Two-Phase Fermentation Systems for Microbial Production of Plant-Derived Terpenes
Abstract
:1. Introduction
2. Types of TPF Systems
2.1. Liquid–Liquid TPF Systems
2.1.1. Aqueous-Organic TPF Systems
2.1.2. Aqueous Two-Phase System
2.2. Liquid–Solid TPF Systems
2.2.1. Immobilized Cells as the Solid Phase
2.2.2. Immobilized Solvent as the Solid Phase
2.2.3. Solid Adsorbents as the Solid Phase
3. The Advantages of TPF Systems
3.1. Enhance Productivity
3.1.1. Reducing Toxicity to Microbial Cells
3.1.2. Alleviating Feedback Inhibition
3.1.3. Preventing Product Degradation and Loss
3.2. Industrial Application: Cost-Effective and Downstream Processing
3.2.1. Increase in Cell Biomass and Recycling of the Second Phase
3.2.2. Reduction of Post-Processing Steps
4. Applications of TPF in Microbial Production of Terpenes
4.1. Microbial Production of Plant-Derived Terpenes
4.1.1. Monoterpenes
4.1.2. Sesquiterpenes
| Sesquiterpenes | Chassis Cells | Fermentation Types and Scales | Second Phases | Titers (mg/L) | References |
|---|---|---|---|---|---|
| amorphadiene | E. coli | flask | none | 112.2 | [130] |
| 14 mL tube | n-dodecane | 300 | [141] | ||
| 250 mL flask | n-dodecane | 1400 | [142] | ||
| 250 mL bioreactor | n-dodecane | 30,000 | [16] | ||
| S. cerevisiae | 250 mL flask | n-dodecane | 497 | [143] | |
| 2 L bioreactor | n-dodecane | 41,000 | [79] | ||
| flask | isopropyl myristate | 4000 | [25] | ||
| 2 L bioreactor | methyl oleate | 40,000 | [25] | ||
| Y. lipolytica | 250 mL flask | n-dodecane | 171.5 | [144] | |
| R. toruloides | 2 L bioreactor | n-dodecane | 36 | [145] | |
| B. subtilis | flask | n-dodecane | 20 | [146] | |
| S. elongatus | 100 mL flask | n-hexadecane | 19.8 | [147] | |
| α-farnesene | E. coli | 500 mL flask | n-decane | 1100 | [133] |
| S. cerevisiae | 250 mL flask | n-dodecane | 1477.2 | [137] | |
| 5 L bioreactor | n-dodecane | 10,400 | [137] | ||
| Y. lipolytica | 300 mL flask | n-dodecane | 1700 | [139] | |
| 1 L bioreactor | n-dodecane | 25,550 | [139] | ||
| S. elongatus | flask | n-dodecane | 4.6 | [148] | |
| Anabaena sp. | 250 mL flask | supelpak 2sv resin columns | 0.3054 | [140] | |
| P. pastoris | flask | n-dodecane | 2560 | [149] | |
| β-farnesene | E. coli | 5 L bioreactor | n-decane | 10,310 | [150] |
| 0.5 L flask | n-decane | 5290 | [151] | ||
| Y. lipolytica | 2.5 mL tubes | n-dodecane | 955 | [152] | |
| 2 L bioreactor | n-decane | 22,800 | [153] | ||
| bisabolene | E. coli | flask | n-dodecane | 1150 | [87] |
| 5 L bioreactor | n-dodecane | 9100 | [154] | ||
| S. cerevisiae | 125 mL flask | n-dodecane | 994 | [155] | |
| 2 L bioreactor | n-dodecane | 5200 | [79] | ||
| Synechococcus sp. | 250 mL flask | n-dodecane | 0.6 | [156] | |
| 3 L bioreactor | n-dodecane | 22.5 | [157] | ||
| R. toruloides | 2 L bioreactor | n-dodecane | 680 | [145] | |
| nerolidol | E. coli | 5 L bioreactor | n-dodecane | 16,000 | [32] |
| S. cerevisiae | 250 mL flask | n-dodecane | 497 | [158] | |
| 5 L bioreactor | n-dodecane | 7010 | [32] | ||
| α-humulene | E. coli | 2 L bioreactor | Amberlite XAD4 resin | 60.2 | [24] |
| bioreactor | n-dodecane | 0.958 | [159] | ||
| S. cerevisiae | 5 L bioreactor | n-dodecane | 1726.78 | [160] | |
| patchoulol | E. coli | 5 L bioreactor | n-dodecane | 970 | [161] |
| S. cerevisiae | 1.1 L flask | n-dodecane | 42.1 | [162] | |
| 5 L bioreactor | n-dodecane | 1632 | [163] | ||
| valencene | S. cerevisiae | 300 mL flask | n-dodecane | 31 | [164] |
| 3 L bioreactor | n-dodecane | 264.6 | [165] | ||
| Y. lipolytica | flask | n-dodecane | 22.8 | [166] | |
| C. glutamicum | 100 mL flask | n-dodecane | 2.41 | [167] | |
| R. sphaeroides | 250 mL flask | n-dodecane | 352 | [168] | |
| Synechocystis sp. | flask | isopropyl myristate | 9.6 | [169] | |
| germacrene A | E. coli | flask | none | 6.325 | [170] |
| 250 mL flask | n-dodecane | 364.26 | [171] | ||
| 4 L bioreactor | n-dodecane | 3520 | [64] | ||
| S. cerevisiae | flask | n-dodecane | 375 | [172] | |
| Y. lipolytica | 5 L bioreactor | isopropyl myristate | 39,000 | [78] | |
| P. pastoris | 1 L bioreactor | n-dodecane | 1900 | [173] | |
| O. polymorpha | 250 mL bioreactor | n-dodecane | 4700 | [174] | |
| α-santalene | E. coli | 1.3 L bioreactor | isopropyl myristate | 2916 | [175] |
| S. cerevisiae | 2.5 L flask | n-dodecane | 92 | [176] | |
| 5 L bioreactor | n-dodecane | 163 | [177] | ||
| Y. lipolytica | 5 L bioreactor | n-dodecane | 27.92 | [178] | |
| β-caryophyllene | E. coli | 25 mL flask | none | 100 | [179] |
| 5 L bioreactor | none | 1520 | [180] | ||
| 5 L bioreactor | n-dodecane | 5142 | [181] | ||
| S. cerevisiae | 1.3 L bioreactor | n-dodecane | 2949.1 | [182] | |
| α-cuprenene | X. dendrorhous | 100 mL flask | n-dodecane | 80 | [183] |
| viridiflorol | E. coli | 250 mL bioreactor | n-dodecane | 25,700 | [16] |
| longifolene | E. coli | 5 L bioreactor | n-decane | 382 | [184] |
| (+)-zizaene | E. coli | 2 L bioreactor | diaion HP20 resin | 211 | [185] |
| valerenadiene | E. coli | flask | n-decane | 62 | [186] |
| protoilludene | E. coli | flask | n-decane | 1199 | [187] |
| farnesol | S. cerevisiae | flask | none | 70 | [188] |
| E. coli | flask | methyl oleate | 1419 | [189] | |
| epi-isozizaene | E. coli | 4 L bioreactor | n-decane | 727.9 | [190] |
| α-isocomene | E. coli | bioreactor | n-decane | 77.5 | [190] |
| pentalenene | E. coli | 2.5 L bioreactor | n-decane | 780.3 | [190] |
| α-neoclovene | S. cerevisiae | 1.3 L bioreactor | n-dodecane | 487.1 | [182] |
| valerenic acid | S. cerevisiae | flask | n-dodecane | 4 | [191] |
| zerumbone | S. cerevisiae | 5 L bioreactor | n-dodecane | 40 | [192] |
| prespatane | R. toruloides | 2 L bioreactor | n-dodecane | 1173.6 | [77] |
| santalols | S. cerevisiae | 5 L bioreactor | n-dodecane | 1300 | [193] |
| z-α-Santalol | S. cerevisiae | 5 L bioreactor | n-dodecane | 1200 | [193] |
| zerumbone | S. cerevisiae | 5 L bioreactor | n-dodecane | 40 | [192] |
4.1.3. Diterpenes
| Diterpenes | Chassis Cells | Fermentation Types and Scales | Second Phases | Titers (mg/L) | References |
|---|---|---|---|---|---|
| miltiradiene | S. cerevisiae | 5 L bioreactor | none | 488 | [195] |
| 10 mL flask | n-dodecane | 550 | [198] | ||
| 5 L bioreactor | n-dodecane | 3500 | [198] | ||
| taxadiene | E. coli | flask | none | 1.3 | [200] |
| 2 L flask | n-dodecane | 570 | [201] | ||
| 1 L bioreactor | n-dodecane | 1020 | [196] | ||
| S. cerevisiae | 500 mL bioreactor | none | 33 | [202] | |
| 500 mL flask | RP18 silica gel | 8 | [66] | ||
| 500 mL bioreactor | n-dodecane | 129 | [197] | ||
| A. fumigatus | 250 mL flask | immobilization | 0.694 | [52] | |
| A.tenuissima | 250 mL flask | immobilization | 0.388 | [52] | |
| oxygenated taxane | S. cerevisiae | 1 L bioreactor | n-dodecane | 78 | [203] |
| ent-Kaurene | E. coli | 1 L bioreactor | none | 578 | [204] |
| 3 L bioreactor | n-dodecane | 624 | [205] | ||
| R. toruloides | 2 L bioreactor | n-dodecane | 1400 | [206] | |
| geranylgeraniol | S. cerevisiae | bioreactor | none | 3300 | [207] |
| flask | n-dodecane | 772.98 | [208] | ||
| 5 L bioreactor | n-dodecane | 5070 | [208] | ||
| steviol | E. coli | 2q L bioreactor | none | 1100 | [209] |
| 3 L bioreactor | n-dodecane | 38.4 | [205] | ||
| sclareol | E. coli | bioreactor | n-dodecane | 1500 | [210] |
| S. cerevisiae | 100 mL flask | n-dodecane | 750 | [211] | |
| 0.4 L bioreactor | n-hexane | 11,400 | [199] | ||
| levopimaradiene | E. coli | 3 L bioreactor | n-dodecane | 700 | [212] |
| levopimaric acid | S. cerevisiae | 5 L bioreactor | n-dodecane | 400.3 | [213] |
| retinoids | E. coli | 14 mL tube | n-dodecane | 33 | [214] |
| retinol | S. cerevisiae | 5 L bioreactor | n-dodecane | 2349 | [215] |
| Y. lipolytica | 5 L bioreactor | n-dodecane | 4860 | [216] | |
| cis-abienol | E. coli | bioreactor | isopropyl myristate | 634 | [217] |
| 13R-manoyl oxide | S. cerevisiae | 5 L bioreactor | n-dodecane | 3000 | [218] |
| forskolin | S. cerevisiae | 5 L flask | n-hexane | 40 | [219] |
| gibberellic acid 3 | Y. lipolytica | 24-roundwell plates | none | 12.8 | [220] |
| gibberellic acid 4 | Y. lipolytica | 24-roundwell plates | none | 17.3 | [220] |
| carnosic acid | S. cerevisiae | 30 mL flask | none | 25 | [221] |
| 5 L bioreactor | none | 75.2 | [221] | ||
| rubusoside | S. cerevisiae | 250 mL bioreactor | none | 1400 | [222] |
| rebaudiosides | S. cerevisiae | 250 mL bioreactor | none | 132.7 | [222] |
4.1.4. Triterpenes and Tetraterpenes
5. Factors Influencing TPF Systems
5.1. Solvent or Adsorbent as the Second Phase
5.1.1. Solvent Selection Considerations
5.1.2. Adsorbent Selection Considerations
5.2. Concentration and Timing of Second Phase Addition
5.3. Economic Considerations and Downstream Processing
6. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gershenzon, J.; Dudareva, N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Cox-Georgian, D.; Ramadoss, N.; Dona, C.; Basu, C. Therapeutic and Medicinal Uses of Terpenes. In Medicinal Plants: From Farm to Pharmacy; Joshee, N., Dhekney, S.A., Parajuli, P., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 333–359. [Google Scholar]
- Brahmkshatriya, P.P.; Brahmkshatriya, P.S. Terpenes: Chemistry, Biological Role, and Therapeutic Applications. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 2665–2691. [Google Scholar]
- Gallego-Jara, J.; Lozano-Terol, G.; Sola-Martinez, R.A.; Canovas-Diaz, M.; de Diego Puente, T. A Compressive Review about Taxol((R)): History and Future Challenges. Molecules 2020, 25, 5986. [Google Scholar] [CrossRef]
- Faurant, C. From bark to weed: The history of artemisinin. Parasite 2011, 18, 215–218. [Google Scholar] [CrossRef]
- Urabe, D.; Asaba, T.; Inoue, M. Convergent Strategies in Total Syntheses of Complex Terpenoids. Chem. Rev. 2015, 115, 9207–9231. [Google Scholar] [CrossRef] [PubMed]
- Hugelshofer, C.L.; Magauer, T. Bioinspired total syntheses of terpenoids. Org. Biomol. Chem. 2016, 15, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Ajikumar, P.K.; Tyo, K.; Carlsen, S.; Mucha, O.; Phon, T.H.; Stephanopoulos, G. Terpenoids: Opportunities for biosynthesis of natural product drugs using engineered microorganisms. Mol. Pharm. 2008, 5, 167–190. [Google Scholar] [CrossRef] [PubMed]
- Mewalal, R.; Rai, D.K.; Kainer, D.; Chen, F.; Kulheim, C.; Peter, G.F.; Tuskan, G.A. Plant-Derived Terpenes: A Feedstock for Specialty Biofuels. Trends Biotechnol. 2017, 35, 227–240. [Google Scholar] [CrossRef]
- Nagegowda, D.A.; Gupta, P. Advances in biosynthesis, regulation, and metabolic engineering of plant specialized terpenoids. Plant Sci. 2020, 294, 110457. [Google Scholar] [CrossRef]
- Belcher, M.S.; Mahinthakumar, J.; Keasling, J.D. New frontiers: Harnessing pivotal advances in microbial engineering for the biosynthesis of plant-derived terpenoids. Curr. Opin. Biotechnol. 2020, 65, 88–93. [Google Scholar] [CrossRef]
- Kirby, J.; Keasling, J.D. Biosynthesis of plant isoprenoids: Perspectives for microbial engineering. Annu. Rev. Plant Biol. 2009, 60, 335–355. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, K.; Zhang, X.; Liu, G.; Zhu, T.; Che, Q.; Li, D.; Zhang, G. Heterologous expression and metabolic engineering tools for improving terpenoids production. Curr. Opin. Biotechnol. 2021, 69, 281–289. [Google Scholar] [CrossRef]
