Recent Advances in Light-Driven Semiconductor-Based Micro/Nanomotors: Optimization Strategies and Emerging Applications
Abstract
:1. Introduction
Propulsion Mechanisms
2. Light-Driven Semiconductor-Based Micro/Nanomotors
2.1. Semiconductors Most Used in the Preparation of Light-Driven Micro/Nanomotors
2.1.1. Light-Driven TiO2-Based Micro/Nanomotors
2.1.2. Light-Driven ZnO-Based Micro/Nanomotors
2.1.3. Light-Driven CuO2-Based Micro/Nanomotors
2.1.4. Hybrid Light-Driven Semiconductor-Based Micro/Nanomotors
Light-Driven Graphene-Based Micro/Nanomotors
Light-Driven MXene-Based Micro/Nanomotors
3. Propulsion Optimization Parameters
3.1. Structural Parameters of LDSM That Affect Propulsion
3.1.1. Scale
3.1.2. Morphology
- Tubular shape
- Spherical shape
- Nanowires and nanorods
3.1.3. Asymmetric Structure (Janus Structure)
3.1.4. Crystallinity
3.1.5. Density
3.2. Surface Parameters of LDSM That Affect Propulsion
3.2.1. Surface Area
3.2.2. Porosity
3.2.3. Functionalization
3.3. External Parameters That Affect the Propulsion of LDSM
3.3.1. Type and Intensity of Light Irradiation
- UV light
- Visible light
- NIR light
- Effect of light intensity
3.3.2. Other External Stimuli
4. Applications
4.1. Environment
4.1.1. Monitoring and Sensing
4.1.2. Remediation
Photocatalytic Degradation of Pollutants
4.2. Biomedicine
5. Conclusions
- Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soto, F.; Karshalev, E.; Zhang, F.; de Avila, B.E.F.; Nourhani, A.; Wang, J. Smart Materials for Microrobots. Chem. Rev. 2022, 122, 5365–5403. [Google Scholar] [CrossRef] [PubMed]
- Somasundar, A.; Sen, A. Chemically Propelled Nano and Micromotors in the Body: Quo Vadis? Small 2021, 17, e2007102. [Google Scholar] [CrossRef] [PubMed]
- Karshalev, E.; de Ávila, B.E.-F.; Wang, J. Micromotors for “Chemistry-on-the-Fly”. J. Am. Chem. Soc. 2018, 140, 3810–3820. [Google Scholar] [CrossRef] [PubMed]
- Büttgenbach, S. Electromagnetic Micromotors—Design, Fabrication and Applications. Micromachines 2014, 5, 929–942. [Google Scholar] [CrossRef]
- Zhou, H.; Mayorga-Martinez, C.C.; Pané, S.; Zhang, L.; Pumera, M. Magnetically Driven Micro and Nanorobots. Chem. Rev. 2021, 121, 4999–5041. [Google Scholar] [CrossRef]
- Wu, Y.; Yakov, S.; Fu, A.; Yossifon, G. A Magnetically and Electrically Powered Hybrid Micromotor in Conductive Solutions: Synergistic Propulsion Effects and Label-Free Cargo Transport and Sensing. Adv. Sci. 2023, 10, 202204931. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, J.; Fang, B.; Zhao, X.; Hao, N. Acoustics-Actuated Microrobots. Micromachines 2022, 13, 481. [Google Scholar] [CrossRef]
- Villa, K.; Pumera, M. Fuel-free light-driven micro/nanomachines: Artificial active matter mimicking nature. Chem. Soc. Rev. 2019, 48, 4966–4978. [Google Scholar] [CrossRef]
- Zhan, Z.; Wei, F.; Zheng, J.; Yang, W.; Luo, J.; Yao, L. Recent advances of light-driven micro/nanomotors: Toward powerful thrust and precise control. Nanotechnol. Rev. 2018, 6, 383–404. [Google Scholar] [CrossRef]
- Yuan, K.; Bujalance-Fernández, J.; Jurado-Sánchez, B.; Escarpa, A. Light-driven nanomotors and micromotors: Envisioning new analytical possibilities for bio-sensing. Microchim. Acta 2020, 187, 132–144. [Google Scholar] [CrossRef]
- Wang, H.; Pumera, M. Emerging materials for the fabrication of micro/nanomotors. Nanoscale 2017, 9, 2109–2116. [Google Scholar] [CrossRef]
