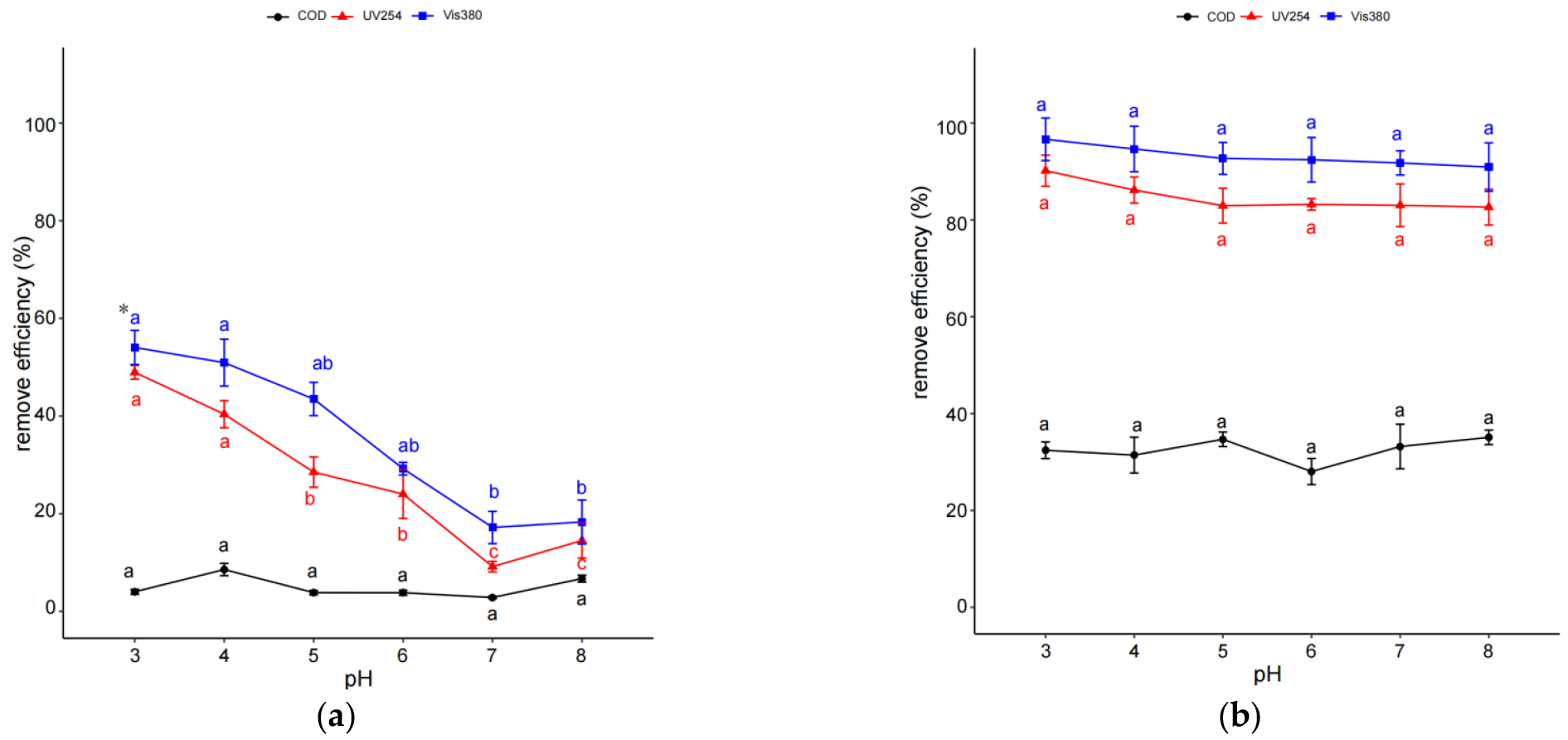
2.2. Effect of pH
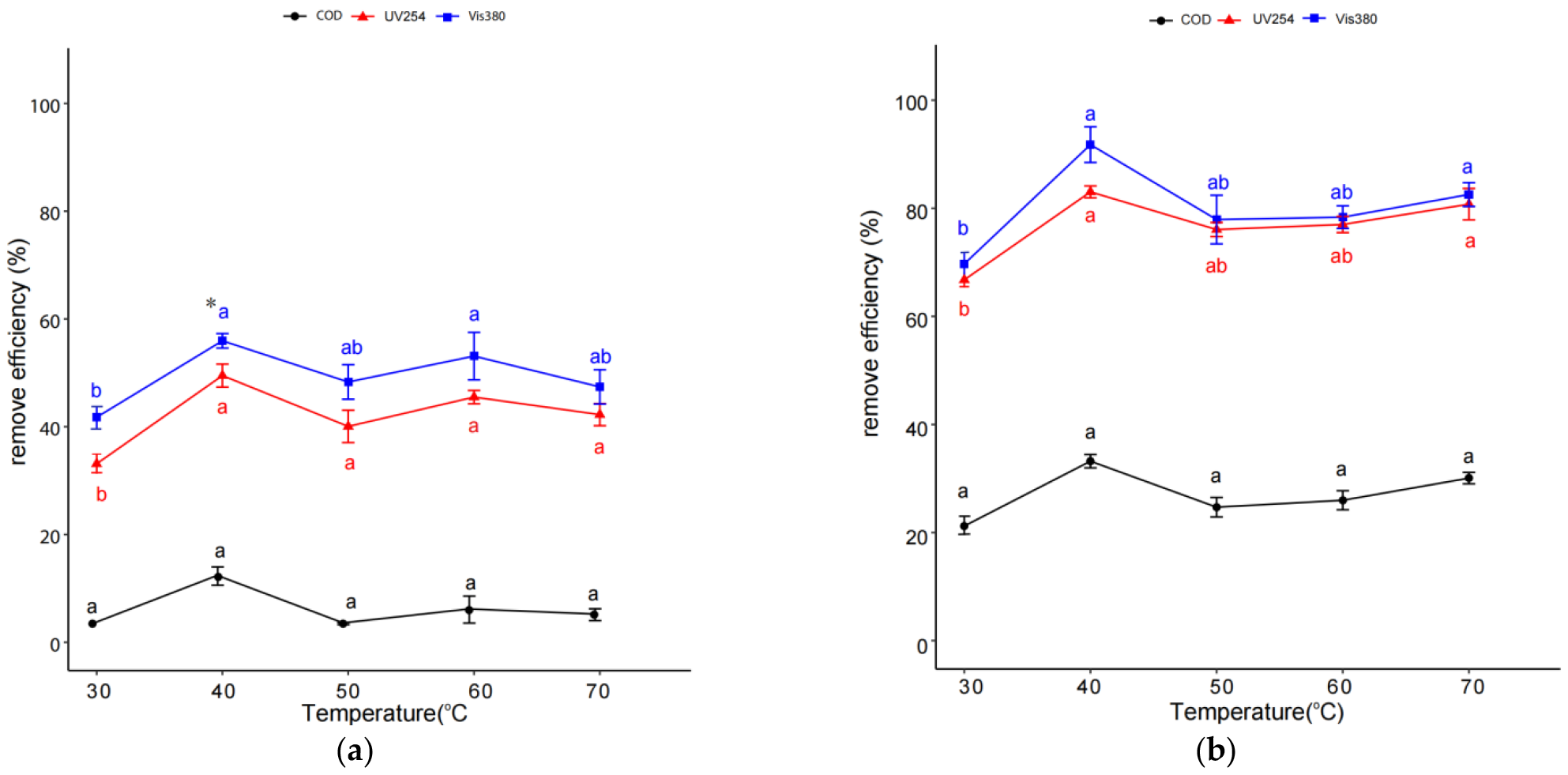
2.3. Effect of Temperature
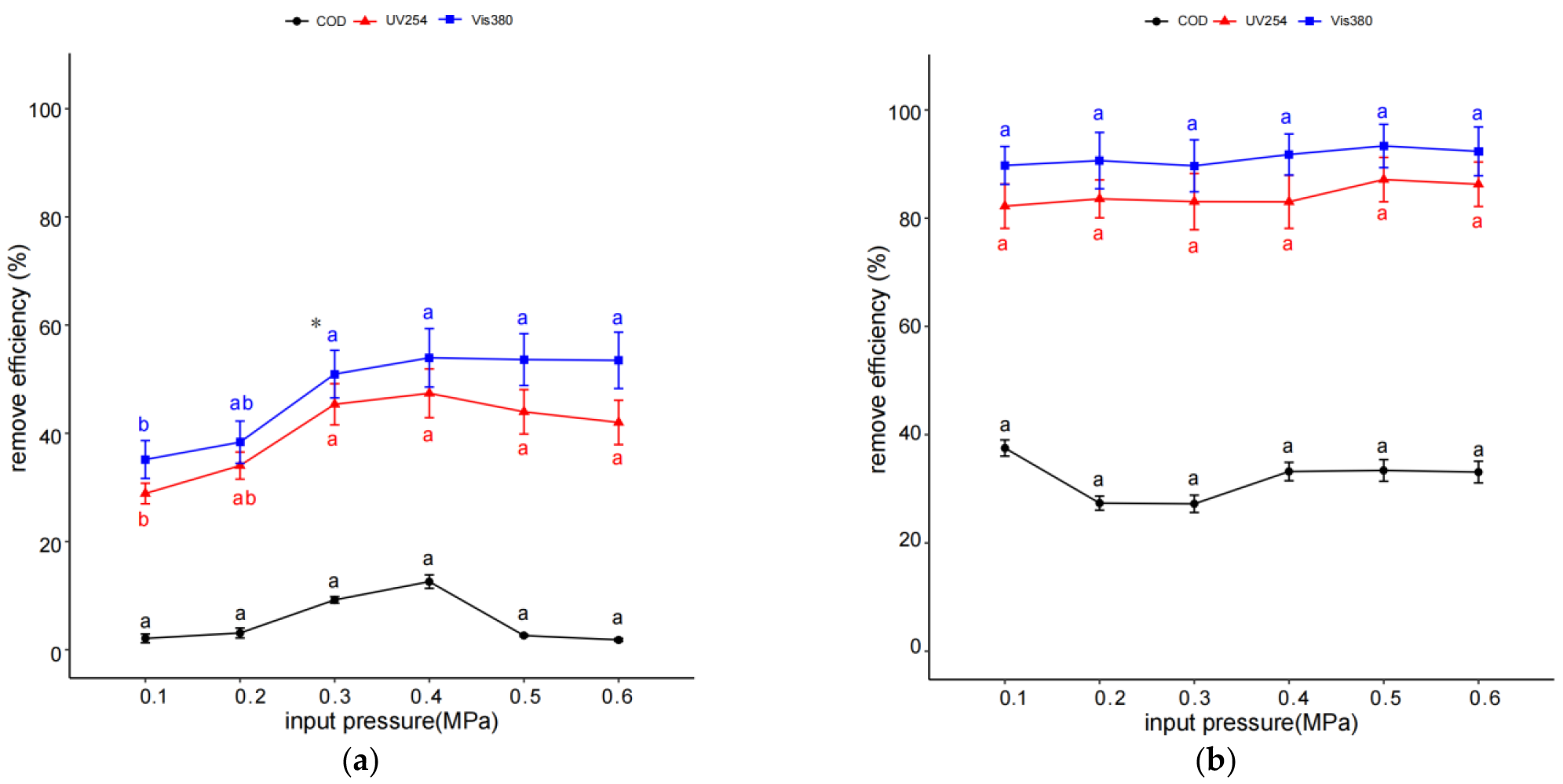
2.4. Effect of Inlet Pressure
2.5. Effects of H2O2 and Fe(II)
2.6. Optimization of Reaction Conditions and the Effect of Interaction between Conditions
2.7. GC–MS Analysis
2.8. Operating Cost
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Characteristics of the Coking Wastewater
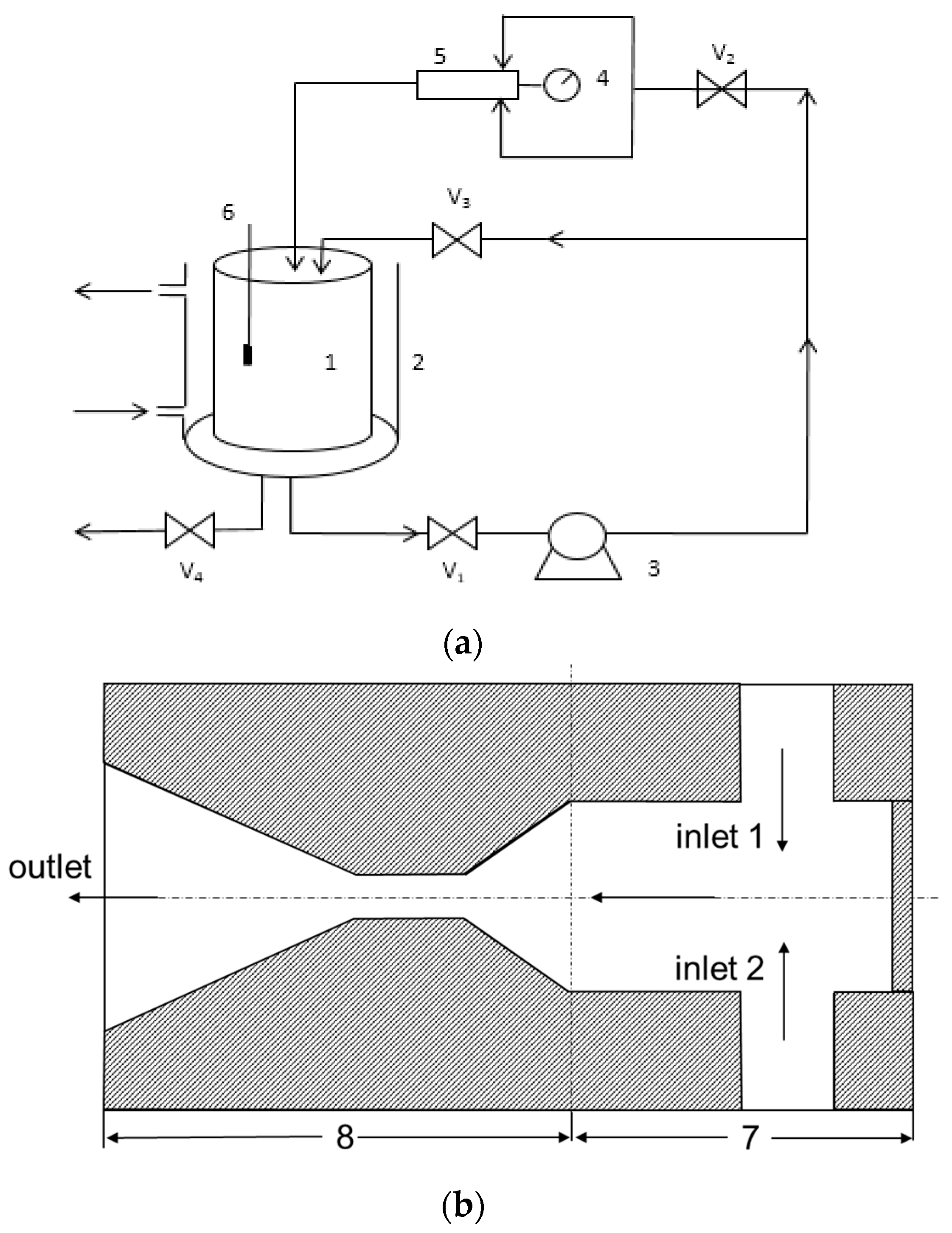
4.3. Experimental Setup
4.4. Comparison of IHC, IHC/FO, and FO at Different Treatment Times
4.5. One-Way Experiments to Optimize Reaction Conditions for IHC and IHC/FO
4.6. Response Surface Methodology (RSM) of IHC and IHC/FO
4.7. Removal Efficiency of Coking Wastewater
4.8. UV-Vis and GC–MS Analysis
4.9. Analysis of One-Way Experiments
4.10. Analysis of RSM
4.11. Operating Cost
5. Conclusions
- (1)
- The combination of IHC with FO results in enhanced extents of degradation of organic pollutants in coking wastewater under similar environment conditions.
- (2)
- The IHC is sensitive to solution pH, reaction time, and inlet pressure in coking wastewater treatment. An acidic condition, long-enough reaction time, and optimum inlet pressure and temperature are recommended for enhancing the extent of degradation through IHC.
- (3)
- Initial pH, inlet pressure, and reaction temperature had smaller effects on the organic pollutant removal efficiency of IHC/FO than IHC alone. By contrast, more significant influences of Fe(II) or H2O2 on pollutant reduction in coking wastewater under IHC/FO treatment were investigated in this study.