- Moser, S.; Pichler, H. Identifying and engineering the ideal microbial terpenoid production host. Appl. Microbiol. Biotechnol. 2019, 103, 5501–5516. [Google Scholar] [CrossRef]
- Daletos, G.; Stephanopoulos, G. Protein engineering strategies for microbial production of isoprenoids. Metab. Eng. Commun. 2020, 11, e00129. [Google Scholar] [CrossRef]
- Shukal, S.; Chen, X.; Zhang, C. Systematic engineering for high-yield production of viridiflorol and amorphadiene in auxotrophic Escherichia coli. Metab. Eng. 2019, 55, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Daugulis, A.J. Partitioning bioreactors. Curr. Opin. Biotechnol. 1997, 8, 169–174. [Google Scholar] [CrossRef]
- Freeman, A.; Woodley, J.M.; Lilly, M.D. In situ product removal as a tool for bioprocessing. Bio/Technology 1993, 11, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, J.J. Two-phase partitioning bioreactors in fermentation technology. Biotechnol. Adv. 2001, 19, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Quijano, G.; Hernandez, M.; Thalasso, F.; Muñoz, R.; Villaverde, S. Two-phase partitioning bioreactors in environmental biotechnology. Appl. Microbiol. Biotechnol. 2009, 84, 829–846. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Hossein Mirjalili, M.; Fett-Neto, A.G.; Mazzafera, P.; Bonfill, M. Living between two worlds: Two-phase culture systems for producing plant secondary metabolites. Crit. Rev. Biotechnol. 2012, 33, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Phillips, T.; Chase, M.; Wagner, S.; Renzi, C.; Powell, M.; DeAngelo, J.; Michels, P. Use of in situ solid-phase adsorption in microbial natural product fermentation development. J. Ind. Microbiol. Biotechnol. 2013, 40, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.D.; Marshall, J.; Chang, M.; Nowroozi, F.; Paradise, E.; Pitera, D.; Newman, K.L.; Keasling, J.D. High-level production of amorpha-4,11-diene in a two-phase partitioning bioreactor of metabolically engineered Escherichia coli. Biotechnol. Bioeng. 2006, 95, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Alemdar, S.; König, J.C.; Hartwig, S.; Frister, T.; Scheper, T.; Beutel, S. Bioproduction of α-humulene in metabolically engineered Escherichia coli and application in zerumbone synthesis. Eng. Life Sci. 2017, 17, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Westfall, P.J.; Pitera, D.J.; Lenihan, J.R.; Eng, D.; Woolard, F.X.; Regentin, R.; Horning, T.; Tsuruta, H.; Melis, D.J.; Owens, A.; et al. Production of amorphadiene in yeast, and its conversion to dihydroartemisinic acid, precursor to the antimalarial agent artemisinin. Proc. Natl. Acad. Sci. USA 2012, 109, E111–E118. [Google Scholar] [CrossRef] [PubMed]
- Drouin, C.M.; Cooper, D.G. Biosurfactants and aqueous two-phase fermentation. Biotechnol. Bioeng. 2004, 40, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, O.; Rito-Palomares, M. Aqueous two-phase systems strategies for the recovery and characterization of biological products from plants. J. Sci. Food Agric. 2010, 90, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Tao, Y.; Xie, S.; Zhu, Y.; Chen, D.; Wang, X.; Huang, L.; Peng, D.; Sattar, A.; Shabbir, M.A.B.; et al. Aqueous two-phase system (ATPS): An overview and advances in its applications. Biol. Proced. Online 2016, 18, 18. [Google Scholar] [CrossRef]
- Rosinha Grundtvig, I.P.; Heintz, S.; Krühne, U.; Gernaey, K.V.; Adlercreutz, P.; Hayler, J.D.; Wells, A.S.; Woodley, J.M. Screening of organic solvents for bioprocesses using aqueous-organic two-phase systems. Biotechnol. Adv. 2018, 36, 1801–1814. [Google Scholar] [CrossRef]
- Inoue, A.; Horikoshi, K. A Pseudomonas thrives in high concentrations of toluene. Nature 1989, 338, 264–266. [Google Scholar] [CrossRef]
- Verhoef, S.; Wierckx, N.; Westerhof, R.G.M.; de Winde, J.H.; Ruijssenaars, H.J. Bioproduction of p-Hydroxystyrene from Glucose by the Solvent-Tolerant Bacterium Pseudomonas putida S12 in a Two-Phase Water-Decanol Fermentation. Appl. Environ. Microbiol. 2009, 75, 931–936. [Google Scholar] [CrossRef]
- Tan, N.; Ong, L.; Shukal, S.; Chen, X.; Zhang, C. High-Yield Biosynthesis of trans-Nerolidol from Sugar and Glycerol. J. Agric. Food Chem. 2023, 71, 8479–8487. [Google Scholar] [CrossRef]
- Freire, M.G.; Cláudio, A.F.M.; Araújo, J.M.M.; Coutinho, J.A.P.; Marrucho, I.M.; Lopes, J.N.C.; Rebelo, L.P.N. Aqueous biphasic systems: A boost brought about by using ionic liquids. Chem. Soc. Rev. 2012, 41, 4966–4995. [Google Scholar] [CrossRef]
- Phong, W.N.; Show, P.L.; Chow, Y.H.; Ling, T.C. Recovery of biotechnological products using aqueous two phase systems. J. Biosci. Bioeng. 2018, 126, 273–281. [Google Scholar] [CrossRef]
- He, A.; Dong, B.; Feng, X.; Yao, S. Extraction of bioactive ginseng saponins using aqueous two-phase systems of ionic liquids and salts. Sep. Purif. Technol. 2018, 196, 270–280. [Google Scholar] [CrossRef]
- González-Valdez, J.; Mayolo-Deloisa, K.; Rito-Palomares, M. Novel aspects and future trends in the use of aqueous two-phase systems as a bioengineering tool. J. Chem. Technol. Biotechnol. 2017, 93, 1836–1844. [Google Scholar] [CrossRef]
- González-Peñas, H.; Lu-Chau, T.A.; Moreira, M.T.; Lema, J.M. Solvent screening methodology for in situ ABE extractive fermentation. Appl. Microbiol. Biotechnol. 2014, 98, 5915–5924. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2020, 121, 1232–1285. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Xu, Q.; Chen, N. Effect of a new ionic liguid on L-valine fermentation. Bull. Ferment. Sci. Technol. 2020, 49, 207–229. [Google Scholar] [CrossRef]
- Badhwar, P.; Kumar, P.; Dubey, K.K. Extractive Fermentation for Process integration and amplified pullulan production by Apullulans in Aqueous Two Phase Systems. Sci. Rep. 2019, 9, 32. [Google Scholar] [CrossRef]
- Haykir, N.I.; Nizan Shikh Zahari, S.M.S.; Harirchi, S.; Sar, T.; Awasthi, M.K.; Taherzadeh, M.J. Applications of ionic liquids for the biochemical transformation of lignocellulosic biomass into biofuels and biochemicals: A critical review. Biochem. Eng. J. 2023, 193, 108850. [Google Scholar] [CrossRef]
- Lapponi, M.J.; Méndez, M.B.; Trelles, J.A.; Rivero, C.W. Cell immobilization strategies for biotransformations. Curr. Opin. Green Sustain. Chem. 2022, 33, 100565. [Google Scholar] [CrossRef]
- Pedro, A.Q.; Coutinho, J.A.P.; Freire, M.G. Immobilization of Ionic Liquids, Types of Materials, and Applications. In Encyclopedia of Ionic Liquids; Springer Nature: Singapore, 2019; pp. 1–12. [Google Scholar]
- Hou, D.; Yu, W.; Zhang, D.; Zhao, L.; Liu, X.; Ma, X. Culture of yeast cells immobilized by alginate-chitosan microcapsules in aqueous-organic solvent biphasic system. J. Oceanol. Limnol. 2019, 37, 863–870. [Google Scholar] [CrossRef]
- Plieva, F.M.; Oknianska, A.; Degerman, E.; Mattiasson, B. Macroporous gel particles as robust macroporous matrices for cell immobilization. Biotechnol. J. 2008, 3, 410–417. [Google Scholar] [CrossRef]
- Lu, J.; Peng, W.; Lv, Y.; Jiang, Y.; Xu, B.; Zhang, W.; Zhou, J.; Dong, W.; Xin, F.; Jiang, M. Application of Cell Immobilization Technology in Microbial Cocultivation Systems for Biochemicals Production. Ind. Eng. Chem. Res. 2020, 59, 17026–17034. [Google Scholar] [CrossRef]
- Brányik, T.; Vicente, A.; Oliveira, R.; Teixeira, J. Physicochemical surface properties of brewing yeast influencing their immobilization onto spent grains in a continuous reactor. Biotechnol. Bioeng. 2004, 88, 84–93. [Google Scholar] [CrossRef]
- Dulieu, C.; Poncelet, D.; Neufeld, R.J. Encapsulation and Immobilization Techniques. In Cell Encapsulation Technology and Therapeutics; Kühtreiber, W.M., Lanza, R.P., Chick, W.L., Eds.; Birkhäuser: Boston, MA, USA, 1999; pp. 3–17. [Google Scholar]
- Coradello, G.; Tirelli, N. Yeast Cells in Microencapsulation. General Features and Controlling Factors of the Encapsulation Process. Molecules 2021, 26, 3123. [Google Scholar] [CrossRef] [PubMed]
- Rathore, S.; Desai, P.M.; Liew, C.V.; Chan, L.W.; Heng, P.W.S. Microencapsulation of microbial cells. J. Food Eng. 2013, 116, 369–381. [Google Scholar] [CrossRef]
- El-Sayed, E.-S.R.; Ahmed, A.S.; Hassan, I.A.; Ismaiel, A.A.; Karam El-Din, A.-Z.A. Strain improvement and immobilization technique for enhanced production of the anticancer drug paclitaxel by Aspergillus fumigatus and Alternaria tenuissima. Appl. Microbiol. Biotechnol. 2019, 103, 8923–8935. [Google Scholar] [CrossRef]
- Darmayanti, R.F.; Tashiro, Y.; Noguchi, T.; Gao, M.; Sakai, K.; Sonomoto, K. Novel biobutanol fermentation at a large extractant volume ratio using immobilized Clostridium saccharoperbutylacetonicum N1-4. J. Biosci. Bioeng. 2018, 126, 750–757. [Google Scholar] [CrossRef]
- Ham, S.; Kim, H.J.; Shin, N.; Hwang, J.H.; Oh, S.J.; Park, J.Y.; Joo, J.C.; Kim, H.T.; Bhatia, S.K.; Yang, Y.-H. Continuous production of gamma aminobutyric acid by engineered and immobilized Escherichia coli whole-cells in a small-scale reactor system. Enzym. Microb. Technol. 2023, 168, 110258. [Google Scholar] [CrossRef]
- de Souza, W.F.C.; Pereira, I.; de Lucena, F.A.; Martins, L.P.; Furtado, R.F.; da Castro, R.J.S.; Sato, H.H. A new system of Erwinia sp. D12 cells immobilized in a matrix of alginate and algaroba gum (Prosopis juliflora): An efficient way to improve isomaltulose production. Process Biochem. 2022, 114, 52–58. [Google Scholar] [CrossRef]
- Carsanba, E.; Pintado, M.; Oliveira, C. Fermentation Strategies for Production of Pharmaceutical Terpenoids in Engineered Yeast. Pharmaceuticals 2021, 14, 295. [Google Scholar] [CrossRef] [PubMed]
- Dusséaux, S.; Wajn, W.T.; Liu, Y.; Ignea, C.; Kampranis, S.C. Transforming yeast peroxisomes into microfactories for the efficient production of high-value isoprenoids. Proc. Natl. Acad. Sci. USA 2020, 117, 31789–31799. [Google Scholar] [CrossRef]
- Li, H.; Bhadury, P.S.; Song, B.; Yang, S. Immobilized functional ionic liquids: Efficient, green, and reusable catalysts. RSC Adv. 2012, 2, 12525–12551. [Google Scholar] [CrossRef]
- Lee, C.; Sandig, B.; Buchmeiser, M.R.; Haumann, M. Supported ionic liquid phase (SILP) facilitated gas-phase enzyme catalysis—CALB catalyzed transesterification of vinyl propionate. Catal. Sci. Technol. 2018, 8, 2460–2466. [Google Scholar] [CrossRef]
- Wang, M.; Wang, M.; Zhang, S.; Chen, J. Pickering gel emulsion stabilized by enzyme immobilized polymeric nanoparticles: A robust and recyclable biocatalyst system for biphasic catalysis. React. Chem. Eng. 2019, 4, 1459–1465. [Google Scholar] [CrossRef]
- Wiese, S.; Spiess, A.C.; Richtering, W. Microgel-Stabilized Smart Emulsions for Biocatalysis. Angew. Chem. Int. Ed. 2012, 52, 576–579. [Google Scholar] [CrossRef]
- Xu, L.-J.; Liu, Y.-S.; Zhou, L.-G.; Wu, J.-Y. Enhanced beauvericin production with in situ adsorption in mycelial liquid culture of Fusarium redolens Dzf2. Process Biochem. 2009, 44, 1063–1067. [Google Scholar] [CrossRef]
