Functionalized Linear Conjugated Polymer/TiO2 Heterojunctions for Significantly Enhancing Photocatalytic H2 Evolution
Abstract
:1. Introduction
2. Results and Discussion
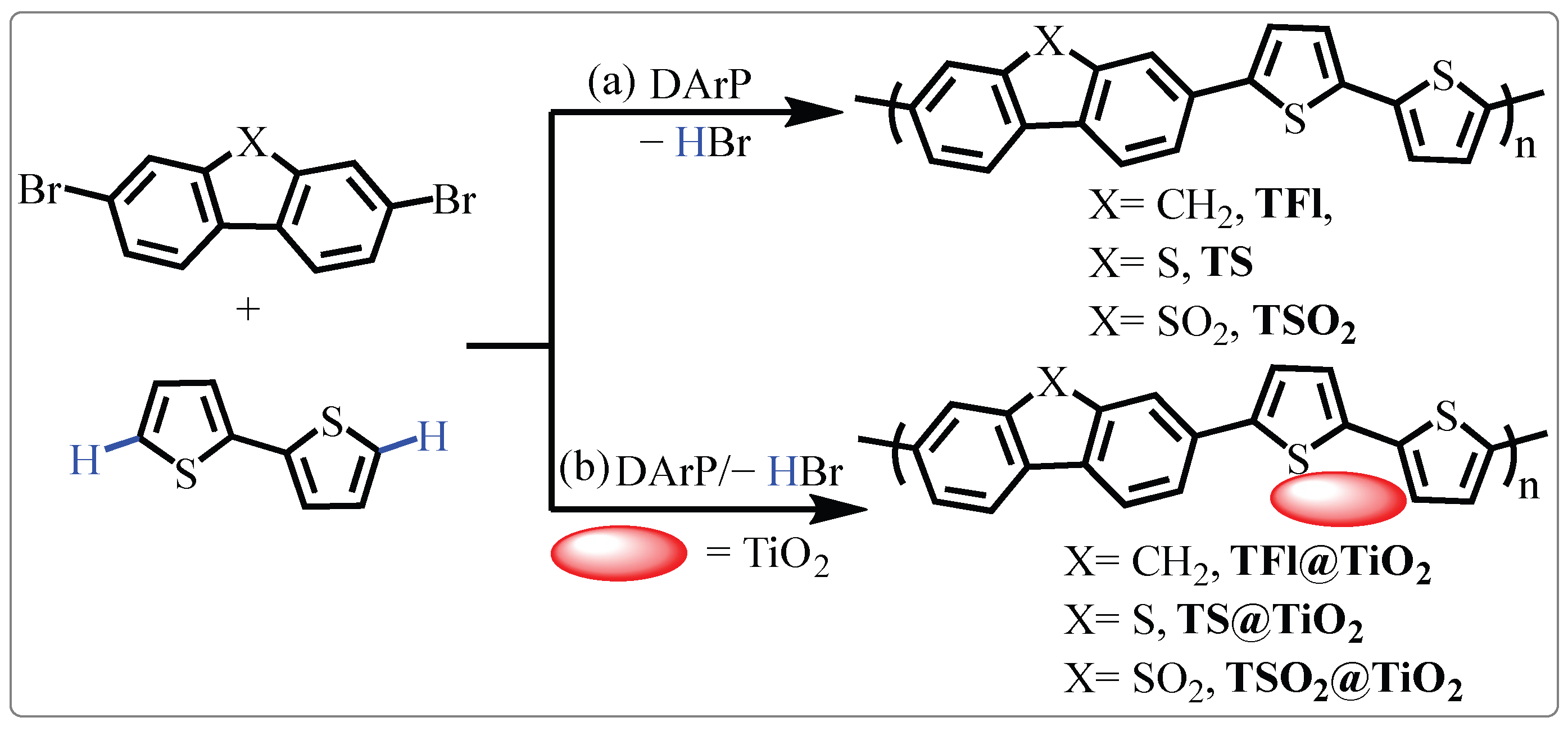
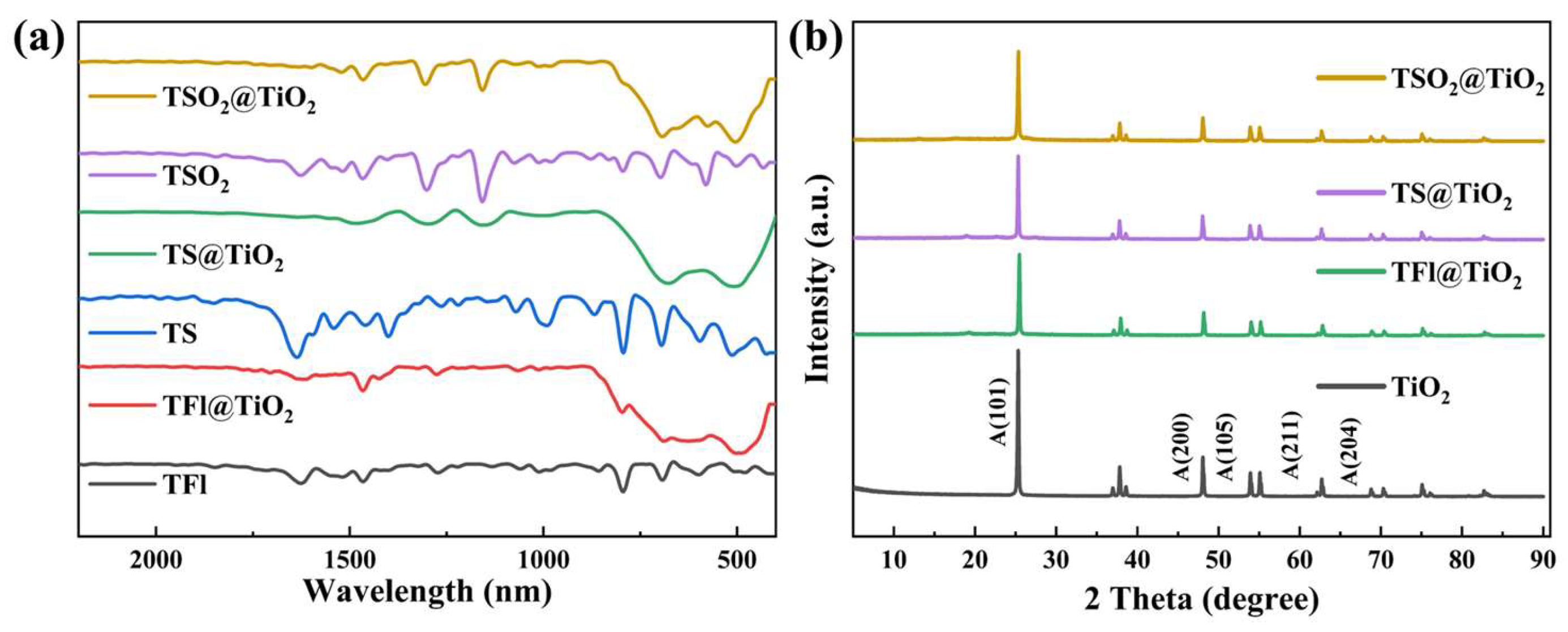
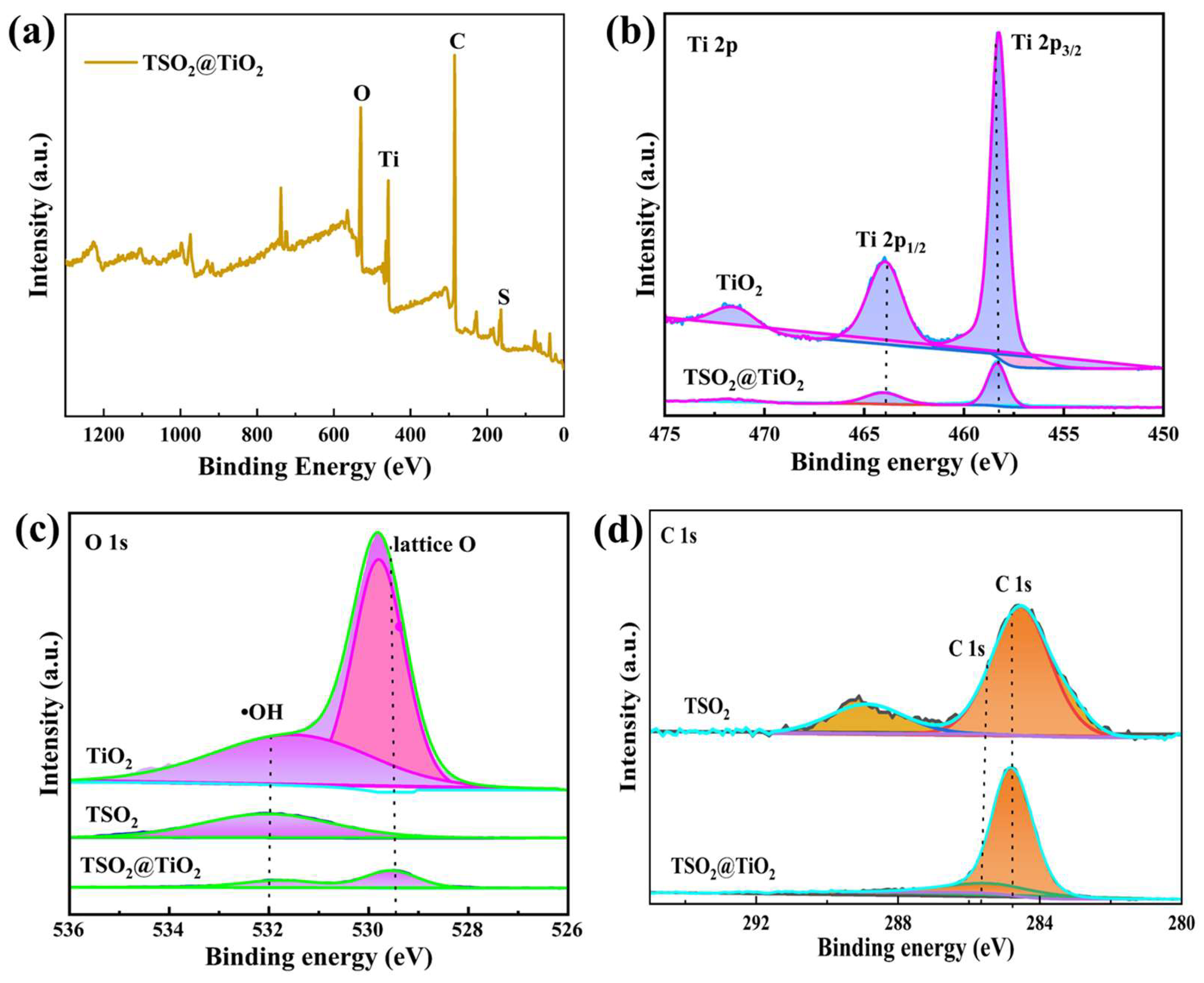
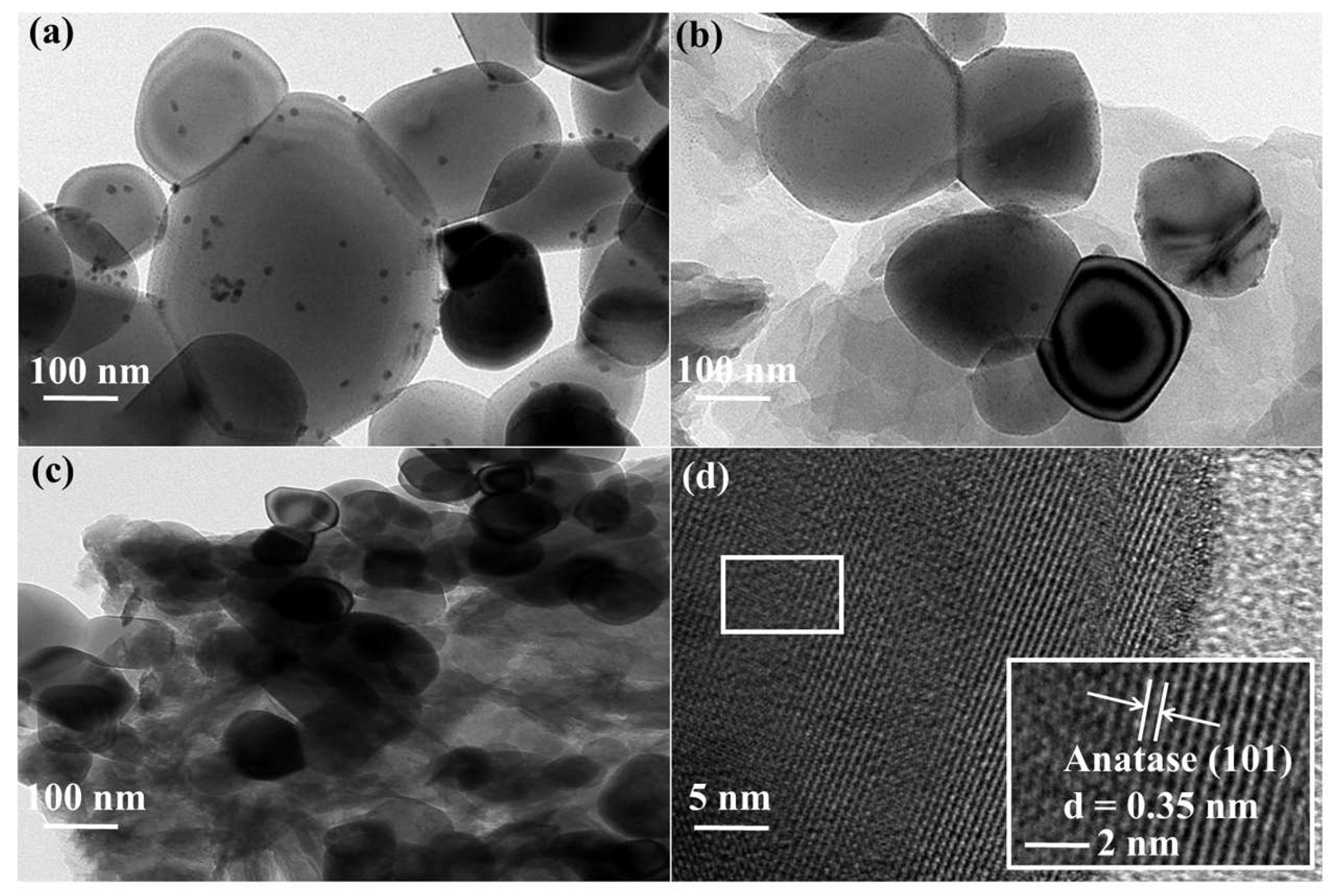
2.1. Synthesis and Characterization of the CPs and CP@TiO2 Heterojunctions
2.2. Opt-Electronic Properties
2.3. Photocatalytic Hydrogen Production Performances
3. Materials and Methods
3.1. Materials and Methods
3.2. Synthesis of 3,7-Dibromodibenzothiophene-S,S-dioxide [63]
3.3. Synthesis of TFl, TS, TSO2, TiO2, TFl@TiO2, TS@TiO2 and TSO2@TiO2
3.4. PHP Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carrillo, A.; González-Aguilar, J.; Romero, M.; Coronado, J. Solar energy on demand: A review on high temperature thermochemical heat storage systems and materials. Chem. Rev. 2019, 119, 4777–4816. [Google Scholar] [CrossRef]
- Wang, Y.O.; Suzuki, H.; Xie, J.J.; Tomita, O.; Martin, D.J.; Higashi, M.; Kong, D.; Abe, R.; Tang, J.W. Mimicking natural photosynthesis: Solar to renewable H2 fuel synthesis by z-scheme water splitting systems. Chem. Rev. 2018, 118, 5201–5241. [Google Scholar] [CrossRef]
- Xu, M.-L.; Li, D.-D.; Sun, K.; Jiao, L.; Xie, C.-F.; Ding, C.-M.; Jiang, H.-L. Interfacial microenvironment modulation boosting electron transfer between metal nanoparticles and MOFs for enhanced photocatalysis. Angew. Chem. Int. Ed. 2021, 60, 16372–16376. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Domen, K. Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting. Chem. Soc. Rev. 2019, 48, 2109–2125. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Bakbolat, B.; Daulbayev, C.; Sultanov, F.; Beissenov, R.; Umirzakov, A.; Mereke, A.; Bekbaev, A.; Chuprakov, I. Recent developments of TiO2-based photocatalysis in the hydrogen evolution and photodegradation: A review. Nanomaterials 2020, 10, 1790. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-K.; Mei, S.-B.; Jiang, H.-P.; Wang, L.-L.; Tang, H.; Liu, Q.-Q. Recent advances in TiO2-based S-scheme heterojunction photocatalysts. Chin. J. Catal. 2023, 5, 137–158. [Google Scholar] [CrossRef]
