Performance Degradation of a Double-Perovskite PrBaCo2O5+δ Cathode Operating under a CO2/H2O-Containing Atmosphere
Abstract
1. Introduction
2. Results and Discussion
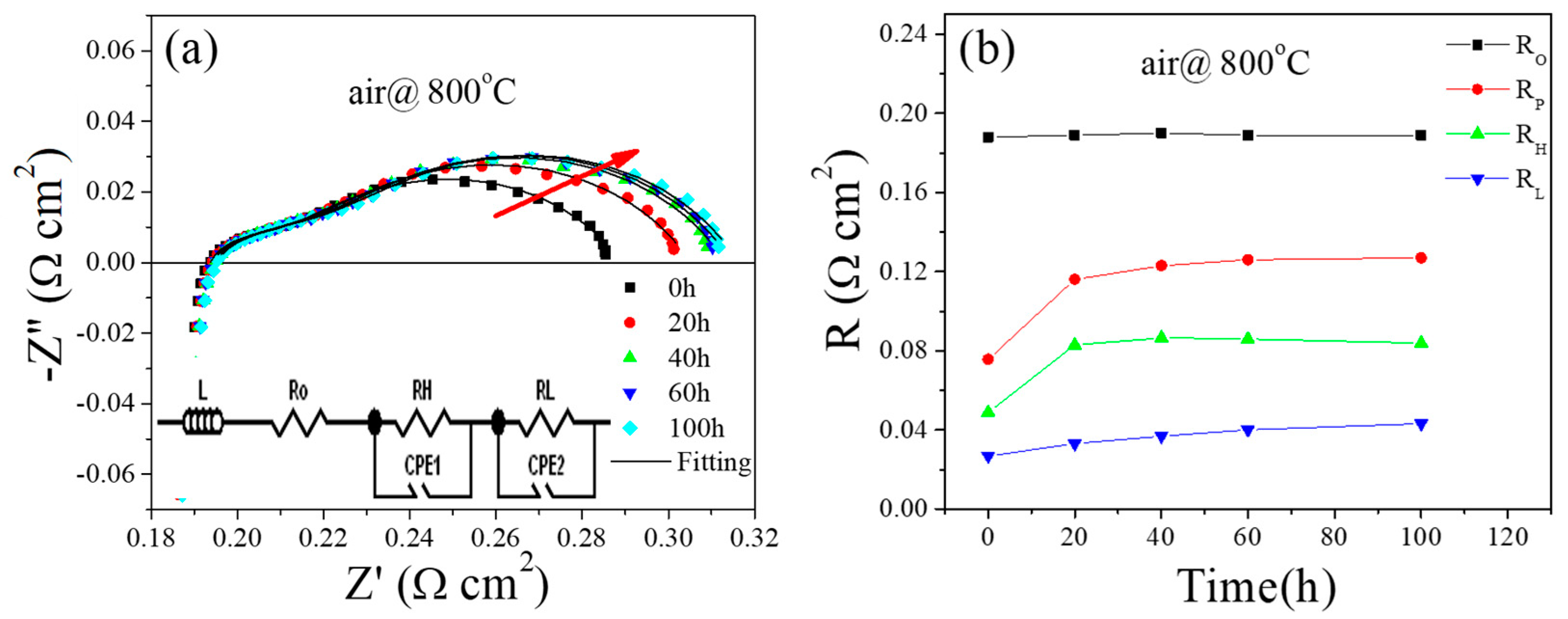
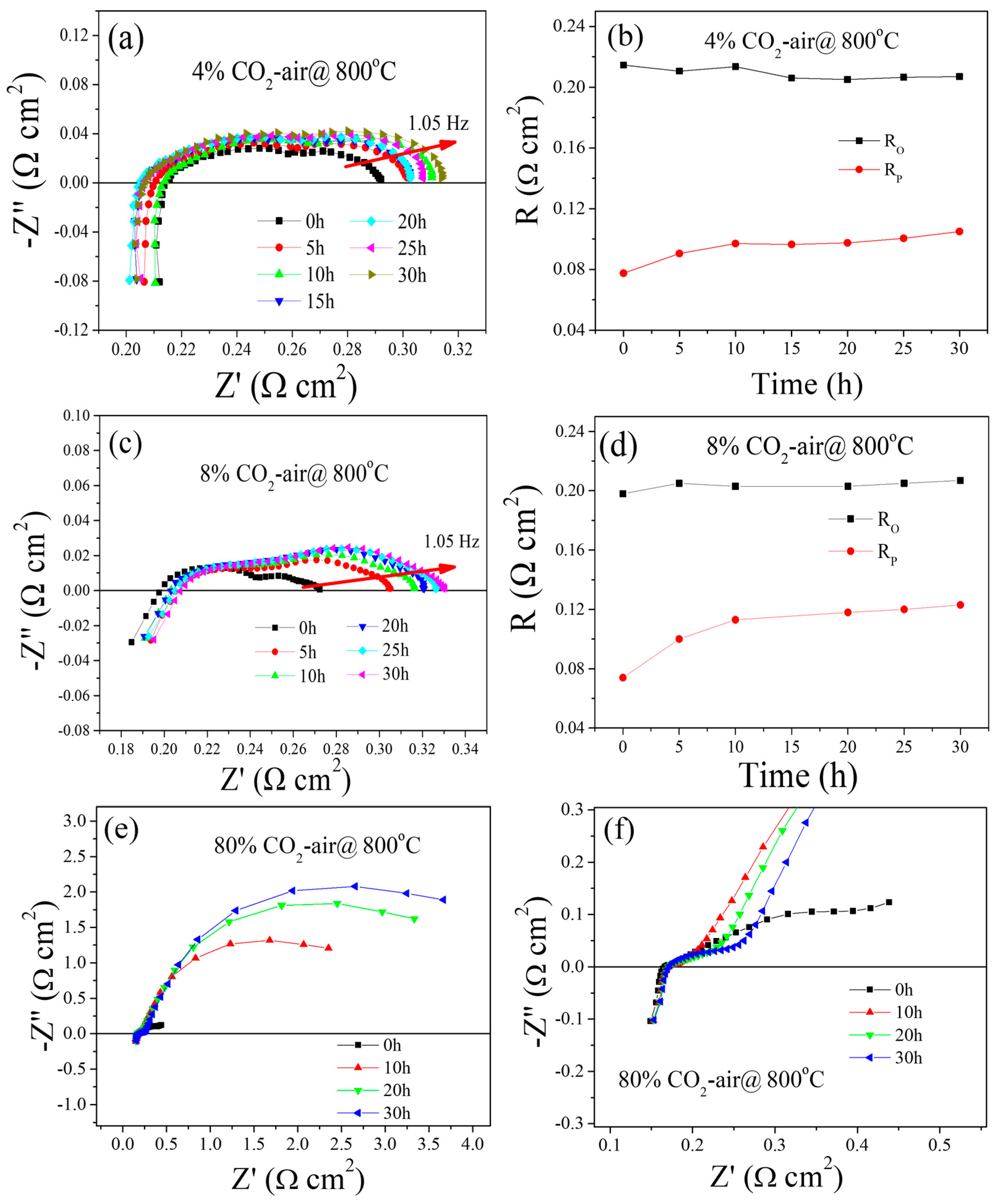
2.1. Electrochemical Performance
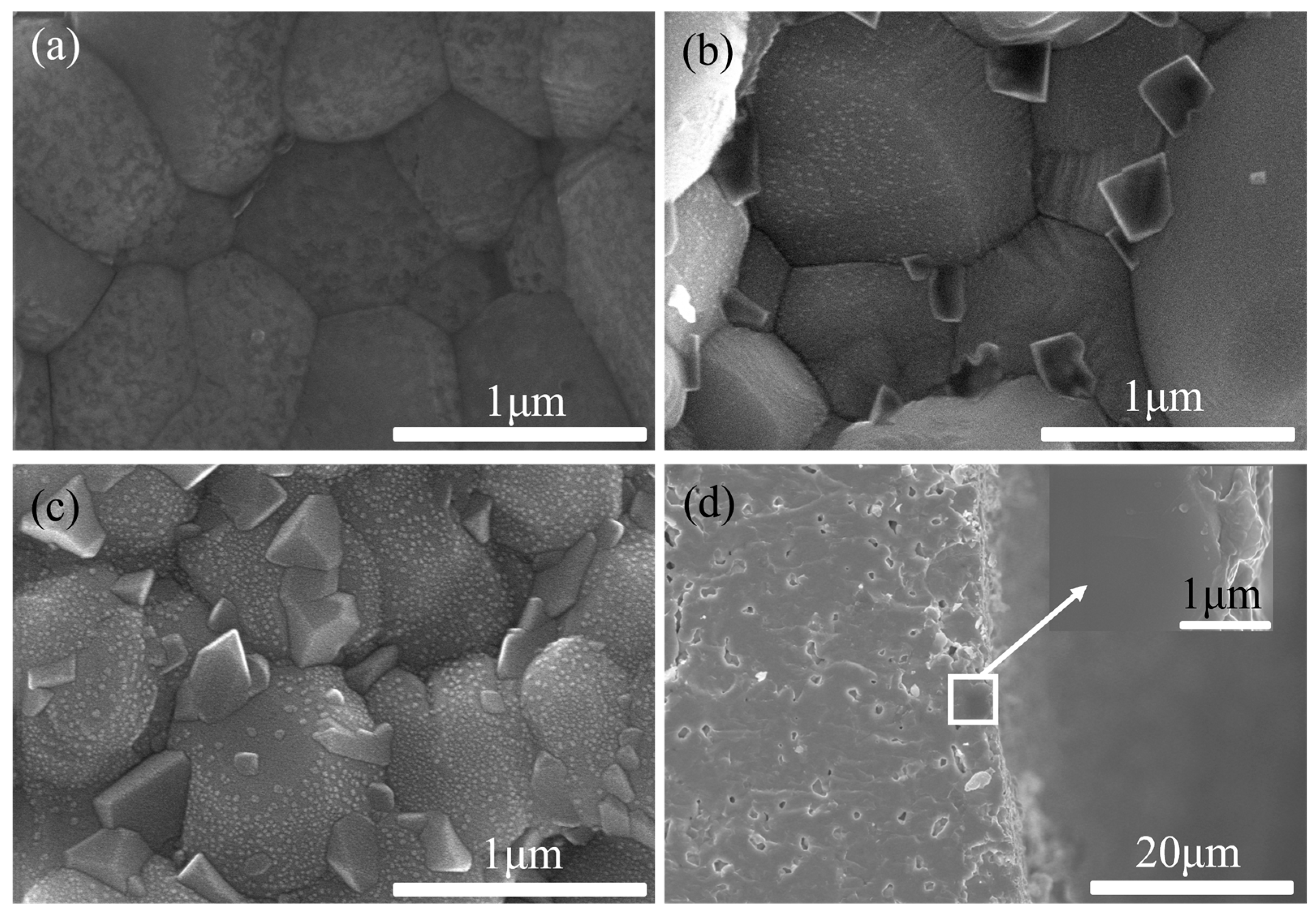
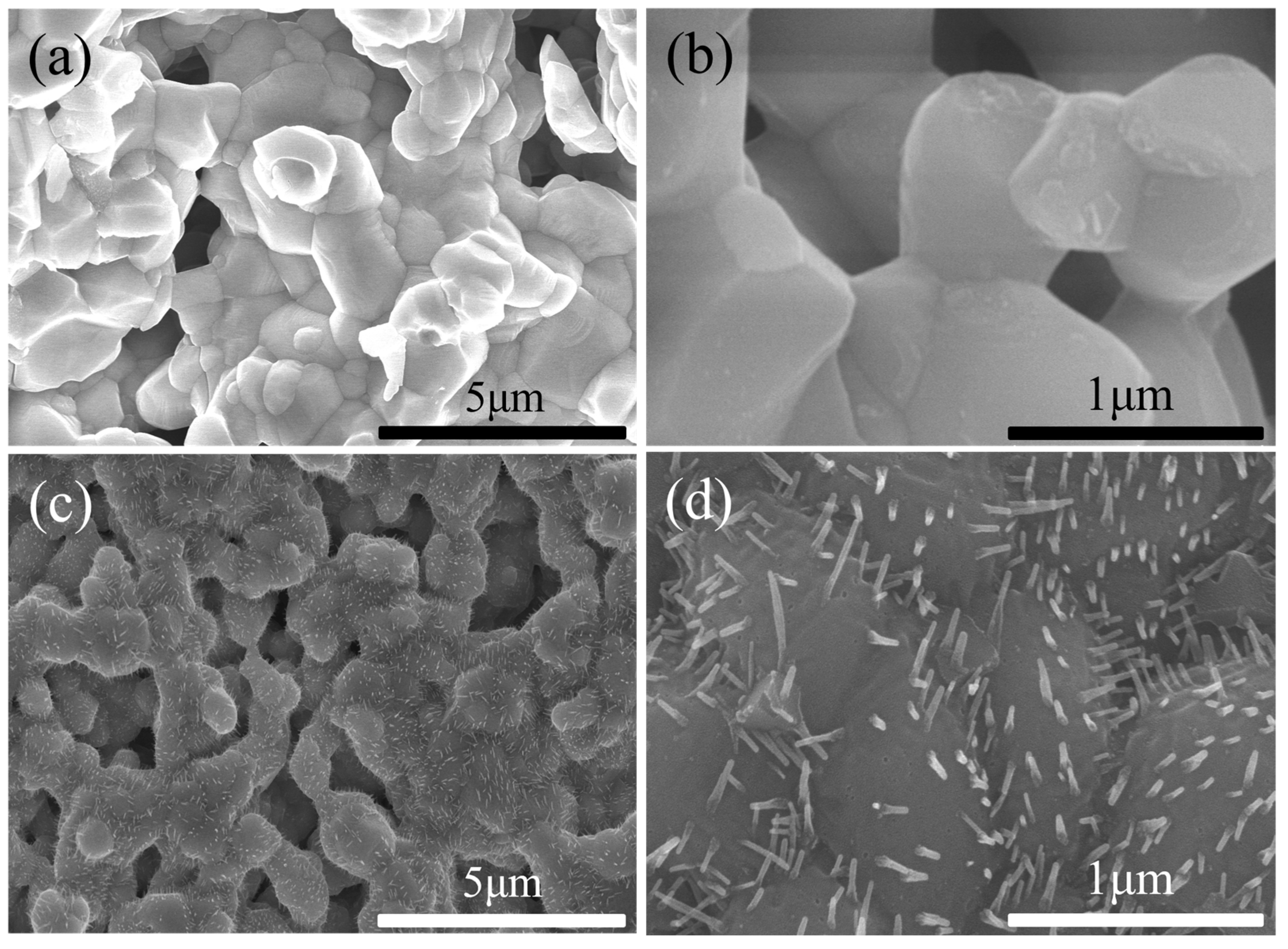
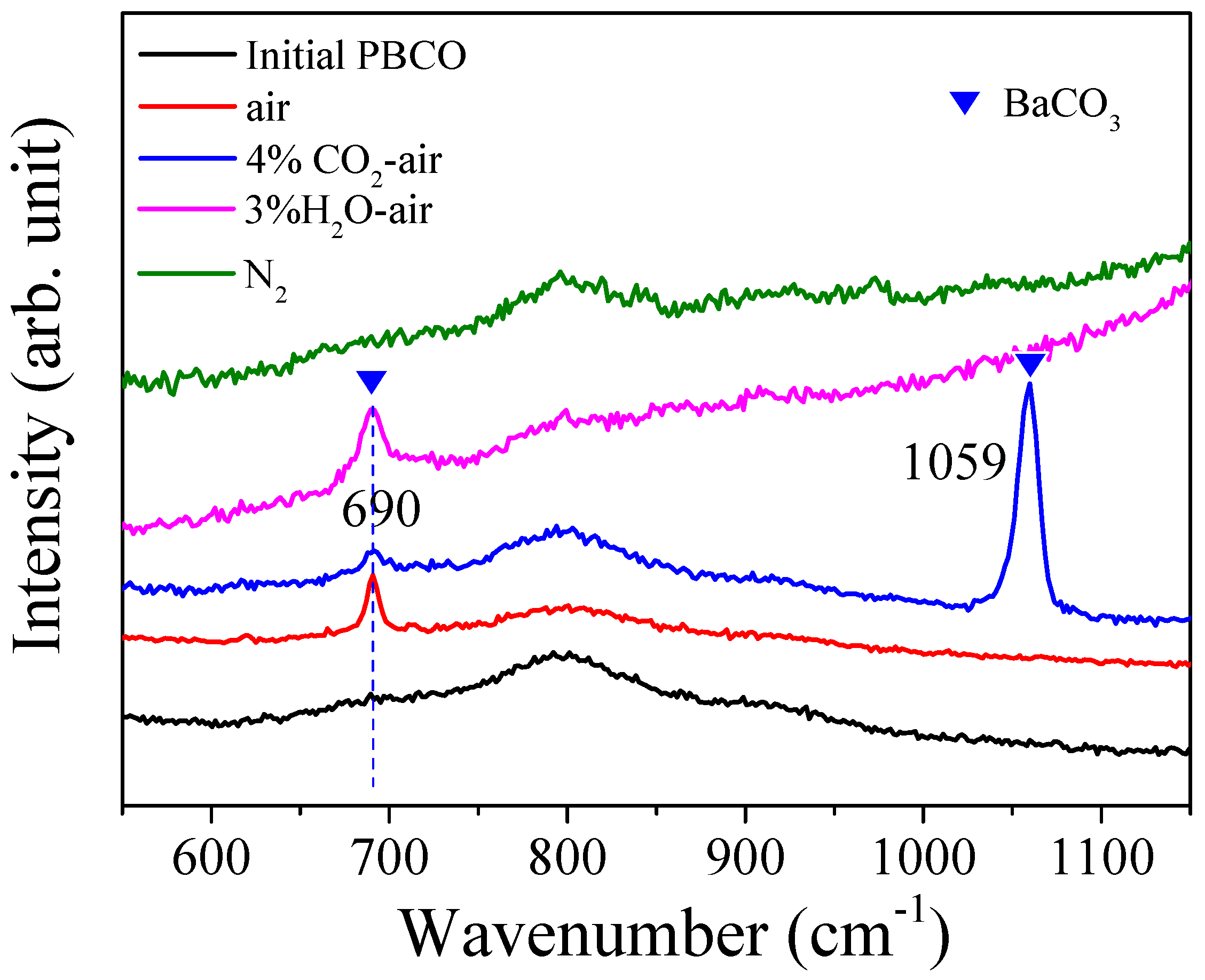
2.2. Structure Characterization
2.3. Discussion
3. Materials and Methods
3.1. Fabrications
3.2. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, Q.; Gu, Z.; Xia, L.; He, Q.; Li, Z.; Bello, I.T.; Zheng, K.; Ni, M. A comprehensive review of solid oxide fuel cells operating on various promising alternative fuels. Energy Convers. Manag. 2022, 253, 115175. [Google Scholar] [CrossRef]
- Marthosa, S.; Suklueng, M.; Niyomwas, S.; Anancharoenwong, E.; Ninwijit, T.; Budmai, N.; Kaewnun, S. Ceria-carbonate Electrolyte Ceramic Membrane for Intermediate and Low Temperature Solid Oxide Fuel Cells: A Review. J. Appl. Membr. Sci. 2020, 24, 1–10. [Google Scholar] [CrossRef]
- Li, Y.H.; Gemmen, R.; Liu, X.B. Oxygen reduction and transportation mechanisms in solide oxide fuel cell cathodes. J. Power Sources 2010, 195, 3345–3358. [Google Scholar] [CrossRef]