- Qi, H.; Zhao, S.; Fu, H.; Wen, J.; Jia, X. Enhancement of ascomycin production in Streptomyces hygroscopicus var. ascomyceticus by combining resin HP20 addition and metabolic profiling analysis. J. Ind. Microbiol. Biotechnol. 2014, 41, 1365–1374. [Google Scholar] [CrossRef]
- Fordjour, E.; Liu, C.L.; Hao, Y.P.; Sackey, I.; Yang, Y.K.; Liu, X.X.; Li, Y.; Tan, T.W.; Bai, Z.H. Engineering Escherichia coli BL21 (DE3) for high-yield production of germacrene A, a precursor of β-elemene via combinatorial metabolic engineering strategies. Biotechnol. Bioeng. 2023, 120, 3039–3056. [Google Scholar] [CrossRef]
- Liu, W.; Xu, X.; Zhang, R.; Cheng, T.; Cao, Y.; Li, X.; Guo, J.; Liu, H.; Xian, M. Engineering Escherichia coli for high-yield geraniol production with biotransformation of geranyl acetate to geraniol under fed-batch culture. Biotechnol. Biofuels 2016, 9, 58. [Google Scholar] [CrossRef]
- Engels, B.; Dahm, P.; Jennewein, S. Metabolic engineering of taxadiene biosynthesis in yeast as a first step towards Taxol (Paclitaxel) production. Metab. Eng. 2008, 10, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Tsueng, G.; Lam, K.S. Stabilization effect of resin on the production of potent proteasome inhibitor NPI-0052 during submerged fermentation of Salinispora tropica. J. Antibiot. 2007, 60, 469–472. [Google Scholar] [CrossRef]
- Pemberton, T.A.; Chen, M.B.; Harris, G.G.; Chou, W.K.W.; Duan, L.; Köksal, M.; Genshaft, A.S.; Cane, D.E.; Christianson, D.W. Exploring the Influence of Domain Architecture on the Catalytic Function of Diterpene Synthases. Biochemistry 2017, 56, 2010–2023. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Yuan, S.Q.; Li, L.S.; Zheng, J.; Zhao, D.; Wang, C.J.; Wang, H.; Liu, X.; Liu, J. Application of Terpenoid Compounds in Food and Pharmaceutical Products. Fermentation 2023, 9, 119. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, X. Biosynthesis of monoterpenoid and sesquiterpenoid as natural flavors and fragrances. Biotechnol. Adv. 2023, 65, 22. [Google Scholar] [CrossRef]
- Ren, Y.Y.; Liu, S.S.; Jin, G.J.; Yang, X.B.; Zhou, Y.J.J. Microbial production of limonene and its derivatives: Achievements and perspectives. Biotechnol. Adv. 2020, 44, 16. [Google Scholar] [CrossRef]
- Daletos, G.; Katsimpouras, C.; Stephanopoulos, G. Novel Strategies and Platforms for Industrial Isoprenoid Engineering. Trends Biotechnol. 2020, 38, 811–822. [Google Scholar] [CrossRef]
- Marienhagen, J.; Bott, M. Metabolic engineering of microorganisms for the synthesis of plant natural products. J. Biotechnol. 2013, 163, 166–178. [Google Scholar] [CrossRef]
- Chen, X.X.; Zhang, C.Q.; Lindley, N.D. Metabolic Engineering Strategies for Sustainable Terpenoid Flavor and Fragrance Synthesis. J. Agric. Food Chem. 2020, 68, 10252–10264. [Google Scholar] [CrossRef]
- Abdel-Mawgoud, A.M.; Markham, K.A.; Palmer, C.M.; Liu, N.; Stephanopoulos, G.; Alper, H.S. Metabolic engineering in the host Yarrowia lipolytica. Metab. Eng. 2018, 50, 192–208. [Google Scholar] [CrossRef]
- Groenewald, M.; Boekhout, T.; Neuvéglise, C.; Gaillardin, C.; van Dijck, P.W.M.; Wyss, M. Yarrowia lipolytica: Safety assessment of an oleaginous yeast with a great industrial potential. Crit. Rev. Microbiol. 2014, 40, 187–206. [Google Scholar] [CrossRef]
- Geiselman, G.M.; Kirby, J.; Landera, A.; Otoupal, P.; Papa, G.; Barcelos, C.; Sundstrom, E.R.; Das, L.; Magurudeniya, H.D.; Wehrs, M.; et al. Conversion of poplar biomass into high-energy density tricyclic sesquiterpene jet fuel blendstocks. Microb. Cell Factories 2020, 19, 16. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, G.; Su, L.Q.; Liu, P.; Jia, S.R.; Wang, Q.H.; Dai, Z.J. Reprogramming the metabolism of oleaginous yeast for sustainably biosynthesizing the anticarcinogen precursor germacrene A. Green Chem. 2023, 25, 7988–7997. [Google Scholar] [CrossRef]
- Özaydin, B.; Burd, H.; Lee, T.S.; Keasling, J.D. Carotenoid-based phenotypic screen of the yeast deletion collection reveals new genes with roles in isoprenoid production. Metab. Eng. 2013, 15, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xiao, L.J.; Zhang, X.Y.; Zhang, J.; Zhang, Y.; Wang, F.; Li, X. Combined bioderivatization and engineering approach to improve the efficiency of geraniol production. Green Chem. 2022, 24, 864–876. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, C.L.; Yang, L.Y.; Choi, E.S.; Kim, S.W. Geranyl diphosphate synthase: An important regulation point in balancing a recombinant monoterpene pathway in Escherichia coli. Enzym. Microb. Technol. 2015, 68, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.Z.; Li, C.; Zhang, Y.; Shen, Y.; Hou, J.; Bao, X.M. Dynamic control of ERG20 expression combined with minimized endogenous downstream metabolism contributes to the improvement of geraniol production in Saccharomyces cerevisiae. Microb. Cell Factories 2017, 16, 11. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, X.; Zhang, J.; Zhou, Y.; Wang, F.; Zhang, Y.; Li, X. Co-localizing key pathway enzymes by protein scaffold to enhance geraniol production in Escherichia coli. Ind. Crops Prod. 2023, 203, 117144. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, W.; Du, G.; Chen, J.; Zhou, J. Overproduction of geraniol by enhanced precursor supply in Saccharomyces cerevisiae. J. Biotechnol. 2013, 168, 446–451. [Google Scholar] [CrossRef]
- Jiang, G.-Z.; Yao, M.-D.; Wang, Y.; Zhou, L.; Song, T.-Q.; Liu, H.; Xiao, W.-H.; Yuan, Y.-J. Manipulation of GES and ERG20 for geraniol overproduction in Saccharomyces cerevisiae. Metab. Eng. 2017, 41, 57–66. [Google Scholar] [CrossRef]
- Li, M.; Xu, S.; Lu, W. Engineering Corynebacterium glutamicum for Geraniol Production. Trans. Tianjin Univ. 2020, 27, 377–384. [Google Scholar] [CrossRef]
- Alonso-Gutierrez, J.; Kim, E.-M.; Batth, T.S.; Cho, N.; Hu, Q.; Chan, L.J.G.; Petzold, C.J.; Hillson, N.J.; Adams, P.D.; Keasling, J.D.; et al. Principal component analysis of proteomics (PCAP) as a tool to direct metabolic engineering. Metab. Eng. 2015, 28, 123–133. [Google Scholar] [CrossRef]
- Wu, J.; Cheng, S.; Cao, J.; Qiao, J.; Zhao, G.-R. Systematic Optimization of Limonene Production in Engineered Escherichia coli. J. Agric. Food Chem. 2019, 67, 7087–7097. [Google Scholar] [CrossRef]
- Willrodt, C.; Hoschek, A.; Bühler, B.; Schmid, A.; Julsing, M.K. Decoupling production from growth by magnesium sulfate limitation boosts de novo limonene production. Biotechnol. Bioeng. 2015, 113, 1305–1314. [Google Scholar] [CrossRef]
- Rolf, J.; Julsing, M.K.; Rosenthal, K.; Lütz, S. A Gram-Scale Limonene Production Process with Engineered Escherichia coli. Molecules 2020, 25, 1881. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Li, H.; Weng, Y.; Li, P.; Zhang, C.; Xiao, D. Improve the production of d-limonene by regulating the mevalonate pathway of Saccharomyces cerevisiae during alcoholic beverage fermentation. J. Ind. Microbiol. Biotechnol. 2020, 47, 1083–1097. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, X.; Meng, Y.; Zhang, L.; Qiao, J.; Zhao, G.-R. Combinatorial engineering of Saccharomyces cerevisiae for improving limonene production. Biochem. Eng. J. 2021, 176, 108155. [Google Scholar] [CrossRef]
- Kong, X.; Wu, Y.; Yu, W.; Liu, Y.; Li, J.; Du, G.; Lv, X.; Liu, L. Efficient Synthesis of Limonene in Saccharomyces cerevisiae Using Combinatorial Metabolic Engineering Strategies. J. Agric. Food Chem. 2023, 71, 7752–7764. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, M.; Ren, Y.; Jin, G.; Tao, Y.; Lyu, L.; Zhao, Z.K.; Yang, X. Engineering Rhodosporidium toruloides for limonene production. Biotechnol. Biofuels 2021, 14, 243. [Google Scholar] [CrossRef]
- Zhao, D.; Gao, Q.; Zheng, X.; Liu, S.; Qi, Q.; Wang, X.; Yang, X. Optimization of Fermentation Conditions for Elevating Limonene Production with Engineered Rhodosporidium toruloides. Fermentation 2023, 9, 431. [Google Scholar] [CrossRef]
- Muñoz-Fernández, G.; Martínez-Buey, R.; Revuelta, J.L.; Jiménez, A. Metabolic engineering of Ashbya gossypii for limonene production from xylose. Biotechnol. Biofuels Bioprod. 2022, 15, 79. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.H.; Lv, Y.-B.; Chen, J.; Imanaka, T.; Wei, L.; Hua, Q. Metabolic engineering of oleaginous yeast Yarrowia lipolytica for limonene overproduction. Biotechnol. Biofuels 2016, 9, 214. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Wei, L.; Lv, Y.-B.; Chen, J.; Hua, Q. Elevating Limonene Production in Oleaginous Yeast Yarrowia lipolytica via Genetic Engineering of Limonene Biosynthesis Pathway and Optimization of Medium Composition. Biotechnol. Bioprocess Eng. 2019, 24, 500–506. [Google Scholar] [CrossRef]
- Lin, P.-C.; Saha, R.; Zhang, F.; Pakrasi, H.B. Metabolic engineering of the pentose phosphate pathway for enhanced limonene production in the cyanobacterium Synechocystis sp. PCC 6803. Sci. Rep. 2017, 7, 17503. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-C.; Zhang, F.; Pakrasi, H.B. Enhanced limonene production in a fast-growing cyanobacterium through combinatorial metabolic engineering. Metab. Eng. Commun. 2021, 12, e00164. [Google Scholar] [CrossRef]
- Sun, C.; Dong, X.; Zhang, R.; Xie, C. Effectiveness of recombinant Escherichia coli on the production of (R)-(+)-perillyl alcohol. BMC Biotechnol. 2020, 21, 3. [Google Scholar] [CrossRef]
- Alonso-Gutiérrez, J.; Chan, R.; Batth, T.S.; Adams, P.D.; Keasling, J.D.; Petzold, C.J.; Lee, T.S. Metabolic engineering of Escherichia coli for limonene and perillyl alcohol production. Metab. Eng. 2013, 19, 33–41. [Google Scholar] [CrossRef]
- Kong, S.; Fu, X.-Z.; Li, X.; Pan, H.; Guo, D. De novo biosynthesis of linalool from glucose in engineered Escherichia coli. Enzym. Microb. Technol. 2020, 140, 109614. [Google Scholar] [CrossRef]
- Ferraz, C.A.; Leferink, N.G.H.; Kosov, I.; Scrutton, N.S. Isopentenol Utilization Pathway for the Production of Linalool in Escherichia coli Using an Improved Bacterial Linalool/Nerolidol Synthase. Chembiochem 2021, 22, 2325–2334. [Google Scholar] [CrossRef]
- Mendez-Perez, D.; Alonso-Gutiérrez, J.; Hu, Q.; Molinas, M.; Baidoo, E.E.K.; Wang, G.; Chan, L.J.G.; Adams, P.D.; Petzold, C.J.; Keasling, J.D.; et al. Production of jet fuel precursor monoterpenoids from engineered Escherichia coli. Biotechnol. Bioeng. 2017, 114, 1703–1712. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Zhang, X.; Zhang, J.; Zhou, Y.; Wang, F.; Li, X. Efficient myrcene production using linalool dehydratase isomerase and rational biochemical process in Escherichia coli. J. Biotechnol. 2023, 371–372, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, X.; Xiao, L.; Wang, F.; Zhang, Y.; Li, X. Synthetic Protein Scaffolds for Improving R-(−)-Linalool Production in Escherichia coli. J. Agric. Food Chem. 2021, 69, 5663–5670. [Google Scholar] [CrossRef] [PubMed]