- Rafique, M.; Hajra, S.; Irshad, M.; Usman, M.; Imran, M.; Assiri, M.-A.; Ashraf, W.-M. Hydrogen production using TiO2-based photocatalysts: A comprehensive review. ACS Omega 2023, 8, 25640–25648. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.-P.; Guo, R.-T.; Bi, Z.-X.; Zhang, Z.-R.; Li, C.-F.; Pan, W.-G. Recent progress of covalent organic frameworks-based materials in photocatalytic applications: A review. Small 2023, 19, 2303632. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.-Q.; Xie, J.; Chen, X.-B.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Park, J. Visible and near infrared light active photocatalysis based on conjugated polymers. J. Ind. Eng. Chem. 2017, 51, 27–43. [Google Scholar] [CrossRef]
- Mansha, M.; Ahmad, T.; Ullah, N.; Khan, S.-A.; Ashraf, M.; Ali, S.; Tan, B.; Khan, I. Photocatalytic water-splitting by organic conjugated polymers: Opportunities and challenges. Chem. Rec. 2022, 22, e202100336. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-C.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.-M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.-H.; Chen, X.-R.; Lin, L.-H.; Fang, Y.-X.; Wang, X.-C. Biomimetic donor–acceptor motifs in conjugated polymers for promoting exciton splitting and charge separation. Angew. Chem. Int. Ed. 2018, 57, 8729–8733. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-H.; Lin, Z.-Y.; Zhang, J.; Cai, X.; Lin, W.; Yu, Z.-Y.; Wang, X.-C. Molecular-level insights on the reactive facet of carbon nitride single crystals photocatalysing overall water splitting. Nat. Catal. 2020, 3, 649–655. [Google Scholar] [CrossRef]
- Han, C.-Z.; Dong, P.-H.; Tang, H.-R.; Zheng, P.-Y.; Zhang, C.; Wang, F.; Huang, F.; Jiang, J.-X. Realizing high hydrogen evolution activity under visible light using narrow band gap organic photocatalysts. Chem. Sci. 2021, 12, 1796–1802. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.-M.; Shu, C.; Zhang, C.; Ma, W.-Y.; Ren, S.-B.; Wang, F.; Chen, Y.; Zeng, J.-H.; Jiang, J.X. Substituent effect of conjugated microporous polymers on the photocatalytic hydrogen evolution activity. J. Mater. Chem. A 2020, 8, 2404–2411. [Google Scholar] [CrossRef]