- (4)
- More kinds and more amounts of organic compounds in coking wastewater were removed through IHC/FO than through IHC, as determined through GC–MS analysis and UV-Vis spectra.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AOP | Advanced oxidation processes |
| HC | Hydrodynamic cavitation |
| IHC | Imping stream hydrodynamic cavitation |
| FO | Fenton oxidation |
| IS | Impinging stream |
| COD | Chemical oxygen demand |
| UV-Vis | Ultraviolet and visible spectrophotometry |
| GC–MS | Gas chromatography and mass spectrometry |
| UV254 | Absorbance of some organic compounds in water under UV light at 254 nm wave length |
| Vis380 | Absorbance of visible light at 380 nm |
| RSM | Response Surface Methodology |
| ANOVA | Analysis of Variance |
| 2-D | Two-dimensional |
| CCD | Central composite design |
| Co | Initial concentration of pollutants |
| Cf | Concentration of pollutant after treatment |
| OC | Operating costs |
| ROS | Reactive oxygen species |
| EPR | Electron paramagnetic resonance |
| 3D-EEM | Three-dimensional excitation-emission matrix spectroscopy |
| SOA | Synchronized oxidation–adsorption |
References
- Zhu, X.P.; Ni, J.R.; Lai, P. Advanced treatment of biologically pretreated coking wastewater by electrochemical oxidation using boron-doped diamond electrodes. Water Res. 2009, 43, 4347–4355. [Google Scholar] [CrossRef]
- He, X.W.; Chai, Z.; Li, F.P.; Zhang, C.H.; Li, D.; Li, J.; Hua, J.L. Advanced treatment of biologically pretreated coking wastewater by electrochemical oxidation using Ti/RuO2–IrO2 electrodes. J. Chem. Technol. Biotechnol. 2013, 88, 1568–1575. [Google Scholar] [CrossRef]
- Yang, W.; Li, X.; Pan, B.; Lv, L.; Zhang, W. Effective removal of effluent organic matter (EfOM) from bio-treated coking wastewater by a recyclable aminated hypercross-linked polymer. Water Res. 2013, 47, 4730–4738. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, J.; Chen, Z. Combination of ozonation and biological aerated filter (BAF) for bio-treated coking wastewater. Sep. Purif. Technol. 2014, 132, 610–615. [Google Scholar] [CrossRef]
- Ou, H.S.; Wei, C.H.; Mo, C.H.; Wu, H.Z.; Ren, Y.; Feng, C.H. Novel insights into anoxic/aerobic1/aerobic2 biological fluidized-bed system for coke wastewater treatment by fluorescence excitation–emission matrix spectra coupled with parallel factor analysis. Chemosphere 2014, 113, 158–164. [Google Scholar] [CrossRef]
- Lai, P.; Zhao, H.Z.; Wang, C.; Ni, J.R. Advanced treatment of coking wastewater by coagulation and zero-valent iron processes. J. Hazard. Mater. 2007, 147, 232–239. [Google Scholar] [CrossRef]
- Jin, X.; Li, E.; Lu, S.; Qiu, Z.; Sui, Q. Coking wastewater treatment for industrial reuse purpose: Combining biological processes with ultrafiltration, nanofiltration and reverse osmosis. J. Environ. Sci. 2013, 25, 1565–1574. [Google Scholar] [CrossRef]
- Chu, L.; Wang, J.; Dong, J.; Liu, H.; Sun, X. Treatment of coking wastewater by an advanced Fenton oxidation process using iron powder and hydrogen peroxide. Chemosphere 2012, 86, 409–414. [Google Scholar] [CrossRef]
- Du, X.; Zhang, R.; Gan, Z.; Bi, J. Treatment of high strength coking wastewater by supercritical water oxidation. Fuel 2013, 104, 77–82. [Google Scholar] [CrossRef]
- Zupanc, M.; Kosjek, T.; Petkovšek, M.; Dular, M.; Kompare, B.; Širok, B.; Blazeka, Z.; Heath, E. Removal of pharmaceuticals from wastewater by biological processes, hydrodynamic cavitation and UV treatment. Ultrason. Sonochem. 2013, 20, 1104–1112. [Google Scholar] [CrossRef]
- Joshi, S.M.; Gogate, P.R. Intensification of industrial wastewater treatment using hydrodynamic cavitation combined with advanced oxidation at operating capacity of 70 L. Ultrason. Sonochem. 2019, 52, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Gogate, P.R.; Pandit, A.B. A review of imperative technologies for wastewater treatment I: Oxidation technologies at ambient conditions. Adv. Environ. Res. 2004, 8, 501–551. [Google Scholar] [CrossRef]