- Amiri, P.; Shahpiri, A.; Asadollahi, M.A.; Momenbeik, F.; Partow, S. Metabolic engineering of Saccharomyces cerevisiae for linalool production. Biotechnol. Lett. 2015, 38, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.C.; Wang, J.; Cao, X.; Liu, W.; Yu, H.; Ye, L. High-level production of linalool by engineered Saccharomyces cerevisiae harboring dual mevalonate pathways in mitochondria and cytoplasm. Enzym. Microb. Technol. 2020, 134, 109462. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Du, Y.; Fang, X.; Xu, N.; Yue, C.; Ye, L. Combinatorial Modulation of Linalool Synthase and Farnesyl Diphosphate Synthase for Linalool Overproduction in Saccharomyces cerevisiae. J. Agric. Food Chem. 2021, 69, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.H.; Wei, L.; Lin, J.; Hua, Q. Enhancing linalool production by engineering oleaginous yeast Yarrowia lipolytica. Bioresour. Technol. 2017, 245 Pt B, 1641–1644. [Google Scholar] [CrossRef]
- Taratynova, M.O.; Tikhonova, E.E.; Fedyaeva, I.M.; Dementev, D.A.; Yuzbashev, T.V.; Solovyev, A.I.; Sineoky, S.P.; Yuzbasheva, E.Y. Boosting Geranyl Diphosphate Synthesis for Linalool Production in Engineered Yarrowia lipolytica. Appl. Biochem. Biotechnol. 2023, 196, 1304–1315. [Google Scholar] [CrossRef]
- Hoshino, Y.; Moriya, M.; Matsudaira, A.; Katashkina, J.I.; Nitta, N.; Nishio, Y.; Usuda, Y. Stereospecific linalool production utilizing two-phase cultivation system in Pantoea ananatis. J. Biotechnol. 2020, 324, 21–27. [Google Scholar] [CrossRef]
- Nitta, N.; Tajima, Y.; Yamamoto, Y.; Moriya, M.; Matsudaira, A.; Hoshino, Y.; Nishio, Y.; Usuda, Y. Fermentative production of enantiopure (S)-linalool using a metabolically engineered Pantoea ananatis. Microb. Cell Factories 2021, 20, 54. [Google Scholar] [CrossRef]
- Karuppiah, V.; Ranaghan, K.E.; Leferink, N.G.H.; Johannissen, L.O.; Shanmugam, M.; Ní Cheallaigh, A.; Bennett, N.J.; Kearsey, L.J.; Takano, E.; Gardiner, J.M.; et al. Structural Basis of Catalysis in the Bacterial Monoterpene Synthases Linalool Synthase and 1,8-Cineole Synthase. ACS Catal. 2017, 7, 6268–6282. [Google Scholar] [CrossRef] [PubMed]
- Ignea, C.; Cvetković, I.; Loupassaki, S.; Kefalas, P.; Johnson, C.B.; Kampranis, S.C.; Makris, A.M. Improving yeast strains using recyclable integration cassettes, for the production of plant terpenoids. Microb. Cell Factories 2011, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Q.; Cao, Y.; Feng, X.; Zheng, Y.; Zou, H.; Liu, H.; Yang, J.; Xian, M. Microbial production of sabinene—A new terpene-based precursor of advanced biofuel. Microb. Cell Factories 2014, 13, 20. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, T.; Zou, H.; Zhang, H.; Xu, X.; Sun, C.; Aboulnaga, E.A.; Cheng, Z.; Zhao, G.; Xian, M. High titer mevalonate fermentation and its feeding as a building block for isoprenoids (isoprene and sabinene) production in engineered Escherichia coli. Process Biochem. 2017, 62, 1–9. [Google Scholar]
- Ignea, C.; Pontini, M.; Maffei, M.E.; Makris, A.M.; Kampranis, S.C. Engineering monoterpene production in yeast using a synthetic dominant negative geranyl diphosphate synthase. ACS Synth. Biol. 2014, 3, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Nie, Q.; Ren, M.-F.; Feng, H.; Jiang, X.; Zheng, Y.; Liu, M.; Zhang, H.; Xian, M. Metabolic engineering of Escherichia coli for the biosynthesis of alpha-pinene. Biotechnol. Biofuels 2013, 6, 60. [Google Scholar] [CrossRef]
- Niu, F.-X.; He, X.; Wu, Y.; Liu, J.-Z. Enhancing Production of Pinene in Escherichia coli by Using a Combination of Tolerance, Evolution, and Modular Co-culture Engineering. Front. Microbiol. 2018, 9, 1623. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, R.S. Metabolic engineering of Saccharomyces cerevisiae for pinene production. CIESC 2019, 70, 179–188. [Google Scholar] [CrossRef]
- Ma, T.; Zong, H.; Lu, X.; Zhuge, B. Synthesis of pinene in the industrial strain Candida glycerinogenes by modification of its mevalonate pathway. J. Microbiol. 2022, 60, 1191–1200. [Google Scholar] [CrossRef]
- Kim, E.-M.; Eom, J.-H.; Um, Y.; Kim, Y.; Woo, H.M. Microbial Synthesis of Myrcene by Metabolically Engineered Escherichia coli. J. Agric. Food Chem. 2015, 63, 4606–4612. [Google Scholar] [CrossRef]
- Chuck, C.J.; Donnelly, J. The compatibility of potential bioderived fuels with Jet A-1 aviation kerosene. Appl. Energy 2014, 118, 83–91. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, C. A new approach for bio-jet fuel generation from palm oil and limonene in the absence of hydrogen. Chem. Commun. 2015, 51, 17249–17252. [Google Scholar] [CrossRef] [PubMed]
- Willrodt, C.; David, C.C.; Cornelissen, S.; Bühler, B.; Julsing, M.K.; Schmid, A. Engineering the productivity of recombinant Escherichia coli for limonene formation from glycerol in minimal media. Biotechnol. J. 2014, 9, 1000–1012. [Google Scholar] [CrossRef] [PubMed]
- Schewe, H.; Holtmann, D.; Schrader, J. P450 BM-3-catalyzed whole-cell biotransformation of α-pinene with recombinant Escherichia coli in an aqueous-organic two-phase system. Appl. Microbiol. Biotechnol. 2009, 83, 849–857. [Google Scholar] [CrossRef]
- Shi, Q.Q.; Tang, J.J.; Gao, J.M. Picrotoxane sesquiterpenoids: Chemistry, chemo- and bio-syntheses and biological activities. Nat. Product Rep. 2022, 39, 2096–2131. [Google Scholar] [CrossRef]
- Martin, V.J.J.; Pitera, D.J.; Withers, S.T.; Newman, J.D.; Keasling, J.D. Engineering a mevalonate pathway in Escherichia coli for production of terpenoids. Nat. Biotechnol. 2003, 21, 796–802. [Google Scholar] [CrossRef]
- Crock, J.; Wildung, M.; Croteau, R. Isolation and bacterial expression of a sesquiterpene synthase cDNA clone from peppermint (Mentha x piperita, L.) that produces the aphid alarm pheromone (E)-beta-farnesene. Proc. Natl. Acad. Sci. USA 1997, 94, 12833–12838. [Google Scholar] [CrossRef]
- Wang, C.; Yoon, S.H.; Jang, H.J.; Chung, Y.R.; Kim, J.Y.; Choi, E.S.; Kim, S.W. Metabolic engineering of Escherichia coli for α-farnesene production. Metab. Eng. 2011, 13, 648–655. [Google Scholar] [CrossRef]
- Zhu, F.; Zhong, X.; Hu, M.; Lu, L.; Deng, Z.; Liu, T. In vitro reconstitution of mevalonate pathway and targeted engineering of farnesene overproduction in Escherichia coli. Biotechnol. Bioeng. 2014, 111, 1396–1405. [Google Scholar] [CrossRef]
- Tippmann, S.; Anfelt, J.; David, F.; Rand, J.M.; Siewers, V.; Uhlen, M.; Nielsen, J.; Hudson, E.P. Affibody Scaffolds Improve Sesquiterpene Production in Saccharomyces cerevisiae. ACS Synth. Biol. 2017, 6, 19–28. [Google Scholar] [CrossRef]
- Tippmann, S.; Ferreira, R.; Siewers, V.; Nielsen, J.; Chen, Y. Effects of acetoacetyl-CoA synthase expression on production of farnesene in Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 2017, 44, 911–922. [Google Scholar] [CrossRef]
- You, S.P.; Yin, Q.D.A.; Zhang, J.Y.; Zhang, C.Y.; Qi, W.; Gao, L.; Tao, Z.P.; Su, R.X.; He, Z.M. Utilization of biodiesel by-product as substrate for high-production of β-farnesene via relatively balanced mevalonate pathway in Escherichia coli. Bioresour. Technol. 2017, 243, 228–236. [Google Scholar] [CrossRef]
- Wang, J.H.; Jiang, W.; Liang, C.J.; Zhu, L.H.; Li, Y.R.; Mo, Q.; Xu, S.; Chu, A.; Zhang, L.; Ding, Z.Y.; et al. Overproduction of α-Farnesene in Saccharomyces cerevisiae by Farnesene Synthase Screening and Metabolic Engineering. J. Agric. Food Chem. 2021, 69, 3103–3113. [Google Scholar] [CrossRef]
- Meadows, A.L.; Hawkins, K.M.; Tsegaye, Y.; Antipov, E.; Kim, Y.; Raetz, L.; Dahl, R.H.; Tai, A.; Mahatdejkul-Meadows, T.; Xu, L.; et al. Rewriting yeast central carbon metabolism for industrial isoprenoid production. Nature 2016, 537, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiang, X.; Cui, Z.; Wang, Z.; Qi, Q.; Hou, J. Engineering the oleaginous yeast Yarrowia lipolytica for production of α-farnesene. Biotechnol. Biofuels 2019, 12, 296. [Google Scholar] [CrossRef] [PubMed]
- Halfmann, C.; Gu, L.P.; Gibbons, W.; Zhou, R.B. Genetically engineering cyanobacteria to convert CO2, water, and light into the long-chain hydrocarbon farnesene. Appl. Microbiol. Biotechnol. 2014, 98, 9869–9877. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Ching, C.B. Combinatorial engineering of mevalonate pathway for improved amorpha-4,11-diene production in budding yeast. Biotechnol. Bioeng. 2013, 111, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Dahl, R.H.; Zhang, F.; Alonso-Gutierrez, J.; Baidoo, E.; Batth, T.S.; Redding-Johanson, A.M.; Petzold, C.J.; Mukhopadhyay, A.; Lee, T.S.; Adams, P.D.; et al. Engineering dynamic pathway regulation using stress-response promoters. Nat. Biotechnol. 2013, 31, 1039–1046. [Google Scholar] [CrossRef]
- Kwak, S.; Yun, E.J.; Lane, S.; Oh, E.J.; Kim, K.H.; Jin, Y.S. Redirection of the Glycolytic Flux Enhances Isoprenoid Production in Saccharomyces cerevisiae. Biotechnol. J. 2019, 15, 1900173. [Google Scholar] [CrossRef] [PubMed]
- Marsafari, M.; Xu, P. Debottlenecking mevalonate pathway for antimalarial drug precursor amorphadiene biosynthesis in Yarrowia lipolytica. Metab. Eng. Commun. 2020, 10, e00121. [Google Scholar] [CrossRef]
- Yaegashi, J.; Kirby, J.; Ito, M.; Sun, J.; Dutta, T.; Mirsiaghi, M.; Sundstrom, E.R.; Rodriguez, A.; Baidoo, E.; Tanjore, D.; et al. Rhodosporidium toruloides: A new platform organism for conversion of lignocellulose into terpene biofuels and bioproducts. Biotechnol. Biofuels 2017, 10, 241. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Zou, R.; Zhang, C.; Stephanopoulos, G.; Too, H.P. Optimization of amorphadiene synthesis in bacillus subtilis via transcriptional, translational, and media modulation. Biotechnol. Bioeng. 2013, 110, 2556–2561. [Google Scholar] [CrossRef]
- Choi, S.Y.; Lee, H.J.; Choi, J.; Kim, J.; Sim, S.J.; Um, Y.; Kim, Y.; Lee, T.S.; Keasling, J.D.; Woo, H.M. Photosynthetic conversion of CO2 to farnesyl diphosphate-derived phytochemicals (amorpha-4,11-diene and squalene) by engineered cyanobacteria. Biotechnol. Biofuels 2016, 9, 202. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, J.; Lee, S.m.; Um, Y.; Kim, Y.; Sim, S.J.; Choi, J.-i.; Woo, H.M. Direct Conversion of CO2 to α-Farnesene Using Metabolically Engineered Synechococcus elongatus PCC 7942. J. Agric. Food Chem. 2017, 65, 10424–10428. [Google Scholar] [PubMed]
- Liu, H.; Chen, S.-L.; Xu, J.-Z.; Zhang, W.-G. Dual Regulation of Cytoplasm and Peroxisomes for Improved A-Farnesene Production in Recombinant Pichia pastoris. ACS Synth. Biol. 2021, 10, 1563–1573. [Google Scholar] [CrossRef]
- Yao, P.; You, S.; Qi, W.; Su, R.; He, Z. Investigation of fermentation conditions of biodiesel by-products for high production of β-farnesene by an engineered Escherichia coli. Environ. Sci. Pollut. Res. 2020, 27, 22758–22769. [Google Scholar] [CrossRef]
- Lv, J.; Wang, Y.; Zhang, C.; You, S.; Qi, W.; Su, R.; He, Z. Highly efficient production of FAMEs and β-farnesene from a two-stage biotransformation of waste cooking oils. Energy Convers. Manag. 2019, 199, 112001. [Google Scholar] [CrossRef]
- Arnesen, J.A.; Kildegaard, K.R.; Cernuda Pastor, M.; Jayachandran, S.; Kristensen, M.; Borodina, I. Yarrowia lipolytica Strains Engineered for the Production of Terpenoids. Front. Bioeng. Biotechnol. 2020, 8, 945. [Google Scholar] [CrossRef]
- Shi, T.; Li, Y.; Zhu, L.; Tong, Y.; Yang, J.; Fang, Y.; Wang, M.; Zhang, J.; Jiang, Y.; Yang, S. Engineering the oleaginous yeast Yarrowia lipolytica for β-farnesene overproduction. Biotechnol. J. 2021, 16, 2100097. [Google Scholar] [CrossRef]
