Bromopyrene Symphony: Synthesis and Characterisation of Isomeric Derivatives at Non-K Region and Nodal Positions for Diverse Functionalisation Strategies
Abstract
:1. Introduction
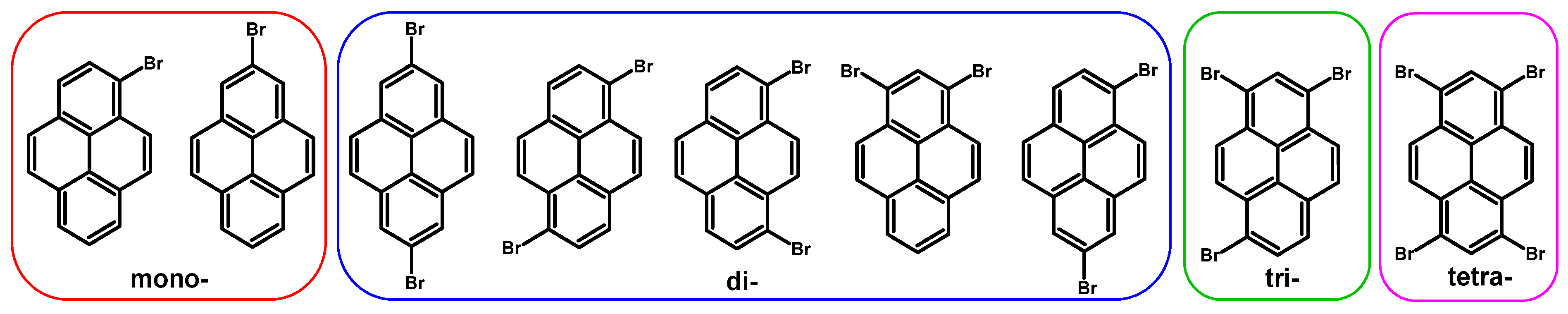
2. Introduction of Bromine
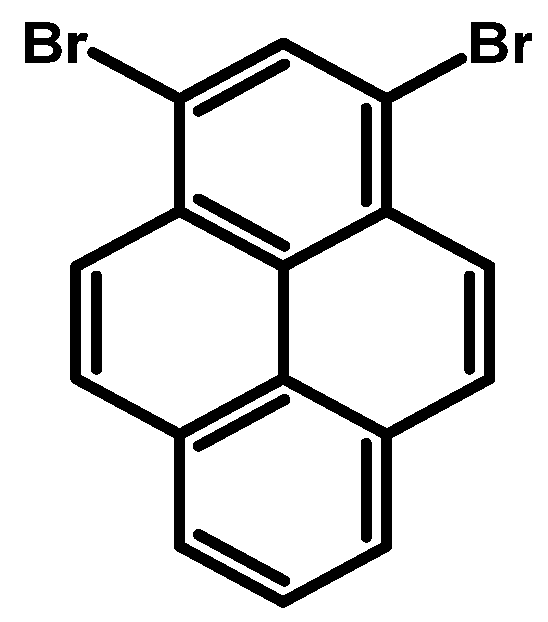
2.1. 1,3,6,8-Tetrabromopyrene
- Procedure for the Synthesis of 1,3,6,8-Tetrabromopyrene: Pyrene (10.00 g, 49.44 mmol) and nitrobenzene (200 mL) were combined in a three-necked round-bottom flask, to which bromine (34.77 g, 11.14 mL, 217.55 mmol) was added dropwise. The resulting mixture was heated at 120 °C overnight under a nitrogen atmosphere. Subsequently, it was allowed to cool to room temperature, followed by filtration and washing with ethanol and diethyl ether. The product obtained was as light green solid (25.09 g, 98% yield).
2.2. 1-Bromopyrene
- Procedure for the Synthesis of 1-Bromopyrene: Pyrene (10.00 g, 49.44 mmol) and a mixture of MeOH/Et2O (125 mL, 1:1 v/v) were combined in a three-necked round-bottom flask, to which HBr (48% w/w aq solution, 9.17 g, 6.15 mL, 54.39 mmol) was added dropwise. The resulting mixture was cooled to 15 °C using an ice-water bath and stirred for 10 min, followed by dropwise addition of H2O2 (30% w/w aq solution, 5.89 g, 5.30 mL, 51.92 mmol) over 30 min. The mixture was stirred overnight under a nitrogen atmosphere. Subsequently, the precipitate was filtrated and washed with a small amount of cold ethanol and diethyl ether. Dichloromethane (100 mL) was added to the filtrate and extracted twice with water. The solvent was evaporated using a rotary evaporator, and the residue was dissolved in hot hexane and placed in a refrigerator overnight. Precipitate was collected by filtration and mixed with the previously obtained solid. The process of dissolving in a small amount of hot hexane was repeated, and the mixture was placed in a refrigerator overnight. After filtration, the product was obtained as a pale yellow solid (10.15 g, 73% yield). 1H NMR (400 MHz, CDCl3) δ 8.42 (d, J = 9.2 Hz, 1H), 8.24–8.18 (m, 3H), 8.15 (d, J = 9.2 Hz, 1H), 8.09–8.02 (m, 2H), 8.02–7.96 (m, 2H).
2.3. 1,6-Dibromopyrene and 1,8-Dibromopyrene
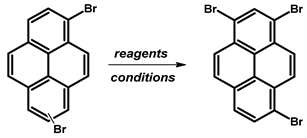
- Procedure for the Synthesis of 1,6-Dibromopyrene and 1,8-Dibromopyrene: Pyrene (10.00 g, 49.44 mmol) was combined with carbon tetrachloride (250 mL) in a three-necked round-bottom flask. Bromine (15.80 g, 5.07 mL, 98.89 mmol) was added dropwise over five h under a nitrogen atmosphere. The resulting mixture was stirred overnight. The precipitate formed was then filtered and washed with diethyl ether and hexane. The obtained solid underwent fractional crystallization from toluene, resulting in the initial formation of the less soluble 1,6-dibromopyrene, which crystallized in needle-like structures. The crystallisation process was repeated using toluene. The products were obtained as beige solids, 1,6-dibromopyrene (7.12 g, 40% yield), and 1,8-dibromopyrene (6.23 g, 35% yield). Importantly, due to cost and environmental considerations, the carbon tetrachloride used after filtration was washed three times with water, dried with magnesium sulphate, and then distilled. This purified solvent was utilised in subsequent bromination reactions. Furthermore, the mixture of 1,6- and 1,8-dibromopyrenes obtained from the last crystallization step was employed to synthesise 1,3,6-tribromopyrene. 1,6-Dibromopyrene: 1H NMR (400 MHz, CDCl3) δ 8.46 (d, J = 9.2 Hz, 2H), 8.27 (d, J = 8.2 Hz, 2H), 8.12 (d, J = 9.2 Hz, 2H), 8.06 (d, J = 8.2 Hz, 2H). 1,8-Dibromopyrene: 1H NMR (400 MHz, CDCl3) δ 8.49 (s, 1H), 8.42 (d, J = 9.2 Hz, 1H), 8.25 (d, J = 8.1 Hz, 2H), 8.08 (d, J = 9.2 Hz, 1H), 8.04–8.01 (m, 1H), 8.01 (d, J = 3.1 Hz, 2H).
2.4. 1,3-Dibromopyrene
- Procedure for the Synthesis of 1,3-dibromo-7-tert-butylpyrene: 2-Tert-butylpyrene (1.00 g, 3.87 mmol) was combined with dichloromethane (40 mL) in a three-necked round-bottom flask. Bromine (1.24 g, 0.40 mL, 7.74 mmol), dissolved in dichloromethane (40 mL), was added dropwise at −78 °C under a nitrogen atmosphere. The reaction mixture was allowed to slowly warm to room temperature and stirred overnight. Afterwards, the organic layer was washed successively with a sodium thiosulfate (0.3 M) solution and water. The solvent was then removed under reduced pressure using a rotary evaporator. The resulting residue was dissolved in hexane, leading to the crystallisation of the product. The precipitate was collected by filtration, yielding a white–silver solid (1.42 g, 88% yield). 1H NMR (400 MHz, CDCl3) δ 8.44 (s, 1H), 8.34 (d, J = 9.2 Hz, 2H), 8.29 (s, 2H), 8.15 (d, J = 9.2 Hz, 2H), 1.60 (s, 9H).