- Sánchez, S.; Soler, L.; Katuri, J. Chemically Powered Micro- and Nanomotors. Angew. Chem. Int. Ed. 2015, 54, 1414–1444. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, H.; Wen, H.; Zhao, H.; Liu, X.; Cai, Y.; Wang, H.; Dong, R. Graphene oxide induced enhancement of light-driven micromotor with biocompatible fuels. Appl. Mater. Today 2021, 22, 100943–1010036. [Google Scholar] [CrossRef]
- Dubal, D.P.; Chodankar, N.R.; Kim, D.-H.; Gomez-Romero, P. Towards flexible solid-state supercapacitors for smart and wearable electronics. Chem. Soc. Rev. 2018, 47, 2065–2129. [Google Scholar] [CrossRef]
- Liang, X.; Mou, F.; Huang, Z.; Zhang, J.; You, M.; Xu, L.; Luo, M.; Guan, J. Hierarchical Microswarms with Leader–Follower-Like Structures: Electrohydrodynamic Self-Organization and Multimode Collective Photoresponses. Adv. Funct. Mater. 2020, 30, 1908602–1908620. [Google Scholar] [CrossRef]
- Ge, Y.; Wang, T.; Zheng, M.; Jiang, Z.; Wang, S. Controlled one-sided growth of Janus TiO2/MnO2 nanomotors. Nanotechnology 2019, 30, 315702. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Z.; Solovev, A.A.; Huang, G.; Mei, Y. Light-controlled two-dimensional TiO2 plate micromotors. RSC Adv. 2019, 9, 29433–29439. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Tang, S.; Teymourian, H.; Karshalev, E.; Zhang, F.; Li, J.; Mou, F.; Liang, Y.; Guan, J.; Wang, J. Chemical/Light-Powered Hybrid Micromotors with “On-the-Fly” Optical Brakes. Angew. Chem. Int. Ed. 2018, 57, 8110–8114. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Athanassiadis, A.G.; Popescu, M.N.; Chikkadi, V.; Güth, A.; Singh, D.P.; Qiu, T.; Fischer, P. Microchannels with Self-Pumping Walls. ACS Nano 2020, 14, 13673–13680. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Du, X.; Guo, J.; Wang, K.; Song, B. Light-Responsive Nanofibrous Motor with Simultaneously Precise Locomotion and Reversible Deformation. ACS Appl. Mater. Interfaces 2021, 13, 8985–8996. [Google Scholar] [CrossRef] [PubMed]
- Maghsoodi, A.; Bhattacharya, K. Light-induced swirling and locomotion. Proc. R. Soc. A Math. Phys. Eng. Sci. 2022, 478. [Google Scholar] [CrossRef]
- Lan, R.; Sun, J.; Shen, C.; Huang, R.; Zhang, Z.; Ma, C.; Bao, J.; Zhang, L.; Wang, L.; Yang, D.; et al. Light-Driven Liquid Crystalline Networks and Soft Actuators with Degree-of-Freedom-Controlled Molecular Motors. Adv. Funct. Mater. 2020, 30, 202000252. [Google Scholar] [CrossRef]
- Liras, M.; Barawi, M.; De La Peña O’Shea, V.A. Hybrid materials based on conjugated polymers and inorganic semiconductors as photocatalysts: From environmental to energy applications. Chem. Soc. Rev. 2019, 48, 5454–5487. [Google Scholar] [CrossRef]
- Li, W.; Wu, X.; Qin, H.; Zhao, Z.; Liu, H. Light-Driven and Light-Guided Microswimmers. Adv. Funct. Mater. 2016, 26, 3164–3171. [Google Scholar] [CrossRef]
- Pimienta, V.; Antoine, C. Self-propulsion on liquid surfaces. Curr. Opin. Colloid Interface Sci. 2014, 19, 290–299. [Google Scholar] [CrossRef]
- Kavokine, N.; Anyfantakis, M.; Morel, M.; Rudiuk, S.; Bickel, T.; Baigl, D. Light-Driven Transport of a Liquid Marble with and against Surface Flows. Angew. Chem. Int. Ed. 2016, 55, 11183–11187. [Google Scholar] [CrossRef]
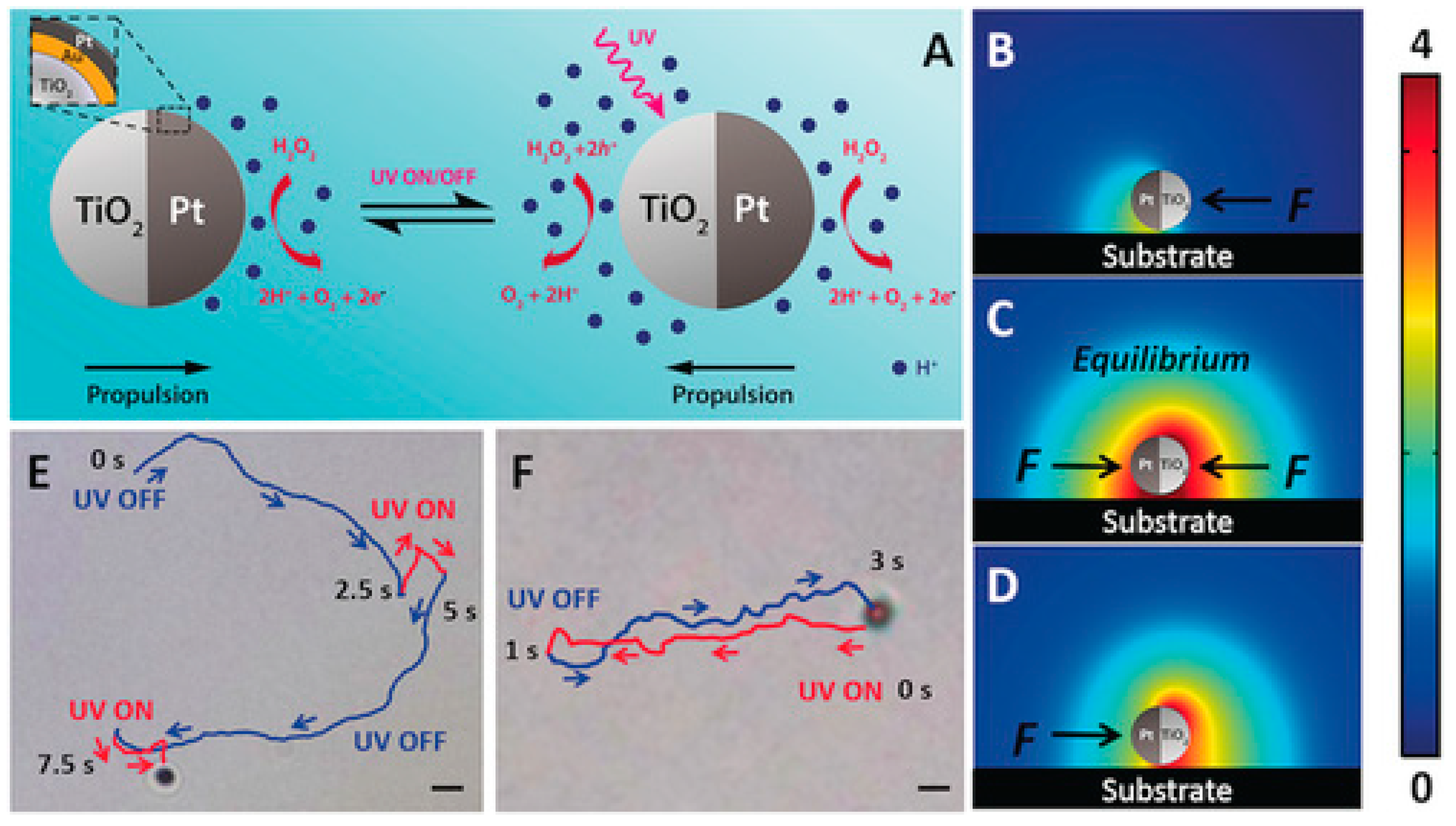
- Sridhar, V.; Park, B.-W.; Sitti, M. Light-Driven Janus Hollow Mesoporous TiO2-Au Microswimmers. Adv. Funct. Mater. 2018, 28, 1704902–1704932. [Google Scholar] [CrossRef]