- Dino, M.; Marina, P.; Mauro, C.; Despina, K.; Pasquale, I.; Silvana, C.; Amedeo, L. Degradation of ibuprofen by hydrodynamic cavitation: Reaction pathways and effect of operational parameters. Ultrason. Sonochem. 2016, 29, 76–83. [Google Scholar]
- Barik, A.J.; Gogate, P.R. Degradation of 4-chloro 2-aminophenol using a novel combined process based on hydrodynamic cavitation, UV photolysis and ozone. Ultrason. Sonochem. 2016, 30, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Dhanke, P.B.; Wagh, S.M. Intensification of the degradation of Acid RED-18 using hydrodynamic cavitation. Emerg. Contam. 2020, 6, 20–32. [Google Scholar] [CrossRef]
- Innocenzi, V.; Prisciandaro, M.; Centofanti, M.; Veglio, F. Comparison of performances of hydrodynamic cavitation in combined treatments based on hybrid induced advanced Fenton process for degradation of azo-dyes. J. Environ. Chem. Eng. 2019, 7, 103171. [Google Scholar] [CrossRef]
- Amin, L.P.; Gogate, P.R.; Burgess, A.E.; Bremner, D.H. Optimization of a hydrodynamic cavitation reactor using salicylic acid dosimetry. Chem. Eng. J. 2010, 156, 165–169. [Google Scholar] [CrossRef]
- Bagal, M.V.; Gogate, P.R. Degradation of 2,4-dinitrophenol using a combination of hydrodynamic cavitation, chemical and advanced oxidation processes. Ultrason. Sonochem. 2013, 20, 1226–1235. [Google Scholar] [CrossRef]
- Cai, M.; Su, J.; Zhu, Y.; Wei, X.; Jin, M.; Zhang, H.; Dong, C. Decolorization of azo dyes Orange G using hydrodynamic cavitation coupled with heterogeneous Fenton process. Ultrason. Sonochem. 2016, 28, 302–310. [Google Scholar] [CrossRef]
- Thanekar, J.P.; Gogate, P.R.; Znak, Z.; Sukhatskiy, Y.; Mnykh, R. Degradation of benzene present in wastewater using hydrodynamic cavitation in combination with air. Ultrason. Sonochem. 2021, 70, 105296. [Google Scholar] [CrossRef]
- Wu, Z.L.; Yuste-Córdobac, F.J.; Cintasd, P.; Wu, Z.S.; Boffaa, L.; Mantegnaa, S.; Cravotto, G. Effects of ultrasonic and hydrodynamic cavitation on the treatment of cork wastewater by flocculation and Fenton processes. Ultrason. Sonochem. 2018, 40, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Lan, Y.; Nie, S.; Qin, T.; Nie, S.; Zhou, J. Synergistic effect of hydrodynamic cavitation characteristics of self-excited oscillation cavity for degradation of dye wastewater. J. Clean. Prod. 2022, 380, 135116. [Google Scholar] [CrossRef]
- Pradhan, A.A.; Gogate, P.R. Degradation of p-nitrophenol using acoustic cavitation and Fenton chemistry. J. Hazard. Mater. 2010, 173, 517–522. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, P.; Yuan, Y.; Yang, F. Synergistic degradation of chitosan by impinging stream and jet cavitation. Ultrason. Sonochem. 2015, 27, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.B.; Tian, J.P.; Liu, R.; Chen, L.J. Optimization of Fenton and electro-Fenton oxidation of biologically treated coking wastewater using response surface methodology. Sep. Purif. 2011, 81, 444–450. [Google Scholar] [CrossRef]
- Verma, V.; Chaudhari, P.K. Optimization of multiple parameters for treatment of coking wastewater using Fenton oxidation. Arab. J. Chem. 2020, 13, 5084–5095. [Google Scholar] [CrossRef]
- Raut-Jadhav, S.; Badve, M.P.; Pinjari, D.V.; Saini, D.R.; Sonawane, S.H.; Pandit, A.B. Treatment of the pesticide industry effluent using hydrodynamic cavitation and its combination with process intensifying additives (H2O2 and ozone). Chem. Eng. J. 2016, 295, 326–335. [Google Scholar] [CrossRef]
- Jiang, W.X.; Zhang, W.; Li, B.J.; Duan, J.; Lv, Y.; Liu, W.D.; Ying, W.C. Combined Fenton oxidation and biological activated carbon process for recycling of coking plant effluent. J. Hazard. Mater. 2011, 189, 308–314. [Google Scholar] [CrossRef]
- Raut-Jadhav, S.; Saharan, V.K.; Pinjari, D.; Sonawane, S.; Saini, D.; Pandit, A. Synergetic effect of combination of AOP’s (hydrodynamic cavitation and H2O2) on the degradation of neonicotinoid class of insecticide. J. Hazard. Mater. 2013, 261, 139–147. [Google Scholar] [CrossRef]