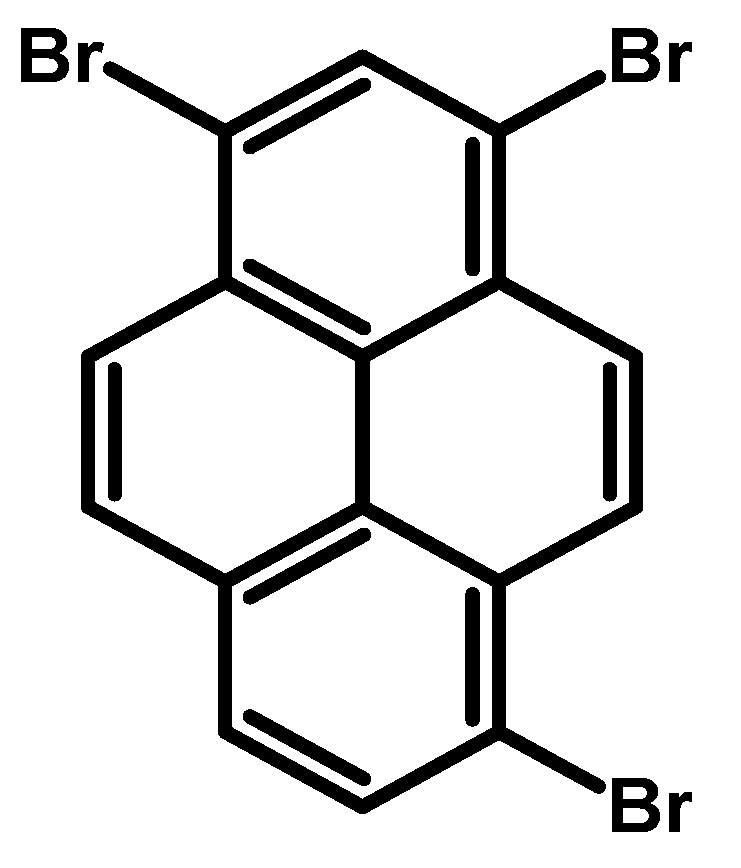
2.5. 1,3,6-Tribromopyrene
- Procedure for the Synthesis of 1,3,6-Tribromopyrene: A mixture of 1,6- and 1,8-dibromopyrene (10.00 g, 27.77 mmol) was combined with nitrobenzene (250 mL) in a three-necked round-bottom flask. Bromine (4.44 g, 1.42 mL, 27.77 mmol) was added dropwise under a nitrogen atmosphere. The resulting mixture was stirred overnight. The precipitate formed was then filtered and washed with diethyl ether and hexane. The obtained solid underwent crystallisation from toluene, resulting in 1,3,6-tribromopyrene as a white solid (9.87 g, 81%). 1H NMR (400 MHz, CDCl3) δ 8.53 (s, 1H), 8.46 (d, J = 9.1 Hz, 2H), 8.27 (d, J = 2.4 Hz, 1H), 8.25 (d, J = 2.4 Hz, 2H), 8.12 (d, J = 9.2 Hz, 2H), 8.06 (d, J = 4.8 Hz, 2H), 8.04 (t, J = 2.4 Hz, 2H).
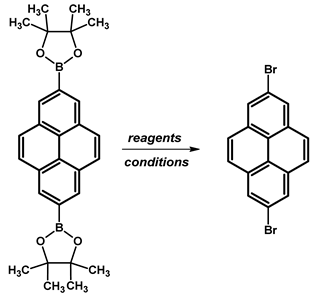
2.6. 1,7-Dibromopyrene
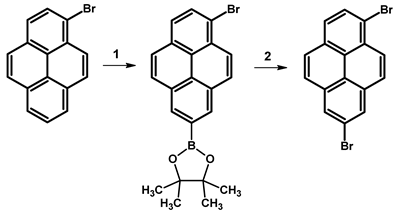
- Procedure for the Synthesis of 1,7-Dibromopyrene: A solution of [Ir(μ-OMe)(cod)]₂ (0.073 g, 0.15 mmol), 4,4′-di-tert-butyl-2,2′-dipyridyl (dtbpy) (0.097 g, 0.36 mmol), and bis(pinacolato)diboron (0.198 g, 0.78 mmol) in hexane (10 mL) was prepared in a Schlenk flask under a nitrogen atmosphere. The mixture was stirred for 10 min. Then, a solution of 1-bromopyrene (5.00 g, 17.78 mmol) and bis(pinacolato)diboron (4.77 g, 18.78 mmol) in hexane (20 mL) was added to the reaction mixture. The Schlenk flask was purged with nitrogen, and the resulting mixture was stirred at 70 °C overnight. The crude product was subsequently extracted with chloroform and water. The organic layer was separated, and the solvent was removed under reduced pressure using a rotary evaporator. The resulting residue was dissolved in dichloromethane and passed through a layer of silica gel. The solvent was then removed under reduced pressure, and the obtained yellow residue was dissolved in a mixture of methanol and tetrahydrofuran (MeOH/THF, 60 mL, 3:1 v/v). Then, a solution of copper(II) bromide (19.86 g, 88.9 mmol) in 30 mL of water was added to the solution. The mixture was stirred overnight at 90 °C under a nitrogen atmosphere. The resulting product was filtered and washed successively with water, diethyl ether, and hexane. The obtained precipitate was then purified by silica gel column chromatography using chloroform as the eluent to obtain a solid, which was crystallised from acetonitrile to yield the desired compound as a white solid (1.98 g, 31%). 1H NMR (400 MHz, CDCl3) δ 8.53 (s, 1H), 8.45 (d, J = 9.2 Hz, 1H), 8.21 (d, J = 8.0 Hz, 1H), 8.13 (d, J = 2.5 Hz, 1H), 8.05 (s, 1H), 7.89 (d, J = 9.2 Hz, 1H), 7.69 (d, J = 9.2 Hz, 1H), 7.63 (d, J = 9.3 Hz, 1H).
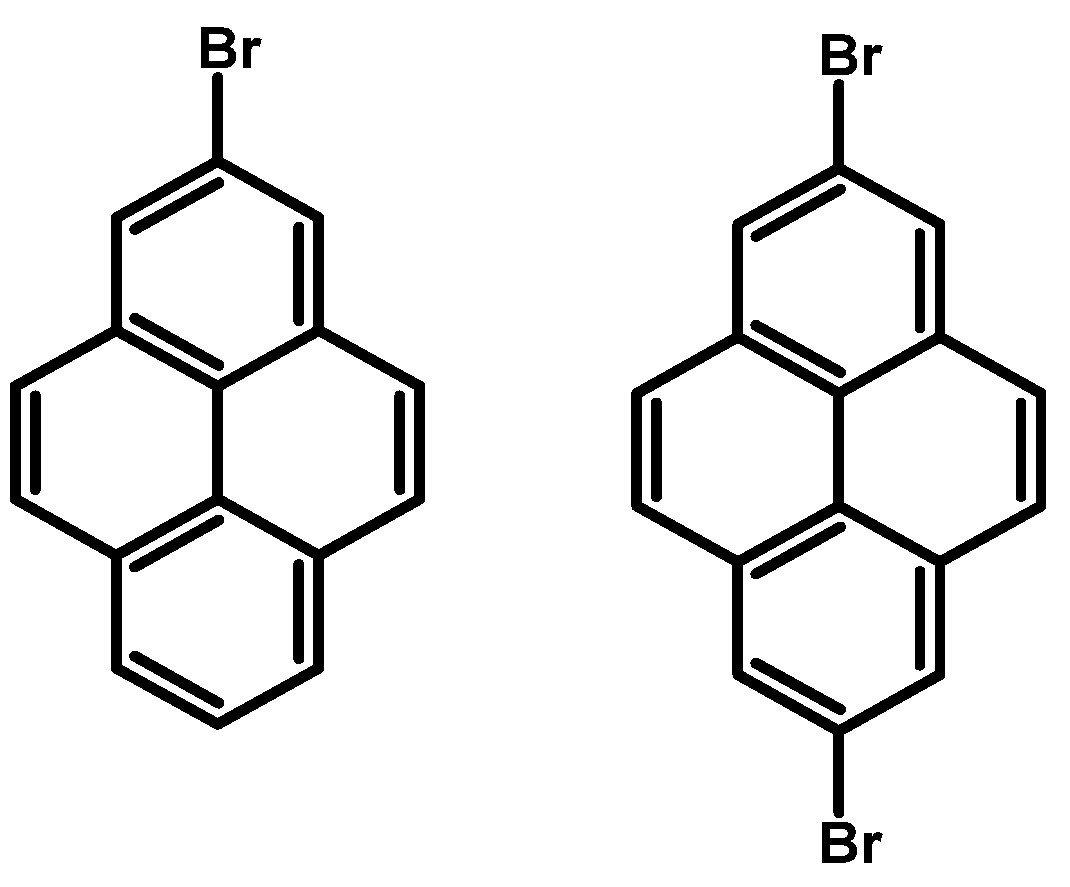
2.7. 2-Bromopyrene and 2,7-Dibromopyrene
- Procedure for the Synthesis of 2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrene and 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrene: A solution of [Ir(μ-OMe)(cod)]₂ (0.044 g, 0.09 mmol), 4,4′-di-tert-butyl-2,2′-dipyridyl (dtbpy) (0.048 g, 0.18 mmol), and bis(pinacolato)diboron (0.102 g, 0.4 mmol) in hexane (5 mL) was prepared in a Schlenk flask under a nitrogen atmosphere. The mixture was stirred for 10 min. Subsequently, a solution of pyrene (2.00 g, 9.89 mmol) and bis(pinacolato)diboron (for monosubstituted: 1.40 g, 5.53 mmol or for disubstituted: 2.79 g, 11.00 mmol) in hexane (for monosubstituted: 10 mL or for disubstituted: 20 mL) was added to the reaction mixture. The Schlenk flask was purged with nitrogen, and the resulting mixture was stirred at 70 °C overnight. The crude product was then extracted with chloroform and water. The organic layer was separated, and the solvent was removed under reduced pressure using a rotary evaporator. The resulting residue was purified by column chromatography on silica gel (eluent: hexane:dichloromethane, 1:1, v/v). The obtained yellowish oil was mixed with hexane, and the obtained precipitate was filtered. 2-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)pyrene was obtained as a white solid (1.69 g, 52%) or 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrene was obtained as a white solid (3.77 g, 84%). 2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrene: 1H NMR (400 MHz, CDCl3) δ 8.67 (s, 2H), 8.17 (d, J = 7.6 Hz, 2H), 8.12 (d, J = 9.0 Hz, 2H), 8.07 (d, J = 9.0 Hz, 2H), 8.03 (d, J = 7.3 Hz, 1H), 1.48 (s, 12H). 13C NMR (101 MHz, CDCl3) δ 131.74, 131.45, 130.52, 127.88, 127.39, 126.49, 126.46, 124.95, 124.71, 84.29, 25.13. 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrene: 1H NMR (400 MHz, CDCl3) δ 8.62 (s, 4H), 8.09 (s, 4H), 1.46 (s, 24H). 13C NMR (101 MHz, CDCl3) δ 131.3, 131.0, 127.8, 126.4, 84.3, 25.1.