- Crumlin, E.J.; Mutoro, E.; Ahn, S.J.; la O’, G.J.; Leonard, D.N.; Borisevich, A.; Biegalski, M.D.; Christen, H.M.; Shao-Horn, Y. Oxygen Reduction Kinetics Enhancement on a Heterostructured Oxide Surface for Solid Oxide Fuel Cells. J. Phys. Chem. Lett. 2010, 1, 3149–3155. [Google Scholar] [CrossRef]
- Shen, M.; Ai, F.; Ma, H.; Xu, H.; Zhang, Y. Progress and prospects of reversible solid oxide fuel cell materials. iScience 2021, 24, 103464–103507. [Google Scholar] [CrossRef]
- Hu, B.; Mahapatra, M.K.; Keane, M.; Zhang, H.; Singh, P. Effect of CO2 on the stability of strontium doped lanthanum manganite cathode. J. Power Sources 2014, 268, 404–413. [Google Scholar] [CrossRef]
- Chen, L.; Lu, C.; Fang, Z.; Lu, Y.; Ni, Y.; Xu, Z. Variable infrared emittance of Sr-incorporated Sm1−xSrxCoO3(0.1 ≤ x ≤ 0.9). Phys. D Appl. Phys. 2013, 46, 105302–105309. [Google Scholar] [CrossRef]
- Fan, L.; Wang, J.; Huang, Z.; Yao, X.; Hou, N.; Gan, T.; Gan, J.; Zhao, Y.; Li, Y. Enhancement of the electrocatalytic activity of La0.6Sr0.4Co0.2Fe0.8O3-δ through surface modification by acid etching. Catal. Today 2021, 364, 97–103. [Google Scholar] [CrossRef]