- Ru, C.-L.; Zhou, T.; Zhang, J.; Wu, X.; Sun, P.-Y.; Chen, P.-Y.; Zhou, L.; Zhao, H.; Wu, J.-C.; Pan, X.-B. Introducing secondary acceptors into conjugated polymers to improve photocatalytic hydrogen evolution. Macromolecules 2021, 54, 8839–8848. [Google Scholar] [CrossRef]
- Wang, J.-L.; Ouyang, G.-C.; Wang, D.-W.; Li, J.; Yao, J.-H.; Li, W.-S.; Li, H.-X. Enhanced photocatalytic performance of donor-acceptor-type polymers based on a thiophene-contained polycyclic aromatic Unit. Macromolecules 2021, 54, 2661–2666. [Google Scholar] [CrossRef]
- Cheng, J.-Z.; Liu, L.-L.; Liao, G.-F.; Shen, Z.-Q.; Tan, Z.-R.; Xing, Y.-Q.; Li, X.-X.; Yang, K.; Chen, L.; Liu, S.-Y. Achieving an unprecedented hydrogen evolution rate by solvent exfoliated CPP-based photocatalysts. J. Mater. Chem. A 2020, 8, 5890–5899. [Google Scholar] [CrossRef]
- Cheng, J.-Z.; Tan, Z.-R.; Xing, Y.-Q.; Shen, Z.-Q.; Zhang, Y.-J.; Liu, L.-L.; Yang, K.; Chen, L.; Liu, S.-Y. Exfoliated conjugated porous polymer nanosheets for highly efficient photocatalytic hydrogen evolution. J. Mater. Chem. A 2021, 9, 5787–5795. [Google Scholar] [CrossRef]
- Liu, M.-Y.; Huang, Q.; Wang, S.-L.; Li, Z.-Y.; Li, B.-Y.; Jin, S.-B.; Tan, B.-E. Crystalline covalent triazine frameworks by in situ oxidation of alcohols to aldehyde monomers. Angew. Chem. Int. Ed. 2018, 57, 11968–11972. [Google Scholar] [CrossRef]
- Wang, K.-W.; Yang, L.-M.; Wang, X.; Guo, L.-P.; Cheng, G.; Zhang, C.; Jin, S.-B.; Tan, B.-E.; Cooper, A. Covalent triazine frameworks via a low-temperature polycondensation approach. Angew. Chem. Int. Ed. 2017, 56, 14149–14153. [Google Scholar] [CrossRef] [PubMed]
- Meier, C.-B.; Clowes, R.; Berardo, E.; Jelfs, K.-E.; Zwijnenburg, M.-A.; Sprick, R.; Cooper, A.-I. Structurally diverse covalent triazine-based framework materials for photocatalytic hydrogen evolution from water. Chem. Mater. 2019, 31, 8830–8838. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qian, C.; Liu, J.; Zeng, Y.-F.; Wang, D.-D.; Zhou, W.-Q.; Gu, L.; Wu, H.-W.; Liu, G.-F.; Zhao, Y.-L. Integrating suitable linkage of covalent organic frameworks into covalently bridged inorganic/organic hybrids toward efficient photocatalysis. J. Am. Chem. Soc. 2020, 142, 4862–4871. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Nakada, A.; Springer, M.-A.; Kawaguchi, T.; Suzuki, K.; Kaji, H.; Baburin, I.; Kuc, A.; Heine, T.; Suzuki, H.; et al. Identification of prime factors to maximize the photocatalytic hydrogen evolution of covalent organic frameworks. J. Am. Chem. Soc. 2020, 142, 9752–9762. [Google Scholar] [CrossRef]