- Procedure for the Synthesis of 2-Bromopyrene and 2,7-Dibromopyrene: 2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrene (0.33 g, 1.00 mmol) or 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrene (0.45 g, 1.00 mmol) was dissolved in a mixture of methanol and tetrahydrofuran (MeOH/THF, 60 mL, 3:1 v/v). Then, a solution of copper(II) bromide (for monosubstituted: 2.23 g, 10.00 mmol or for disubstituted: 4.47 g, 20.00 mmol) in 30 mL of water was added to a solution. The mixture was stirred overnight at 90 °C under a nitrogen atmosphere. The resulting product was filtered and washed successively with water, diethyl ether, and hexane. The obtained precipitate was crystallised from hot hexane to yield the desired compound as a beige solid 2-bromopyrene (0.24 g, 86%) and 2,7-dibromopyrene (0.32 g, 89%). 2-Bromopyrene: 1H NMR (400 MHz, CDCl3) δ 8.16 (s, 2H), 8.12 (d, J = 7.5 Hz, 2H), 8.00–7.94 (m, 2H), 7.83 (d, J = 9.0 Hz, 2H), 7.80–7.72 (m, 1H). 2,7-Dibromopyrene: 1H NMR (400 MHz, CDCl3) δ 8.31 (s, 4H), 8.01 (s, 4H).
2.8. NMR Spectra of Bromopyrenes
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kowser, Z.; Rayhan, U.; Akther, T.; Redshaw, C.; Yamato, T. A Brief Review on Novel Pyrene Based Fluorometric and Colorimetric Chemosensors for the Detection of Cu2+. Mater. Chem. Front. 2021, 5, 2173–2200. [Google Scholar] [CrossRef]
- Qutob, M.; Rafatullah, M.; Muhammad, S.A.; Alosaimi, A.M.; Alorfi, H.S.; Hussein, M.A. A Review of Pyrene Bioremediation Using Mycobacterium Strains in a Different Matrix. Fermentation 2022, 8, 260. [Google Scholar] [CrossRef]
- Figueira-Duarte, T.M.; Müllen, K. Pyrene-Based Materials for Organic Electronics. Chem. Rev. 2011, 111, 7260–7314. [Google Scholar] [CrossRef] [PubMed]
- Kinik, F.P.; Ortega-Guerrero, A.; Ongari, D.; Ireland, C.P.; Smit, B. Pyrene-Based Metal Organic Frameworks: From Synthesis to Applications. Chem. Soc. Rev. 2021, 50, 3143–3177. [Google Scholar] [CrossRef]
- Feng, X.; Hu, J.-Y.; Redshaw, C.; Yamato, T. Functionalization of Pyrene To Prepare Luminescent Materials—Typical Examples of Synthetic Methodology. Chem. A Eur. J. 2016, 22, 11898–11916. [Google Scholar] [CrossRef] [PubMed]
- Bally, O.; Scholl, R. Einwirkung von Glycerin Und Schwefelsäure Auf Amidierte Und Auf Stickstofffreie Verbindungen Der Anthracen-Reihe: Benzanthron Und Seine Reduktionsprodukte, Nebst Bemerkungen Über Namenbildung Und Ortsbezeichnung Hochgegliederter Ringsysteme Der Anthracen-Reihe. Berichte Dtsch. Chem. Ges. 1911, 44, 1656–1670. [Google Scholar] [CrossRef]
- Clar, E. Nomenclature of Polycyclic Hydrocarbons. In Polycyclic Hydrocarbons: Volume 1; Clar, E., Ed.; Springer: Berlin/Heidelberg, Germany, 1964; pp. 3–11. ISBN 978-3-662-01665-7. [Google Scholar]
- Patterson, A.M. Proposed international rules for numbering organic ring systems. J. Am. Chem. Soc. 1925, 47, 543–561. [Google Scholar] [CrossRef]
- Chinchilla, R.; Nájera, C. The Sonogashira Reaction: A Booming Methodology in Synthetic Organic Chemistry. Chem. Rev. 2007, 107, 874–922. [Google Scholar] [CrossRef]
- Farhang, M.; Akbarzadeh, A.R.; Rabbani, M.; Ghadiri, A.M. A Retrospective-Prospective Review of Suzuki–Miyaura Reaction: From Cross-Coupling Reaction to Pharmaceutical Industry Applications. Polyhedron 2022, 227, 116124. [Google Scholar] [CrossRef]
- Dorel, R.; Grugel, C.P.; Haydl, A.M. The Buchwald–Hartwig Amination after 25 Years. Angew. Chem. Int. Ed. 2019, 58, 17118–17129. [Google Scholar] [CrossRef]
- Heravi, M.M.; Kheilkordi, Z.; Zadsirjan, V.; Heydari, M.; Malmir, M. Buchwald-Hartwig Reaction: An Overview. J. Organomet. Chem. 2018, 861, 17–104. [Google Scholar] [CrossRef]
- Zych, D. Non-K Region Disubstituted Pyrenes (1,3-, 1,6- and 1,8-) by (Hetero)Aryl Groups—Review. Molecules 2019, 24, 2551. [Google Scholar] [CrossRef]
- Dewar, M.J.S.; Dennington, R.D.I. DEWAR-PI Study of Electrophilic Substitution in Selected Polycyclic Fluoranthene Hydrocarbons. J. Am. Chem. Soc. 1989, 111, 3804–3808. [Google Scholar] [CrossRef]
- Cerfontain, H.; Laali, K.; Lambrechts, H.J.A. Aromatic Sulfonation 86. Sulfonation of Pyrene, 1-Methylpyrene and Perylene. Recl. Trav. Chim. Pays-Bas 1983, 102, 210–214. [Google Scholar] [CrossRef]
- Vollmann, H.; Becker, H.; Corell, M.; Streeck, H. Beiträge Zur Kenntnis Des Pyrens Und Seiner Derivate. Justus Liebigs Ann. Chem. 1937, 531, 1–159. [Google Scholar] [CrossRef]
- Maeda, H.; Shoji, T.; Segi, M. Effects of Substituents on Silicon Atoms upon Absorption and Fluorescence Properties of 1,3,6,8-Tetrakis(Silylethynyl)Pyrenes. Tetrahedron Lett. 2017, 58, 4372–4376. [Google Scholar] [CrossRef]
- Bernhardt, S.; Kastler, M.; Enkelmann, V.; Baumgarten, M.; Müllen, K. Pyrene as Chromophore and Electrophore: Encapsulation in a Rigid Polyphenylene Shell. Chem. A Eur. J 2006, 12, 6117–6128. [Google Scholar] [CrossRef] [PubMed]
- Zych, D.; Kurpanik, A.; Slodek, A.; Maroń, A.; Pająk, M.; Szafraniec-Gorol, G.; Matussek, M.; Krompiec, S.; Schab-Balcerzak, E.; Kotowicz, S.; et al. NCN-Coordinating Ligands Based on Pyrene Structure with Potential Application in Organic Electronics. Chem. A Eur. J. 2017, 23, 15746–15758. [Google Scholar] [CrossRef] [PubMed]
- Venkataramana, G.; Sankararaman, S. Synthesis, Absorption, and Fluorescence-Emission Properties of 1,3,6,8-Tetraethynylpyrene and Its Derivatives. Eur. J. Org. Chem. 2005, 2005, 4162–4166. [Google Scholar] [CrossRef]
- Moorthy, J.N.; Natarajan, P.; Venkatakrishnan, P.; Huang, D.-F.; Chow, T.J. Steric Inhibition of π-Stacking: 1,3,6,8-Tetraarylpyrenes as Efficient Blue Emitters in Organic Light Emitting Diodes (OLEDs). Org. Lett. 2007, 9, 5215–5218. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Hiyoshi, H.; Do, J.-H.; Yamato, T. Synthesis and Fluorescence Emission Properties of 1,3,6,8-Tetrakis(9H-Fluoren-2-Yl)Pyrene Derivative. J. Chem. Res. 2010, 34, 278–282. [Google Scholar] [CrossRef]
