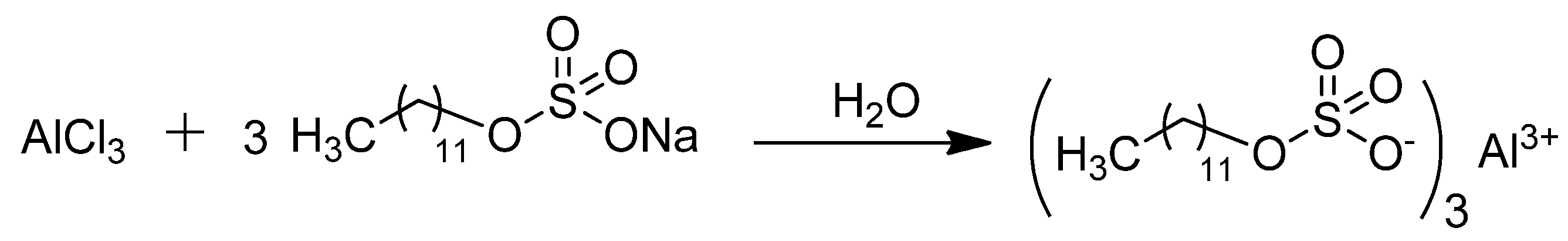Abstract
The current study describes a novel and eco-conscious method to synthesize 1,4-dihydropyridine derivatives utilizing an aqueous micellar solution containing aluminum dodecyl sulfate, Al(DS)3, using readily available starting material. The final products were synthesized with excellent yields within remarkably quick reaction durations, promoting remarkable atom economy and minimizing environmental impacts. The present protocol has several advantages over other methodologies in terms of high yield (up to 97%) with excellent purity. Further, the synthesized 1,4-DHPs exhibit favorable to excellent resistance against examined bacterial and fungal species. Intriguingly, polar groups on the phenyl ring (5b, 5c, 5i and 5j) make the 1,4-DHPs equally potent against the microbes as compared to the standard drugs.
1. Introduction
Researchers have long been intrigued by the biological properties of 1,4-dihydropyridines (1,4-DHPs). 1,4-DHPs constitute a significant category within the N-heterocyclic ring, consisting of a biologically vital nucleus that exhibits various important pharmacological properties and is used as an antibacterial, anticancer [1], anticoagulant, antileishmanial, anticonvulsant, antitubercular [2], antioxidant [3], antiulcer, antifertility, neuroprotection properties, antimalarials, HIV-1 protease inhibitor, antihypertensive [4], anti-atherosclerotic, hepato-protective, vasodilator, anti-mutagenic, bronchodilator, geroprotective, anti-tumor [5], and anti-diabetic agent [6]. Moreover, they constitute an essential category of agents that modulate calcium channels and have been widely employed in the treatment of cardiovascular disorders, exhibiting functions such as antihypertensive, antianginal, vasodilator, and cardiac depressant activities [7]. 1,4-DHP attracts more attention, due to its bioactivity and presence in coenzymes, diphosphate pyridine nucleotide (DPNH). In the pharmaceutical field, DHPs are already commercialized as amlodipine, felodipine, isradipine [8], lacidipine [9], nicardipine [10], nitrendipine [11], nifedipine, and nemadipine B [12], among them, nitrendipine and nemadipine B stand out for their potent calcium channel blocking activities.
In general, 1,4-DHPs are synthesized using multicomponent reactions, where a combination of β-keto-ester, aldehyde, as well as ammonia undergoes reaction to produce the required DHPs. Arthur Hantzsch described the first one-pot formation of symmetrically substituted 1,4-DHP in the year 1882 [13]. The Hantzsch reaction proved to be the most widely used protocol to access 1,4-DHPs and its numerous biologically active derivatives [14]. But due to drawbacks such as harsh reaction conditions, long reaction time, and generally low yield of products, new modifications were made to it to obtain the targeted compounds,1,4-DHPs, in good to excellent yields [15]. It has been reported that in multicomponent reactions (MCRs), a wide variety of catalysts has been studied for the production of 1,4-DHP derivatives, encompassing various Lewis or Bronsted acids such as Y(OTf)3 [16], free nano-Fe2O3 [17], TMU-33 [18], InCl3 [19]; heterogeneous catalyst such as HClO4–SiO2 [20], PdRuNi@GO NPs [7], zeolite [21], IRMOF-3 [22]; organ catalyst, CAN [23], Montmorillonite K10 clay [24], HClO4-SiO2 [25], tetrabutylammonium hydrogen sulfate, sodium- and Cs-Norit carbons, fermenting Baker’s yeast [26], metal triflates with the incorporation of numerous methodologies such as stirring [2], conventional heating [27], refluxing [22], microwave [1], visible light [4], and ultrasound irradiations [21]. Researchers are now focusing on the green aspects of this reaction leading to the usage of various green solvents such as glycerol [18], water [19], and ionic liquids [6]. Unless otherwise stated, aqueous surfactant solutions, as well as neat conditions, have also been incorporated to obtain the required products.
Persisting on our work, in the present work, we describe the synthesis of novel 1,4-DHP scaffolds using a one-pot method using an aqueous micellar solution as a greener solvent. The microwave-assisted reaction method stands out as superior due to its rapid reaction times, cost-effectiveness, and straightforward operational procedures compared to other methods. To begin our investigation into a sustainable and effective approach for synthesizing 1,4-DHPs, we commenced by employing a catalytic quantity of Al(DS)3 with a combination of substituted benzaldehyde, diethyl acetylene dicarboxylate, and either ammonium acetate or aniline, along with malononitrile under microwave irradiations at ambient temperature.
2. Result and Discussion
2.1. Characterization of Aluminum Dodecyl Sulfate, Al(DS)3
2.1.1. SEM Characterization
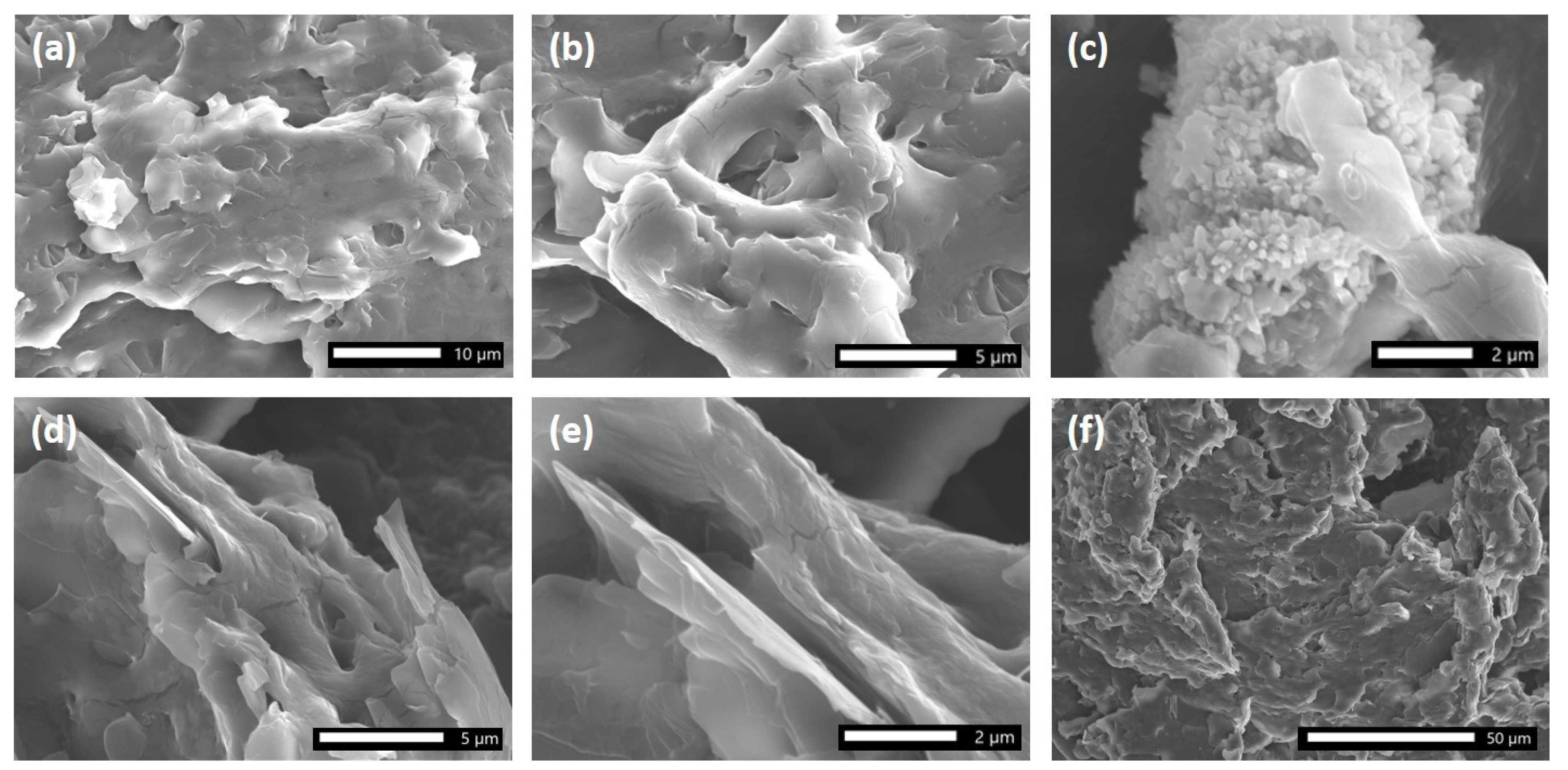
The SEM images depicted in Figure 1a–f, magnified from 500 to 7500 times, provide valuable insights into the morphology of the Al(DS)3 surfactant. This compound was produced as dense aggregates (Figure 1a) which are irregular in shape. At higher magnifications, it becomes evident that randomly organized microplates arranged to compose the micro-objects are in an edge-to-face style (Figure 1a–d).