- Han, G.H.; Kim, S.K.; Yoon, P.K.-S.; Kang, Y.; Kim, B.S.; Fu, Y.; Sung, B.H.; Jung, H.C.; Lee, D.-H.; Kim, S.-W.; et al. Fermentative production and direct extraction of (−)-α-bisabolol in metabolically engineered Escherichia coli. Microb. Cell Factories 2016, 15, 185. [Google Scholar] [CrossRef]
- Peralta-Yahya, P.P.; Ouellet, M.; Chan, R.; Mukhopadhyay, A.; Keasling, J.D.; Lee, T.S. Identification and microbial production of a terpene-based advanced biofuel. Nat. Commun. 2011, 2, 483. [Google Scholar] [CrossRef]
- Davies, F.K.; Work, V.H.; Beliaev, A.S.; Posewitz, M.C. Engineering Limonene and Bisabolene Production in Wild Type and a Glycogen-Deficient Mutant of Synechococcus sp. PCC 7002. Front. Bioeng. Biotechnol. 2014, 2, 21. [Google Scholar] [CrossRef]
- Sebesta, J.; Peebles, C.A.M. Improving heterologous protein expression in Synechocystis sp. PCC 6803 for alpha-bisabolene production. Metab. Eng. Commun. 2020, 10, e00117. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Zhang, L.; Zhu, S.; Yuan, W.; Hang, J.; Yin, D.; Tang, X.; Zheng, J.; Wang, Z.; Sun, J. Overexpression of the transcription factor HAC1 improves nerolidol production in engineered yeast. Enzym. Microb. Technol. 2020, 134, 109485. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; Yu, F.; Okamoto, S.; Kuzuyama, T.; Utsumi, R.; Misawa, N. Efficient synthesis of functional isoprenoids from acetoacetate through metabolic pathway-engineered Escherichia coli. Appl. Microbiol. Biotechnol. 2009, 81, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, M.; Zhao, G.-R.; Lu, W. Harnessing Yeast Peroxisomes and Cytosol Acetyl-CoA for Sesquiterpene α-Humulene Production. J. Agric. Food Chem. 2020, 68, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, Y.; Han, L.; Wang, Q.; Liu, H.; Cheng, P.; Li, R.; Guo, X.; Zhou, Z. Enhancement of Patchoulol Production in Escherichia coli via Multiple Engineering Strategies. J. Agric. Food Chem. 2021, 69, 7572–7580. [Google Scholar] [CrossRef] [PubMed]
- Albertsen, L.; Chen, Y.; Bach, L.S.; Rattleff, S.; Maury, J.; Brix, S.; Nielsen, J.; Mortensen, U.H. Diversion of Flux toward Sesquiterpene Production in Saccharomyces cerevisiae by Fusion of Host and Heterologous Enzymes. Appl. Environ. Microbiol. 2011, 77, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lin, Y.-C.; Guo, J.-J.; Du, M.-M.; Tao, X.; Gao, B.; Zhao, M.; Ma, Y.; Wang, F.-Q.; Wei, D.-Z. High-Level Production of Sesquiterpene Patchoulol in Saccharomyces cerevisiae. ACS Synth. Biol. 2021, 10, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Emmerstorfer, A.; Wimmer-Teubenbacher, M.; Wriessnegger, T.; Leitner, E.; Müller, M.; Kaluzna, I.A.; Schürmann, M.; Mink, D.G.; Zellnig, G.n.; Schwab, H.; et al. Over-expression of ICE2 stabilizes cytochrome P450 reductase in Saccharomyces cerevisiae and Pichia pastoris. Biotechnol. J. 2015, 10, 623–635. [Google Scholar] [CrossRef]
- Ouyang, X.; Li, W.; Cha, Y.; Zhu, C.; Li, S. Production of valencene and its derivatives by recombinant brewer’s yeast fermentation. Food Ferment. Ind. Editor. Staff 2019, 45, 9. [Google Scholar]
- Guo, X.; Sun, J.; Li, D.; Lu, W. Heterologous biosynthesis of (+)-nootkatone in unconventional yeast Yarrowia lipolytica. Biochem. Eng. J. 2018, 137, 125–131. [Google Scholar] [CrossRef]
- Frohwitter, J.; Heider, S.A.E.; Peters-Wendisch, P.; Beekwilder, J.; Wendisch, V.F. Production of the sesquiterpene (+)-valencene by metabolically engineered Corynebacterium glutamicum. J. Biotechnol. 2014, 191, 205–213. [Google Scholar] [CrossRef]
- Beekwilder, J.; van Houwelingen, A.; Cankar, K.; van Dijk, A.D.J.; de Jong, R.M.; Stoopen, G.; Bouwmeester, H.; Achkar, J.; Sonke, T.; Bosch, D. Valencene synthase from the heartwood of Nootka cypress (Callitropsis nootkatensis) for biotechnological production of valencene. Plant Biotechnol. J. 2013, 12, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Matsudaira, A.; Hoshino, Y.; Uesaka, K.; Takatani, N.; Omata, T.; Usuda, Y. Production of glutamate and stereospecific flavors, (S)-linalool and (+)-valencene, by Synechocystis sp. PCC6803. J. Biosci. Bioeng. 2020, 130, 464–470. [Google Scholar] [CrossRef]
- Gao, Y. The Study of Microbial Synthesis of Germacrene A the Precursor of β-Elemene. Master’s Thesis, Hangzhou Normal University, Hangzhou, China, 2012. [Google Scholar]
- Li, M.; Wen, Q.; Lv, S.; Yang, R.; Cheng, T.; Wang, Z.; Yang, J. Co-biosynthesis of germacrene A, a precursor of β-elemene, and lycopene in engineered Escherichia coli. Appl. Microbiol. Biotechnol. 2022, 106, 8053–8066. [Google Scholar] [CrossRef]
- Bröker, J.N.; Müller, B.; Prüfer, D.; Schulze Gronover, C. Combinatorial Metabolic Engineering in Saccharomyces cerevisiae for the Enhanced Production of the FPP-Derived Sesquiterpene Germacrene. Bioengineering 2020, 7, 135. [Google Scholar] [CrossRef]
- Cheng, J.; Zuo, Y.; Liu, G.; Li, D.; Gao, J.; Xiao, F.; Huang, L.; Xu, Z.; Lian, J. Development of a Pichia pastoris cell factory for efficient production of germacrene A: A precursor of β-elemene. Bioresour. Bioprocess. 2023, 10, 38. [Google Scholar] [CrossRef]
- Ye, M.; Gao, J.; Zhou, Y.J. Global metabolic rewiring of the nonconventional yeast Ogataea polymorpha for biosynthesis of the sesquiterpenoid β-elemene. Metab. Eng. 2023, 76, 225–231. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Zhang, X.; Zhang, Y.; Wang, F.; Li, X. Sesquiterpene Synthase Engineering and Targeted Engineering of α-Santalene Overproduction in Escherichia coli. J. Agric. Food Chem. 2022, 70, 5377–5385. [Google Scholar] [CrossRef]
- Scalcinati, G.; Knuf, C.; Partow, S.; Chen, Y.; Maury, J.; Schalk, M.; Daviet, L.; Nielsen, J.; Siewers, V. Dynamic control of gene expression in Saccharomyces cerevisiae engineered for the production of plant sesquitepene α-santalene in a fed-batch mode. Metab. Eng. 2012, 14, 91–103. [Google Scholar] [CrossRef]
- Asadollahi, M.A.; Maury, J.; Møller, K.; Nielsen, K.F.; Schalk, M.; Clark, A.; Nielsen, J. Production of plant sesquiterpenes in Saccharomyces cerevisiae: Effect of ERG9 repression on sesquiterpene biosynthesis. Biotechnol. Bioeng. 2007, 99, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Xu, S.; Sun, J.; Zhang, C.; Li, D.; Lu, W. Yarrowia lipolytica construction for heterologous synthesis of α-santalene and fermentation optimization. Appl. Microbiol. Biotechnol. 2019, 103, 3511–3520. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Liu, F.; Davis, R.W. Engineering Escherichia coli for the production of terpene mixture enriched in caryophyllene and caryophyllene alcohol as potential aviation fuel compounds. Metab. Eng. Commun. 2018, 6, 13–21. [Google Scholar] [CrossRef]
- Yang, J.; Li, Z.; Guo, L.; Du, J.; Bae, H.-J. Biosynthesis of β-caryophyllene, a novel terpene-based high-density biofuel precursor, using engineered Escherichia coli. Renew. Energy 2016, 99, 216–223. [Google Scholar] [CrossRef]
- Cheng, T.; Zhang, K.; Guo, J.; Yang, Q.; Li, Y.; Xian, M.; Zhang, R. Highly efficient biosynthesis of β-caryophyllene with a new sesquiterpene synthase from tobacco. Biotechnol. Biofuels Bioprod. 2022, 15, 39. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, C.; Wang, P.; Yan, X.; Zhou, Z. Production of sesquiterpenoids α-neoclovene and β-caryophyllene by engineered Saccharomyces cerevisiae. Synth. Biol. J. 2021, 2, 792. [Google Scholar]
- Melillo, E.; Setroikromo, R.; Quax, W.J.; Kayser, O. Production of α-cuprenene in Xanthophyllomyces dendrorhous: A step closer to a potent terpene biofactory. Microb. Cell Factories 2013, 12, 13. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, R.; Liu, W.; Zhao, G.; Niu, W.; Guo, J.; Xian, M.; Liu, H. Manipulation of the precursor supply for high-level production of longifolene by metabolically engineered Escherichia coli. Sci. Rep. 2019, 9, 95. [Google Scholar] [CrossRef]
- Aguilar, F.; Scheper, T.; Beutel, S. Improved Production and In Situ Recovery of Sesquiterpene (+)-Zizaene from Metabolically-Engineered E. coli. Molecules 2019, 24, 3356. [Google Scholar] [CrossRef]
- Nybo, S.E.; Saunders, J.; McCormick, S.P. Metabolic engineering of Escherichia coli for production of valerenadiene. J. Biotechnol. 2017, 262, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, C.; Zhou, J.; Kim, S.-W. Combinatorial engineering of hybrid mevalonate pathways in Escherichia coli for protoilludene production. Microb. Cell Factories 2016, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Chappell, J. Building terpene production platforms in yeast. Biotechnol. Bioeng. 2015, 112, 1854–1864. [Google Scholar] [CrossRef]
- Zada, B.; Wang, C.; Park, J.-B.; Jeong, S.-H.; Park, J.-E.; Singh, H.B.; Kim, S.-W. Metabolic engineering of Escherichia coli for production of mixed isoprenoid alcohols and their derivatives. Biotechnol. Biofuels 2018, 11, 210. [Google Scholar] [CrossRef]
- Liu, C.-L.; Tian, T.; Alonso-Gutierrez, J.; Garabedian, B.; Wang, S.; Baidoo, E.E.K.; Benites, V.; Chen, Y.; Petzold, C.J.; Adams, P.D.; et al. Renewable production of high density jet fuel precursor sesquiterpenes from Escherichia coli. Biotechnol. Biofuels 2018, 11, 285. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; d’Espaux, L.; Dev, I.; van der Horst, C.; Keasling, J. De novo synthesis of the sedative valerenic acid in Saccharomyces cerevisiae. Metab. Eng. 2018, 47, 94–101. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, J.; Zhao, F.; Lu, C.; Zhao, G.-R.; Lu, W. Production of sesquiterpenoid zerumbone from metabolic engineered Saccharomyces cerevisiae. Metab. Eng. 2018, 49, 28–35. [Google Scholar] [CrossRef]
- Zha, W.; An, T.; Li, T.; Zhu, J.; Gao, K.; Sun, Z.; Xu, W.; Lin, P.; Zi, J. Reconstruction of the Biosynthetic Pathway of Santalols under Control of the GAL Regulatory System in Yeast. ACS Synth. Biol. 2020, 9, 449–456. [Google Scholar] [CrossRef]
- Adio, A.M. (-)trans-β-Elemene and related compounds: Occurrence, synthesis, and anticancer activity. Tetrahedron 2009, 65, 5145–5159. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, Y.; Huang, L.; Zhang, X. Production of miltiradiene by metabolically engineered Saccharomyces cerevisiae. Biotechnol. Bioeng. 2012, 109, 2845–2853. [Google Scholar] [CrossRef]
- Ajikumar, P.K.; Xiao, W.H.; Tyo, K.E.J.; Wang, Y.; Simeon, F.; Leonard, E.; Mucha, O.; Phon, T.H.; Pfeifer, B.; Stephanopoulos, G. Isoprenoid Pathway Optimization for Taxol Precursor Overproduction in Escherichia coli. Science 2010, 330, 70–74. [Google Scholar] [CrossRef]
- Nowrouzi, B.; Li, R.A.; Walls, L.E.; d’Espaux, L.; Malci, K.; Liang, L.G.; Jonguitud-Borrego, N.; Lerma-Escalera, A.I.; Morones-Ramirez, J.R.; Keasling, J.D.; et al. Enhanced production of taxadiene in Saccharomyces cerevisiae. Microb. Cell Factories 2020, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.Y.; Zhou, J.W.; Tong, Y.R.; Su, P.; Li, X.L.; Liu, Y.; Liu, N.; Wu, X.Y.; Zhang, Y.F.; Wang, J.D.; et al. Engineering chimeric diterpene synthases and isoprenoid biosynthetic pathways enables high-level production of miltiradiene in yeast. Metab. Eng. 2020, 60, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Yu, W.; Chen, Y.; Yang, S.; Zhao, Z.K.; Nielsen, J.; Luan, H.W.; Zhou, Y.J. Engineering yeast for high-level production of diterpenoid sclareol. Metab. Eng. 2023, 75, 19–28. [Google Scholar] [CrossRef]