- Lock, G. Über Abkömmlinge Des Pyrens. Berichte Dtsch. Chem. Ges. (A B Ser.) 1937, 70, 926–930. [Google Scholar] [CrossRef]
- Schulze, M. Synthesis of 1-Bromopyrene and 1-Pyrenecarbaldehyde. Org. Synth. 2016, 93, 100–114. [Google Scholar] [CrossRef]
- He, C.; He, Q.; Chen, Q.; Shi, L.; Cao, H.; Cheng, J.; Deng, C.; Lin, T. Highly Fluorescent Intramolecular Dimmers of Two Pyrenyl-Substituted Fluorenes Bridged by 1,6-Hexanyl: Synthesis, Spectroscopic, and Self-Organized Properties. Tetrahedron Lett. 2010, 51, 1317–1321. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, N.; Zhang, K.; Dai, J.; Bai, W.; Bai, R. Pyrene Boronic Acid Cyclic Ester: A New Fast Self-Recovering Mechanoluminescent Material at Room Temperature. Chem. Commun. 2016, 52, 9679–9682. [Google Scholar] [CrossRef] [PubMed]
- Chidirala, S.; Ulla, H.; Valaboju, A.; Kiran, M.R.; Mohanty, M.E.; Satyanarayan, M.N.; Umesh, G.; Bhanuprakash, K.; Rao, V.J. Pyrene–Oxadiazoles for Organic Light-Emitting Diodes: Triplet to Singlet Energy Transfer and Role of Hole-Injection/Hole-Blocking Materials. J. Org. Chem. 2016, 81, 603–614. [Google Scholar] [CrossRef]
- Yu, L.; Lo, K.C.; Xi, J.; Phillips, D.L.; Chan, W.K. Photo-Induced Electron Transfer in a Pyrenylcarbazole Containing Polymer–Multiwalled Carbon Nanotube Composite. New J. Chem. 2013, 37, 1833. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, L.; Zhuo, J.; Xu, B.; Li, X.; Zhang, J.; Zhang, Z.; Chi, H.; Dong, Y.; Lu, G. A Pyrene-Based Dual Chemosensor for Colorimetric Detection of Cu2+ and Fluorescent Detection of Fe3+. Tetrahedron Lett. 2017, 58, 3951–3956. [Google Scholar] [CrossRef]
- Harsha, K.G.; Appalanaidu, E.; Rao, B.A.; Baggi, T.R.; Rao, V.J. ON–OFF Fluorescent Imidazole Derivative for Sensitive and Selective Detection of Copper(II) Ions. Russ. J. Org. Chem. 2020, 56, 158–168. [Google Scholar] [CrossRef]
- Vyas, P.V.; Bhatt, A.K.; Ramachandraiah, G.; Bedekar, A.V. Environmentally Benign Chlorination and Bromination of Aromatic Amines, Hydrocarbons and Naphthols. Tetrahedron Lett. 2003, 44, 4085–4088. [Google Scholar] [CrossRef]
- Sharif, M.; Reimann, S.; Wittler, K.; Knöpke, L.R.; Surkus, A.; Roth, C.; Villinger, A.; Ludwig, R.; Langer, P. 1-(Arylalkenyl)Pyrenes—Synthetic, Structural, Photophysical, Theoretical, and Electrochemical Investigations. Eur. J. Org. Chem. 2011, 2011, 5261–5271. [Google Scholar] [CrossRef]
- Reimann, S.; Sharif, M.; Wittler, K.; Knöpke, L.R.; Surkus, A.-E.; Roth, C.; Ludwig, R.; Langer, P. 3-Pyrenylacrylates: Synthetic, Photophysical, Theoretical and Electrochemical Investigations. Z. Naturforschung B 2013, 68, 367–377. [Google Scholar] [CrossRef]
- Yang, L.; Finney, N.S. A Mechanistically-Distinct Approach to Fluorescence Visualization of Singlet Oxygen. Chem. Commun. 2017, 53, 11449–11452. [Google Scholar] [CrossRef]
- Kathayat, R.S.; Finney, N.S. Sulfoxides as Response Elements for Fluorescent Chemosensors. J. Am. Chem. Soc. 2013, 135, 12612–12614. [Google Scholar] [CrossRef]
- Đorđević, L.; Milano, D.; Demitri, N.; Bonifazi, D. O-Annulation to Polycyclic Aromatic Hydrocarbons: A Tale of Optoelectronic Properties from Five- to Seven-Membered Rings. Org. Lett. 2020, 22, 4283–4288. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.; Lin, H.; Yan, X.; Huang, T.; Su, Y.; Whang, T. Fast Thermal Evaporation in Purification of 1,4-Di(Pyren-1-ly)Benzene. J. Chin. Chem. Soc. 2012, 59, 289–296. [Google Scholar] [CrossRef]
- Doan, B.; Nguyen, C.; Bui, T.; Tran, T.; Huynh, H.; Nguyen, Q.-T.; Cu, S.; Nguyen, L.-T.; Tran, C.; Mai, P.; et al. Synthesis of Conjugated Molecules Based on Dithienopyrrole Derivatives and Pyrene as Chemosensor for Mesotrione Detection. J. Braz. Chem. Soc. 2022, 33, 1106–1116. [Google Scholar] [CrossRef]
- Keshtov, M.L.; Sharma, G.D.; Godovskii, D.Y.; Belomoina, N.M.; Geng, Y.; Zou, Y.; Kochurov, V.S.; Stakhanov, A.I.; Khokhlov, A.R. Novel Electron-Withdrawing π-Conjugated Pyrene-Containing Poly(Phenylquinoxaline)s. Dokl Chem 2014, 456, 65–71. [Google Scholar] [CrossRef]
- Hu, J.-Y.; Feng, X.; Seto, N.; Do, J.-H.; Zeng, X.; Tao, Z.; Yamato, T. Synthesis, Structural and Spectral Properties of Diarylamino-Functionalized Pyrene Derivatives via Buchwald–Hartwig Amination Reaction. J. Mol. Struct. 2013, 1035, 19–26. [Google Scholar] [CrossRef]
- Wu, W.; Wu, X.; Zhao, J.; Wu, M. Synergetic Effect of C*N^N/C^N^N Coordination and the Arylacetylide Ligands on the Photophysical Properties of Cyclometalated Platinum Complexes. J. Mater. Chem. C 2015, 3, 2291–2301. [Google Scholar] [CrossRef]
- Mitchell, R.H.; Lai, Y.-H.; Williams, R.V. N-Bromosuccinimide-Dimethylformamide: A Mild, Selective Nuclear Monobromination Reagent for Reactive Aromatic Compounds. J. Org. Chem. 1979, 44, 4733–4735. [Google Scholar] [CrossRef]
- Kovalev, I.S.; Kopchuk, D.S.; Khasanov, A.F.; Zyryanov, G.V.; Rusinov, V.L.; Chupakhin, O.N. The Synthesis of Polyarene-Modified 5-Phenyl-2,2′-Bipyridines via the Methodology and Aza-Diels–Alder Reaction. Mendeleev Commun. 2014, 24, 117–118. [Google Scholar] [CrossRef]
- Zhang, Q.; Chan, Y.; Zhang, M.; Yeung, Y.; Ke, Z. Hypervalent Chalcogenonium⋯π Bonding Catalysis. Angew. Chem. Int. Ed. 2022, 61, e202208009. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, X.; Ke, Z.; Yeung, Y.-Y. Bis-Selenonium Cations as Bidentate Chalcogen Bond Donors in Catalysis. ACS Catal. 2021, 11, 12632–12642. [Google Scholar] [CrossRef]
- He, X.; Wang, X.; Tse, Y.; Ke, Z.; Yeung, Y. Applications of Selenonium Cations as Lewis Acids in Organocatalytic Reactions. Angew. Chem. Int. Ed. 2018, 57, 12869–12873. [Google Scholar] [CrossRef] [PubMed]
- Batabyal, M.; Upadhyay, A.; Kadu, R.; Birudukota, N.C.; Chopra, D.; Kumar, S. Tetravalent Spiroselenurane Catalysts: Intramolecular Se···N Chalcogen Bond-Driven Catalytic Disproportionation of H2O2 to H2O and O2 and Activation of I2 and NBS. Inorg. Chem. 2022, 61, 8729–8745. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Luo, G.; Cai, X.; Wu, H.; Su, S.-J.; Cao, Y. Pyrene Terminal Functionalized Perylene Diimide as Non-Fullerene Acceptors for Bulk Heterojunction Solar Cells. RSC Adv. 2015, 5, 83155–83163. [Google Scholar] [CrossRef]