- Yang, T.; Su, C.; Wang, W.; Meng, L.J.; Deng, J.G.; Liu, Y.; Rathore, S.S.; Shao, Z.P. Evaluation of the CO2 tolerant cathode for solid oxide fuel cells: Praseodymium oxysulfates/Ba0.5Sr0.5Co0.8Fe0.2O3-δ. Appl. Surf. Sci. 2019, 472, 10–15. [Google Scholar] [CrossRef]
- Xu, X.; Pan, Y.; Zhong, Y.; Ran, R.; Shao, Z. Ruddlesden–Popper perovskites in electrocatalysis. Mater. Horiz. 2020, 7, 2519–2565. [Google Scholar] [CrossRef]
- Xu, X.; Wang, W.; Zhou, W.; Shao, Z. Recent Advances in Novel Nanostructuring Methods of Perovskite Electrocatalysts for Energy-Related Applications. Small Methods 2018, 2, 1800071. [Google Scholar] [CrossRef]
- Zou, J.; Park, J.; Kwak, B.; Yoon, H.; Chung, J. Effect of Fe doping on PrBaCo2O5+δ as cathode for intermediate-temperature solid oxide fuel cells. Solid State Ion. 2012, 206, 112–119. [Google Scholar] [CrossRef]
- Muñoz-Gil, D.; Pérez-Coll, D.; Peña-Martínez, J.; Garcia-Martín, S. New insights into the GdBaCo2O5+δ material: Crystal structure, electrical and electrochemical properties. J. Power Sources 2014, 263, 90–97. [Google Scholar] [CrossRef]