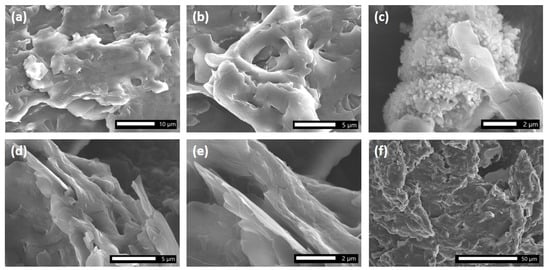
Figure 1.
SEM images of Al(DS)3.
Based on the elemental investigation conducted on microscopic sectors of Al(DS)3 using EDS (as shown in Figure S1), the atomic percentages of Al and S were determined to be 22.40% and 77.60%, respectively, which indicates a 1:3 stoichiometry.
2.1.2. XRD Characterization
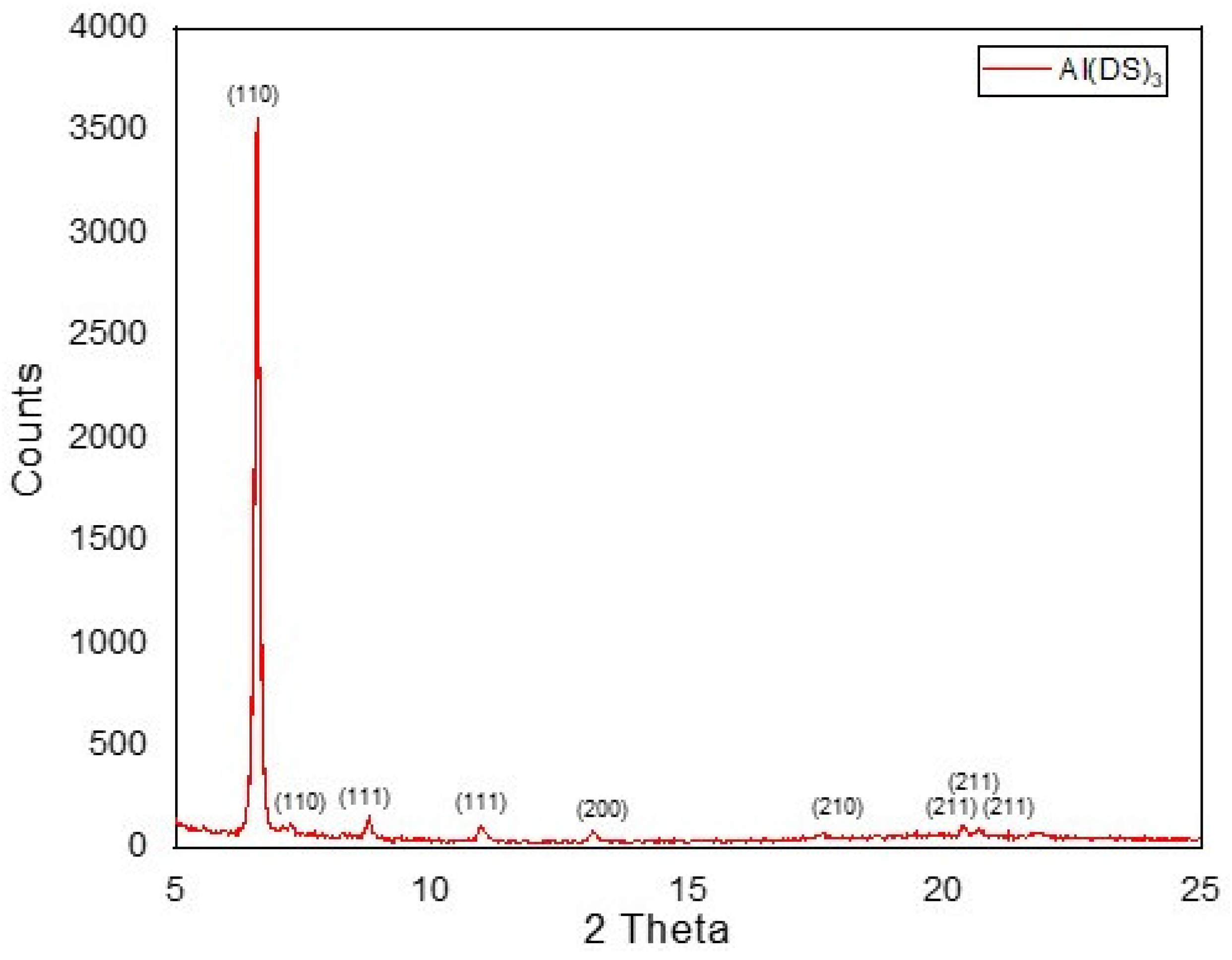
The XRD patterns in the range of 2θ = 5–25° for the Al(DS)3 sample are shown in Figure 2. The prepared sample of Al(DS)3 gave rise to XRD peaks at 2θ = 6.596, 7.184, 10.965, 13.166, 17.587, 20.387, 20.674, and 21.875, corresponding to (110), (110), (111), (111), (200), (210), (211), (211), and (211) diffraction peaks, respectively. According to the above data, the XRD patterns of Al(DS)3 show a prominent diffraction peak at (110) with preferential orientation around 2θ = 6.596° with 100% relative intensity, which shows a reasonable degree of crystallinity of 65.3%.
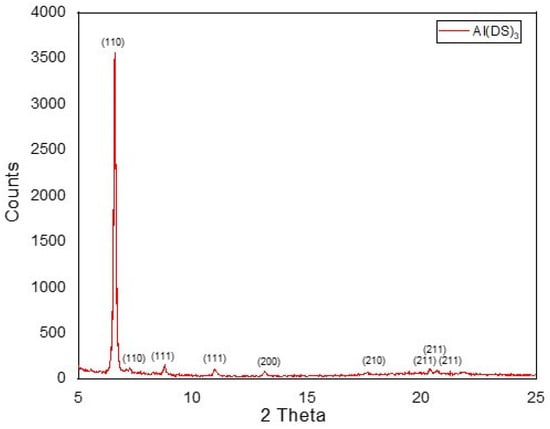
Figure 2.
XRD images of Al(DS)3.
2.2. Preparation of [1,4-DHPs] 10-Amino-3,3,6,6-Tetramethyl-9-Aryl-3,4,6,7,9,10-Hexa-Hydroacridine-1,8(2H,5H)-Dione Derivatives (5a–n) Using Al(DS)3
Various solvents, including water, ethanol, ethanolic solution of p-TSA, glycerol, aqueous solution of SDS, and aqueous solution Al(DS)3, were employed for the condensation of benzaldehyde 1, dialkyl acetylene dicarboxylate 2, ammonium acetate 3, and malononitrile 4. Among these, water as a solvent proved to be the most effective, as indicated in Table 1. The maximum yield was achieved through an Al(DS)3 + water catalyst system.