- Chow, A.L.; So, M.; Lu, W.; Zhu, N.; Che, C. Synthesis, Photophysical Properties, and Molecular Aggregation of Gold(I) Complexes Containing Carbon-Donor Ligands. Chem. Asian J. 2011, 6, 544–553. [Google Scholar] [CrossRef]
- Maeda, H.; Tanaka, K.; Aratani, M.; Segi, M. Ethynylpyrene Linked Benzocrown Ethers as Fluorescent Sensors for Metal Ions. Photochem. Photobiol. 2019, 95, 762–772. [Google Scholar] [CrossRef]
- Murai, M.; Ogita, T.; Takai, K. Regioselective Arene Homologation through Rhenium-Catalyzed Deoxygenative Aromatization of 7-Oxabicyclo[2.2.1]Hepta-2,5-Dienes. Chem. Commun. 2019, 55, 2332–2335. [Google Scholar] [CrossRef]
- Wang, D.; Jin, Z.; Tang, J.; Liang, P.; Mi, Y.; Miao, Z.; Zhang, Y.; Yang, H. Photophysical and Self-Assembly Properties of Asymmetrical Multi-Aralkyl and Arylaldehyde Substituted Pyrene Derivatives. Tetrahedron 2012, 68, 6338–6342. [Google Scholar] [CrossRef]
- Yamato, T.; Fujimoto, M.; Nagano, Y.; Miyazawa, A.; Tashiro, M. Electrophilic Substitution of 7- tert -Butyl-1-Substituted Pyrenes. A New Route for the Preparation of 1,3-Disubstituted Pyrenes. Org. Prep. Proced. Int. 1997, 29, 321–330. [Google Scholar] [CrossRef]
- Niamnont, N.; Kimpitak, N.; Wongravee, K.; Rashatasakhon, P.; Baldridge, K.K.; Siegel, J.S.; Sukwattanasinitt, M. Tunable Star-Shaped Triphenylamine Fluorophores for Fluorescence Quenching Detection and Identification of Nitro-Aromatic Explosives. Chem. Commun. 2013, 49, 780–782. [Google Scholar] [CrossRef]
- Grimshaw, J.; Trocha-Grimshaw, J. Characterisation of 1,6- and 1,8-Dibromopyrenes. J. Chem. Soc. Perkin Trans. 1 1972, 1622. [Google Scholar] [CrossRef]
- Bheemireddy, S.R.; Ubaldo, P.C.; Finke, A.D.; Wang, L.; Plunkett, K.N. Contorted Aromatics via a Palladium-Catalyzed Cyclopentannulation Strategy. J. Mater. Chem. C 2016, 4, 3963–3969. [Google Scholar] [CrossRef]
- Gong, X.; Xie, X.; Chen, N.; Zheng, C.; Zhu, J.; Chen, R.; Huang, W.; Gao, D. Two Symmetrically Bis-Substituted Pyrene Derivatives: Synthesis, Photoluminescence, and Electroluminescence. Chin. J. Chem. 2015, 33, 967–973. [Google Scholar] [CrossRef]
- Minglun, L.; Gong, X.; Zheng, C.; Gao, D. Development of Pyrene Derivatives as Promising N-Type Semiconductors: Synthesis, Structural and Spectral Properties. Asian J. Org. Chem. 2017, 6, 1903–1913. [Google Scholar] [CrossRef]
- Lee, H.; Kim, B.; Kim, S.; Kim, J.; Lee, J.; Shin, H.; Lee, J.-H.; Park, J. Synthesis and Electroluminescence Properties of Highly Efficient Dual Core Chromophores with Side Groups for Blue Emission. J. Mater. Chem. C 2014, 2, 4737–4747. [Google Scholar] [CrossRef]
- Kim, B.; Park, Y.; Lee, J.; Yokoyama, D.; Lee, J.-H.; Kido, J.; Park, J. Synthesis and Electroluminescence Properties of Highly Efficient Blue Fluorescence Emitters Using Dual Core Chromophores. J. Mater. Chem. C 2012, 1, 432–440. [Google Scholar] [CrossRef]
- Lungerich, D.; Papaianina, O.; Feofanov, M.; Liu, J.; Devarajulu, M.; Troyanov, S.I.; Maier, S.; Amsharov, K. Dehydrative π-Extension to Nanographenes with Zig-Zag Edges. Nat. Commun. 2018, 9, 4756. [Google Scholar] [CrossRef]
- Jung, M.; Lee, J.; Jung, H.; Park, J. Synthesis and Physical Properties of New Pyrene Derivative with Bulky Side Groups for Blue Emission. J. Nanosci. Nanotechnol. 2016, 16, 8796–8799. [Google Scholar] [CrossRef]
- Jung, M.; Lee, J.; Jung, H.; Kang, S.; Wakamiya, A.; Park, J. Highly Efficient Pyrene Blue Emitters for OLEDs Based on Substitution Position Effect. Dye. Pigment. 2018, 158, 42–49. [Google Scholar] [CrossRef]
- Gong, X.; Zheng, C.; Feng, X.; Huan, Y.; Li, J.; Yi, M.; Fu, Z.; Huang, W.; Gao, D. 1,8-Substituted Pyrene Derivatives for High-Performance Organic Field-Effect Transistors. Chem. Asian J. 2018, 13, 3920–3927. [Google Scholar] [CrossRef] [PubMed]
- Ðorđević, L.; Valentini, C.; Demitri, N.; Mézière, C.; Allain, M.; Sallé, M.; Folli, A.; Murphy, D.; Mañas-Valero, S.; Coronado, E.; et al. O-Doped Nanographenes: A Pyrano/Pyrylium Route Towards Semiconducting Cationic Mixed-Valence Complexes. Angew. Chem. Int. Ed. 2020, 59, 4106–4114. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, S.; Kang, I.-N.; Park, M.-J.; Hwang, D.-H. Photovoltaic Devices Using Semiconducting Polymers Containing Head-to-Tail-Structured Bithiophene, Pyrene, and Benzothiadiazole Derivatives. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 3415–3424. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, H.; Kang, I.-N.; Lee, S.; Moon, S.-J.; Shin, W.S.; Hwang, D.-H. Incorporation of Pyrene Units to Improve Hole Mobility in Conjugated Polymers for Organic Solar Cells. Macromolecules 2012, 45, 8628–8638. [Google Scholar] [CrossRef]
- Connor, D.M.; Kriegel, R.M.; Collard, D.M.; Liotta, C.L.; Schiraldi, D.A. Pyrene and Anthracene Dicarboxylic Acids as Fluorescent Brightening Comonomers for Polyester. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 1291–1301. [Google Scholar] [CrossRef]
- Suenaga, H.; Nakashima, K.; Mizuno, T.; Takeuchi, M.; Hamachi, I.; Shinkai, S. Pyrenylboronic Acids as a Novel Entry for Photochemical DNA Cleavage: Diradical-Forming Pyrene-1,6-Diyldiboronic Acid Mimics the Cleavage Mechanism of Enediyne Antitumor Antibiotics. J. Chem. Soc. Perkin Trans. 1 1998, 1263–1268. [Google Scholar] [CrossRef]
- Kaplunov, M.G.; Yakushchenko, I.K.; Krasnikova, S.S.; Echmaev, S.B. Novel 1,8-Bis(Diarylamino)Pyrenes as OLED Materials. Mendeleev Commun. 2016, 26, 437–439. [Google Scholar] [CrossRef]