- Huang, Q.; Roessner, C.A.; Croteau, R.; Scott, A.I. Engineering Escherichia coli for the synthesis of taxadiene, a key intermediate in the biosynthesis of taxol. Bioorg. Med. Chem. 2001, 9, 2237–2242. [Google Scholar] [CrossRef] [PubMed]
- Biggs, B.W.; Lim, C.G.; Sagliani, K.; Shankar, S.; Stephanopoulos, G.; De Mey, M.; Ajikumar, P.K. Overcoming heterologous protein interdependency to optimize P450-mediated Taxol precursor synthesis in Escherichia coli. Proc. Natl. Acad. Sci. USA 2016, 113, 3209–3214. [Google Scholar] [CrossRef]
- Zhou, K.; Qiao, K.; Edgar, S.; Stephanopoulos, G. Distributing a metabolic pathway among a microbial consortium enhances production of natural products. Nat. Biotechnol. 2015, 33, 377–383. [Google Scholar] [CrossRef]
- Walls, L.E.; Malcı, K.; Nowrouzi, B.; Li, R.A.; d’Espaux, L.; Wong, J.; Dennis, J.A.; Semião, A.J.C.; Wallace, S.; Martinez, J.L.; et al. Optimizing the biosynthesis of oxygenated and acetylated Taxol precursors in Saccharomyces cerevisiae using advanced bioprocessing strategies. Biotechnol. Bioeng. 2020, 118, 279–293. [Google Scholar] [CrossRef]
- Kong, M.K.; Kang, H.-J.; Kim, J.H.; Oh, S.H.; Lee, P.C. Metabolic engineering of the Stevia rebaudiana ent-kaurene biosynthetic pathway in recombinant Escherichia coli. J. Biotechnol. 2015, 214, 95–102. [Google Scholar] [CrossRef]
- Moon, J.H.; Lee, K.; Lee, J.H.; Lee, P.C. Redesign and reconstruction of a steviol-biosynthetic pathway for enhanced production of steviol in Escherichia coli. Microb. Cell Factories 2020, 19, 20. [Google Scholar] [CrossRef]
- Geiselman, G.M.; Zhuang, X.; Kirby, J.; Tran-Gyamfi, M.B.; Prahl, J.-P.; Sundstrom, E.R.; Gao, Y.; Munoz Munoz, N.; Nicora, C.D.; Clay, D.M.; et al. Production of ent-kaurene from lignocellulosic hydrolysate in Rhodosporidium toruloides. Microb. Cell Factories 2020, 19, 24. [Google Scholar] [CrossRef]
- Tokuhiro, K.; Muramatsu, M.; Ohto, C.; Kawaguchi, T.; Obata, S.; Muramoto, N.; Hirai, M.; Takahashi, H.; Kondo, A.; Sakuradani, E.; et al. Overproduction of Geranylgeraniol by Metabolically Engineered Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2009, 75, 5536–5543. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, L.; Li, Y.; Xu, S.; Jiang, W.; Liang, C.; Fang, Y.; Chu, A.; Zhang, L.; Ding, Z.; et al. Enhancing Geranylgeraniol Production by Metabolic Engineering and Utilization of Isoprenol as a Substrate in Saccharomyces cerevisiae. J. Agric. Food Chem. 2021, 69, 4480–4489. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, Z.; Wang, G.; Lv, H.; Mao, Y.; Ma, K.; Wang, Y. De novo production of versatile oxidized kaurene diterpenes in Escherichia coli. Metab. Eng. 2022, 73, 201–213. [Google Scholar] [CrossRef]
- Schalk, M.; Pastore, L.; Mirata, M.A.; Khim, S.; Schouwey, M.; Deguerry, F.; Pineda, V.; Rocci, L.; Daviet, L. Toward a Biosynthetic Route to Sclareol and Amber Odorants. J. Am. Chem. Soc. 2012, 134, 18900–18903. [Google Scholar] [CrossRef]
- Ignea, C.; Trikka, F.A.; Nikolaidis, A.K.; Georgantea, P.; Ioannou, E.; Loupassaki, S.; Kefalas, P.; Kanellis, A.K.; Roussis, V.; Makris, A.M.; et al. Efficient diterpene production in yeast by engineering Erg20p into a geranylgeranyl diphosphate synthase. Metab. Eng. 2015, 27, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Leonard, E.; Ajikumar, P.K.; Thayer, K.; Xiao, W.-H.; Mo, J.D.; Tidor, B.; Stephanopoulos, G.; Prather, K.L.J. Combining metabolic and protein engineering of a terpenoid biosynthetic pathway for overproduction and selectivity control. Proc. Natl. Acad. Sci. USA 2010, 107, 13654–13659. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, C.; Lu, W. Heterologous production of levopimaric acid in Saccharomyces cerevisiae. Microb. Cell Factories 2018, 17, 114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, X.; Lindley, N.; Too, H.-P. A “plug-n-play” modular metabolic system for the production of apocarotenoids. Biotechnol. Bioeng. 2018, 115, 174–183. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, T.; Yu, H.; Ye, L. Selective biosynthesis of retinol in S. cerevisiae. Bioresour. Bioprocess. 2022, 9, 22. [Google Scholar] [CrossRef]
- Park, H.; Lee, D.; Kim, J.-E.; Park, S.; Park, J.H.; Ha, C.W.; Baek, M.; Yoon, S.-H.; Park, K.H.; Lee, P.; et al. Efficient production of retinol in Yarrowia lipolytica by increasing stability using antioxidant and detergent extraction. Metab. Eng. 2022, 73, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, X.; Li, X.; Shi, H.; Wang, F.; Zhang, Y.; Li, X. Combinatorial Engineering of Mevalonate Pathway and Diterpenoid Synthases in Escherichia coli for cis-Abienol Production. J. Agric. Food Chem. 2019, 67, 6523–6531. [Google Scholar] [CrossRef]
- Zhang, C.; Ju, H.; Lu, C.-Z.; Zhao, F.; Liu, J.; Guo, X.; Wu, Y.; Zhao, G.-R.; Lu, W. High-titer production of 13R-manoyl oxide in metabolically engineered Saccharomyces cerevisiae. Microb. Cell Factories 2019, 18, 73. [Google Scholar] [CrossRef]
- Pateraki, I.; Andersen-Ranberg, J.; Jensen, N.B.; Wubshet, S.G.; Heskes, A.M.; Forman, V.; Hallström, B.; Hamberger, B.; Motawia, M.S.; Olsen, C.E.; et al. Total biosynthesis of the cyclic AMP booster forskolin from Coleus forskohlii. eLife 2017, 6, 23001. [Google Scholar] [CrossRef] [PubMed]
- Kildegaard, K.R.; Arnesen, J.A.; Adiego-Pérez, B.; Rago, D.; Kristensen, M.; Klitgaard, A.K.; Hansen, E.H.; Hansen, J.; Borodina, I. Tailored biosynthesis of gibberellin plant hormones in yeast. Metab. Eng. 2021, 66, 1–11. [Google Scholar] [CrossRef]
- Wei, P.; Zhang, C.; Bian, X.; Lu, W. Metabolic Engineering of Saccharomyces cerevisiae for Heterologous Carnosic Acid Production. Front. Bioeng. Biotechnol. 2022, 10, 916605. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, X.; Zhang, C.; Zhou, X.; Xu, X.; Han, L.; Lv, X.; Liu, Y.; Liu, S.; Li, J.; et al. De novo biosynthesis of rubusoside and rebaudiosides in engineered yeasts. Nat. Commun. 2022, 13, 3040. [Google Scholar] [CrossRef]
- Yendo, A.C.A.; de Costa, F.; Gosmann, G.; Fett-Neto, A.G. Production of Plant Bioactive Triterpenoid Saponins: Elicitation Strategies and Target Genes to Improve Yields. Mol. Biotechnol. 2010, 46, 94–104. [Google Scholar] [CrossRef]
- Zhao, Y.-j.; Li, C. Biosynthesis of Plant Triterpenoid Saponins in Microbial Cell Factories. J. Agric. Food Chem. 2018, 66, 12155–12165. [Google Scholar] [CrossRef]
- Foong, L.C.; Loh, C.W.L.; Ng, H.S.; Lan, J.C.-W. Recent development in the production strategies of microbial carotenoids. World J. Microbiol. Biotechnol. 2021, 37, 12. [Google Scholar] [CrossRef]
- Dai, Z.B.; Liu, Y.; Zhang, X.A.; Shi, M.Y.; Wang, B.B.; Wang, D.; Huang, L.Q.; Zhang, X.L. Metabolic engineering of Saccharomyces cerevisiae for production of ginsenosides. Metab. Eng. 2013, 20, 146–156. [Google Scholar] [CrossRef]
- Luo, Z.S.; Liu, N.; Lazar, Z.; Chatzivasileiou, A.; Ward, V.; Chen, J.; Zhou, J.W.; Stephanopoulos, G. Enhancing isoprenoid synthesis in Yarrowia lipolytica by expressing the isopentenol utilization pathway and modulating intracellular hydrophobicity. Metab. Eng. 2020, 61, 344–351. [Google Scholar] [CrossRef]
- Li, T.; Liu, G.-S.; Zhou, W.; Jiang, M.; Ren, Y.-H.; Tao, X.-Y.; Liu, M.; Zhao, M.; Wang, F.-Q.; Gao, B.; et al. Metabolic Engineering of Saccharomyces cerevisiae To Overproduce Squalene. J. Agric. Food Chem. 2020, 68, 2132–2138. [Google Scholar] [CrossRef]
- Ke, D.; Caiyin, Q.; Zhao, F.; Liu, T.; Lu, W. Heterologous biosynthesis of triterpenoid ambrein in engineered Escherichia coli. Biotechnol. Lett. 2017, 40, 399–404. [Google Scholar] [CrossRef]
- Moser, S.; Strohmeier, G.A.; Leitner, E.; Plocek, T.J.; Vanhessche, K.; Pichler, H. Whole-cell (+)-ambrein production in the yeast Pichia pastoris. Metab. Eng. Commun. 2018, 7, e00077. [Google Scholar] [CrossRef]
- Huang, J.; Zha, W.; An, T.; Dong, H.; Huang, Y.; Wang, D.; Yu, R.; Duan, L.; Zhang, X.; Peters, R.J.; et al. Identification of RoCYP01 (CYP716A155) enables construction of engineered yeast for high-yield production of betulinic acid. Appl. Microbiol. Biotechnol. 2019, 103, 7029–7039. [Google Scholar] [CrossRef]
- Li, W.; Ma, X.; Li, G.; Zhang, A.; Wang, D.; Fan, F.; Ma, X.; Zhang, X.; Dai, Z.; Qian, Z. De Novo Biosynthesis of the Oleanane-Type Triterpenoids of Tunicosaponins in Yeast. ACS Synth. Biol. 2021, 10, 1874–1881. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Zhou, Z.; Liang, Q.; Mosongo, I.; Li, C.; Zhang, Y. Improving lupeol production in yeast by recruiting pathway genes from different organisms. Sci. Rep. 2019, 9, 2992. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Rasool, A.; Liu, H.; Lv, B.; Chang, P.; Song, H.; Wang, Y.; Li, C. Engineering Saccharomyces cerevisiae for high yield production of α-amyrin via synergistic remodeling of α-amyrin synthase and expanding the storage pool. Metab. Eng. 2020, 62, 72–83. [Google Scholar] [CrossRef]
- Zhang, G.; Cao, Q.; Liu, J.; Liu, B.-x.; Li, J.; Li, C. Refactoring β-amyrin synthesis in Saccharomyces cerevisiae. Aiche J. 2015, 61, 3172–3179. [Google Scholar] [CrossRef]
- Kirby, J.; Romanini, D.W.; Paradise, E.M.; Keasling, J.D. Engineering triterpene production in Saccharomyces cerevisiae–β-amyrin synthase from Artemisia annua. FEBS J. 2008, 275, 1852–1859. [Google Scholar] [CrossRef]
- Gao, H.; Shao, M.; Zhang, X.; Yang, T.; Xu, M.; Gao, X.; Rao, Z. Construction of Saccharomyces cerevisiae cell factories for the production of pentacyclic triterpenoids. Food Ferment. Ind. 2021, 47, 8–14. [Google Scholar]
- Lu, C.; Zhang, C.; Zhao, F.; Li, D.; Lu, W. Biosynthesis of ursolic acid and oleanolic acid in Saccharomyces cerevisiae. AIChE J. 2018, 64, 3794–3802. [Google Scholar] [CrossRef]
- Jin, C.C.; Zhang, J.L.; Song, H.; Cao, Y.X. Boosting the biosynthesis of betulinic acid and related triterpenoids in Yarrowia lipolytica via multimodular metabolic engineering. Microb. Cell Factories 2019, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Fan, J.; Wang, C.; Feng, X.; Li, C. Enhancing oleanolic acid production in engineered Saccharomyces cerevisiae. Bioresour. Technol. 2018, 257, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.F.; Xiao, H.; Zhong, J.J. Biosynthesis of a ganoderic acid in Saccharomyces cerevisiae by expressing a cytochrome P450 gene from Ganoderma lucidum. Biotechnol. Bioeng. 2018, 115, 1842–1854. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Liu, Y.; Sun, Z.; Wang, D.; Qu, G.; Ma, X.; Fan, F.; Zhang, L.; Li, S.; Zhang, X. Identification of a novel cytochrome P450 enzyme that catalyzes the C-2α hydroxylation of pentacyclic triterpenoids and its application in yeast cell factories. Metab. Eng. 2019, 51, 70–78. [Google Scholar] [CrossRef]