Table 1.
Impact of solvents on production of the 5a–h.
Table 2 illustrates the optimal temperature for a reaction carried out in an aqueous micellar solution and it was also observed that increasing the temperature has a significant increase in yield with time. Conversely, further temperature increases, result in a decrease in product yield as the products start decomposing at higher temperatures.

Table 2.
Impact of pressure on the percentage yield of 5a–h using Al(DS)3 in water.
Similarly, various other aldehydes 3b–g will undergo reactions with diethyl acetylene dicarboxylate, malononitrile, and either ammonium acetate or aniline using the same procedure, with the formation monitored by TLC and melting point analysis. The reactions proceed smoothly even in the company of various electron-donating (-Me and -OMe) as well as electron-withdrawing substituents (-NO2) on the aldehyde, facilitating efficient reactions. The effectiveness of this method is notable, yielding high percentages (92–97%) for the final products.
2.3. Characterization of Diethyl 6-Amino-5-Cyano-4-Phenyl-1,4-Dihydropyridine-2,3-Dicarboxylate (5a) Using Al(DS)3
The structural elucidation of the novel scaffold is accomplished by using various spectroscopic methods such as FTIR, 1H NMR, 13C NMR, Mass, and elemental analysis. In the series, the IR spectra of compound 5a reveal three significant absorptions at 3360.7, 2250.6, and 1740.5 cm−1 corresponding to the N-H, C≡N, and C=O groups stretching. In 1H NMR spectra (500 MHz, CDCl3) of compound 5a, three singulets at δ 11.94, 6.59, and 4.54 were observed for NH, NH2, and CH, respectively, confirming the formation of 1,4-DHP. The multiplet for 5 protons was at δ 7.13–7.27 for the phenyl ring attached at the C-4 position. One quadruplet and one triplet at δ 4.17–4.13 and 0.90–0.91 were observed for CH2 and CH3 protons of ester linkage.
In 13C, NMR spectra of molecule 5a, most down-shielded peaks at δ 190.26 and 189.73 ppm were observed for the C=O group. Peaks at δ 160.57, 157.91, 154.74, 153.24, and 54.77 ppm were observed for C-2, C-6, C-3, C-5, and C-4 carbon atoms of the main 1,4-DHP skeleton, whereas carbon atoms belonging to the aromatic region were observed at δ 136.15 (C-1′), 135.43 (C-2′ and C-6′), 128.34 (C-3′ and C-5′), and 120.71 (C-4′) ppm. The peaks for C≡N and other sp3 carbons were observed at the appropriate position. Furthermore, ESI-MS fragmentation Pattern and Elemental analysis also support the formation of final 1,4-DHP derivatives. The spectral data of compound 5a strongly corroborates the assigned structure.
2.4. Anti-Microbial Activity
The novel synthesized compounds 5a–n underwent testing against two Gram-positive bacteria (Streptococcus pyogenes MTCC 442, and Bacillus subtilis MTCC 441), three Gram-negative bacteria (Klebsiella pneumonia MTCC 3384, Escherichia coli MTCC 443, and Staphylococcus aureus MTCC 96), as well as three fungal strains (Aspergillus niger MTCC 281, Aspergillus janus MTCC 2751, and Aspergillus sclerotiorum MTCC 1008). Fungal strains were cultivated in malt extract medium prior to inoculation for a duration of 72 h at 28 °C, whereas bacterial samples were grown in nutrient broth for 24 h at 37 °C. Each synthesized chemical underwent triplicate testing after being dissolved in DMSO at concentrations of 2, 4, 8, 16, 32, 64, and 128 g/mL using a serial dilution procedure.
3. Experimental Section
3.1. Materials and Methods
3.1.1. Chemicals
All chemicals used in this study were obtained from Sigma-Aldrich (St. Louis, MO, USA) which were employed without additional distillation, while the solvents were ordered from Loba Chemie (Mumbai, India).
3.1.2. Analytical Instruments
The digital melting point apparatus was employed to measure the melting point of all the resulting products via the open capillary method. IR spectra of the targeted compound were taken using ATR mode on Perkin Elmer (Waltham, MA, USA) Spectrum II. NMR such as 1H and 13C are collected on a Bruker (Billerica, MA, USA) Avance NEO 500 MHz NMR spectrometer using DMSO as solvent. Chemical shifts (δ) are accounted for in ppm relative to that of TMS as an internal standard. The mass spectroscopy was recorded on LC-MS Spectrometer Model Q-ToF Micromass Thermo Scientific (Waltham, MA, USA) (FLASH 2000) CHN Elemental Analyser is used for fundamental analysis. The thin layer chromatographic (TLC) technique was used to observe the reaction time as well as to check the purity of the compound, and then the visualization of TLC was performed with the help of a UV chamber. XRD patterns of the dried (lyophilized) samples were captured at room temperature using a Bruker D8 advance. The compounds were exposed to monochromatic Cu-Kα radiation (λ = 1.5418 Å, 50 kV, 40 mA) across the 2θ range between <1 and >150°, with steps of 0.02°. SEM micrographs were obtained utilizing a JSM IT500 scanning electron microscope. Elemental analysis on microscopic sections of the Al(DS)3 sample was conducted via EDS. SEM images were acquired under high vacuum mode, ranging from 30 nm (30 kV) to 15.0 nm (1.0 kV).
3.2. Synthesis of Aluminum Dodecyl Sulfate
The preparation of aluminum dodecyl sulfate was conducted following the procedure outlined in the literature (Scheme 1) [28]. Anhydrous AlCl3 (0.1 mol) and sodium dodecyl sulfate (SDS) (0.3 mol) were dissolved using the minimum amount of water required. The solutions were gradually diluted with continuous stirring at room temperature, resulting in the precipitation of a colorless solid. This solid was filtered to isolate the solid Al(DS)3.
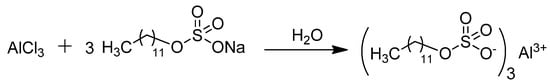
Scheme 1.
Preparation of Al(DS)3 in water.
3.3. Synthesis of 1,4-DHPs Derivatives (5a–n)
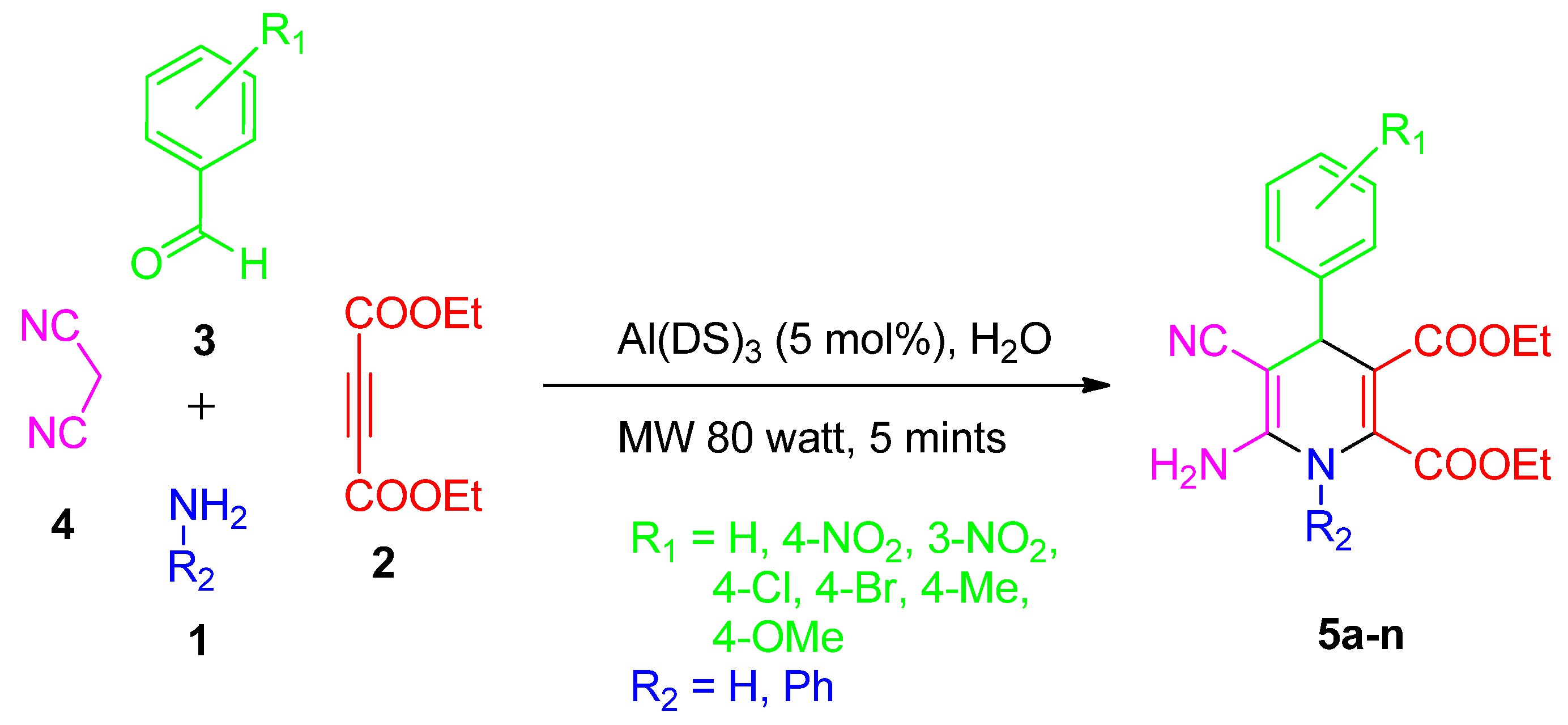
Using Al(DS)3 catalyst (5 mole%), a mixture composed of substituted benzaldehyde (5 mmol), diethyl acetylene dicarboxylate (5 mmol), and either ammonium acetate or aniline (5 mmol), along with malononitrile (5 mmol), was subjected to MW irradiation at 80 watts for 5 min in H2O (Scheme 2). The reaction’s progress was monitored using TLC (Merck, Darmstadt, Germany) (EtOAc: Toluene; 8:2). After determining that the reaction had concluded, the mixture was cooled to ambient temperature, filtered, rinsed with water, and subsequently subjected to extraction using ethyl acetate. Subsequently, the resulting solid was recrystallized using ethyl alcohol to yield colorless crystals with an efficiency of 93–97%.