- Li, R.; Xu, H.; Zhang, Y.; Chang, L.; Ma, Y.; Hou, Y.; Miao, S.; Wang, C. Electrochromic Properties of Pyrene Conductive Polymers Modified by Chemical Polymerization. RSC Adv. 2021, 11, 39291–39305. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, Y.; Zhang, T.; Xu, L.; Ni, Z.-H. A Series of Short Axially Symmetrically 1,3,6,8-Tetrasubstituted Pyrene-Based Green and Blue Emitters with 4-Tert-Butylphenyl and Arylamine Attachments. Dye. Pigment. 2016, 130, 106–115. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, T.; Xu, L.; Han, F.; Zhao, Y.; Ni, Z.-H. A New Series of Short Axially Symmetrically and Asymmetrically 1,3,6,8-Tetrasubstituted Pyrenes with Two Types of Substituents: Syntheses, Structures, Photophysical Properties and Electroluminescence. J. Mol. Struct. 2016, 1127, 237–246. [Google Scholar] [CrossRef]
- Arai, R.; Uemura, S.; Irie, M.; Matsuda, K. Reversible Photoinduced Change in Molecular Ordering of Diarylethene Derivatives at a Solution−HOPG Interface. J. Am. Chem. Soc. 2008, 130, 9371–9379. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Tomonaga, Y.; Miyata, K.; Uchida, M.; Terao, Y. Reaction of Polycyclic Aromatic Hydrocarbons Adsorbed on Silica in Aqueous Chlorine. Environ. Sci. Technol. 2007, 41, 2190–2195. [Google Scholar] [CrossRef] [PubMed]
- Minabe, M.; Takeshige, S.; Soeda, Y.; Kimura, T.; Tsubota, M. Electrophilic Substitution of Monosubstituted Pyrenes. Bull. Chem. Soc. Jpn. 1994, 67, 172–179. [Google Scholar] [CrossRef]
- Gerasimenko, Y.E.; Poteleshchenko, V.P. Some substituted pyrene-2-carboxylic acid. J. Org. Chem. USSR 1972, 8, 1084–1087. [Google Scholar]
- Nielsen, T.; Siigur, K.; Helweg, C.; Jørgensen, O.; Hansen, P.E.; Kirso, U. Sorption of Polycyclic Aromatic Compounds to Humic Acid As Studied by High-Performance Liquid Chromatography. Environ. Sci. Technol. 1997, 31, 1102–1108. [Google Scholar] [CrossRef]
- Casas-Solvas, J.; Mooibroek, T.; Sandramurthy, S.; Howgego, J.; Davis, A. A Practical, Large-Scale Synthesis of Pyrene-2-Carboxylic Acid. Synlett 2014, 25, 2591–2594. [Google Scholar] [CrossRef]
- Gooßen, L.J.; Thiel, W.R.; Rodríguez, N.; Linder, C.; Melzer, B. Copper-Catalyzed Protodecarboxylation of Aromatic Carboxylic Acids. Adv. Synth. Catal. 2007, 349, 2241–2246. [Google Scholar] [CrossRef]
- Stock, L.M.; Obeng, M. Oxidation and Decarboxylation. A Reaction Sequence for the Study of Aromatic Structural Elements in Pocahontas No. 3 Coal. Energy Fuels 1997, 11, 987–997. [Google Scholar] [CrossRef]
- Goossen, L.J.; Manjolinho, F.; Khan, B.A.; Rodríguez, N. Microwave-Assisted Cu-Catalyzed Protodecarboxylation of Aromatic Carboxylic Acids. J. Org. Chem. 2009, 74, 2620–2623. [Google Scholar] [CrossRef]
- Maeda, H.; Hironishi, M.; Ishibashi, R.; Mizuno, K.; Segi, M. Synthesis and Fluorescence Properties of Dioxa-, Dithia-, and Diselena-[3.3](1,3)Pyrenophanes. Photochem. Photobiol. Sci. 2017, 16, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Xie, X.; Gong, X.J.; Liu, M.L.; Chen, R.F.; Gao, D.Q.; Huang, W. Two Bipolar Blue-Emitting Fluorescent Materials Based on 1,3,5-Triazine and Peripheral Pyrene for Organic Light-Emitting Diodes. Dye. Pigment. 2017, 145, 43–53. [Google Scholar] [CrossRef]
- Ding, Z.; Ma, B.; Zhou, Z.; Zhang, S.; Pan, J.; Zhu, W.; Liu, Y. Efficient Solution-Processed OLEDs Featuring Deep-Blue Triplet-Triplet Annihilation Emitter Based on Pyrene Derivative. Dye. Pigment. 2023, 216, 111346. [Google Scholar] [CrossRef]
- Feng, X.; Hu, J.-Y.; Tomiyasu, H.; Tao, Z.; Redshaw, C.; Elsegood, M.R.J.; Horsburgh, L.; Teat, S.J.; Wei, X.-F.; Yamato, T. Iron(III) Bromide Catalyzed Bromination of 2-Tert-Butylpyrene and Corresponding Position-Dependent Aryl-Functionalized Pyrene Derivatives. RSC Adv. 2015, 5, 8835–8848. [Google Scholar] [CrossRef]
- Feng, X.; Hu, J.-Y.; Yi, L.; Seto, N.; Tao, Z.; Redshaw, C.; Elsegood, M.R.J.; Yamato, T. Pyrene-Based Y-Shaped Solid-State Blue Emitters: Synthesis, Characterization, and Photoluminescence. Chem. Asian J. 2012, 7, 2854–2863. [Google Scholar] [CrossRef]
- Feng, X.; Iwanaga, F.; Hu, J.-Y.; Tomiyasu, H.; Nakano, M.; Redshaw, C.; Elsegood, M.R.J.; Yamato, T. An Efficient Approach to the Synthesis of Novel Pyrene-Fused Azaacenes. Org. Lett. 2013, 15, 3594–3597. [Google Scholar] [CrossRef] [PubMed]
- Figueira-Duarte, T.M.M.; Simon, S.C.C.; Wagner, M.; Druzhinin, S.I.I.; Zachariasse, K.A.A.; Müllen, K. Polypyrene Dendrimers. Angew. Chem. Int. Ed. 2008, 47, 10175–10178. [Google Scholar] [CrossRef]
- Hashikawa, Y.; Murata, M.; Wakamiya, A.; Murata, Y. Palladium-Catalyzed Cyclization: Regioselectivity and Structure of Arene-Fused C60 Derivatives. J. Am. Chem. Soc. 2017, 139, 16350–16358. [Google Scholar] [CrossRef]
- Wang, C.-Z.; Ichiyanagi, H.; Sakaguchi, K.; Feng, X.; Elsegood, M.R.J.; Redshaw, C.; Yamato, T. Pyrene-Based Approach to Tune Emission Color from Blue to Yellow. J. Org. Chem. 2017, 82, 7176–7182. [Google Scholar] [CrossRef]
- Lorbach, D.; Keerthi, A.; Figueira-Duarte, T.M.; Baumgarten, M.; Wagner, M.; Müllen, K. Cyclization of Pyrene Oligomers: Cyclohexa-1,3-Pyrenylene. Angew. Chem. Int. Ed. 2016, 55, 418–421. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wei, J.-H.; Xu, L.-H.; Jiang, Y.; Miao, B.-X.; Ni, Z.-H. Temperature- and Solvent-Induced Solid-State Emission Changes and AIEE Property of a Pyrene-Based Sulfide Compound. Dye. Pigment. 2020, 177, 108299. [Google Scholar] [CrossRef]
- Wang, C.-Z.; Pang, Z.-J.; Yu, Z.-D.; Zeng, Z.-X.; Zhao, W.-X.; Zhou, Z.-Y.; Redshaw, C.; Yamato, T. Short Axially Asymmetrically 1,3-Disubstituted Pyrene-Based Color-Tunable Emitters: Synthesis, Characterization and Optical Properties. Tetrahedron 2021, 78, 131828. [Google Scholar] [CrossRef]
- Li, F.; Pan, M.; He, Q.; Zhou, Q.; Tang, Q.; Gong, C. Ester-Functionalized Pyrene Derivatives: Effects of Ester Substituents on Photophysical, Electrochemical, Electrochromic, and Electrofluorochromic Properties. Dye. Pigment. 2022, 201, 110203. [Google Scholar] [CrossRef]
- Idzik, K.R.; Licha, T.; Lukeš, V.; Rapta, P.; Frydel, J.; Schaffer, M.; Taeuscher, E.; Beckert, R.; Dunsch, L. Synthesis and Optical Properties of Various Thienyl Derivatives of Pyrene. J. Fluoresc. 2014, 24, 153–160. [Google Scholar] [CrossRef]