- Li, P.; Song, Y.; Wang, S.; Tao, Z.; Yu, S.L.; Liu, Y.N. Enhanced decolorization of methyl orange using zero-valent copper nanoparticles under assistance of hydrodynamic cavitation. Ultrason. Sonochem. 2015, 22, 132–138. [Google Scholar] [CrossRef]
- Bagal, M.V.; Gogate, P.R. Wastewater treatment using hybrid treatment schemes based on cavitation and Fenton chemistry: A review. Ultrason. Sonochem. 2014, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Korpe, S.; Rao, P.V.; Sonawane, S.H. Performance evaluation of hydrodynamic cavitation in combination with AOPs for degradation of tannery wastewater. J. Environ. Chem. Eng. 2023, 11, 109731. [Google Scholar] [CrossRef]
- Tao, Y.Q.; Cai, J.; Huai, X.L.; Liu, B.; Guo, Z.X. Application of hydrodynamic cavitation to wastewater treatment. Chem. Eng. Technol. 2016, 39, 1363–1376. [Google Scholar] [CrossRef]
- Patil, P.N.; Bote, S.D.; Gogate, P.R. Degradation of imidacloprid using combined advanced oxidation processes based on hydrodynamic cavitation. Ultrason. Sonochem. 2014, 21, 1770–1777. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.K.; Zhang, Y. Degradation of alachlor in aqueous solution by using hydrodynamic cavitation. J. Hazard. Mater. 2009, 161, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Saharan, V.K.; Pandit, A.B.; Kumar, P.S.S.; Anandan, S. Hydrodynamic cavitation as an advanced oxidation technique for the degradation of Acid Red 88 Dye. Ind. Eng. Chem. Res. 2012, 51, 1981–1989. [Google Scholar] [CrossRef]
- Gore, M.M.; Saharan, V.K.; Pinjari, D.V.; Chavan, P.V.; Pandit, A.B. Degradation of reactive orange 4 dye using hydrodynamic cavitation based hybrid techniques. Ultrason. Sonochem. 2014, 21, 1075–1082. [Google Scholar] [CrossRef]
- Chakinala, A.G.; Gogate, P.R.; Burgess, A.E.; Bremner, D.H. Treatment of industrial wastewater effluents using hydrodynamic cavitation and the advanced Fenton process. Ultrason. Sonochem. 2008, 15, 49–54. [Google Scholar] [CrossRef]
- Sawant, S.S.; Anil, A.C.; Krishnamurthy, V.; Gaonkar, C.; Kolwalkar, J.; Khandeparker, L.; Desai, D.; Mahulkar, A.V.; Ranade, V.V.; Pandit, A.B. Effect of hydrodynamic cavitation on zooplankton: A tool for disinfection. Biochem. Eng. J. 2008, 42, 320–328. [Google Scholar] [CrossRef]
- Angaji, M.T.; Ghiaee, R. Decontamination of unsymmetrical dimethylhydrazine waste water by hydrodynamic cavitation-induced advanced Fenton process. Ultrason. Sonochem. 2015, 23, 257–265. [Google Scholar] [CrossRef]
- Goel, M.; Hu, H.; Mujumdar, A.S.; Ray, M.B. Sonochemical decomposition of volatile and non-volatile organic compounds—A comparative study. Water Res. 2004, 38, 4247–4261. [Google Scholar] [CrossRef]
- Deng, Y.; Englehardt, J.D. Treatment of landfill leachate by the Fenton process. Water Res. 2006, 40, 3683–3694. [Google Scholar] [CrossRef]
- Golash, N.; Gogate, P.R. Degradation of dichlorvos containing wastewaters using sonochemical reactors. Ultrason. Sonochem. 2012, 19, 1051–1060. [Google Scholar] [CrossRef]
- Saharan, V.K.; Badve, M.P.; Pandit, A.B. Degradation of Reactive Red 120 dye using hydrodynamic cavitation. Chem. Eng. J. 2011, 178, 100–107. [Google Scholar] [CrossRef]
- Joshi, R.K.; Gogate, P.R. Degradation of dichlorvos using hydrodynamic cavitation based treatment strategies. Ultrason. Sonochem. 2012, 19, 532–539. [Google Scholar] [CrossRef]
- Dubber, D.; Gray, N.F. Replacement of chemical oxygen demand (COD) with total organic carbon (TOC) for monitoring wastewater treatment performance to minimize disposal of toxic analytical waste. J. Environ. 2010, 45, 1595–1600. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.W.; Su, H.J.; Zhang, B. Hydrodynamic cavitation as a promising route for wastewater treatment—A review. Chem. Eng. J. 2021, 412, 128685. [Google Scholar] [CrossRef]