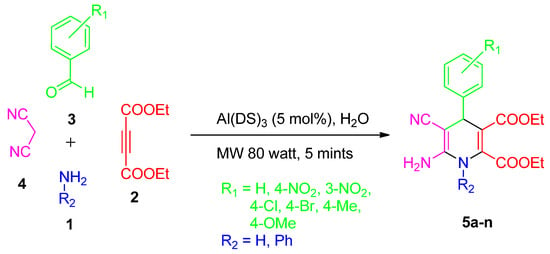
Scheme 2.
Synthesis of 1,4-DHPs using Al(DS)3 in water under microwave radiations.
By the optimized reaction conditions, a diversity of 1,4-DHP was prepared using various substituted aldehydes in aqueous micellar solution under MW irradiations at 80 watts for 5 min (Table 3).

Table 3.
1,4-DHP derivatives 5a–n synthesis using Al(DS)3 + water for 5 mints under MW radiations.
diethyl 6-amino-5-cyano-4-phenyl-1,4-dihydropyridine-2,3-dicarboxylate (5a): Yield 97%, colorless crystals, mp 227–228 °C. IR spectrum, ν, cm−1: 3360.7 (N-H stretch, -NH2), 2250.6 (C≡N), 1740.5 (C=O stretch). 1H NMR spectrum, δ, ppm (J, Hz): 11.94 (s, 1H, NH), 7.13–7.27 (m, 5H, Ar-H), 4.54 (s, 1H, CH), 6.59 (s, 1H, NH2), 4.17–4.13 (q, 4H, CH2), 0.90–0.91 (t, 6H, CH3). 13C NMR spectrum, δ, ppm: 190.26, 189.73, 160.57, 157.91, 154.74, 153.24, 136.47, 135.43, 128.34, 120.71, 113.47, 54.77, 57.95, 57.96, 13.72, 13.51. Mass spectrum, m/z (Irel, %): 342.138 (M + 1), Found: 342.136. Anal. calcd. for C18H19N3O4: C, 63.33; H, 5.61; N, 12.31%.
diethyl 6-amino-5-cyano-4-(4-nitrophenyl)-1,4-dihydropyridine-2,3-dicarboxylate (5b): Yield 96%, colorless crystals, mp 240 °C. IR spectrum, ν, cm−1: 3361.1 (N-H stretch, -NH2), 2251.3 (C≡N), 1740.7 (C=O stretch). 1H NMR spectrum, δ, ppm (J, Hz): 12.23 (s, 1H, NH), 7.66–7.97 (m, 4H, Ar-H), 4.46 (s, 1H, CH), 6.89 (s, 1H, NH2), 4.16–4.12 (q, 4H, CH2), 1.19–1.20 (t, 6H, CH3). 13C NMR spectrum, δ, ppm: 190.52, 190.07, 160.85, 158.23, 155.07, 153.57, 136.77, 135.78, 128.63, 121.03, 113.75, 55.03, 58.28, 58.27, 14.03, 13.86. Mass spectrum, m/z (Irel, %): 387.123 (M + 1), Found: 387.122. Anal. calcd. for C18H18N4O6: C, 55.96; H, 4.70; N, 14.50%.
diethyl 6-amino-5-cyano-4-(3-nitrophenyl)-1,4-dihydropyridine-2,3-dicarboxylate (5c): Yield 94%, colorless crystals, mp 242 °C. IR spectrum, ν, cm−1: 3361.3 (N-H stretch, -NH2), 2251.6 (C≡N), 1741.1 (C=O stretch). 1H NMR spectrum, δ, ppm (J, Hz): 12.10 (s, 1H, NH), 7.58–8.12 (m, 4H, Ar-H), 4.81 (s, 1H, CH), 6.85 (s, 1H, NH2), 4.49–4.53 (q, 4H, CH2), 1.14–1.15 (t, 6H, CH3). 13C NMR spectrum, δ, ppm: 190.41, 189.99, 160.74, 158.17, 154.93, 153.42, 136.64, 135.63, 128.55, 120.97, 113.61, 58.11, 58.10, 54.92, 13.94, 13.74. Mass spectrum, m/z (Irel, %): 387.123 (M + 1), Found: 387.122. Anal. calcd. for C18H18N4O6: C, 55.96; H, 4.70; N, 14.50%.
diethyl 6-amino-4-(4-chlorophenyl)-5-cyano-1,4-dihydropyridine-2,3-dicarboxylate (5d): Yield 94%, colorless crystals, mp 237–239 °C. IR spectrum, ν, cm−1:3361.6 (N-H stretch, -NH2), 2251.7 (C≡N), 1741.5 (C=O stretch). 1H NMR spectrum, δ, ppm (J, Hz): 12.31 (s, 1H, NH), 7.60–7.87 (m, 4H, Ar-H), 4.76 (s, 1H, CH), 6.93 (s, 1H, NH2), 4.09–4.13 (q, 4H, CH2), 1.16–1.17 (t, 6H, CH3). 13C NMR spectrum, δ, ppm: 190.68, 190.18, 160.96, 158.35, 155.09, 153.65, 136.91, 135.95, 128.81, 121.18, 113.97, 58.35, 58.37, 55.14, 14.26, 14.04. Mass spectrum, m/z (Irel, %): 376.099 (M + 1);377.099 (M + 2), Found: 376.098(M + 1); 377.098 (M + 2). Anal. calcd. for C18H18N3BrO4: C, 57.53; H, 4.83; N, 11.18%.
diethyl 6-amino-4-(4-bromophenyl)-5-cyano-1,4-dihydropyridine-2,3-dicarboxylate (5e): Yield 95%, colorless crystals, mp 267–268 °C. IR spectrum, ν, cm−1: 3361.9 (N-H stretch, -NH2), 2251.9 (C≡N), 1741.6 (C=O stretch). 1H NMR spectrum, δ, ppm (J, Hz): 12.27 (s, 1H, NH), 7.52–7.73 (m, 4H, Ar-H), 4.85 (s, 1H, CH), 6.90 (s, 1H, NH2), 4.51–4.54 (q, 4H, CH2), 1.13–1.12 (t, 6H, CH3). 13C NMR spectrum, δ, ppm: 190.62, 190.05, 160.91, 158.30, 155.01, 153.61, 136.73, 135.72, 128.58, 121.11, 113.79, 58.31, 58.32, 55.11, 14.20, 13.96. Mass spectrum, m/z (Irel, %): 420.048 (M + 1); 421.048 (M + 2), Found: 420.047; 421.047 (M + 2). Anal. calcd. for C18H18N3ClO4: C, 51.44; H, 4.32; N, 10.00%.