- Wang, Q.-L.; Wang, C.; Dong, R.-F.; Pang, Q.-Q.; Cai, Y.-P. Steerable light-driven TiO2-Fe Janus micromotor. Inorg. Chem. Commun. 2018, 91, 1–4. [Google Scholar] [CrossRef]
- Chen, B.; Ding, M.; Tan, H.; Wang, S.; Liu, L.; Wang, F.; Tian, H.; Gao, J.; Ye, Y.; Fu, D.; et al. Visible-light-driven TiO2@N-Au nanorobot penetrating the vitreous. Appl. Mater. Today 2022, 27, 101455. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, F.; Zhao, J.; Talaat, W.; Soto, F.; Karshalev, E.; Chen, C.; Hu, Z.; Lu, X.; Li, J.; et al. Structure-Dependent Optical Modulation of Propulsion and Collective Behavior of Acoustic/Light-Driven Hybrid Microbowls. Adv. Funct. Mater. 2019, 29, 1809003–1809019. [Google Scholar] [CrossRef]
- Wang, X.; Sridhar, V.; Guo, S.; Talebi, N.; Miguel-López, A.; Hahn, K.; van Aken, P.A.; Sánchez, S. Fuel-Free Nanocap-Like Motors Actuated Under Visible Light. Adv. Funct. Mater. 2018, 28, 1705862–1705899. [Google Scholar] [CrossRef]
- Wittmann, M.; Ali, A.; Gemming, T.; Stavale, F.; Simmchen, J. Semiconductor-Based Microswimmers: Attention to Detail Matters. J. Phys. Chem. Lett. 2021, 12, 9651–9656. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhu, H.; Zhao, Z.; You, C.; Kong, Y.; Zhao, Y.; Liu, J.; Chen, H.; Shi, X.; Makarov, D.; et al. Hydrogel-Based Janus Micromotors Capped with Functional Nanoparticles for Environmental Applications. Adv. Mater. Technol. 2020, 5, 2000279–2000299. [Google Scholar] [CrossRef]
- Nicholls, D.; DeVerse, A.; Esplin, R.; Castañeda, J.; Loyd, Y.; Nair, R.; Voinescu, R.; Zhou, C.; Wang, W.; Gibbs, J.G. Shape-Dependent Motion of Structured Photoactive Microswimmers. ACS Appl. Mater. Interfaces 2018, 10, 18050–18056. [Google Scholar] [CrossRef] [PubMed]
- Holterhoff, A.L.; Li, M.; Gibbs, J.G. Self-Phoretic Microswimmers Propel at Speeds Dependent upon an Adjacent Surface’s Physicochemical Properties. J. Phys. Chem. Lett. 2018, 9, 5023–5028. [Google Scholar] [CrossRef]
- Wang, L.; Popescu, M.N.; Stavale, F.; Ali, A.; Gemming, T.; Simmchen, J. Cu@TiO2 Janus microswimmers with a versatile motion mechanism. Soft Matter 2018, 14, 6969–6973. [Google Scholar] [CrossRef]
- Dai, J.; Cheng, X.; Li, X.; Wang, Z.; Wang, Y.; Zheng, J.; Liu, J.; Chen, J.; Wu, C.; Tang, J. Solution-Synthesized Multifunctional Janus Nanotree Microswimmer. Adv. Funct. Mater. 2021, 31, 2106204. [Google Scholar] [CrossRef]
- Deng, Z.; Mou, F.; Tang, S.; Xu, L.; Luo, M.; Guan, J. Swarming and collective migration of micromotors under near infrared light. Appl. Mater. Today 2018, 13, 45–53. [Google Scholar] [CrossRef]
- Sridhar, V.; Park, B.-W.; Guo, S.; van Aken, P.A.; Sitti, M. Multiwavelength-Steerable Visible-Light-Driven Magnetic CoO–TiO2 Microswimmers. ACS Appl. Mater. Interfaces 2020, 12, 24149–24155. [Google Scholar] [CrossRef]
- Maric, T.; Nasir, M.Z.M.; Webster, R.D.; Pumera, M. Tailoring Metal/TiO2 Interface to Influence Motion of Light-Activated Janus Micromotors. Adv. Funct. Mater. 2020, 30, 1908614–1908629. [Google Scholar] [CrossRef]
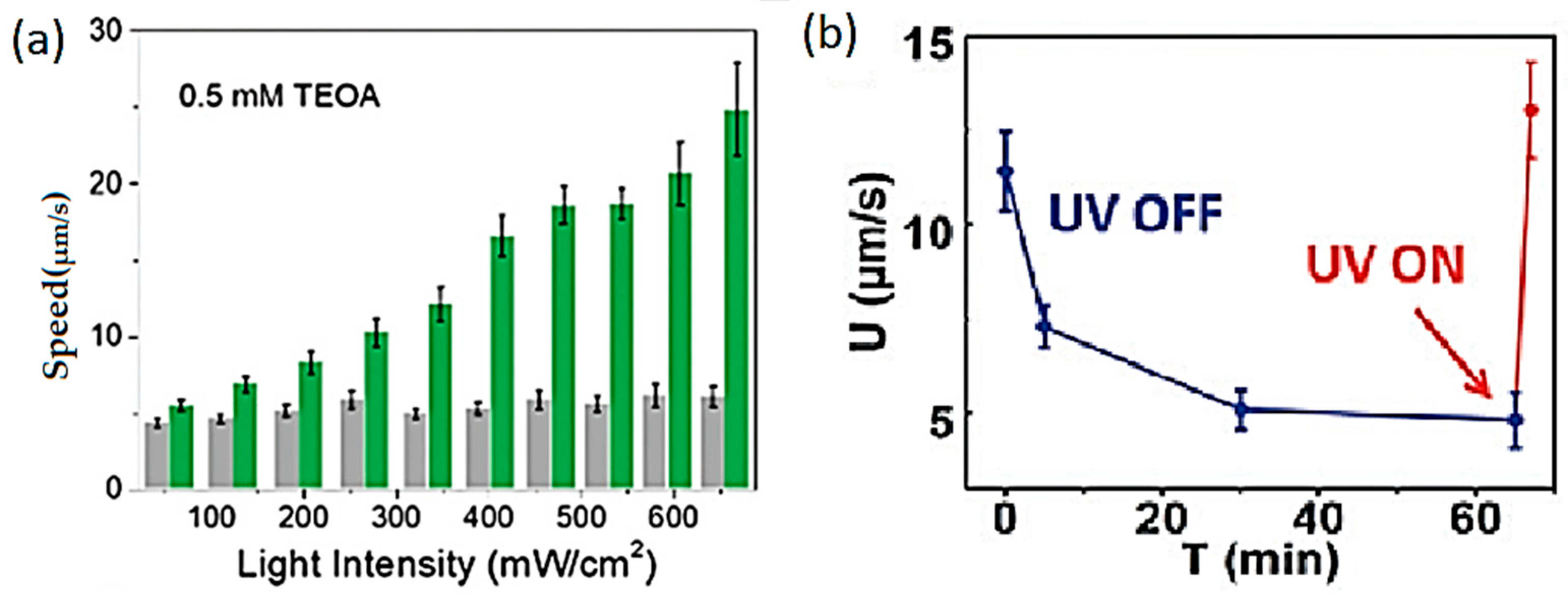
- Duan, S.; Xu, P.; Wang, W. Better fuels for photocatalytic micromotors: A case study of triethanolamine. Chem. Commun. 2021, 57, 9902–9905. [Google Scholar] [CrossRef]
- Zhang, J.; Mou, F.; Tang, S.; Kauffman, J.E.; Sen, A.; Guan, J. Photochemical micromotor of eccentric core in isotropic hollow shell exhibiting multimodal motion behavior. Appl. Mater. Today 2022, 26, 101371. [Google Scholar] [CrossRef]