- Chen, W.-B.; Wang, L.; Mo, D.-Z.; He, F.; Wen, Z.-L.; Wu, X.-J.; Xu, H.-X.; Chen, L. Modulating benzothiadiazole-based covalent organic frameworks via halogenation for enhanced photocatalytic water splitting. Angew. Chem. Int. Ed. 2020, 59, 16902–16909. [Google Scholar] [CrossRef]
- Zhao, Z.-F.; Zheng, Y.-L.; Wang, C.; Zhang, S.-N.; Song, J.; Li, Y.-F.; Ma, S.-Q.; Cheng, P.; Zhang, Z.-J.; Chen, Y. Fabrication of robust covalent organic frameworks for enhanced visible-light-driven H2 evolution. ACS Catal. 2021, 11, 2098–2107. [Google Scholar] [CrossRef]
- Hu, Z.-C.; Wang, Z.-F.; Zhang, X.; Tang, H.-R.; Liu, X.-C.; Huang, F.; Cao, Y. Conjugated polymers with oligoethylene glycol side chains for improved photocatalytic hydrogen evolution. iScience 2019, 13, 33–42. [Google Scholar] [CrossRef]
- Wang, W.-H.; Ting, L.-Y.; Ayakumar, J.; Chang, J.-L.; Lin, W.-C.; Chung, C.-C.; Elsayed, M.-H.; Lu, C.-Y.; Elewa, A.-M.; Chou, H.-H. Design and synthesis of phenylphosphine oxide-based polymer photocatalysts for highly efficient visible-light-driven hydrogen evolution. Sustain. Energy Fuels 2020, 4, 5264–5270. [Google Scholar] [CrossRef]
- Lin, W.-C.; Jayakumar, J.; Chang, C.-L.; Ting, L.-Y.; Elsayed, M.-H.; Abdellah, M.; Zheng, K.-B.; Elewa, A.-M.; Lin, Y.-T.; Liu, J.-J.; et al. Effect of energy bandgap and sacrificial agents of cyclopentadithiophene-based polymers for enhanced photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2021, 298, 120577. [Google Scholar] [CrossRef]
- Tan, Z.-R.; Xing, Y.-Q.; Cheng, J.-Z.; Zhang, G.; Shen, Z.-Q.; Zhang, Y.-J.; Liao, G.; Chen, L.; Liu, S.-Y. EDOT-based conjugated polymers accessed via C-H direct arylation for efficient photocatalytic hydrogen production. Chem. Sci. 2022, 13, 1725–1733. [Google Scholar] [CrossRef]
- Zhang, G.-G.; Lan, Z.-A.; Wang, X.-C. Conjugated polymers: Catalysts for photocatalytic hydrogen evolution. Angew. Chem. Int. Ed. 2016, 55, 15712–15727. [Google Scholar] [CrossRef]