- Mutoro, E.; Crumlin, E.J.; Biegalski, M.D.; Christen, H.M.; Shao-Horn, Y. Enhanced oxygen reduction activity on surface decorated perovskite thin films for solid oxide fuel cells. Energy Environ. Sci. 2011, 4, 3689–3696. [Google Scholar] [CrossRef]
- Yi, K.; Sun, L.; Li, Q.; Xia, T.; Huo, L.; Zhao, H.; Li, J.; Lü, Z.; Bassat, J.-M.; Rougier, A.; et al. Effect of Nd-deficiency on electrochemical properties of NdBaCo2O6−δ cathode for intermediate-temperature solid oxide fuel cells. Int. J. Hydrog. Energy 2016, 41, 10228–10238. [Google Scholar] [CrossRef]
- De Souza, R.A.; Chater, R.J. Oxygen exchange and diffusion measurements: The importance of extracting the correct initial and boundary conditions. Solid State Ion. 2005, 176, 1915–1920. [Google Scholar] [CrossRef]
- Téllez, H.; Druce, J.; Ju, Y.-W.; Kilner, J.; Ishihara, T. Surface chemistry evolution in LnBaCo2O5+δ double perovskites for oxygen electrodes. Int. J. Hydrog. Energy 2014, 39, 20856–20863. [Google Scholar] [CrossRef]
- Yu, A.; Xia, T.; Sun, L.; Li, Q.; Huo, L.; Zhao, H. Effects of rare earth doping on electrochemical properties of NdBaCo2O6-δ cathode materials. J. Alloys Compd. 2020, 837, 155563. [Google Scholar]
- Burriel, M.; Peça-Martínez, J.; Chater, R.J.; Fearn, S.; Berenov, A.V.; Skinner, S.J.; Kilner, J.A. Anisotropic Oxygen Ion Diffusion in Layered PrBaCo2O5+δ. Chem. Mater. 2012, 24, 613–621. [Google Scholar] [CrossRef]