- Étude O’Neel-Judy, J.G.G.; Nicholls, D.; Castañeda, J.; Gibbs, J.G. Light-Activated, Multi-Semiconductor Hybrid Microswimmers. Small 2018, 14, e1801860. [Google Scholar] [CrossRef]
- Ikram, M.; Hu, F.; Peng, G.; Basharat, M.; Jabeen, N.; Pan, K.; Gao, Y. Light-Activated Fuel-Free Janus Metal Organic Framework Colloidal Motors for the Removal of Heavy Metal Ions. ACS Appl. Mater. Interfaces 2021, 13, 51799–51806. [Google Scholar] [CrossRef]
- Liu, M.; Jiang, J.; Tan, H.; Chen, B.; Ou, J.; Wang, H.; Sun, J.; Liu, L.; Wang, F.; Gao, J.; et al. Light-driven Au–ZnO nanorod motors for enhanced photocatalytic degradation of tetracycline. Nanoscale 2022, 14, 12804–12813. [Google Scholar] [CrossRef]
- He, X.; Jiang, H.; Li, J.; Ma, Y.; Fu, B.; Hu, C. Dipole-Moment Induced Phototaxis and Fuel-Free Propulsion of ZnO/Pt Janus Micromotors. Small 2021, 17, 2101388. [Google Scholar] [CrossRef]
- Zhan, X.; Zheng, J.; Zhao, Y.; Zhu, B.; Cheng, R.; Wang, J.; Liu, J.; Tang, J.; Tang, J. From Strong Dichroic Nanomotor to Polarotactic Microswimmer. Adv. Mater. 2019, 31, e1903329. [Google Scholar] [CrossRef] [PubMed]
- Pourrahimi, A.M.; Villa, K.; Ying, Y.; Sofer, Z.; Pumera, M. ZnO/ZnO2/Pt Janus Micromotors Propulsion Mode Changes with Size and Interface Structure: Enhanced Nitroaromatic Explosives Degradation under Visible Light. ACS Appl. Mater. Interfaces 2018, 10, 42688–42697. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Zhou, D.; Zhang, G.; Li, L. Numerical Analysis of Visible Light Driven Gold/Ferric Oxide Nanomotors. IEEE Trans. Nanotechnol. 2018, 17, 692–696. [Google Scholar] [CrossRef]
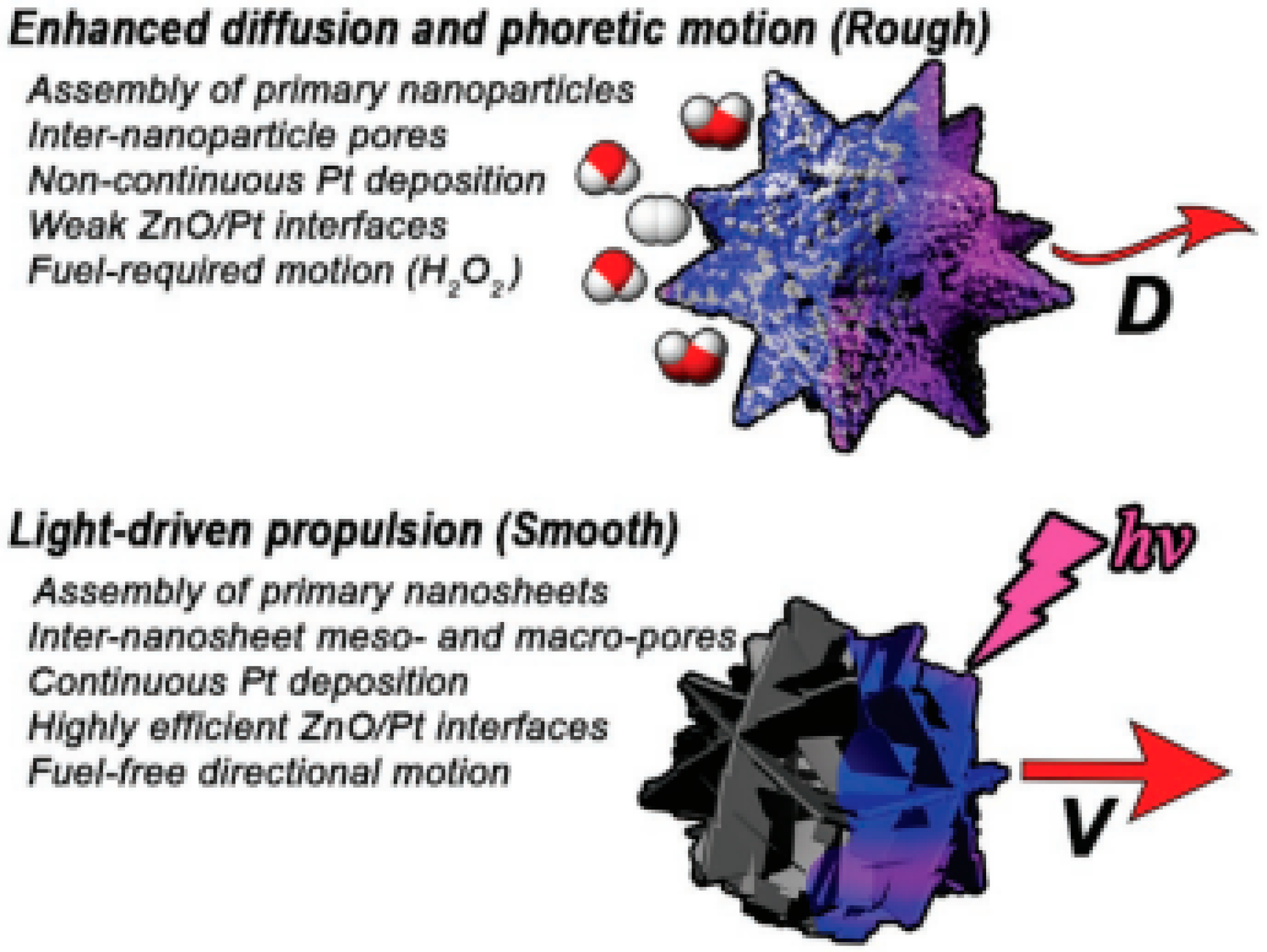
- Pourrahimi, A.M.; Villa, K.; Palenzuela, C.L.M.; Ying, Y.; Sofer, Z.; Pumera, M. Catalytic and Light-Driven ZnO/Pt Janus Nano/Micromotors: Switching of Motion Mechanism via Interface Roughness and Defect Tailoring at the Nanoscale. Adv. Funct. Mater. 2019, 29, 1808678–1808685. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Z.; Tan, L.; Zhang, Y.; Jiao, Y. Near-Infrared Light-Steered Graphene Aerogel Micromotor with High Speed and Precise Navigation for Active Transport and Microassembly. ACS Appl. Mater. Interfaces 2020, 12, 23134–23144. [Google Scholar] [CrossRef]
- Maria-Hormigos, R.; Jurado-Sánchez, B.; Escarpa, A. Graphene quantum dot based micromotors: A size matter. Chem. Commun. 2019, 55, 6795–6798. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Dong, R.; Yang, Q.; Wang, J.; Xu, S.; Cai, Y. Highly efficient visible-light-driven oxygen-vacancy-based Cu2+1O micromotors with biocompatible fuels. Nanoscale Horiz. 2020, 5, 325–330. [Google Scholar] [CrossRef]
- Chen, X.; Ding, X.; Liu, Y.; Li, J.; Liu, W.; Lu, X.; Gu, Z. Highly efficient visible-light-driven Cu2O@CdSe micromotors adsorbent. Appl. Mater. Today 2021, 25, 101200. [Google Scholar] [CrossRef]
- Liu, W.; Chen, X.; Ding, X.; Long, Q.; Lu, X.; Wang, Q.; Gu, Z. Visible-light-driven cuprous oxide nanomotors with surface-heterojunction-induced propulsion. Nanoscale Horiz. 2021, 6, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Jiang, J.; Wang, X.; Zhang, H.; Song, B.; Dong, B. Visible light-regulated BiVO4-based micromotor with biomimetic ‘predator-bait’ behavior. J. Mater. Sci. 2022, 57, 4092–4103. [Google Scholar] [CrossRef]