- Guo, S.; Chen, M.; Wei, Y.; You, L.M.; Cai, C.; Wei, Q.S.; Zhou, K. Designing hierarchically porous zero-valent iron via 3D printing to degrade organic pollutants by activating peroxymonosulfate using high-valent iron-oxo species. Chem. Eng. J. 2023, 476, 146523. [Google Scholar] [CrossRef]
- Sun, G.; Zhang, Y.; Gao, Y.; Han, X.; Yang, M. Removal of hard COD from biological effluent of coking wastewater using synchronized oxidation-adsorption technology: Performance, mechanism, and full-scale application. Water Res. 2020, 173, 115517. [Google Scholar] [CrossRef]
- Tijing, L.D.; Woo, Y.C.; Choi, J.S.; Lee, S.; Kim, S.H.; Shon, H.K. Fouling and its control in membrane distillation—A review. J. Membr. Sci. 2015, 475, 215–244. [Google Scholar] [CrossRef]
- Tang, H.; Sha, J.P.; Liu, G.Z.; Ou, Y.L. Advanced treatment of biologically pretreated coking wastewater by electro-coagulation: Degradation behavior and mechanism. Pol. J. Environ. Stud. 2015, 24, 1355–1362. [Google Scholar]
- Wei, X.X.; Zhang, Z.Y.; Fan, Q.L.; Yuan, X.Y.; Guo, D.S. The effect of treatment stages on the coking wastewater hazardous compounds and their toxicity. J. Hazard. Mater. 2012, 239–240, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Ramteke, L.P.; Gogate, P.R. Treatment of toluene, benzene, naphthalene and xylene (BTNXs) containing wastewater using improved biological oxidation with pretreatment using Fenton/ultrasound based processes. J. Environ. Chem. Eng. 2015, 28, 247–260. [Google Scholar] [CrossRef]
- GB 16171-2012; Emission Standard of Pollutants for Coking Chemical Industry. National Standards of People’s Republic of China: Beijing, China, 2012.
- Singh, S.; Randhavane, S. Hydrodynamic cavitation: Its optimization and potential application in treatment of pigment industry wastewater. Mater. Today Proc. 2022, 61, 523–529. [Google Scholar] [CrossRef]

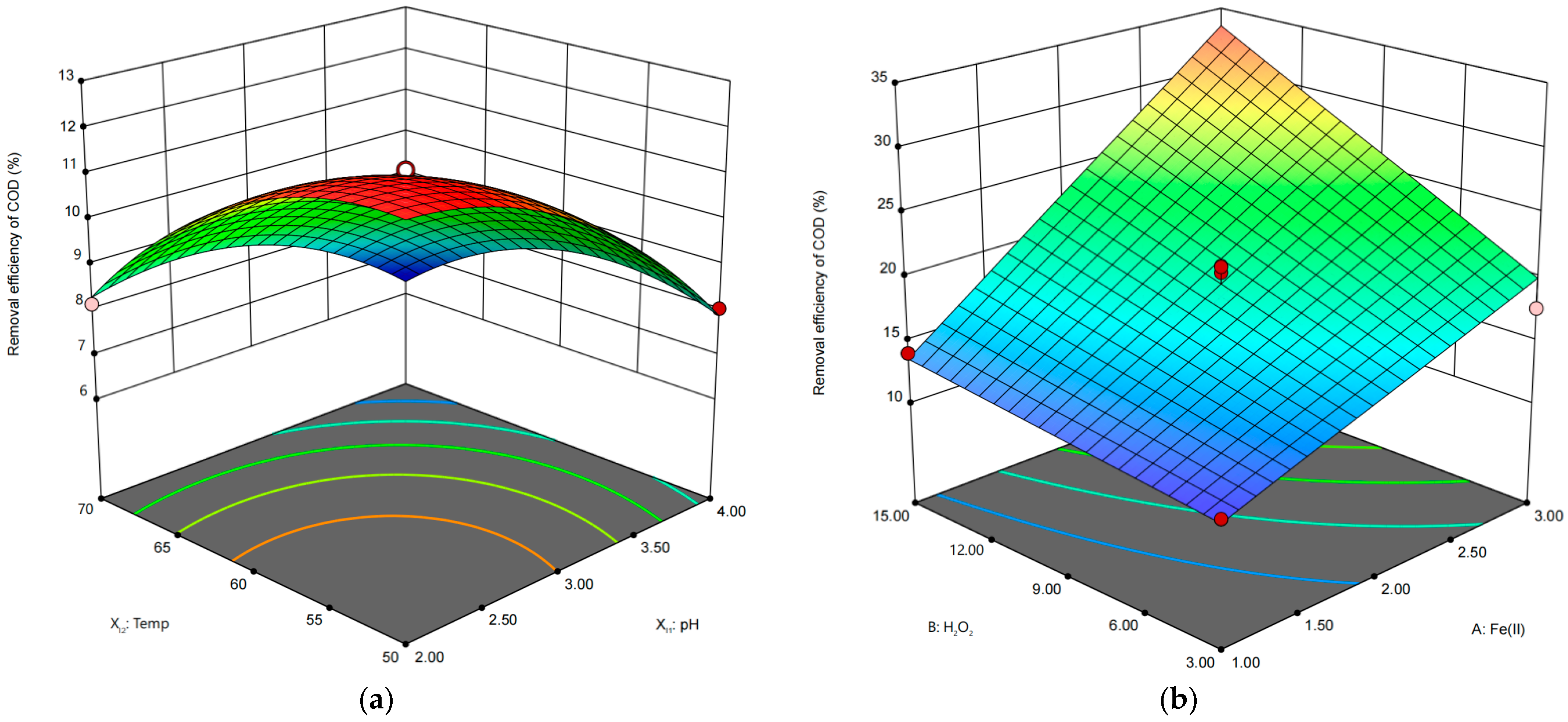
| Variables for IHC | Range and Level | Variables for IHC/FO | Range and Level | ||||
|---|---|---|---|---|---|---|---|
| −1 | 0 | 1 | −1 | 0 | 1 | ||
| XI1: initial pH | 2 | 3 | 4 | XIF1: initial Fe(II) (mmol/L) | 1 | 2 | 3 |
| XI2: temperature (°C) | 50 | 60 | 70 | XIF2: initial H2O2 (mmol/L) | 3 | 9 | 15 |
| XI3: reaction time (min) | 20 | 40 | 60 | XIF3: initial pH | 5 | 6 | 7 |
| Variables | Removal Efficiency of COD (%) | Variables | Removal Efficiency of COD (%) | |||||
|---|---|---|---|---|---|---|---|---|
| XI1 | XI2 | XI3 | XIF1 | XIF2 | XIF3 | |||
| Initial pH | Temperature (°C) | Reaction Time (min) | Initial Fe(II) (mmol/L) | Initial H2O2 (mmol/L) | Initial pH | |||
| 1 | 2 | 50 | 40 | 11.98 | 2 | 15 | 7 | 19.65 |
| 2 | 3 | 60 | 40 | 10.86 | 1 | 9 | 5 | 12.13 |
| 3 | 4 | 50 | 40 | 8.05 | 2 | 9 | 6 | 20.44 |
| 4 | 4 | 60 | 20 | 7.63 | 2 | 9 | 6 | 18.20 |
| 5 | 3 | 60 | 40 | 11.04 | 3 | 9 | 7 | 24.56 |
| 6 | 3 | 50 | 60 | 10.77 | 3 | 3 | 6 | 17.62 |
| 7 | 4 | 70 | 40 | 6.51 | 1 | 15 | 6 | 14.01 |
| 8 | 2 | 70 | 40 | 8.15 | 3 | 9 | 5 | 34.26 |
| 9 | 3 | 60 | 40 | 10.51 | 2 | 3 | 5 | 15.29 |
| 10 | 2 | 60 | 60 | 11.06 | 2 | 9 | 6 | 20.97 |
| 11 | 3 | 60 | 40 | 11.12 | 2 | 9 | 6 | 18.90 |
| 12 | 3 | 70 | 20 | 8.73 | 2 | 15 | 5 | 25.89 |
| 13 | 2 | 60 | 20 | 11.94 | 1 | 9 | 7 | 12.35 |
| 14 | 4 | 60 | 60 | 8.36 | 3 | 15 | 6 | 31.30 |
| 15 | 3 | 60 | 40 | 10.78 | 2 | 3 | 7 | 14.51 |
| 16 | 3 | 50 | 20 | 11.33 | 2 | 9 | 6 | 20.90 |
| 17 | 3 | 70 | 60 | 7.77 | 1 | 3 | 6 | 11.41 |
| Source in IHC | Sum of Squares | Df | Mean Square | F-Value | Prob > F |
|---|---|---|---|---|---|
| Model | 47.98 | 9 | 5.33 | 51.52 | <0.0001 |
| XI1 | 19.78 | 1 | 19.78 | 191.2 | <0.0001 |
| XI2 | 15.04 | 1 | 15.04 | 145.39 | <0.0001 |
| XI3 | 0.3486 | 1 | 0.3486 | 3.37 | 0.109 |
| XI1XI2 | 1.31 | 1 | 1.31 | 12.67 | 0.0092 |
| XI1XI3 | 0.648 | 1 | 0.648 | 6.26 | 0.0508 |
| XI2XI3 | 0.04 | 1 | 0.04 | 0.3866 | 0.5538 |
| Residual | 0.5004 | 3 | 0.1668 | 2.98 | 0.1594 |
| Lack of fit | 0.2238 | 4 | 0.0559 | ||