- Souza, R.A.D.; Kilner, J.A.; Walker, J.F. A SIMS study of oxygen tracer diffusion and surface exchange in La0.8Sr0.2MnO3+δ. Mater. Lett. 2000, 43, 43–52. [Google Scholar] [CrossRef]
- Zhang, K.; Ge, L.; Ran, R.; Shao, Z.; Liu, S. Synthesis, characterization and evaluation of cation-ordered LnBaCo2O5+δ as materials of oxygen permeation membranes and cathodes of SOFCs. Acta Mater. 2008, 56, 4876–4889. [Google Scholar] [CrossRef]
- Zhou, Q.J.; Wang, F.; Shen, Y.; He, T.M. CO2-tolerant Sr2CoTaO6−δ double perovskite oxide as a novel cathode for intermediate-temperature solid oxide fuel cell. J. Power Sources 2010, 195, 2174–2181. [Google Scholar] [CrossRef]
- Zhu, L.; Wei, B.; Lü, Z.; Feng, J.; Xu, L.; Gao, H.; Zhang, Y.; Huang, X. Performance degradation of double-perovskite PrBaCo2O5+δ oxygen electrode in CO2 containing atmospheres. Appl. Surf. Sci. 2017, 416, 649–655. [Google Scholar] [CrossRef]
- Druce, J.; Téllez, H.; Burriel, M.; Sharp, M.D.; Fawcett, L.J.; Cook, S.N.; McPhail, D.S.; Ishihara, T.; Brongersma, H.H.; Kilner, J.A. Surface termination and subsurface restructuring of perovskite-based solid oxide electrode materials. Energy Environ. Sci. 2014, 7, 3593–3599. [Google Scholar] [CrossRef]