- Villa, K.; Novotný, F.; Zelenka, J.; Browne, M.P.; Ruml, T.; Pumera, M. Visible-Light-Driven Single-Component BiVO4 Micromotors with the Autonomous Ability for Capturing Microorganisms. ACS Nano 2019, 13, 8135–8145. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Mayorga-Martinez, C.C.; Guan, J.; Pumera, M. Fuel-Free Light-Powered TiO2/Pt Janus Micromotors for Enhanced Nitroaromatic Explosives Degradation. ACS Appl. Mater. Interfaces 2018, 10, 22427–22434. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Fu, D.; Xie, D.; Liu, L.; Chen, B.; Ye, Y.; Wilson, D.A.; Peng, F. Light driven micromotor swarm for tumor photothermal therapy. Appl. Mater. Today 2022, 26, 101348. [Google Scholar] [CrossRef]
- Zhang, J.; Mou, F.; Wu, Z.; Tang, S.; Xie, H.; You, M.; Liang, X.; Xu, L.; Guan, J. Simple-Structured Micromotors Based on Inherent Asymmetry in Crystalline Phases: Design, Large-Scale Preparation, and Environmental Application. ACS Appl. Mater. Interfaces 2019, 11, 16639–16646. [Google Scholar] [CrossRef]
- Peng, X.; Urso, M.; Pumera, M. Metal oxide single-component light-powered micromotors for photocatalytic degradation of nitroaromatic pollutants. npj Clean Water 2023, 6, 1–7. [Google Scholar] [CrossRef]
- Hong, Y.; Diaz, M.; Córdova-Figueroa, U.M.; Sen, A. Light-Driven Titanium-Dioxide-Based Reversible Microfireworks and Micromotor/Micropump Systems. Adv. Funct. Mater. 2010, 20, 1568–1576. [Google Scholar] [CrossRef]
- Yu, T.; Chuphal, P.; Thakur, S.; Reigh, S.Y.; Singh, D.P.; Fischer, P. Chemical micromotors self-assemble and self-propel by spontaneous symmetry breaking. Chem. Commun. 2018, 54, 11933–11936. [Google Scholar] [CrossRef] [PubMed]
- 64. She, P.; Qin, J.S.; Rao, H.; Guan, B.; Yu, J. Spatially separated bimetallic cocatalysts on hollow-structured TiO2 for photocatalytic hydrogen generation. Mater. Chem. Front. 2020, 4, 1671–1678. [Google Scholar] [CrossRef]
- Zheng, J.; Dai, B.; Wang, J.; Xiong, Z.; Yang, Y.; Liu, J.; Zhan, X.; Wan, Z.; Tang, J. Orthogonal navigation of multiple visible-light-driven artificial microswimmers. Nat. Commun. 2017, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.; Hong, A.; Kang, H.E.; Alcantara, C.; Charreyron, S.; Mushtaq, F.; Pellicer, E.; Büchel, R.; Sort, J.; Lee, S.S.; et al. Multiwavelength Light-Responsive Au/B-TiO2 Janus Micromotors. ACS Nano 2017, 11, 6146–6154. [Google Scholar] [CrossRef] [PubMed]
- Pourrahimi, A.M.; Villa, K.; Sofer, Z.; Pumera, M. Light-Driven Sandwich ZnO/TiO2/Pt Janus Micromotors: Schottky Barrier Suppression by Addition of TiO2 Atomic Interface Layers into ZnO/Pt Micromachines Leading to Enhanced Fuel-Free Propulsion. Small Methods 2019, 3, 1900258. [Google Scholar] [CrossRef]
- Ke, H.; Ye, S.; Carroll, R.L.; Showalter, K. Motion Analysis of Self-Propelled Pt−Silica Particles in Hydrogen Peroxide Solutions. J. Phys. Chem. A 2010, 114, 5462–5467. [Google Scholar] [CrossRef]
- Mbayachi, V.B.; Ndayiragije, E.; Sammani, T.; Taj, S.; Mbuta, E.R.; Khan, A.U. Graphene synthesis, characterization and its applications: A review. Results Chem. 2021, 3, 100163. [Google Scholar] [CrossRef]
- Orozco, J.; Mercante, L.A.; Pol, R.; Merkoçi, A. Graphene-based Janus micromotors for the dynamic removal of pollutants. J. Mater. Chem. A 2016, 4, 3371–3378. [Google Scholar] [CrossRef]
- Mayorga-Martinez, C.C.; Vyskočil, J.; Novotný, F.; Pumera, M. Light-driven Ti3C2 MXene micromotors: Self-propelled autonomous machines for photodegradation of nitroaromatic explosives. J. Mater. Chem. A 2021, 9, 14904–14910. [Google Scholar] [CrossRef]