- Hansen, N.L.; Miettinen, K.; Zhao, Y.; Ignea, C.; Andreadelli, A.; Raadam, M.H.; Makris, A.M.; Møller, B.L.; Stærk, D.; Bak, S.; et al. Integrating pathway elucidation with yeast engineering to produce polpunonic acid the precursor of the anti-obesity agent celastrol. Microb. Cell Factories 2020, 19, 15. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, C.; Sun, W.; Zhou, A.; Wang, Y.; Zhang, G.; Zhou, X.; Huo, Y.; Li, C. Boosting 11-oxo-β-amyrin and glycyrrhetinic acid synthesis in Saccharomyces cerevisiae via pairing novel oxidation and reduction system from legume plants. Metab. Eng. 2018, 45, 43–50. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Y.; Xu, J.; Wang, J.; Dai, Z.; Zhang, X.; Huang, L. Construction of efficient yeast cell factories for production of ginsenosides precursor dammarenediol-II. Acta Pharm. Sin. B 2018, 53, 1233–1241. [Google Scholar]
- Zhang, X. Performance Optimization of Saccharomyces cerevisiae for Dammarendiol-II Production. Master’s Thesis, Tianjin University, Tianjin, China, 2015. [Google Scholar]
- Li, D.; Zhang, Q.; Zhou, Z.; Zhao, F.; Lu, W. Heterologous biosynthesis of triterpenoid dammarenediol-II in engineered Escherichia coli. Biotechnol. Lett. 2016, 38, 603–609. [Google Scholar] [CrossRef]
- Dai, Z.; Wang, B.; Liu, Y.; Shi, M.; Wang, D.; Zhang, X.; Liu, T.; Huang, L.; Zhang, X. Producing aglycons of ginsenosides in bakers’ yeast. Sci. Rep. 2014, 4, 3698. [Google Scholar] [CrossRef]
- Wang, P.; Wei, W.; Ye, W.; Li, X.; Zhao, W.; Yang, C.; Li, C.; Yan, X.; Zhou, Z. Synthesizing ginsenoside Rh2 in Saccharomyces cerevisiae cell factory at high-efficiency. Cell Discov. 2019, 5, 5. [Google Scholar] [CrossRef]
- Wang, P.; Wei, Y.; Fan, Y.; Liu, Q.; Wei, W.; Yang, C.; Zhang, L.; Zhao, G.; Yue, J.; Yan, X.; et al. Production of bioactive ginsenosides Rh2 and Rg3 by metabolically engineered yeasts. Metab. Eng. 2015, 29, 97–105. [Google Scholar] [CrossRef]
- Jung, S.C.; Kim, W.; Park, S.C.; Jeong, J.; Park, M.K.; Lim, S.; Lee, Y.; Im, W.T.; Lee, J.H.; Choi, G.; et al. Two Ginseng UDP-Glycosyltransferases Synthesize Ginsenoside Rg3 and Rd. Plant Cell Physiol. 2014, 55, 2177–2188. [Google Scholar] [CrossRef]
- Wei, W.; Wang, P.; Wei, Y.; Liu, Q.; Yang, C.; Zhao, G.; Yue, J.; Yan, X.; Zhou, Z. Characterization of Panax ginseng UDP-Glycosyltransferases Catalyzing Protopanaxatriol and Biosyntheses of Bioactive Ginsenosides F1 and Rh1 in Metabolically Engineered Yeasts. Mol. Plant 2015, 8, 1412–1424. [Google Scholar] [CrossRef]
- Yoon, S.H.; Park, H.M.; Kim, J.E.; Lee, S.H.; Choi, M.S.; Kim, J.Y.; Oh, D.K.; Keasling, J.D.; Kim, S.W. Increased β-carotene production in recombinant Escherichia coli harboring an engineered isoprenoid precursor pathway with mevalonate addition. Biotechnol. Progress 2007, 23, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Guo, L. Biosynthesis of β-carotene in engineered E. coli using the MEP and MVA pathways. Microb. Cell Factories 2014, 13, 160. [Google Scholar] [CrossRef] [PubMed]
- Fathi, Z.; Tramontin, L.R.R.; Ebrahimipour, G.; Borodina, I.; Darvishi, F. Metabolic engineering of Saccharomyces cerevisiae for production of β-carotene from hydrophobic substrates. FEMS Yeast Res. 2020, 21, foaa068. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shen, H.; Cui, Y.-Y.; Chen, S.-G.; Weng, Z.; Zhao, M.; Liu, J.-Z. Chromosomal evolution of Escherichia coli for the efficient production of lycopene. BMC Biotechnol. 2013, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Miao, L.; Li, Q.; Dai, G.; Lu, F.; Liu, T.; Zhang, X.; Ma, Y. Production of lycopene by metabolically-engineered Escherichia coli. Biotechnol. Lett. 2014, 36, 1515–1522. [Google Scholar] [CrossRef]
- Ma, T.; Shi, B.; Ye, Z.; Li, X.; Liu, M.; Chen, Y.; Xia, J.; Nielsen, J.; Deng, Z.; Liu, T. Lipid engineering combined with systematic metabolic engineering of Saccharomyces cerevisiae for high-yield production of lycopene. Metab. Eng. 2019, 52, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Nicolás-Molina, F.E.; Navarro, E.; Ruiz-Vázquez, R.M. Lycopene over-accumulation by disruption of the negative regulator gene crgA in Mucor circinelloides. Appl. Microbiol. Biotechnol. 2008, 78, 131–137. [Google Scholar] [CrossRef]
- Wang, G.-S.; Grammel, H.; Abou-Aisha, K.; Sägesser, R.; Ghosh, R. High-Level Production of the Industrial Product Lycopene by the Photosynthetic Bacterium Rhodospirillum rubrum. Appl. Environ. Microbiol. 2012, 78, 7205–7215. [Google Scholar] [CrossRef]
- Su, A.; Chi, S.; Li, Y.; Tan, S.; Qiang, S.; Chen, Z.; Meng, Y. Metabolic Redesign of Rhodobacter sphaeroides for Lycopene Production. J. Agric. Food Chem. 2018, 66, 5879–5885. [Google Scholar] [CrossRef]
- Zuo, Z.-Q.; Xue, Q.; Zhou, J.; Zhao, D.-H.; Han, J.; Xiang, H. Engineering Haloferax mediterranei as an Efficient Platform for High Level Production of Lycopene. Front. Microbiol. 2018, 9, 2893. [Google Scholar] [CrossRef]
- Bhataya, A.; Schmidt-Dannert, C.; Lee, P.C. Metabolic engineering of Pichia pastoris X-33 for lycopene production. Process Biochem. 2009, 44, 1095–1102. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.; Duan, Y.; Zheng, X.; Lin, Y.; Liang, S. Production of lycopene by metabolically engineered Pichia pastoris. Biosci. Biotechnol. Biochem. 2020, 84, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gong, Z.; Tang, J.; Lu, F.; Li, Q.; Zhang, X. Improving astaxanthin production in Escherichia coli by co-utilizing CrtZ enzymes with different substrate preference. Microb. Cell Factories 2022, 21, 71. [Google Scholar] [CrossRef]
- Chai, F.; Wang, Y.; Mei, X.; Yao, M.; Chen, Y.; Liu, H.; Xiao, W.; Yuan, Y. Heterologous biosynthesis and manipulation of crocetin in Saccharomyces cerevisiae. Microb. Cell Factories 2017, 16, 54. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-R.; Tian, G.-Q.; Shen, H.-J.; Liu, J.-Z. Metabolic engineering of Escherichia coli to produce zeaxanthin. J. Ind. Microbiol. Biotechnol. 2015, 42, 627–636. [Google Scholar] [CrossRef]
- Shen, H.-J.; Cheng, B.-Y.; Zhang, Y.-M.; Tang, L.; Li, Z.; Bu, Y.-F.; Li, X.-R.; Tian, G.-Q.; Liu, J.-Z. Dynamic control of the mevalonate pathway expression for improved zeaxanthin production in Escherichia coli and comparative proteome analysis. Metab. Eng. 2016, 38, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Ning, J.C.; Zhao, H. Coordinated induction of multi-gene pathways in Saccharomyces cerevisiae. Nucleic Acids Res. 2013, 41, e54. [Google Scholar] [CrossRef] [PubMed]
- Beuttler, H.; Hoffmann, J.; Jeske, M.; Hauer, B.; Schmid, R.D.; Altenbuchner, J.; Urlacher, V.B. Biosynthesis of zeaxanthin in recombinant Pseudomonas putida. Appl. Microbiol. Biotechnol. 2010, 89, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Ventura, S.P.M.; e Silva, F.A.; Quental, M.V.; Mondal, D.; Freire, M.G.; Coutinho, J.A.P. Ionic-Liquid-Mediated Extraction and Separation Processes for Bioactive Compounds: Past, Present, and Future Trends. Chem. Rev. 2017, 117, 6984–7052. [Google Scholar] [CrossRef]
- Bardi, L.; Mattei, A.; Steffan, S.; Marzona, M. Hydrocarbon degradation by a soil microbial population with beta-cyclodextrin as surfactant to enhance bioavailability. Enzym. Microb. Technol. 2000, 27, 709–713. [Google Scholar] [CrossRef]
- Wu, M.; Li, W.; Dick, W.A.; Ye, X.; Chen, K.; Kost, D.; Chen, L. Bioremediation of hydrocarbon degradation in a petroleum-contaminated soil and microbial population and activity determination. Chemosphere 2017, 169, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Benfenati, E.; Gini, G.; Piclin, N.; Roncaglioni, A.; Vari, M.R. Predicting logP of pesticides using different software. Chemosphere 2003, 53, 1155–1164. [Google Scholar] [CrossRef]
- Akira Inoue, K.H. Estimation of Solvent-Tolerance of Bacteria by the Solvent Parameter Log P. J. Ferment. Bioeng. 1991, 71, 193–196. [Google Scholar]
- Laane, C.; Boeren, S.; Vos, K.; Veeger, C. Rules for optimization of biocatalysis in organic solvents. Biotechnol. Bioeng. 1987, 30, 81–87. [Google Scholar] [CrossRef]
- Demling, P.; von Campenhausen, M.; Grütering, C.; Tiso, T.; Jupke, A.; Blank, L.M. Selection of a recyclablein situliquid–liquid extraction solvent for foam-free synthesis of rhamnolipids in a two-phase fermentation. Green Chem. 2020, 22, 8495–8510. [Google Scholar] [CrossRef]
- Kujawski, J.; Bernard, M.K.; Janusz, A.; Kuźma, W. Prediction of log P: ALOGPS Application in Medicinal Chemistry Education. J. Chem. Educ. 2011, 89, 64–67. [Google Scholar] [CrossRef]
- Yadav, D.K.; Yadav, M.; Rani, P.; Yadav, A.; Bhardwaj, N.; Bishnoi, N.R.; Singh, A. Demonstration of n-dodecane suitability for milking lipids from Chlorella vulgaris for the production of biodiesel. Bioresour. Technol. Rep. 2023, 23, 101550. [Google Scholar] [CrossRef]
- Zhang, C.; Seow, V.Y.; Chen, X.; Too, H.-P. Multidimensional heuristic process for high-yield production of astaxanthin and fragrance molecules in Escherichia coli. Nat. Commun. 2018, 9, 1858. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Braune, M.; Gröngröft, A. Liquid-Liquid Extraction of Caproic and Caprylic Acid: Solvent Properties and pH. Chem. Ing. Tech. 2023, 95, 1573–1579. [Google Scholar] [CrossRef]
- Wang, A.; Sun, Y.; Sun, Z.; Liu, X.; Yu, X.; Li, K.; Zhang, X.; Xu, Y.; Mu, W.; Li, B. Modification of sedimentation and bioaccumulation behavior as an efficient strategy to modulate the toxicity of pyraclostrobin to zebrafish (Danio rerio). Environ. Pollut. 2023, 322, 121164. [Google Scholar] [CrossRef] [PubMed]
- Ruelle, P. The n-octanol and n-hexane/water partition coefcient ofenvironmentally relevant chemicals predicted from the mobileorder and disorder(MOD)thermodynamics. Chemosphere 2000, 40, 457–512. [Google Scholar] [CrossRef]
- Gill, U.S.; Craan, A.G.; Subramanian, K.S.; Chu, I. Diisononyl Phthalate: Chemistry, Environmental Path, and Toxicology. In Reviews of Environmental Contamination and Toxicology; Ware, G.W., Ed.; Springer: Berlin/Heidelberg, Germany, 2001; Volume 172, pp. 87–127. [Google Scholar]
- Frykman, S.; Tsuruta, H.; Galazzo, J.; Licari, P. Characterization of product capture resin during microbial cultivations. J. Ind. Microbiol. Biotechnol. 2006, 33, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Pellenz, L.; de Oliveira, C.R.S.; da Silva Júnior, A.H.; da Silva, L.J.S.; da Silva, L.; Ulson de Souza, A.A.; de Souza, S.M.d.A.G.U.; Borba, F.H.; da Silva, A. A comprehensive guide for characterization of adsorbent materials. Sep. Purif. Technol. 2023, 305, 122435. [Google Scholar] [CrossRef]
- Jatoi, A.S.; Baloch, H.A.; Mazari, S.A.; Mubarak, N.M.; Sabzoi, N.; Aziz, S.; Soomro, S.A.; Abro, R.; Shah, S.F. A review on extractive fermentation via ion exchange adsorption resins opportunities, challenges, and future prospects. Biomass Convers. Biorefinery 2021, 13, 3543–3554. [Google Scholar] [CrossRef]
- Luongo, V.; Palma, A.; Rene, E.R.; Fontana, A.; Pirozzi, F.; Esposito, G.; Lens, P.N.L. Lactic acid recovery from a model of Thermotoga neapolitana fermentation broth using ion exchange resins in batch and fixed-bed reactors. Sep. Sci. Technol. 2018, 54, 1008–1025. [Google Scholar] [CrossRef]
- Beschkov, V.N. Ion exchange in downstream processing in biotechnology. Phys. Sci. Rev. 2020, 5, 20180066. [Google Scholar] [CrossRef]