- Idzik, K.R.; Cywiński, P.J.; Kuznik, W.; Frydel, J.; Licha, T.; Ratajczyk, T. The Optical Properties and Quantum Chemical Calculations of Thienyl and Furyl Derivatives of Pyrene. Phys. Chem. Chem. Phys. 2015, 17, 22758–22769. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Ueno, R.; Furuyama, T.; Segi, M. Effects of Substituents on Absorption and Fluorescence Properties of Trimethylsilylethynyl- and Tert-Butylethynyl-Pyrenes. J. Photochem. Photobiol. A Chem. 2020, 392, 112428. [Google Scholar] [CrossRef]
- Thakur, K.; Wang, D.; Mirzaei, S.; Rathore, R. Electron-Transfer-Induced Self-Assembly of a Molecular Tweezer Platform. Chem. A Eur. J. 2020, 26, 14085–14089. [Google Scholar] [CrossRef]
- Ahn, Y.; Kim, S.; Song, J.H.; Yeom, W.; Lee, J.; Suh, M.C. The Positional Effect of Arylamines on Pyrene Core in a Blue Fluorescent Dopant Significantly Affecting the Performance of Organic Light Emitting Diodes. Dye. Pigment. 2022, 205, 110505. [Google Scholar] [CrossRef]
- Streitwieser, A., Jr.; Lawler, R.G.; Schwaab, D. On the Bromopyrenes1. J. Org. Chem. 1965, 30, 1470–1473. [Google Scholar] [CrossRef]
- Lee, H.; Harvey, R.G. Synthesis of 2,7-Dibromopyrene. J. Org. Chem. 1986, 51, 2847–2848. [Google Scholar] [CrossRef]
- Foroozesh, M.; Primrose, G.; Guo, Z.; Bell, L.C.; Alworth, W.L.; Guengerich, F.P. Aryl Acetylenes as Mechanism-Based Inhibitors of Cytochrome P450-Dependent Monooxygenase Enzymes. Chem. Res. Toxicol. 1997, 10, 91–102. [Google Scholar] [CrossRef]
- Harvey, R.G.; Schmolka, S.; Cortez, C.; Lee, H. Syntheses of 2-Bromopyrene and 2-Hydroxypyrene. Synth. Commun. 1988, 18, 2207–2209. [Google Scholar] [CrossRef]
- El-Ballouli, A.O.; Khnayzer, R.S.; Khalife, J.C.; Fonari, A.; Hallal, K.M.; Timofeeva, T.V.; Patra, D.; Castellano, F.N.; Wex, B.; Kaafarani, B.R. Diarylpyrenes vs. Diaryltetrahydropyrenes: Crystal Structures, Fluorescence, and Upconversion Photochemistry. J. Photochem. Photobiol. A Chem. 2013, 272, 49–57. [Google Scholar] [CrossRef]
- Godinez, C.E.; Zepeda, G.; Garcia-Garibay, M.A. Molecular Compasses and Gyroscopes. II. Synthesis and Characterization of Molecular Rotors with Axially Substituted Bis[2-(9-Triptycyl)Ethynyl]Arenes. J. Am. Chem. Soc. 2002, 124, 4701–4707. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tan, L.; Shi, J.; Ji, L. Iridium-Catalysed Borylation of Pyrene—A Powerful Route to Novel Optoelectronic Materials. New J. Chem. 2021, 45, 14869–14878. [Google Scholar] [CrossRef]
- Cirera, B.; Đorđević, L.; Otero, R.; Gallego, J.M.; Bonifazi, D.; Miranda, R.; Ecija, D. Dysprosium-Carboxylate Nanomeshes with Tunable Cavity Size and Assembly Motif through Ionic Interactions. Chem. Commun. 2016, 52, 11227–11230. [Google Scholar] [CrossRef] [PubMed]
- Crawford, A.G.; Liu, Z.; Mkhalid, I.A.I.; Thibault, M.-H.; Schwarz, N.; Alcaraz, G.; Steffen, A.; Collings, J.C.; Batsanov, A.S.; Howard, J.A.K.; et al. Synthesis of 2- and 2,7-Functionalized Pyrene Derivatives: An Application of Selective C-H Borylation. Chemistry 2012, 18, 5022–5035. [Google Scholar] [CrossRef]
- Mitchell, R.H.; Chen, Y.; Zhang, J. ChemInform Abstract: N-Bromosuccinimide—Chloroform, a More Convenient Method to Nuclear Brominate Reactive Aromatic Hydrocarbons. ChemInform 1998, 29. [Google Scholar] [CrossRef]
- Sasaki, S.; Suzuki, S.; Igawa, K.; Morokuma, K.; Konishi, G. The K-Region in Pyrenes as a Key Position to Activate Aggregation-Induced Emission: Effects of Introducing Highly Twisted N,N-Dimethylamines. J. Org. Chem. 2017, 82, 6865–6873. [Google Scholar] [CrossRef]




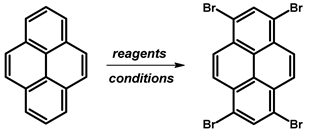 | ||||
|---|---|---|---|---|
| Brominating Agent | Solvent | Reaction Conditions | Yield [%] | Ref. |
| Br2 | PhNO2 | 80 °C, 12 h | 92 | [17] |
| 160 °C, 3 h | 90 | [18] | ||
| 120 °C, 16 h | 98 | [19] | ||
| 120 °C, 4 h | 94 | [20] | ||
| 120 °C, 2 h | 99 | [21] | ||
| 120 °C, 12 h | 96 | [22] | ||
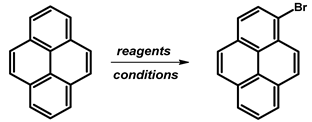 | ||||
|---|---|---|---|---|
| Brominating Agent | Solvent | Reaction Conditions | Yield [%] | Ref. |
| HBr 40%, H2O2 30% | MeOH/Et2O (1:1) | rt, 12 h | 96 | [25] |
| HBr 48%, H2O2 30% | MeOH/Et2O (1:1) | 30 °C, 12 h | 90 | [26] |
| rt, 14 h | 86 | [27] | ||
| rt, 12 h | 84.4 | [28] | ||
| rt, 12 h | 84.7 | [29] | ||
| rt, 16 h | 77 | [24] | ||
| rt, 24 h | 90 | [30] | ||
| HBr 48%, H2O2 35% | MeOH/Et2O (1:1) | rt, 12 h | 95 | [31,32] |
| rt, 14 h | 86 | [33] | ||
| NBS | DCM | rt, 2 h | 95 | [34] |
| rt, 6 h | 90 | [35] | ||
| rt, 6 h | 88 | [36] | ||
| NBS, benzoyl peroxide | DMF | rt | 96 | [37] |
| NBS | DMF | rt, over a night | 94 | [38] |
| rt, over a night | 78 | [39] | ||
| rt, 24 h | 85 | [22,40,41] | ||
| rt | 65–70 | [42,43] | ||
| NBS, additive 1 | DCM | −40 °C, darkness | 82 | [44] |
| NBS, C28H28Se2(BF4)2 | DCM | −40 °C, darkness | 95 | [45] |
| NBS, additive 2 | DCM | −40 °C, 72 h, darkness | 91 | [46] |
| NBS, additive 3 | DCM | rt, 6 h, darkness | 85 | [47] |
| Br2 | DCM | rt, overnight | 75 | [48] |
| Br2 | CCl4 | rt, overnight | 86 | [49] |
| 82 | [50] | |||
| Br2 | CHCl3 | 80 °C, 24 h | 81 | [51] |
| Br2 | PhNO2 | 120 °C, reflux, 10 h | - | [52] |
| BTMABr3, CaCO3 | DCM/MeOH (1:3) | rt, 4 h | 80.3 | [53] |
| BTMABr3, ZnCl2 | AcOH | rt, 12 h | 67 | [54] |
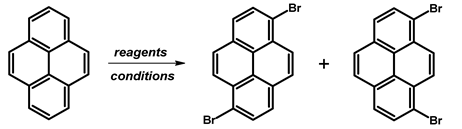 | |||||
|---|---|---|---|---|---|
| Brominating Agent | Solvent | Reaction Conditions | Yield [%] | Ref. | |
| 1,6- | 1,8- | ||||
| Br2 | CH2Cl2 | rt, 24 h | 15 | - | [56] |
| rt, 2 h | 50 | - | [57] | ||
| rt, 20 h | 25 | 9 | [58] | ||
| Br2 | CHCl3 | rt, 17 h | 33 | - | [59,60] |
| rt, 24 h | 36 | - | [61] | ||
| rt, 5 h | 14 | - | [62] | ||
| rt, 17 h | 14 | 6 | [63] | ||
| rt, 5 h | 14 | 6 | [63] | ||
| 20 °C, 4 h | 25 | 9 | [58,64] | ||
| rt, 24 h | 32 | - | [65] | ||
| Br2 | CCl4 | 110 °C, 12 h, darkness | 63 | - | [66] |
| rt, 16 h | 21 | - | [67] | ||
| rt, 17 h | 44 | 45 | [55] | ||