- Zhu, L.; Wei, B.; Wang, Z.; Chen, K.; Zhang, H.; Zhang, Y.; Huang, X.; Lu, Z. Electrochemically Driven Deactivation and Recovery in PrBaCo2O5+δ Oxygen Electrodes for Reversible Solid Oxide Fuel Cells. ChemSusChem 2016, 9, 2443–2450. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H.; Chen, F.L.; Xia, C.R. Electrochemical characteristics of nano-structured PrBaCo2O5+x cathodes fabricated with ion impregnation process. J. Power Sources 2012, 203, 34–41. [Google Scholar] [CrossRef]
- Oh, D.; Gostovic, D.; Wachsman, E.D. Mechanism of La0.6Sr0.4Co0.2Fe0.8O3 cathode degradation. J. Mater. Res. 2012, 27, 1992–1999. [Google Scholar] [CrossRef]
- Jiang, S.P.; Love, J.G. Origin of the initial polarization behavior of Sr-doped LaMnO3 for O2 reduction in solid oxide fuel cells. Solid State Ion. 2001, 138, 183–190. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, L.; Zhang, X.; Wu, W.; Tu, B.; Cui, D.; Ou, D.; Cheng, M. High- and low-temperature behaviors of La0.6Sr0.4Co0.2Fe0.8O3−δ cathode operating under CO2/H2O-containing atmosphere. Int. J. Hydrog. Energy 2013, 38, 15361–15370. [Google Scholar] [CrossRef]