- Do, H.-H.; Nguyen, D.-L.T.; Nguyen, X.-C.; Le, T.-H.; Nguyen, T.-P.; Trinh, Q.-T.; Ahn, S.-H.; Vo, D.-V.N.; Kim, S.-Y.; Le, Q.-V. Recent progress in TiO2-based photocatalysts for hydrogen evolution reaction: A review. Arab. J. Chem. 2020, 13, 3653–3671. [Google Scholar] [CrossRef]
- Lettieri, S.; Pavone, M.; Ioravanti, A.; Amato, F.-S.; Maddalena, P. Charge carrier processes and optical properties in TiO2 and TiO2-based heterojunction photocatalysts: A review. Materials 2021, 14, 1645. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.-Q.; Wang, W.-K.; Wang, L.-L.; Tang, H.; Hu, J.; Liu, Q.-Q. Organic-inorganic heterojunction photocatalysts: From organic molecules to frameworks. Mater. Sci. Semicond. Process. 2023, 164, 107623. [Google Scholar] [CrossRef]
- Wei, G.; Niu, F.; Wang, Z.; Liu, X.; Feng, S.; Hu, K.; Gong, X.; Hua, J. Enhanced photocatalytic H2 evolution based on a polymer/TiO2 film heterojunction. Mater. Today Chem. 2022, 26, 101075. [Google Scholar] [CrossRef]
- Zhang, Y.-P.; Han, W.; Yang, Y.; Zhang, H.-Y.; Wang, Y.; Wang, L.; Sun, X.-J.; Zhang, F.-M. S-scheme heterojunction of black TiO2 and covalent-organic framework for enhanced photocatalytic hydrogen evolution. Chem. Eng. J. 2022, 446, 137213. [Google Scholar] [CrossRef]
- Xing, Y.-Q.; Tan, Z.-R.; Cheng, J.-Z.; Shen, Z.-Q.; Zhang, Y.-J.; Chen, L.; Liu, S.-Y. In situ C-H activation-derived polymer@TiO2 p-n heterojunction for photocatalytic hydrogen evolution. Sustain. Energy Fuels 2021, 5, 5166–5174. [Google Scholar] [CrossRef]
- Ye, H.-N.; Wang, Z.-Q.; Yu, F.-T.; Zhang, S.-C.; Kong, K.-Y.; Gong, X.-Q.; Hua, J.-L.; Tian, H. Fluorinated conjugated poly(benzotriazole)/g-C3N4 heterojunctions for significantly enhancing photocatalytic H2 evolution. Appl. Catal. B Environ. 2020, 267, 118577. [Google Scholar] [CrossRef]
- Shen, H.-Q.; Shang, D.-D.; Li, L.-H.; Li, D.; Shi, W.-D. Rational design of 2D/2D covalent-organic framework/TiO2 nanosheet heterojunction with boosted photocatalytic H2 evolution. Appl. Surf. Sci. 2022, 578, 152024. [Google Scholar] [CrossRef]