- Urso, M.; Ussia, M.; Novotný, F.; Pumera, M. Trapping and detecting nanoplastics by MXene-derived oxide microrobots. Nat. Commun. 2022, 13, 3573. [Google Scholar] [CrossRef] [PubMed]
- Urso, M.; Bruno, L.; Dattilo, S.; Carroccio, S.C.; Mirabella, S. Band Engineering versus Catalysis: Enhancing the Self-Propulsion of Light-Powered MXene-Derived Metal–TiO2 Micromotors to Degrade Polymer Chains. ACS Appl. Mater. Interfaces 2023, 16, 1293–1307. [Google Scholar] [CrossRef]
- 74. Mou, F.; Kong, L.; Chen, C.; Chen, Z.; Xu, L.; Guan, J. Light-controlled propulsion, aggregation and separation of water-fuelled TiO2/Pt Janus submicromotors and their “on-the-fly” photocatalytic activities. Nanoscale 2016, 8, 4976–4983. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Yang, M.; Liu, H.; Zhang, Z.; Hu, Y.; Shi, J.; Wang, Z.-H. Light-driven micro/nanomotors in biomedical applications. Nanoscale 2023, 15, 18550–18570. [Google Scholar] [CrossRef] [PubMed]
- Šípová-Jungová, H.; Andrén, D.; Jones, S.; Käll, M. Nanoscale Inorganic Motors Driven by Light: Principles, Realizations, and Opportunities. Chem. Rev. 2020, 120, 269–287. [Google Scholar] [CrossRef]
- Katuri, J.; Ma, X.; Stanton, M.M.; Sánchez, S. Designing Micro- and Nanoswimmers for Specific Applications. Acc. Chem. Res. 2017, 50, 2–11. [Google Scholar] [CrossRef]
- Zha, F.; Wang, T.; Luo, M.; Guan, J. Tubular Micro/Nanomotors: Propulsion Mechanisms, Fabrication Techniques and Applications. Micromachines 2018, 9, 2699–2706. [Google Scholar] [CrossRef]
- Yuan, K.; Pacheco, M.; Jurado-Sánchez, B.; Escarpa, A. Design and Control of the Micromotor Swarm Toward Smart Applications. Adv. Intell. Syst. 2021, 3, 2100002. [Google Scholar] [CrossRef]
- Parmar, J.; Ma, X.; Katuri, J.; Simmchen, J.; Stanton, M.M.; Trichet-Paredes, C.; Soler, L.; Sanchez, S. Nano and micro architectures for self-propelled motors. Sci. Technol. Adv. Mater. 2015, 16, 14802. [Google Scholar] [CrossRef]
- Ferreira, V.; Santos, P.; Silva, C.; Azenha, M. Latest developments on TiO2-based photocatalysis: A special focus on selectivity and hollowness for enhanced photonic efficiency. Appl. Catal. A Gen. 2021, 623, 118243–118299. [Google Scholar] [CrossRef]
- Jiang, H.; He, X.; Yang, M.; Hu, C. Visible Light-Driven Micromotors in Fuel-Free Environment with Promoted Ion Tolerance. Nanomaterials 2023, 13, 15987–15999. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Gao, J.; Wilson, D.A.; Tu, Y.; Peng, F. Fuel-Free Micro-/Nanomotors as Intelligent Therapeutic Agents. Chem. Asian J. 2019, 14, 2325–2335. [Google Scholar] [CrossRef]
- Pourrahimi, A.M.; Pumera, M. Multifunctional and self-propelled spherical Janus nano/micromotors: Recent advances. Nanoscale 2018, 10, 16398–16415. [Google Scholar] [CrossRef]
- Wang, J.; Xiong, Z.; Zhan, X.; Dai, B.; Zheng, J.; Liu, J.; Tang, J. A Silicon Nanowire as a Spectrally Tunable Light-Driven Nanomotor. Adv. Mater. 2017, 29, 1701451. [Google Scholar] [CrossRef] [PubMed]
- Marschelke, C.; Fery, A.; Synytska, A. Janus particles: From concepts to environmentally friendly materials and sustainable applications. Colloid Polym. Sci. 2020, 298, 841–865. [Google Scholar] [CrossRef]
- Gai, M.; Frueh, J.; Hu, N.; Si, T.; Sukhorukov, G.B.; He, Q. Self-propelled two dimensional polymer multilayer plate micromotors. Phys. Chem. Chem. Phys. 2016, 18, 3397–3401. [Google Scholar] [CrossRef]