- Yousuf, A.; Bonk, F.; Bastidas-Oyanedel, J.-R.; Schmidt, J.E. Recovery of carboxylic acids produced during dark fermentation of food waste by adsorption on Amberlite IRA-67 and activated carbon. Bioresour. Technol. 2016, 217, 137–140. [Google Scholar] [CrossRef]
- Wo, J. Biosynthetic Mechanism of Streptonigrin and High Production of 10′-Desmethoxystreptonigrin. Ph.D. Thesis, Shanghai Jiaotong University, Shanghai, China, 2016. [Google Scholar]
- Isken, S.; Heipieper, H.J. Toxicity of Organic Solvents to Microorganisms; Wiley: New York, NY, USA, 2003. [Google Scholar]
- Osborne, S.J.; Leaver, J.; Turner, M.K.; Dunnill, P. Correlation of biocatalytic activity in an organic-aqueous two-liquid phase system with solvent concentration in the cell membrane. Enzym. Microb. Technol. 1990, 12, 281–291. [Google Scholar] [CrossRef]
- Huang, W.-C.; Tang, I.C. Chapter 8—Bacterial and Yeast Cultures—Process Characteristics, Products, and Applications. In Bioprocessing for Value-Added Products from Renewable Resources; Yang, S.-T., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 185–223. [Google Scholar]
- Rosano, G.L.; Ceccarelli, E.A. Recombinant protein expression in Escherichia coli: Advances and challenges. Front. Microbiol. 2014, 5, 172. [Google Scholar] [CrossRef] [PubMed]
- Marbach, A.; Bettenbrock, K. lac operon induction in Escherichia coli: Systematic comparison of IPTG and TMG induction and influence of the transacetylase LacA. J. Biotechnol. 2012, 157, 82–88. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, J.; Zheng, Z.; Chen, N.; Luo, X.; Tang, H. Engineering an Efficient Expression Using Heterologous GAL Promoters and Transcriptional Activators in Saccharomyces cerevisiae. ACS Synth. Biol. 2023, 12, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Compagno, C.; Dashko, S.; Piškur, J. Introduction to Carbon Metabolism in Yeast. In Molecular Mechanisms in Yeast Carbon Metabolism; Piškur, J., Compagno, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–19. [Google Scholar]
- Lopez-Cuellar, M.R.; Alba-Flores, J.; Rodriguez, J.N.; Perez-Guevara, F. Production of polyhydroxyalkanoates (PHAs) with canola oil as carbon source. Int. J. Biol. Macromol. 2011, 48, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Ravera, F.; Dziza, K.; Santini, E.; Cristofolini, L.; Liggieri, L. Emulsification and emulsion stability: The role of the interfacial properties. Adv. Colloid Interface Sci. 2021, 288, 102344. [Google Scholar] [CrossRef]
- Chen, L.; Pang, Y.; Luo, Y.; Cheng, X.; Lv, B.; Li, C. Separation and purification of plant terpenoids from biotransformation. Eng. Life Sci. 2021, 21, 724–738. [Google Scholar] [CrossRef]
- Barton, D.; Chickos, J. The vapor pressure and vaporization enthalpy of (−) β-Elemene and (−) β-Bisabolene by correlation gas chromatography. J. Chem. Thermodyn. 2020, 148, 106139. [Google Scholar] [CrossRef]



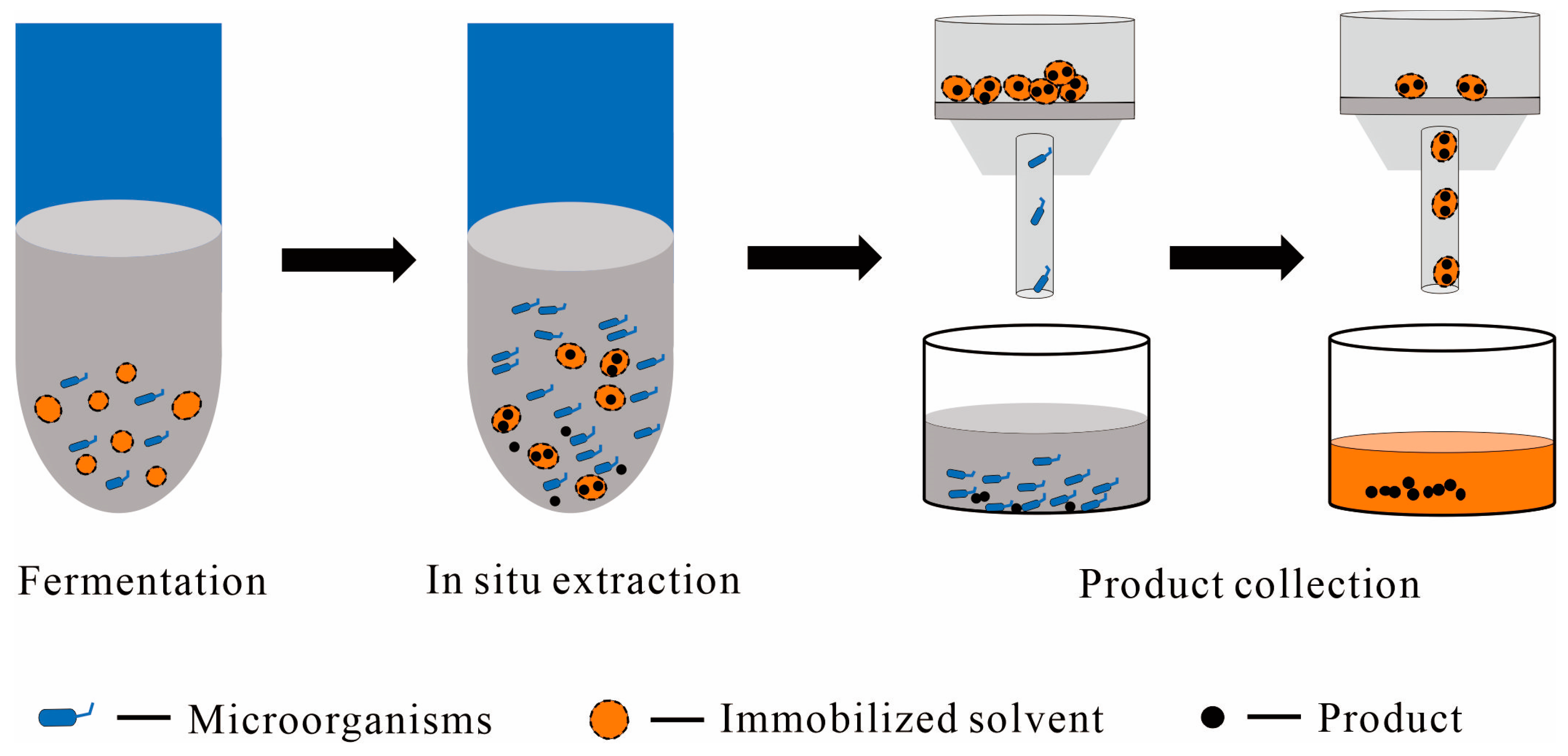


| Monoterpenes | Chassis Cells | Fermentation Types and Scales | Second Phases | Titers (mg/L) | References |
|---|---|---|---|---|---|
| geraniol | E. coli | 5 L bioreactor | none | 78.8 | [65] |
| flask | n-decane | 1119 | [81] | ||
| flask | isopropyl myristate | 2102.5 | [83] | ||
| 10 L bioNreactor | isopropyl myristate | 13,190 | [80] | ||
| S. cerevisiae | flask | none | 36.04 | [84] | |
| 5 L bioreactor | isopropyl myristate | 1680 | [85] | ||
| 1 L bioreactor | n-dodecane | 1690 | [82] | ||
| C. glutamicum | 250 mL flask | n-dodecane | 15.2 | [86] | |
| limonene | E. coli | flask | n-dodecane | 605 | [87] |
| 250 mL flask | isopropyl myristate | 1290 | [88] | ||
| 250 mL flask | diisononyl phthalate | 37.8 | [89] | ||
| 3.1 L bioreactor | diisononyl phthalate | 3630 | [90] | ||
| S. cerevisiae | flask | none | 62.31 | [91] | |
| flask | isopropyl myristate | 2230 | [92] | ||
| flask | n-dodecane | 2580 | [57] | ||
| 3 L bioreactor | n-dodecane | 2630 | [93] | ||
| R. toruloides | 250 mL tube | n-dodecane | 393.5 | [94] | |
| 250 mL flask | n-dodecane | 358.1 | [95] | ||
| Ashbya gossypii | 40 mL flask | n-dodecane | 336.4 | [96] | |
| Y. lipolytica | flask | n-dodecane | 23.56 | [97] | |
| 1.5 L bioreactor | n-dodecane | 165.3 | [98] | ||
| Synechococcus sp. | 250 mL flask | n-dodecane | 6.7 | [99] | |
| cyanobacteria | flask | isopropyl myristate | 16.4 | [100] | |
| perillyl alcohol | E. coli | 5 L bioreactor | n-dodecane | 87 | [101] |
| flask | anion exchange column with Amberlite resin | 105 | [102] | ||
| linalool | E. coli | 500 mL flask | none | 63 | [103] |
| flask | n-nonane | 1054 | [104] | ||
| 250 mL flask | n-dodecane | 505 | [105] | ||
| flask | isopropyl myristate | 1250 | [106] | ||
| 1.3 L bioreactor | isopropyl myristate | 1523.2 | [107] | ||
| S. cerevisiae | 500 mL flask | none | 0.095 | [108] | |
| 2 L bioreactor | none | 23.45 | [109] | ||
| flask | isopropyl myristate | 80.9 | [110] | ||
| Y. lipolytica | flask | n-dodecane | 6.96 | [111] | |
| flask | isopropyl myristate | 109.6 | [112] | ||
| Pantoea ananatis | tube | isopropyl myristate | 5600 | [113] | |
| bioreactor | isopropyl myristate | 10,900 | [114] | ||
| cineole | E. coli | flask | n-nonane | 116.8 | [115] |
| flask | n-dodecane | 653 | [105] | ||
| S. cerevisiae | bioreactor | none | 1100 | [116] | |
| sabinene | E. coli | 5 L flask | none | 2650 | [117] |
| 5 L bioreactor | none | 150 | [118] | ||
| S. cerevisiae | flask | n-dodecane | 17.5 | [119] | |
| pinene | E. coli | 5 L bioreactor | none | 970 | [120] |
| 50 mL flask | n-dodecane | 166.5 | [121] | ||
| S. cerevisiae | 50 mL flask | isopropyl myristate | 11.7 | [122] | |
| C. glycerinogenes | flask | n-dodecane | 6 | [123] | |
| myrcene | E. coli | 250 mL flask | n-dodecane | 58.19 | [124] |
| 1 L flask | isopropyl myristate | 1250 | [106] |
| Triterpenes and Tetraterpenes | Chassis Cells | Fermentation Types and Scales | Second Phases | Titers (mg/L) | References |
|---|---|---|---|---|---|
| squalene | S. cerevisiae | 5 L bioreactor | none | 9472 | [228] |
| 5 L bioreactor | n-dodecane | 207.02 | [137] | ||
| ambrein | E. coli | flask | none | 2.6 | [229] |
| P. pastoris | 5 L bioreactor | none | 100 | [230] | |
| betulin | S. cerevisiae | 5 L flask | none | 59.5 | [231] |
| gypsogenin | S. cerevisiae | bioreactor | none | 146.84 | [232] |
| lupeol | S. cerevisiae | flask | none | 200.1 | [233] |
| α-amyrin | S. cerevisiae | 20 mL flask | none | 213.7 | [234] |
| 5 L bioreactor | none | 1100 | [234] | ||
| β-amyrin | S. cerevisiae | 5 L bioreactor | none | 138.8 | [235] |
| tube | none | 6 | [236] | ||
| ursolic acid | S. cerevisiae | 10 mL flask | none | 101.4 | [237] |
| bioreactor | none | 123.27 | [238] | ||
| betulinic acid | S. cerevisiae | 50 mL flask | none | 91.6 | [237] |
| 5 L bioreactor | none | 1000 | [231] | ||
| Y. lipolytica | flask | isopropyl myristate | 51.87 | [239] | |
| morolic acid | S. cerevisiae | 50 mL flask | none | 68.3 | [237] |
| bioreactor | none | 34.1 | [237] | ||
| oleanolic acid | S. cerevisiae | 5 L bioreactor | none | 606.9 | [240] |
| S. cerevisiae | flask | none | 186.1 | [240] | |
| ganoderic acid | S. cerevisiae | flask | none | 14.5 | [241] |
| maslinic acid | S. cerevisiae | 5 L bioreactor | none | 384 | [242] |
| corosolic acid | S. cerevisiae | 5 L bioreactor | none | 141 | [242] |
| alphitolic acid | S. cerevisiae | 5 L bioreactor | none | 23 | [242] |
| quillaic acid | S. cerevisiae | bioreactor | none | 314.01 | [232] |
| polpunonic acid | S. cerevisiae | tube | none | 1.4 | [243] |
| glycyrrhetinic acid | S. cerevisiae | 5 L bioreactor | none | 18.9 | [244] |
| dammarenediol-II | S. cerevisiae | 7 L bioreactor | none | 15,000 | [245] |
| 50 mL flask | none | 211.52 | [246] | ||
| 7.5 L bioreactor | n-dodecane /methyl oleate | 1548 | [226] | ||
| E. coli | 250 mL flask | none | 8.63 | [247] | |
| protopanaxadiol | S. cerevisiae | 250 mL flask | none | 17.2 | [248] |
| 10 L bioreactor | none | 9054.5 | [249] | ||
| 7.5 L bioreactor | n-dodecane /methyl oleate | 1189 | [226] | ||
| protopanaxatriol | S. cerevisiae | 250 mL flask | none | 15.9 | [248] |
| ginsenoside Rh2 | S. cerevisiae | 50 mL flask | none | 16.9 | [250] |
| 10 L bioreactor | none | 2250 | [249] | ||
| ginsenoside Rg3 | S. cerevisiae | 1.5 L bioreactor | none | 1.3 | [251] |
| 50 mL flask | none | 51.8 | [250] | ||
| ginsenoside RF1 | S. cerevisiae | flask | none | 42.1 | [252] |
| ginsenoside Rh1 | S. cerevisiae | flask | none | 92.8 | [252] |
| β-carotene | E. coli | flask | none | 503 | [253] |
| 5 L bioreactor | none | 3200 | [254] | ||
| S. cerevisiae | 2 mL tube | none | 477.9 | [255] | |
| lycopene | E. coli | 5 mL tube | none | 77.85 | [256] |
| 7 L bioreactor | none | 3520 | [257] | ||
| S. cerevisiae | 7 L bioreactor | none | 2370 | [258] | |
| Y. lipolytica | 3 L bioreactor | none | 4200 | [227] | |
| Mucor circinelloides | 500 mL flask | none | 54,000 | [259] | |
| R. rubrum | 100 mL flask | none | 15 | [260] | |
| Rhodobacter sphaeroides | 250 mL flask | none | 66.05 | [261] | |
| Haloferax mediterranei | 5 L flask | none | 429.41 | [262] | |
| P. pastoris | 4 L bioreactor | none | 73.9 | [263] | |
| 3 L flask | none | 714 | [264] | ||
| astaxanthin | E. coli | 5 L bioreactor | none | 1820 | [265] |
| crocetin | S. cerevisiae | 5 L bioreactor | none | 6.278 | [266] |
| zeaxanthin | E. coli | 250 mL flask | none | 43.46 | [267] |
| 5 L bioreactor | none | 722.46 | [268] | ||
| S. cerevisiae | tube | none | 1.5 | [269] | |
| Pseudomonas putida | flask | none | 51.3 | [270] |
| Name | CAS Number | Chemical Structure and Formula | Log p | Boiling Point b (°C) | References |
|---|---|---|---|---|---|
| n-dodecane | 112-40-3 |  C12H26 | 6.6 | 216.3 | [279] |
| isopropyl myristate | 110-27-0 |  C17H34O2 | 7.02 | 315.0 | [280] |
| n-decane | 124-18-5 |  C10H22 | 5.6 | 174.1 | [281] |
| oleyl alcohol | 143-28-2 |  C18H36O | 7.5 | 305-370 | [281] |
| 2,2,4-trimethylpentane | 540-84-1 |  C8H18 | 4.49 a | 99.2 | [278] |
| n-hexane | 110-54-3 |  C6H14 | 3.5 | 68.8 | [281] |
| methyl oleate | 112-62-9 |  C19H36O2 | 11.2 | 218.5 | [282] |
| n-nonane | 111-84-2 |  C9H20 | 5.65 | 150.7 | [283] |
| diisononyl phthalate | 28553-12-0 |  C26H42O4 | 9.37 | 77.7 | [284] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Liu, X.; Xiang, H.; Zhu, H.; Lu, X.; Feng, B. Two-Phase Fermentation Systems for Microbial Production of Plant-Derived Terpenes. Molecules 2024, 29, 1127. https://doi.org/10.3390/molecules29051127
Li T, Liu X, Xiang H, Zhu H, Lu X, Feng B. Two-Phase Fermentation Systems for Microbial Production of Plant-Derived Terpenes. Molecules. 2024; 29(5):1127. https://doi.org/10.3390/molecules29051127
Chicago/Turabian StyleLi, Tuo, Ximeng Liu, Haoyu Xiang, Hehua Zhu, Xuan Lu, and Baomin Feng. 2024. "Two-Phase Fermentation Systems for Microbial Production of Plant-Derived Terpenes" Molecules 29, no. 5: 1127. https://doi.org/10.3390/molecules29051127
APA StyleLi, T., Liu, X., Xiang, H., Zhu, H., Lu, X., & Feng, B. (2024). Two-Phase Fermentation Systems for Microbial Production of Plant-Derived Terpenes. Molecules, 29(5), 1127. https://doi.org/10.3390/molecules29051127