- Liu, S.-J.; Zou, Q.-C.; Ma, Y.; Chi, D.-J.; Chen, R.; Fang, H.-X.; Hu, W.; Zhang, K.; Chen, L.-F. Metal-organic frameworks derived TiO2/carbon nitride heterojunction photocatalyst with efficient catalytic performance under visible light. Inorg. Chem. 2022, 536, 120918. [Google Scholar] [CrossRef]
- Tatykayev, B.; Chouchene, B.; Balan, L.; Gries, T.; Medjahdi, G.; Girot, M.; Uralbekov, B.; Schneider, R. Heterostructured g-CN/TiO2 photocatalysts prepared by thermolysis of g-CN/MIL-125(Ti) composites for efficient pollutant degradation and hydrogen production. Nanomaterials 2020, 10, 1387. [Google Scholar] [CrossRef] [PubMed]
- Acharya, R.; Parida, K. A review on TiO2/g-C3N4 visible-light- responsive photocatalysts for sustainable energy generation and environmental remediation. J. Environ. Chem. Eng. 2020, 8, 103896. [Google Scholar] [CrossRef]
- Zhang, J.-L.; Yang, H.-G.; Xu, S.-B.; Yang, L.; Song, Y.-Q.; Jiang, L.; Dan, Y. Dramatic enhancement of visible light photocatalysis due to strong interaction between TiO2 and end-group functionalized P3HT. Appl. Catal. B Environ. 2015, 174–175, 193–202. [Google Scholar] [CrossRef]
- Hou, H.-J.; Zhang, X.-H.; Huang, D.-K.; Ding, X.; Wang, S.-Y.; Yang, X.-L.; Li, S.-Q.; Xiang, Y.-G.; Chen, H. Conjugated microporous poly(benzothiadiazole)/TiO2 heterojunction for visible-light-driven H2 production and pollutant removal. Appl. Catal. B Environ. 2017, 203, 563–571. [Google Scholar] [CrossRef]
- Shu, G.; Wang, Y.; Li, Y.-D.; Zhang, S.; Jiang, J.-X.; Wang, F. A high performance and low-cost poly (dibenzothiophene-S, S-dioxide) @TiO2 composite with hydrogen evolution rate up to 51.5 mmol h−1 g−1. J. Mater. Chem. A 2020, 8, 18292–18301. [Google Scholar] [CrossRef]
- Sheng, Z.Q.; Xing, Y.Q.; Chen, Y.; Zhang, G.; Liu, S.Y.; Chen, L. Nanoporous and nonporous conjugated donor-acceptor polymer semiconductors for photocatalytic hydrogen production. Beilstein J. Nanotechnol. 2021, 12, 607–623. [Google Scholar] [CrossRef]
- Zhao, Y.-H.; Sheng, J.-F.; Zhao, X.-B.; Mo, J.; Wang, J.-L.; Chen, Z.; Dong, H.-J.; Li, C.M. Recent progress in conjugated polymers-based donor–acceptor semiconductor materials for photocatalytic hydrogen evolution from water splitting. Catalysts 2023, 13, 850. [Google Scholar] [CrossRef]