- Mueller, D.N.; Souza, R.A.D.; Weirich, T.E.; Roehrens, D.; Mayer, J.; Martin, M. A kinetic study of the decomposition of the cubic perovskite-type oxide BaxSr1−xCo0.8Fe0.2O3+δ (BSCF) (x = 0.1 and 0.5). Phys. Chem. Chem. Phys. 2010, 12, 10320–10328. [Google Scholar] [CrossRef]
- Van Der Heide, P.A.W. Systematic X-ray photoelectron spectroscopic study of La1−xSrx-based perovskite-type oxides. Surf. Interface Anal. 2002, 33, 414–425. [Google Scholar] [CrossRef]
- Frost, R.L.; Bouzaid, J.M. Raman spectroscopy of dawsonite NaAl(CO3)(OH)2. J. Raman Spectrosc. 2007, 38, 873–879. [Google Scholar] [CrossRef]
- Lee, W.; Han, J.W.; Chen, Y.; Cai, Z.; Yildiz, B. Cation Size Mismatch and Charge Interactions Drive Dopant Segregation at the Surfaces of Manganite Perovskites. J. Am. Chem. Soc. 2013, 135, 7909–7925. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.; Tuller, H.L. Investigation of surface Sr segregation in model thin film solid oxidefuel cell perovskite electrodes. Energy Environ. Sci. 2012, 5, 5370–5378. [Google Scholar] [CrossRef]