diethyl 6-amino-5-cyano-4-p-tolyl-1,4-dihydropyridine-2,3-dicarboxylate (5f): Yield 93%, colorless crystals, mp 230–231 °C. IR spectrum, ν, cm−1: 3361.0 (N-H stretch, -NH2), 2250.9 (C≡N), 1740.9 (C=O stretch). 1H NMR spectrum, δ, ppm (J, Hz): 12.15 (s, 1H, NH), 7.09–7.41 (m, 4H, Ar-H), 4.87 (s, 1H, CH), 6.87 (s, 1H, NH2), 4.50–4.52 (q, 4H, CH2), 2.15 (s, 3H, CH3), 0.89–0.91 (t, 6H, CH3). 13C NMR spectrum, δ, ppm: 190.33, 189.78, 160.79, 158.09, 154.87, 153.48, 136.51, 135.57, 128.42, 120.83, 113.56, 58.01, 58.03, 54.96, 13.95, 13.71. Mass spectrum, m/z (Irel, %): 356.153 (M + 1), Found: 356.151. Anal. calcd. for C19H21N3O4: C, 64.21; H, 5.96; N, 11.82%.
diethyl 6-amino-5-cyano-4-(4-methoxyphenyl)-1,4-dihydropyridine-2,3-dicarboxylate (5g): Yield 94%, colorless crystals, mp 238–240 °C. IR spectrum, ν, cm−1: 3360.9 (N-H stretch, -NH2), 2250.8 (C≡N), 1741.3 (C=O stretch). 1H NMR spectrum, δ, ppm (J, Hz): 12.01 (s, 1H, NH), 7.19–7.49 (m, 4H, Ar-H), 4.79 (s, 1H, CH), 6.67 (s, 1H, NH2), 4.07–4.09 (q, 4H, CH2), 3.89 (s, 3H, OCH3), 1.11–1.13 (t, 6H, CH3). 13C NMR spectrum, δ, ppm: 190.09, 189.63, 160.47, 157.83, 154.65, 153.13, 136.38, 135.32, 128.28, 120.64, 113.39, 57.83, 57.81, 54.65, 13.66, 13.50. Mass spectrum, m/z (Irel, %): 372.148 (M + 1) Found: 372.146. Anal. calcd. for C19H21N3O5: C, 61.45; H, 5.70; N, 11.31%.
diethyl 6-amino-5-cyano-1,4-diphenyl-1,4-dihydropyridine-2,3-dicarboxylate (5h): Yield 96%, colorless crystals, mp 170–172 °C. IR spectrum, ν, cm−1: 3362.3 (N-H stretch, -NH2), 2252.5 (C≡N), 1742.1 (C=O stretch). 1H NMR spectrum, δ, ppm (J, Hz):6.85–8.12 (m, 10H, Ar-H), 4.58 (s, 1H, CH), 6.95 (s, 1H, NH2), 4.40–4.47 (q, 4H, CH2),2.13–2.14 (t, 6H, CH3). 13C NMR spectrum, δ, ppm: 190.49, 189.99, 160.93, 158.33, 155.12, 153.65, 137.20, 136.87, 136.24, 135.83, 129.15, 128.74, 121.23,121.13, 113.81, 58.36, 58.35, 54.17, 14.12, 13.92. Mass spectrum, m/z (Irel, %): 417.169 (M + 1) Found: 417.167. Anal. calcd. for C24H23N3O4: C, 69.05; H, 5.55; N, 10.07%.
diethyl 6-amino-5-cyano-4-(4-nitrophenyl)-1-phenyl-1,4-dihydropyridine-2,3-dicarboxylate (5i): Yield 94%, colorless crystals, mp 171–173 °C. IR spectrum, ν, cm−1: 3362.6 (N-H stretch, -NH2), 2252.8 (C≡N), 1742.5 (C=O stretch). 1H NMR spectrum, δ, ppm (J, Hz): 7.33–8.63 (m, 9H, Ar-H), 4.51 (s, 1H, CH), 7.31 (s, 1H, NH2), 4.46–4.50 (q, 4H, CH2), 2.15–2.16 (t, 6H, CH3). 13C NMR spectrum, δ, ppm: 190.96, 190.47, 161.24, 158.61, 155.48, 153.94, 137.47, 137.17, 136.51, 136.17, 129.31, 129.03, 121.55,121.45, 114.12, 58.68, 58.67, 55.43, 14.43, 14.27. Mass spectrum, m/z (Irel, %): 462.154 (M + 1) Found: 462.153. Anal. calcd. for C24H22N4O6: C, 62.33; H, 4.79; N, 12.12%.
diethyl 6-amino-5-cyano-4-(3-nitrophenyl)-1-phenyl-1,4-dihydropyridine-2,3-dicarboxylate (5j): Yield 95%, colorless crystals, mp 270–272 °C. IR spectrum, ν, cm−1: 3362.8 (N-H stretch, -NH2), 2252.9 (C≡N), 1742.7 (C=O stretch). 1H NMR spectrum, δ, ppm (J, Hz): 7.27–8.15 (m, 9H, Ar-H), 4.92 (s, 1H, CH), 7.27 (s, 1H, NH2), 4.47–4.51 (q, 4H, CH2), 1.52–1.53 (t, 6H, CH3). 13C NMR spectrum, δ, ppm: 190.81, 190.41, 161.17, 158.54, 155.36, 153.87, 137.45, 137.04, 136.57, 136.12, 129.26, 128.96, 121.49, 121.40, 114.07, 58.52, 58.51, 55.32, 14.31, 14.19. Mass spectrum, m/z (Irel, %): 463.154 (M + 1) Found: 463.153. Anal. calcd. for C24H22N4O6: C, 62.33; H, 4.79; N, 12.12%.
diethyl 6-amino-4-(4-chlorophenyl)-5-cyano-1-phenyl-1,4-dihydropyridine-2,3-dicarboxylate (5k): Yield 96%, colorless crystals, mp 186–187 °C. IR spectrum, ν, cm−1: 3363.2 (N-H stretch, -NH2), 2253.1 (C≡N), 1742.9 (C=O stretch). 1H NMR spectrum, δ, ppm (J, Hz): 7.30–7.81 (m, 9H, Ar-H), 4.86 (s, 1H, CH), 7.34 (s, 1H, NH2), 4.48–4.52 (q, 4H, CH2), 1.78–1.79 (t, 6H, CH3). 13C NMR spectrum, δ, ppm: 191.07, 190.58, 161.33, 158.79, 155.49, 154.16, 137.74, 137.32, 136.89, 136.36, 129.38, 129.27, 121.71, 121.62, 114.35, 58.75, 58.74, 55.54,14.66, 14.41. Mass spectrum, m/z (Irel, %): 452.130 (M + 1); 453.130 (M + 1) Found: 452.128 (M + 1); 453.128 (M + 2). Anal. calcd. for C24H22ClN3O4: C, 63.79; H, 4.91; N, 9.30%.
diethyl 6-amino-4-(4-bromophenyl)-5-cyano-1-phenyl-1,4-dihydropyridine-2,3-dicarboxylate (5l): Yield 96%, colorless crystals, mp 151–153 °C. IR spectrum, ν, cm−1:3363.5 (N-H stretch, -NH2), 2253.3 (C≡N), 1743.1 (C=O stretch). 1H NMR spectrum, δ, ppm (J, Hz): 7.21–7.69 (m, 9H, Ar-H), 4.95 (s, 1H, CH), 7.32 (s, 1H, NH2), 4.48–4.51 (q, 4H, CH2), 1.67–1.68 (t, 6H, CH3). 13C NMR spectrum, δ, ppm: 191.02, 190.45, 161.21, 158.72, 155.41, 154.02, 137.52, 137.15, 136.50, 136.19, 129.24, 128.98, 121.68, 121.56, 114.29, 58.72, 58.71, 55.51, 14.60, 14.31. Mass spectrum, m/z (Irel, %): 496.079 (M + 1); 497.079 (M + 2) Found: 496.075(M + 1); 497.075 (M + 2). Anal. calcd. for C24H22BrN3O4: C, 58.07; H, 4.47; N, 8.47%.