- Gai, M.; Frueh, J.; Si, T.; Hu, N.; Sukhorukov, G.B.; He, Q. The collision phenomena of Janus polymer micro-plate motors propelled by oscillating micro-bubbles. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 510, 113–121. [Google Scholar] [CrossRef]
- Su, H.; Price, C.-A.H.; Jing, L.; Tian, Q.; Liu, J.; Qian, K. Janus particles: Design, preparation, and biomedical applications. Mater. Today Bio 2019, 4, 100033. [Google Scholar] [CrossRef]
- Cui, X.; Ruan, Q.; Zhuo, X.; Xia, X.; Hu, J.; Fu, R.; Li, Y.; Wang, J.; Xu, H. Photothermal Nanomaterials: A Powerful Light-to-Heat Converter. Chem. Rev. 2023, 123, 6891–6952. [Google Scholar] [CrossRef]
- Córdoba, A.; Schieber, J.D. The effects of hydrodynamic interactions on the swimming velocity and stability of a swarm of microswimmers. Phys. Fluids 2022, 35, 111901–111911. [Google Scholar] [CrossRef]
- Giudicatti, S.; Marz, S.M.; Soler, L.; Madani, A.; Jorgensen, M.R.; Sanchez, S.; Schmidt, O.G. Photoactive rolled-up TiO2microtubes: Fabrication, characterization and applications. J. Mater. Chem. C 2014, 2, 5892–5901. [Google Scholar] [CrossRef]
- Chen, M.; Lin, Z.; Xuan, M.; Lin, X.; Yang, M.; Dai, L.; He, Q. Programmable Dynamic Shapes with a Swarm of Light-Powered Colloidal Motors. Angew. Chem. Int. Ed. 2021, 60, 16674–16679. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Ibarlucea, B.; Caspari, A.; Synytska, A.; Cuniberti, G.; de Graaf, J.; Baraban, L. Impact of surface charge on the motion of light-activated Janus micromotors. Eur. Phys. J. E 2021, 44, 6586–6599. [Google Scholar] [CrossRef] [PubMed]
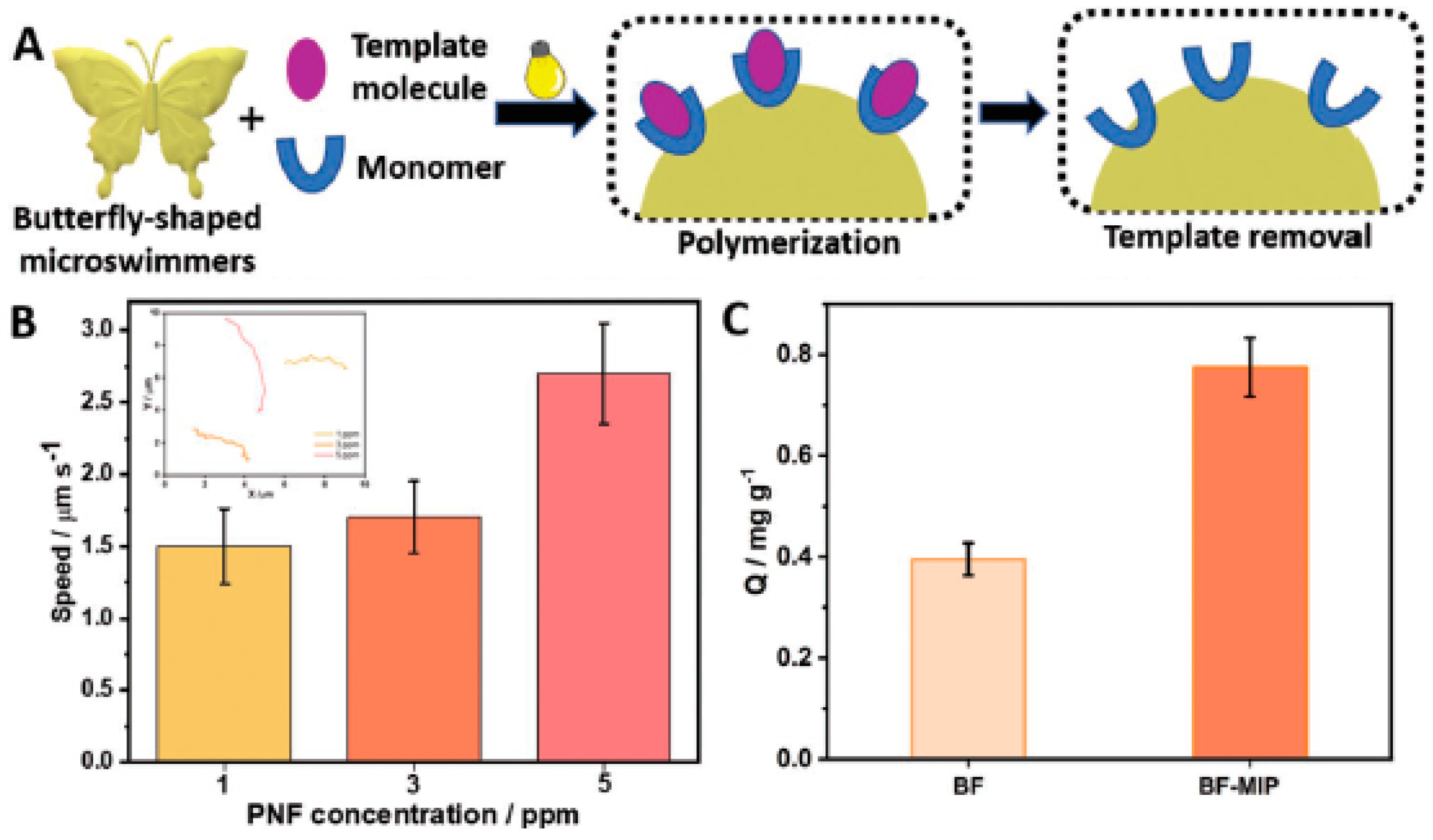
- Arabi, M.; Ostovan, A.; Li, J.; Wang, X.; Zhang, Z.; Choo, J.; Chen, L. Molecular Imprinting: Green Perspectives and Strategies. Adv. Mater. 2021, 33, 2100543. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ji, F.; Ng, D.H.; Liu, J.; Bing, X.; Wang, P. Bioinspired Pt-free molecularly imprinted hydrogel-based magnetic Janus micromotors for temperature-responsive recognition and adsorption of erythromycin in water. Chem. Eng. J. 2019, 369, 611–620. [Google Scholar] [CrossRef]
- Bing, X.; Zhang, X.; Li, J.; Ng, D.H.L.; Yang, W.; Yang, J. 3D hierarchical tubular micromotors with highly selective recognition and capture for antibiotics. J. Mater. Chem. A 2020, 8, 2809–2819. [Google Scholar] [CrossRef]
- Orozco, J.; Cortés, A.; Cheng, G.; Sattayasamitsathit, S.; Gao, W.; Feng, X.; Shen, Y.; Wang, J. Molecularly Imprinted Polymer-Based Catalytic Micromotors for Selective Protein Transport. J. Am. Chem. Soc. 2013, 135, 5336–5339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, J.; Fu, L.; Liu, D.; Chen, L. Magnetic molecularly imprinted microsensor for selective recognition and transport of fluorescent phycocyanin in seawater. J. Mater. Chem. A 2015, 3, 7437–7444. [Google Scholar] [CrossRef]
- Yuan, X.; Ferrer-Campos, R.; Garcés-Pineda, F.A.; Villa, K. Molecular Imprinted BiVO4 Microswimmers for Selective Target Recognition and Removal. Small 2023, 12, e2207303. [Google Scholar] [CrossRef]
- Heckel, S.; Simmchen, J. Photocatalytic BiVO4 Microswimmers with Bimodal Swimming Strategies. Adv. Intell. Syst. 2019, 1, 1900093. [Google Scholar] [CrossRef]
- Jurado-Sánchez, B.; Wang, J. Micromotors for environmental applications: A review. Environ. Sci. Nano 2018, 5, 1530–1544. [Google Scholar] [CrossRef]