- Wei, B.; Chen, K.F.; Wang, C.C.; Lü, Z.; Jiang, S.P. Performance degradation of SmBaCo2O5+δ cathode induced by chromium deposition for solid oxide fuel cells. Electrochim. Acta 2015, 174, 327–331. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Li, P.; Li, Y.; Fu, X.; Qi, Y.; Wang, J.; Liu, Z.; Yang, H. Performance Degradation of a Double-Perovskite PrBaCo2O5+δ Cathode Operating under a CO2/H2O-Containing Atmosphere. Molecules 2024, 29, 1063. https://doi.org/10.3390/molecules29051063
Zhu L, Li P, Li Y, Fu X, Qi Y, Wang J, Liu Z, Yang H. Performance Degradation of a Double-Perovskite PrBaCo2O5+δ Cathode Operating under a CO2/H2O-Containing Atmosphere. Molecules. 2024; 29(5):1063. https://doi.org/10.3390/molecules29051063
Chicago/Turabian StyleZhu, Lin, Pengzhang Li, Yuanyuan Li, Xiaonan Fu, Yuanyuan Qi, Juntao Wang, Zaixu Liu, and Hongyan Yang. 2024. "Performance Degradation of a Double-Perovskite PrBaCo2O5+δ Cathode Operating under a CO2/H2O-Containing Atmosphere" Molecules 29, no. 5: 1063. https://doi.org/10.3390/molecules29051063
APA StyleZhu, L., Li, P., Li, Y., Fu, X., Qi, Y., Wang, J., Liu, Z., & Yang, H. (2024). Performance Degradation of a Double-Perovskite PrBaCo2O5+δ Cathode Operating under a CO2/H2O-Containing Atmosphere. Molecules, 29(5), 1063. https://doi.org/10.3390/molecules29051063