diethyl 6-amino-5-cyano-1-phenyl-4-p-tolyl-1,4-dihydropyridine-2,3-dicarboxylate (5m): Yield 93%, colorless crystals, mp 286–287 °C. IR spectrum, ν, cm−1:3362.5 (N-H stretch, -NH2), 2252.1 (C≡N), 1742.6 (C=O stretch). 1H NMR spectrum, δ, ppm (J, Hz): 6.97–7.93 (m, 9H, Ar-H), 4.96 (s, 1H, CH), 7.29 (s, 1H, NH2), 4.47–4.49 (q, 4H, CH2), 2.10–2.12 (t, 6H, CH3), 2.17 (s, 3H, CH3). 13C NMR spectrum, δ, ppm: 190.73, 190.13, 161.11, 158.44, 155.28, 153.89, 137.33, 136.93, 136.32, 135.95, 129.17, 128.82, 121.37, 121.21, 113.96, 58.43, 58.41, 55.37, 14.35, 14.10. Mass spectrum, m/z (Irel, %): 432.185 (M + 1) Found: 432.176. Anal. calcd. for C25H25N3O4: C, 69.59; H, 5.84; N, 9.74%.
diethyl 6-amino-5-cyano-4-(4-methoxyphenyl)-1-phenyl-1,4-dihydropyridine-2,3-dicarboxylate (5n): Yield 92%, colorless crystals, mp 253–255 °C. IR spectrum, ν, cm−1: 3362.7 (N-H stretch, -NH2), 2252.4 (C≡N), 1742.3 (C=O stretch). 1H NMR spectrum, δ, ppm (J, Hz): 7.08–7.95 (m, 9H, Ar-H), 4.89 (s, 1H, CH), 7.05 (s, 1H, NH2), 4.39–4.45 (q, 4H, CH2),3.95 (s, 3H, OCH3), 1.93–1.94 (t, 6H, CH3). 13C NMR spectrum, δ, ppm: 190.47, 190.04, 160.93, 158.25, 155.07, 153.43, 137.19, 136.79, 136.18, 135.71, 128.99, 128.68, 121.16, 121.09, 113.79, 58.23, 58.21, 55.05, 14.08, 13.87. Mass spectrum, m/z (Irel, %): 448.179 (M + 1) Found: 448.166. Anal. calcd. for C25H25N3O5: C, 67.10; H, 5.63; N, 9.39%.
3.4. Plausible Mechanism
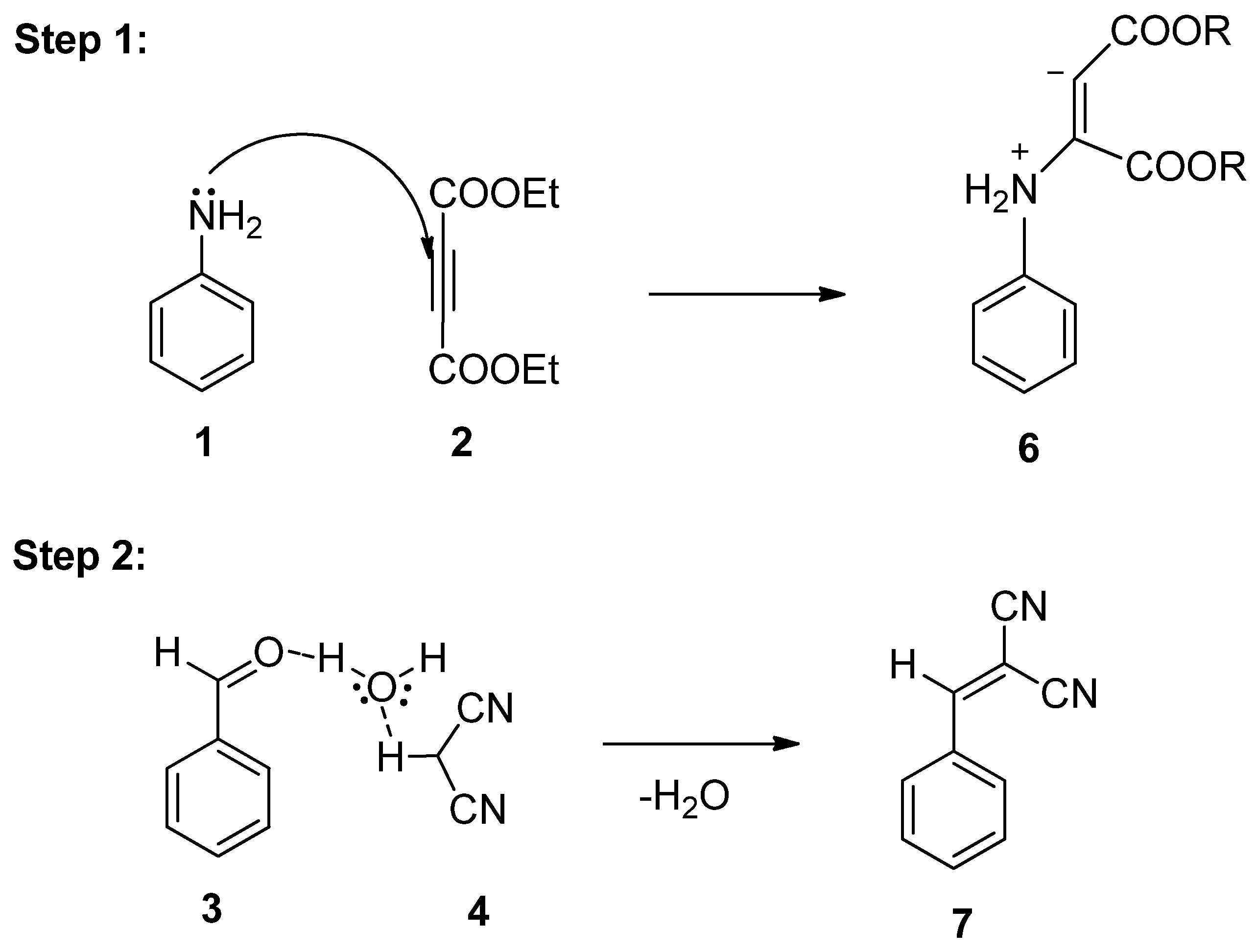
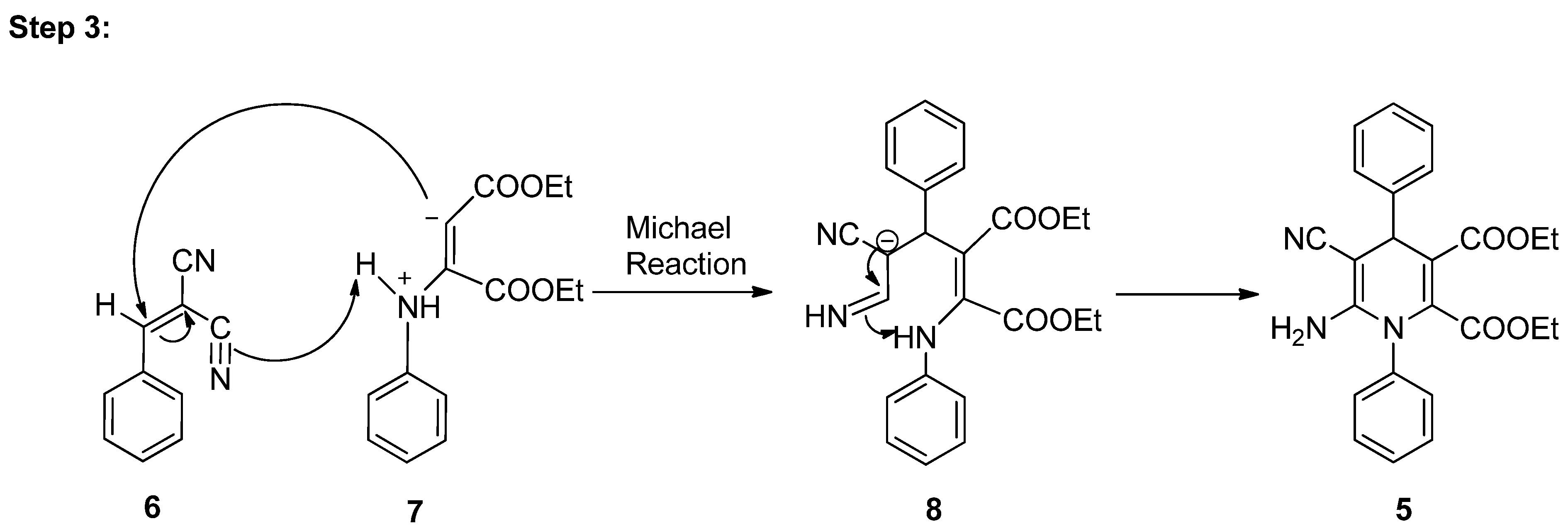
As per literature [32], a conceivable mechanism can be reasonably suggested for the production of pyran pyrazole 5a from the four-component reaction between aniline 1, diethyl acetylene dicarboxylate 2, substituted benzaldehyde 3, and malononitrile 4 (Scheme 3). The hypothesis suggests that the substituted benzaldehyde 3 may undergo activation by water, leading to increased electrophilicity of the carbonyl carbon. It is suggested that this process involves the establishment of hydrogen bonds between the oxygen atom of the carbonyl group and water molecules, while simultaneously, hydrogen bonds form between the acidic hydrogen of malononitrile and the oxygen of water. Following this, Knoevenagel condensation takes place [33], resulting in the generation of an intermediate 7. Subsequently, aniline 1 reacts with diethyl acetylene dicarboxylate 2, resulting in the generation of enolate intermediate 6. Afterward, the Michael reaction takes place between intermediate 6 and intermediate 7, which leads to the generation of transient intermediate [34] 8 which undergoes intramolecular cyclization, followed by tautomerization, ultimately resulting in the formation of the target compound, the 1,4-dihydropyridine derivative 9.