- Yang, C.; Cheng, B.; Xu, J.-S.; Yu, J.-G.; Cao, S.-W. Donor-acceptor-based conjugated polymers for photocatalytic energy conversion. EnergyChem 2023, 29, 100116. [Google Scholar] [CrossRef]
- Pati, P.-B.; Damas, G.; Tian, L.; Fernandes, D.-L.A.; Zhang, L.; Pehlivan, I.-B.; Edvinsson, T.; Araujo, C.-M.; Tian, H.-N. An experimental and theoretical study of an efficient polymer nano-photocatalyst for hydrogen evolution. Energy Environ. Sci. 2017, 10, 1372–1376. [Google Scholar] [CrossRef]
- Reyes, Y.I.A.; Ting, L.-Y.; Tu, X.; Chen, H.-Y.T.; Chou, H.-H.; Coluccini, C. Mechanistic studies of hydrogen evolution reaction on donor-acceptor conjugated polymer photocatalysts. Appl. Sci. 2020, 10, 7017. [Google Scholar] [CrossRef]
- Brédas, J.-L.; Beljonne, D.; Coropceanu, V.; Cornil, J. Charge-transfer and energy-transfer processes in π-conjugated oligomers and polymers: A molecular picture. Chem. Rev. 2004, 104, 4971–5004. [Google Scholar] [CrossRef]
- Xiang, Y.-G.; Wang, X.-P.; Zhang, X.-H.; Hou, H.-J.; Dai, K.; Huang, Q.-Y.; Chen, H. Enhanced visible light photocatalytic activity of TiO2 assisted by organic semiconductors: A structure optimization strategy of conjugated polymers. J. Mater. Chem. A 2018, 6, 153–159. [Google Scholar] [CrossRef]
- Chen, B.; Wang, X.-P.; Dong, W.-B.; Zhang, X.-H.; Rao, L.; Chen, H.; Huang, D.-K.; Xiang, Y.-G. Enhanced light-driven hydrogen-production activity induced by accelerated interfacial charge transfer in donor–acceptor conjugated polymers/TiO2 hybrid. Chem. Eur. J. 2019, 25, 3362–3368. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-Y.; Shen, Z.-Q.; Cheng, J.-Z.; Liu, L.-L.; Yang, K.; Wen, H.; Liu, S.-Y. C-H activation derived CPPs for photocatalytic hydrogen production excellently accelerated by a DMF cosolvent. J. Mater. Chem. A 2019, 7, 24222–24229. [Google Scholar] [CrossRef]
- Wu, Y.; He, X.; Huang, X.-M.; Yang, L.-J.; Liu, P.; Chen, N.; Li, C.-Z.; Liu, S.-Y. Synthesis of Long-Chain Oligomeric Donor and Acceptors via Direct Arylation for Organic Solar Cells. Chin. J. Chem. 2024, 42, 523–532. [Google Scholar] [CrossRef]