| Pure error | 48.7 | 16 | |||
| Cor total | 0.5004 | 3 | 0.1668 | 2.98 | 0.1594 |
| R2 | 0.9850 | Adjusted R2 | 0.9658 | Adeq. Precision | 23.86 |
| C.V. (%) | 3.29 | Predicted R2 | 0.8289 |
| Source in IHC | Sum of Squares | Df | Mean Square | F-value | Prob > F |
|---|---|---|---|---|---|
| Model | 643.12 | 6 | 107.19 | 30.95 | <0.0001 |
| XIF1 | 418.18 | 1 | 418.18 | 120.76 | <0.0001 |
| XIF2 | 128.16 | 1 | 128.16 | 37.01 | 0.0001 |
| XIF3 | 34.03 | 1 | 34.03 | 9.83 | 0.0106 |
| XIF1XIF2 | 30.69 | 1 | 30.69 | 8.86 | 0.0139 |
| XIF1XIF3 | 24.6 | 1 | 24.6 | 6.1 | 0.0537 |
| XIF2XIF3 | 7.45 | 1 | 7.45 | 2.15 | 0.1731 |
| Residual | 34.63 | 10 | 3.46 | ||
| Lack of fit | 28.3 | 6 | 4.72 | 2.98 | 0.1548 |
| Pure error | 6.32 | 4 | 1.58 | ||
| Cor total | 677.75 | 16 | |||
| R2 | 0.9489 | Adjusted R2 | 0.9182 | Adeq. Precision | 18.81 |
| C.V. (%) | 9.52 | Predicted R2 | 0.7781 |
| Treat | Time (min) | Condition | Removal Efficiency of COD(%) | Consumption | Costs (CNY/g COD) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Input Pressure (MPa) | pH | H2O2 (mmol/L) | FeSO4 (mmol/L) | ENC (kwh) | H2O2 (g/g COD) | FeSO4 (g/g COD) | H2SO4 (g/g COD) | H2SO4 | ENC | H2O2 | FeSO4 | Total | |||
| FO | 15 | 0.01 | 3.00 | 12.00 | 3.00 | 18.41 | 0.000 | 8.865 | 9.908 | 0.222 | 0.000 | 0.000 | 0.024 | 0.002 | 0.026 |
| 30 | 0.01 | 3.00 | 12.00 | 3.00 | 19.49 | 0.001 | 8.374 | 9.359 | 0.222 | 0.000 | 0.000 | 0.023 | 0.002 | 0.025 | |
| 45 | 0.01 | 3.00 | 12.00 | 3.00 | 19.72 | 0.001 | 8.276 | 9.249 | 0.222 | 0.000 | 0.000 | 0.022 | 0.002 | 0.024 | |
| 60 | 0.01 | 3.00 | 12.00 | 3.00 | 20.10 | 0.002 | 8.119 | 9.075 | 0.222 | 0.000 | 0.000 | 0.022 | 0.002 | 0.024 | |
| IHC | 45 | 0.50 | 3.00 | 0.00 | 0.00 | 10.67 | 0.024 | 0.000 | 0.000 | 0.222 | 0.000 | 0.024 | 0.000 | 0.000 | 0.024 |
| 60 | 0.50 | 3.00 | 0.00 | 0.00 | 12.57 | 0.027 | 0.000 | 0.000 | 0.222 | 0.000 | 0.027 | 0.000 | 0.000 | 0.027 | |
| 60 | 0.40 | 3.00 | 0.00 | 0.00 | 12.57 | 0.022 | 0.000 | 0.000 | 0.222 | 0.000 | 0.022 | 0.000 | 0.000 | 0.022 | |
| IHC/FO | 15 | 0.10 | 3.00 | 12.00 | 3.00 | 32.43 | 0.001 | 5.032 | 5.624 | 0.222 | 0.000 | 0.001 | 0.014 | 0.001 | 0.016 |
| 15 | 0.10 | 4.00 | 12.00 | 3.00 | 31.43 | 0.001 | 5.192 | 5.803 | 0.020 | 0.000 | 0.001 | 0.014 | 0.001 | 0.016 | |
| 15 | 0.10 | 5.00 | 12.00 | 3.00 | 34.69 | 0.000 | 4.705 | 5.258 | 0.000 | 0.000 | 0.000 | 0.013 | 0.001 | 0.015 | |
| 15 | 0.10 | 7.00 | 12.00 | 3.00 | 33.20 | 0.001 | 4.916 | 5.494 | 0.000 | 0.000 | 0.001 | 0.013 | 0.001 | 0.015 | |
| 15 | 0.10 | 8.00 | 12.00 | 3.00 | 35.10 | 0.000 | 4.650 | 5.197 | 0.000 | 0.000 | 0.000 | 0.013 | 0.001 | 0.014 | |
| 15 | 0.10 | 7.00 | 12.00 | 3.00 | 33.20 | 0.001 | 4.916 | 5.494 | 0.000 | 0.000 | 0.001 | 0.013 | 0.001 | 0.015 | |
| 15 | 0.10 | 7.00 | 12.00 | 3.50 | 32.52 | 0.001 | 5.018 | 6.544 | 0.000 | 0.000 | 0.001 | 0.014 | 0.002 | 0.016 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, D.; Huang, T.; Li, Q.; Huang, Y.; Sun, Y.; Liang, J.; Li, J. Treatment of Coking Wastewater Using Hydrodynamic Cavitation Coupled with Fenton Oxidation Process. Molecules 2024, 29, 1057. https://doi.org/10.3390/molecules29051057
Deng D, Huang T, Li Q, Huang Y, Sun Y, Liang J, Li J. Treatment of Coking Wastewater Using Hydrodynamic Cavitation Coupled with Fenton Oxidation Process. Molecules. 2024; 29(5):1057. https://doi.org/10.3390/molecules29051057
Chicago/Turabian StyleDeng, Dongmei, Ting Huang, Qing Li, Yongchun Huang, Yufei Sun, Jieliang Liang, and Jintian Li. 2024. "Treatment of Coking Wastewater Using Hydrodynamic Cavitation Coupled with Fenton Oxidation Process" Molecules 29, no. 5: 1057. https://doi.org/10.3390/molecules29051057
APA StyleDeng, D., Huang, T., Li, Q., Huang, Y., Sun, Y., Liang, J., & Li, J. (2024). Treatment of Coking Wastewater Using Hydrodynamic Cavitation Coupled with Fenton Oxidation Process. Molecules, 29(5), 1057. https://doi.org/10.3390/molecules29051057