| rt, 17 h | 61 | - | [39] | ||
| rt, 24 h | 28 | 13 | [68] | ||
| rt, 48 h | 38 | - | [69] | ||
| rt, 54 h | 25 | 50 | [70] | ||
| rt, 12 h | 43 | - | [71] | ||
| Br2 | CS2 | rt, 17 h | 15 | 85 | [72,73] |
| DBMH | CH2Cl2 | rt, 1 h | 97 | [22] | |
| BTMABr3 + ZnCl2 | CH2Cl2 MeOH | rt, 16 h | quant. | [74] | |
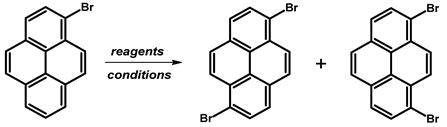 | |||||
|---|---|---|---|---|---|
| Brominating Agent | Solvent | Reaction Conditions | Yield [%] | Ref. | |
| 1,6- | 1,8- | ||||
| KBr + NaClO | HCl, MeOH | rt, 24 h | 43 | [75] | |
| Br2 | CH2Cl2 | rt, 6 h | 35 | 36 | [76] |
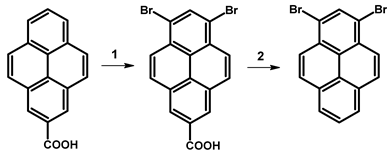 | |||||
|---|---|---|---|---|---|
| Reagents | Solvent | Reaction Conditions | Yield [%] | Ref. | |
| 1 | Br2 | PhNO2 | 120 °C | 80 | [77] |
| 2 | CaO, Ca(OH)2 | DMF, H2O | reflux | 9.3 | |
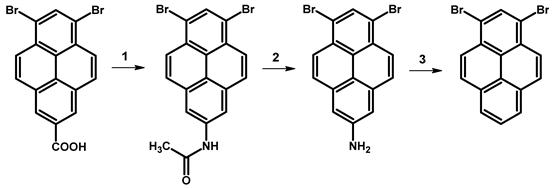 | ||||
|---|---|---|---|---|
| Reagents | Reaction Conditions | Yield [%] | Ref. | |
| 1 | PCl5, py, NaN3, Ac2O, H2O | 100 °C, 1 h | 48.5 | [77] |
| 2 | HCl, AcOH | reflux | 98 | |
| 3 | NaNO2, H2SO4, AcOH | reflux | 19.6 | |
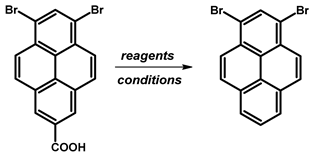 | |||
|---|---|---|---|
| Substrates | Reaction Conditions | Yield [%] | Ref. |
| Cu, quinoline | reflux, 1.5 h | ≈0 | [78] |
| Cu2O, TMEDA, NMP | 120 °C, 4 h | ≈0 | [80] |
| Cu2O, quinoline, NMP | 180 °C, 12 h | ≈0 | [81] |
| Cu2O, 4,7-diphenyl-1,10-phenanthroline, NMP | 170 °C, 12 h | ≈0 | [82] |
| Cu2O, 1,10-phenanthroline, quinoline, NMP | 180 °C, 1 h, MW | ≈0 | [82] |
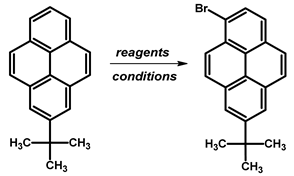 | ||||
|---|---|---|---|---|
| Brominating Agent | Solvent | Reaction Conditions | Yield [%] | Ref. |
| Br2 | CH2Cl2 | −78 °C to rt, overnight | 88 | [84] |
| Br2 | CH2Cl2 | −78 °C to rt, overnight | 75 | [48,85,89] |
| Br2 | CH2Cl2 | −78 °C to rt, 10 h | 72 | [93] |
| Br2 | CH2Cl2 | −78 °C to rt, 14 h | 80 | [90] |
| Br2, Fe | CH2Cl2 | 0 °C to 28 °C, 5 h | 83 | [86] |
| BTMABr3 | CH2Cl2 | 0 °C to rt, 3 h | 84 | [86] |
| NBS | THF | 0 °C to rt, overnight | 94 | [94] |
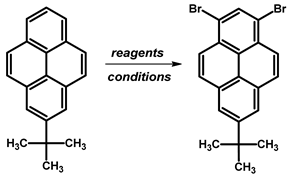 | ||||
|---|---|---|---|---|
| Brominating Agent | Solvent | Reaction Conditions | Yield [%] | Ref. |
| Br2 | CCl4 | rt, 16 h | 68 | [83] |
| Br2 | CH2Cl2 | −78 °C | 89 | [89] |
| Br2, Fe | CH2Cl2 | 0 °C to 28 °C, 5 h | 35 | [86] |
| NBS | THF | 30 °C, overnight | 91 | [65] |
| BTMABr3, CaCO3 | CH2Cl2/MeOH | 0 °C, 1 h rt, overnight | 76 | [87,88,91] |
| BTMABr3 | CH2Cl2 | 0 °C to rt, overnight | 76 | [86] |
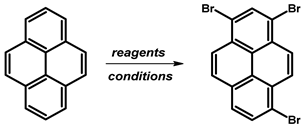 | ||||
|---|---|---|---|---|
| Brominating Agent | Solvent | Reaction Conditions | Yield [%] | Ref. |
| Br2 | PhNO2 | rt, 96 h | 14 | [55] |
| Br2 | nitrotoluene | - | - | [95,96,97] |
| Br2 | PhNO2 | 80 °C, 12 h | 87 | [98] |
 | ||||
|---|---|---|---|---|
| Brominating Agent | Solvent | Reaction Conditions | Yield [%] | Ref. |
| Br2 | PhNO2 | rt, 12 h | 82 | [99] |
 | |||||
|---|---|---|---|---|---|
| Reagents | Solvent | Reaction Conditions | Yield [%] | Ref. | |
| 1 | B2pin2, [Ir(μ-OMe)(cod)]2, dtbpy | cyclohexane | 70 °C, overnight | 22 | [100] |
| 2 | CuBr2 | isopropanol:DMF (1:1), H2O | 110 °C, 6 h | 66 | |
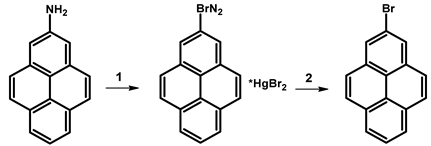 | ||||
|---|---|---|---|---|
| Reagents | Reaction Conditions | Yield [%] | Ref. | |
| 1 | NaNO2, H2SO4, H2O; CO(NH₂)₂, H2O; HgBr2, KBr, H2O | 2 h; 1 h; 1 h | 120 (C16H9BrN2*HgBr2) | [101] |
| 2 | KBr | 120 °C, 0.5 h | 32 | |
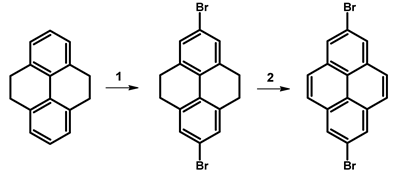 | ||||
|---|---|---|---|---|
| Reagents | Reaction Conditions | Yield [%] | Ref. | |
| 1 | Br2, FeCl3·H2O, H2O | rt, overnight | 99 | [102] |
| 2 | Br2, CS2 | rt, 4 h | 73 | |
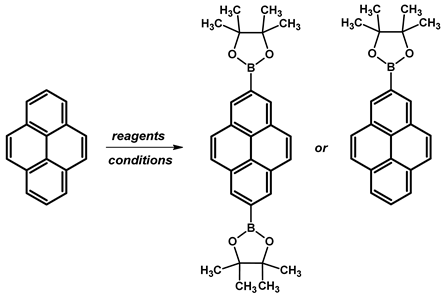 | ||||
|---|---|---|---|---|
| Reagents | Reaction Conditions | Yield [%] | Ref. | |
| 2,7-di | B2pin2, [Ir(μ-OMe)(cod)]2, dtbpy, THF | 80 °C, 16 h | 81 | [108] |
| 94 | [109] | |||
| 2-mono | B2pin2, [Ir(μ-OMe)(cod)]2, dtbpy, hexane | 80 °C, 16 h | 65 | [109] |
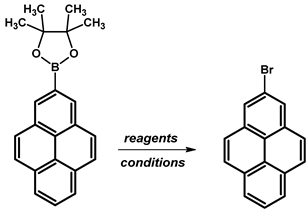 | ||||
|---|---|---|---|---|
| Brominating Agent | Solvent | Reaction Conditions | Yield [%] | Ref. |
| NBS | CHCl3 | 15–20 °C, 24 h | 96 | [110] |
| CuBr2 | MeOH/H2O (1:1) | 90 °C, 16 h | 83 | [109] |
 | ||||
|---|---|---|---|---|
| Brominating Agent | Solvent | Reaction Conditions | Yield [%] | Ref. |
| CuBr2 | THF/MeOH (1:3), H2O | 90 °C, overnight | 64 | [108] |
| CuBr2 | THF/MeOH/H2O (1:3:3) | 90 °C, 16 h | 70 | [109] |
| CuBr2 | THF/MeOH/H2O (1:3:3) | 90 °C, 12 h | 98 | [111] |
| 1,3,6-triBr | 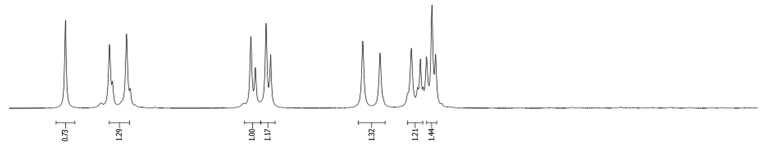 |
| 1,3-diBr-7-tert-butyl | 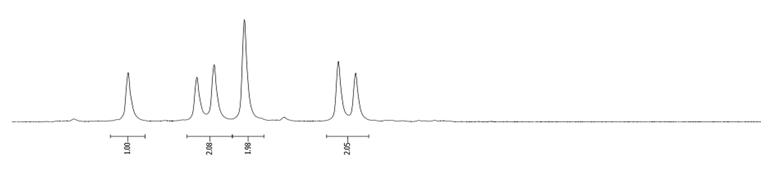 |
| 2,7-diBr | 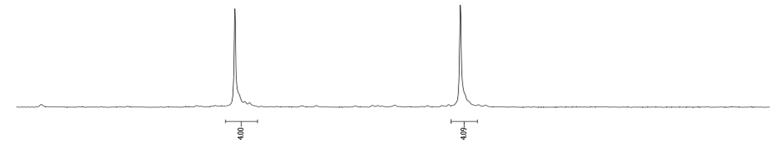 |
| 1,7-diBr |  |
| 1,8-diBr |  |
| 1,6-diBr | 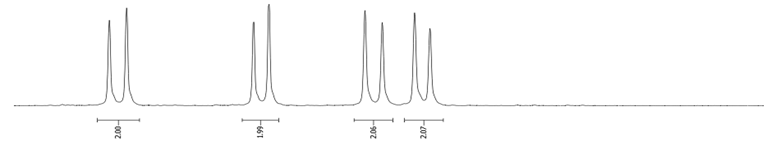 |
| 2-Br | 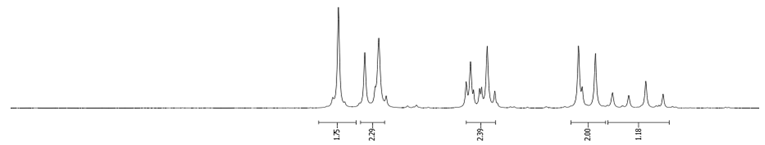 |
| 1-Br | 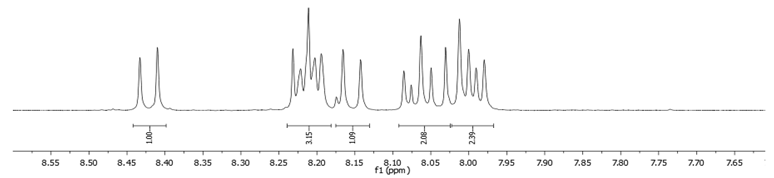 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zych, D.; Kubis, M. Bromopyrene Symphony: Synthesis and Characterisation of Isomeric Derivatives at Non-K Region and Nodal Positions for Diverse Functionalisation Strategies. Molecules 2024, 29, 1131. https://doi.org/10.3390/molecules29051131
Zych D, Kubis M. Bromopyrene Symphony: Synthesis and Characterisation of Isomeric Derivatives at Non-K Region and Nodal Positions for Diverse Functionalisation Strategies. Molecules. 2024; 29(5):1131. https://doi.org/10.3390/molecules29051131
Chicago/Turabian StyleZych, Dawid, and Martyna Kubis. 2024. "Bromopyrene Symphony: Synthesis and Characterisation of Isomeric Derivatives at Non-K Region and Nodal Positions for Diverse Functionalisation Strategies" Molecules 29, no. 5: 1131. https://doi.org/10.3390/molecules29051131
APA StyleZych, D., & Kubis, M. (2024). Bromopyrene Symphony: Synthesis and Characterisation of Isomeric Derivatives at Non-K Region and Nodal Positions for Diverse Functionalisation Strategies. Molecules, 29(5), 1131. https://doi.org/10.3390/molecules29051131