- Yu, F.-T.; Wang, Z.-Q.; Zhang, S.-C.; Ye, H.-N.; Kong, K.-Y.; Gong, X.-Q.; Hua, J.-L.; Tian, H. Molecular engineering of donor–acceptor conjugated polymer/g-C3N4 heterostructures for significantly enhanced hydrogen evolution under visible-light irradiation. Adv. Funct. Mater. 2018, 28, 1804512. [Google Scholar] [CrossRef]
- Guo, Y.-X.; Sun, J.-Y.; Guo, T.; Liu, Y.; Yao, Z.-Y. Emerging Light-Harvesting Materials Based on Organic Photovoltaic D/A Heterojunctions for Efficient Photocatalytic Water Splitting. Angew. Chem. Int. Ed. 2024, 19, e202319664. [Google Scholar]
- Hu, N.-N.; Cai, Y.-L.; Li, L.; Wang, X.-S.; Gao, J.-K. Amino-functionalized titanium-based metal-organic framework for photocatalytic hydrogen production. Molecules 2022, 27, 4241. [Google Scholar] [CrossRef]
- Ye, D.-N.; Liu, L.; Peng, Q.-M.; Qiu, J.-B.; Gong, H.; Zhong, A.-G.; Liu, S.-Y. Effect of Controlling Thiophene Rings on D-A Polymer Photocatalysts Accessed via Direct Arylation for Hydrogen Production. Molecules 2023, 28, 4507. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.-N.; Liu, L.; Zhang, Y.-J.; Qiu, J.-B.; Tan, Z.-R.; Xing, Y.-Q.; Liu, S.-Y. Tunable donor-acceptor linear conjugated polymers involving cyanostyrylthiophene linkages for visible-light-driven hydrogen production. Molecules 2023, 28, 2203. [Google Scholar] [CrossRef] [PubMed]
- Han, C.-Z.; Xiang, S.-H.; Jin, S.-L.; Zhang, C.; Jiang, J.-X. Rational design of conjugated microporous polymer photocatalysts with definite D−π—A structures for ultrahigh photocatalytic hydrogen evolution activity under natural sunlight. ACS Catal. 2023, 13, 204–212. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, H.; Xing, Y.; Li, J.; Liu, S. Functionalized Linear Conjugated Polymer/TiO2 Heterojunctions for Significantly Enhancing Photocatalytic H2 Evolution. Molecules 2024, 29, 1103. https://doi.org/10.3390/molecules29051103
Gong H, Xing Y, Li J, Liu S. Functionalized Linear Conjugated Polymer/TiO2 Heterojunctions for Significantly Enhancing Photocatalytic H2 Evolution. Molecules. 2024; 29(5):1103. https://doi.org/10.3390/molecules29051103
Chicago/Turabian StyleGong, Hao, Yuqin Xing, Jinhua Li, and Shiyong Liu. 2024. "Functionalized Linear Conjugated Polymer/TiO2 Heterojunctions for Significantly Enhancing Photocatalytic H2 Evolution" Molecules 29, no. 5: 1103. https://doi.org/10.3390/molecules29051103