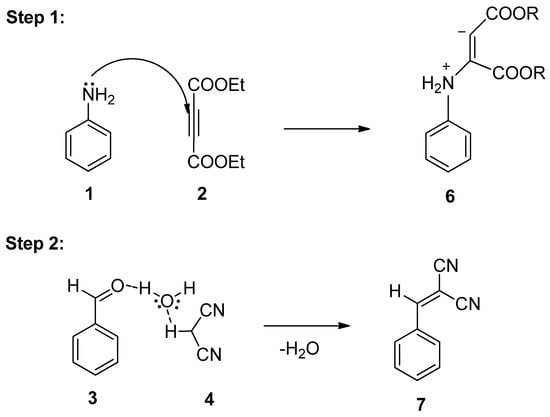
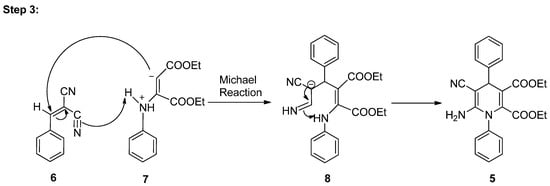
Scheme 3.
Plausible mechanism for synthesis of 1,4-DHPs using Al(DS)3 in water under microwave radiations.
3.5. Anti-Microbial Activity
The antimicrobial activities of the synthesized compounds 5a–n were evaluated using the Minimum Inhibitory Concentration (MIC) method. The results were compared to the reference drugs Fluconazole and Amoxicillin, with concentrations of 4 g/mL and 2 g/mL, respectively, in their respective areas of application. Table 4 shows that compound 5a–n exhibits moderate to excellent resistance against the tested strains. 1,4-DHPs with polar electron-withdrawing groups (5b, 5c, 5i, and 5j) attached to the phenyl ring at position 4 exhibited effectiveness against all the tested strains, comparable to the standard drugs Amoxicillin (MIC 4 μg/mL) and Fluconazole (MIC 2 μg/mL) this may be due the formation of H-bonds with the different parts of the protein of microbes. However, the resistance efficiency against the tested microbes decreases if the same phenyl ring is substituted with less polar groups such as Me (5f and 5m), OMe (5g and 5n), and Halogens (5d, 5e, 5k, and 5l) this is due to their less or no ability of formation of H-Bonds.

Table 4.
Anti-microbial activity of synthesized 1,4-DHP derivatives 5a–n.
4. Conclusions
An environmentally green procedure was performed for the synthesis of a novel 1,4-DHP scaffold via a one-pot, four-component reaction using aqueous micellar solution under microwave irradiation by treatment of a mixture of substituted benzaldehyde, diethyl acetylene dicarboxylate, and either ammonium acetate or aniline, along with malononitrile in an equivalent ratio. In summary, the outlined procedure illustrates remarkable efficacy in generating 1,4-DHP derivatives from easily accessible starting materials in a single step, utilizing a micellar solution of Al(DS)3 in water. The resulting crops are swiftly attained with adaptability and variety, achieving outstanding yields and purity. This approach demonstrates efficiency in terms of labor, cost-effectiveness, and minimal waste generation while operating under mild reaction conditions. Furthermore, all synthesized 1,4-DHPs display potent activity against the evaluated microbial strains. On a note, it finds that the presence of polar groups such as NO2 on the phenyl ring imparts comparable resistance to standard drugs such as Amoxicillin and Fluconazole.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29051115/s1, Scheme S1: Synthesis of 1,4-DHPs using Al(DS)3 in water under microwave radiations; Table S1: Synthesis of 1,4-DHP derivatives 5a-n using Al(DS)3 in water for 5 mints under microwave radiations; Table S2: Indexing of XRD using the Debye Scherrer Method; Figure S1: Energy-Dispersive X-ray Spectroscopy (EDS) data; Figure S2: 1H-NMR of diethyl 6-amino-5-cyano-4-phenyl-1,4-dihydropyridine-2,3-dicarboxylate (5a); Figure S3: 1H-NMR of diethyl 6-amino-5-cyano-4-(3-nitrophenyl)-1,4-dihydropyridine-2,3-dicarboxylate (5c); Figure S4: 1H-NMR of diethyl 6-amino-5-cyano-1,4-diphenyl-1,4-dihydropyridine-2,3-dicarboxylate (5h); Figure S5: 1H-NMR of diethyl 6-amino-5-cyano-4-(3-nitrophenyl)-1-phenyl-1,4-dihydropyridine-2,3-dicarboxylate (5j).
Author Contributions
Methodology, P.K.B.; Investigation, H.H.; Writing—original draft, A.G. and H.S.S.; Writing—review & editing, N.K., K.S., H.H. and P.K.B.; Supervision, M.K. and H.S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
The authors express sincere gratitude to Chandigarh University, Gharuan, Punjab, India, for providing all the essential facilities to conduct this research. The publication fees for this article were supported by the UNLV University Libraries Open Article Fund.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Sirisha, K.; Achaiah, G.; Reddy, V.M. Facile synthesis and antibacterial, antitubercular, and anticancer activities of novel 1,4-dihydropyridines. Arch. Pharm. 2010, 343, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, A.; Dodiya, D.; Dholariya, B.; Kataria, V.; Bhuva, V.; Shah, V. Synthesis and biological evaluation of some novel 1,4-dihydropyridines as potential antitubercular agents. Chem. Biol. Drug Des. 2011, 78, 881–886. [Google Scholar] [CrossRef]
- Kumar, A.; Maurya, R.A.; Sharma, S.; Kumar, M.; Bhatia, G. Synthesis and biological evaluation of N-aryl-1,4-dihydropyridines as novel antidyslipidemic and antioxidant agents. Eur. J. Med. Chem. 2010, 45, 501–509. [Google Scholar] [CrossRef]
- De Luca, M.; Ioele, G.; Ragno, G. 1,4-dihydropyridine antihypertensive drugs: Recent advances in photostabilization strategies. Pharmaceutics 2019, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.F.; Darweesh, A.F.; Elwahy, A.H.M.; Abdelhamid, I.A. Synthesis, characterization and antitumor activity of novel tetrapodal 1,4-dihydropyridines: P53 induction, cell cycle arrest and low damage effect on normal cells induced by genotoxic factor H2O2. RSC Adv. 2016, 6, 40900–40910. [Google Scholar] [CrossRef]
- Tajbakhsh, M.; Alinezhad, H.; Norouzi, M.; Baghery, S.; Akbari, M. Protic pyridinium ionic liquid as a green and highly efficient catalyst for the synthesis of polyhydroquinoline derivatives via Hantzsch condensation in water. J. Mol. Liq. 2013, 177, 44–48. [Google Scholar] [CrossRef]
- Mathur, R.; Negi, K.S.; Shrivastava, R.; Nair, R. Recent developments in the nanomaterial-catalyzed green synthesis of structurally diverse 1,4-dihydropyridines. RSC Adv. 2021, 11, 1376–1393. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.; A Johnson, B.; Ait-Daoud, N.; Wells, L.T. Effects of isradipine, a dihydropyridine-class calcium channel antagonist, on D-methamphetamine-induced cognitive and physiological changes in humans. Neuropsychopharmacology 2000, 22, 504–512. [Google Scholar] [CrossRef]
- Huai, Y.; Wang, H.; Zhao, L.; Zhao, L.; Pei, J. Lacidipine inhibits endoplasmic reticulum stress and myocardial remodeling induced by pressure overload in rat heart. Eur. J. Pharmacol. 2013, 718, 441–447. [Google Scholar] [CrossRef]
- Amenta, F.; Tomassoni, D.; Traini, E.; Mignini, F.; Veglio, F. Nicardipine: A hypotensive dihydropyridine-type calcium antagonist with a peculiar cerebrovascular profile. Clin. Exp. Hypertens. 2008, 30, 808–826. [Google Scholar] [CrossRef]
- Wu, J.Y.; Zhang, L.; Cai, L.L.; Zhang, Y. Catalyzing synthesis of chiral nitrendipine. Adv. Mater. Res. 2012, 518–523, 3943–3946. [Google Scholar] [CrossRef]
- Affeldt, R.F.; Benvenutti, E.V.; Russowsky, D. A new In-SiO2 composite catalyst in the solvent-free multicomponent synthesis of Ca2+ channel blockers nifedipine and nemadipine B. New J. Chem. 2012, 36, 1502–1511. [Google Scholar] [CrossRef]
- Hantzsch, A. Ueber die Synthese pyridinartiger Verbindungen aus Acetessigäther und Aldehydammoniak. Justus Liebigs Ann. Chem. 1882, 215, 1–82. [Google Scholar] [CrossRef]
- Jassem, A.M.; Almashal, F.A.K.; Mohammed, M.Q.; Jabir, H.A.S. A catalytic and green method for one-pot synthesis of new Hantzsch 1,4-dihydropyridines. SN Appl. Sci. 2020, 2, 359. [Google Scholar] [CrossRef]
- Machado, I.V.; dos Santos, J.R.; Januario, M.A.; Corrêa, A.G. Greener organic synthetic methods: Sonochemistry and heterogeneous catalysis promoted multicomponent reactions. Ultrason. Sonochem. 2021, 78, 105704. [Google Scholar] [CrossRef] [PubMed]
- Davarpanah, J.; Ghahremani, M.; Najafi, O. Synthesis of 1,4-dihydropyridine and polyhydroquinoline derivatives via Hantzsch reaction using nicotinic acid as a green and reusable catalyst. J. Mol. Struct. 2019, 1177, 525–535. [Google Scholar] [CrossRef]
- Koukabi, N.; Kolvari, E.; Khazaei, A.; Zolfigol, M.A.; Shirmardi-Shaghasemi, B.; Khavasi, H.R. Hantzsch reaction on free nano-Fe2O3 catalyst: Excellent reactivity combined with facile catalyst recovery and recyclability. Chem. Commun. 2011, 47, 9230–9232. [Google Scholar] [CrossRef]
- Shaibuna, M.; Sreekumar, K. Dual solvent-catalyst role of deep eutectic solvents in Hantzsch dihydropyridine synthesis. Synth. Commun. 2021, 51, 1742–1753. [Google Scholar] [CrossRef]
- Elumalai, D.; Gnanasekaran, R.; Leelakrishnan, S.; Nachimuthu, G.; Kannan, T.; Paramasivam, T.P.; Jayabal, K. InCl3-Assisted Eco-Friendly Approach for N-Fused 1,4-Dihydropyridine Scaffolds via Ring Opening Michael Addition of Cyclic Nitroketene and Iminocoumarin: Synthesis and DFT Studies. ChemistrySelect 2018, 3, 2070–2079. [Google Scholar] [CrossRef]
- Sharma, V.K.; Singh, S.K. Synthesis, utility and medicinal importance of 1,2- & 1,4-dihydropyridines. RSC Adv. 2017, 7, 2682–2732. [Google Scholar] [CrossRef]
- Sutarni, Y.D.S.Y.D.; Purwono, B.; Kunarti, E.S.K.E.S.; Mardjan, M.I.D. Fe/ZSM-5-Catalyzed-Synthesis of 1,4-Dihydropyridines under Ultrasound Irradiation and Their Antioxidant Activities. Sains Malays. 2023, 52, 1189–1202. [Google Scholar] [CrossRef]
- Rostamnia, S.; Morsali, A. Basic isoreticular nanoporous metal-organic framework for Biginelli and Hantzsch coupling: IRMOF-3 as a green and recoverable heterogeneous catalyst in solvent-free conditions. RSC Adv. 2014, 4, 10514–10518. [Google Scholar] [CrossRef]
- Parthiban, A.; Makam, P. 1,4-Dihydropyridine: Synthetic advances, medicinal and insecticidal properties. RSC Adv. 2022, 12, 29253–29290. [Google Scholar] [CrossRef]
- Zonouz, A.M.; Hosseini, S.B. Montmorillonite K10 clay: An efficient catalyst for Hantzsch synthesis of 1,4-dihydropyridine derivatives. Synth. Commun. 2008, 38, 290–296. [Google Scholar] [CrossRef]
- Sadeghi, B.; Namakkoubi, A.; Hassanabadi, A. BF3.SiO2 nanoparticles: A solid phase acidic catalyst for efficient one-pot Hantzsch synthesis of 1,4-dihydropyridines. J. Chem. Res. 2013, 37, 11–13. [Google Scholar] [CrossRef]
- Lee, J.H. Synthesis of Hantsch 1,4-dihydropyridines by fermenting bakers’ yeast. Tetrahedron Lett. 2005, 46, 7329–7330. [Google Scholar] [CrossRef]
- Sivamurugan, V.; Kumar, R.S.; Palanichamy, M.; Murugesan, V. Synthesis of Hantzsch 1,4-dihydropyridines under solvent-free condition using Zn[(L)proline]2 as Lewis acid catalyst. J. Heterocycl. Chem. 2005, 42, 969–974. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Basak, A.K.; Narsaiah, A.V. Three-component coupling reactions in ionic liquids: An improved protocol for the synthesis of 1,4-dihydropyridines. Green Chem. 2003, 5, 60–63. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S. A grinding-induced catalyst- and solvent-free synthesis of highly functionalized 1,4-dihydropyridines via a domino multicomponent reaction. Green Chem. 2011, 13, 2017–2020. [Google Scholar] [CrossRef]
- Hesari, Z.; Hadavand, B.S. Deep Eutectic Solvent Based on Choline Chloride/Urea as an Efficient Catalytic System for the One-Pot Synthesis of Highly Functionalized 1,4-Dihydropyridines and Polysubstituted 4H- Chromenes. J. Appl. Chem. Res. 2019, 13, 97–106. [Google Scholar]
- Jeffrey, K.; Elizabeth, R.; Samuel, B.; Shannon, S.; Beata, D.; Bojarski, A.J.; Strekowski, L. Synthesis of 4-Substituted 2-(4-Methylpiperazino)pyrimidines and Quinazoline Analogs as Serotonin 5-HT 2A Receptor Ligands. J. Heterocycl. Chem. 2009, 46, 1259–1265. [Google Scholar] [CrossRef]
- Ramesh, R.; Lalitha, A. Facile and Green Chemistry Access to 5-aryl-1,2,4-Triazolidine-3-thiones in Aqueous Medium. ChemistrySelect 2016, 1, 2085–2089. [Google Scholar] [CrossRef]
- Kerru, N.; Gummidi, L.; Bhaskaruni, S.V.H.S.; Maddila, S.N.; Jonnalagadda, S.B. Green synthesis and characterization of novel 1,2,4,5-tetrasubstituted imidazole derivatives with eco-friendly red brick clay as efficacious catalyst. Mol. Divers. 2020, 24, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Maddila, S.; Nagaraju, K.; Chinnam, S.; Jonnalagadda, S.B. Microwave-Assisted Multicomponent Reaction: A Green and Catalyst-Free Method for the Synthesis of Poly-Functionalized 1,4-Dihydropyridines. ChemistrySelect 2019, 4, 9451–9454. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).