- Kagan, D.; Calvo-Marzal, P.; Balasubramanian, S.; Sattayasamitsathit, S.; Manesh, K.M.; Flechsig, G.-U.; Wang, J. Chemical Sensing Based on Catalytic Nanomotors: Motion-Based Detection of Trace Silver. J. Am. Chem. Soc. 2009, 131, 12082–12083. [Google Scholar] [CrossRef]
- Guix, M.; Orozco, J.; García, M.; Gao, W.; Sattayasamitsathit, S.; Merkoçi, A.; Escarpa, A.; Wang, J. Superhydrophobic Alkanethiol-Coated Microsubmarines for Effective Removal of Oil. ACS Nano 2012, 6, 4445–4451. [Google Scholar] [CrossRef]
- Soler, L.; Magdanz, V.; Fomin, V.M.; Sanchez, S.; Schmidt, O.G. Self-Propelled Micromotors for Cleaning Polluted Water. ACS Nano 2013, 7, 9611–9620. [Google Scholar] [CrossRef]
- Ye, H.; Wang, Y.; Xu, D.; Liu, X.; Liu, S.; Ma, X. Design and fabrication of micro/nano-motors for environmental and sensing applications. Appl. Mater. Today 2021, 23, 101007. [Google Scholar] [CrossRef]
- Jones, R.A.L. Challenges in soft nanotechnology. Faraday Discuss. 2009, 143, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Sánchez, B.; Escarpa, A.; Wang, J. Lighting up micromotors with quantum dots for smart chemical sensing. Chem. Commun. 2015, 51, 14088–14091. [Google Scholar] [CrossRef]
- Pacheco, M.; Jurado-Sánchez, B.; Escarpa, A. Sensitive Monitoring of Enterobacterial Contamination of Food Using Self-Propelled Janus Microsensors. Anal. Chem. 2018, 90, 2912–2917. [Google Scholar] [CrossRef] [PubMed]
- Maric, T.; Nasir, M.Z.M.; Wang, Y.; Khezri, B.; Pumera, M. Corrosion due to ageing influences the performance of tubular platinum microrobots. Nanoscale 2018, 10, 1322–1325. [Google Scholar] [CrossRef]
- Zhao, G.; Khezri, B.; Sanchez, S.; Schmidt, O.G.; Webster, R.D.; Pumera, M. Corrosion of self-propelled catalytic microengines. Chem. Commun. 2013, 49, 9125–9127. [Google Scholar] [CrossRef]
- Wang, J.; Dong, R.; Yang, Q.; Wu, H.; Bi, Z.; Liang, Q.; Wang, Q.; Wang, C.; Mei, Y.; Cai, Y. One body, two hands: Photocatalytic function- and Fenton effect-integrated light-driven micromotors for pollutant degradation. Nanoscale 2019, 11, 16592–16598. [Google Scholar] [CrossRef]
- Li, X.; Sun, Y.-M.; Zhang, Z.-Y.; Feng, N.-X.; Song, H.; Liu, Y.-L.; Hai, L.; Cao, J.-M.; Wang, G.P. Visible light-driven multi-motion modes CNC/TiO2 nanomotors for highly efficient degradation of emerging contaminants. Carbon 2019, 155, 195–203. [Google Scholar] [CrossRef]
- Chistè, E.; Ghafarinazari, A.; Donini, M.; Cremers, V.; Dendooven, J.; Detavernier, C.; Benati, D.; Scarpa, M.; Dusi, S.; Daldosso, N. TiO2-coated luminescent porous silicon micro-particles as a promising system for nanomedicine. J. Mater. Chem. B 2018, 6, 1815–1824. [Google Scholar] [CrossRef]
- Ge, Y.; Cao, R.; Ye, S.; Chen, Z.; Zhu, Z.; Tu, Y.; Ge, D.; Yang, X. A bio-inspired homogeneous graphene oxide actuator driven by moisture gradients. Chem. Commun. 2018, 54, 3126–3129. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-H.; Chu, M.; Yin, N.; Huang, W.; Liu, W.; Zhang, Z.; Liu, J.; Shi, J. Biological chemotaxis-guided self-thermophoretic nanoplatform augments colorectal cancer therapy through autonomous mucus penetration. Sci. Adv. 2022, 8, eabn3917. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, W.; Zhang, S.; Chu, M.; Yin, N.; Zhu, C.; Zhang, Z.; Shi, J.; Liu, J. Self-Thermophoretic Nanoparticles Enhance Intestinal Mucus Penetration and Reduce Pathogenic Bacteria Interception in Colorectal Cancer. Adv. Funct. Mater. 2023, 33, 2212013–2212041. [Google Scholar] [CrossRef]
- Wang, W.; Ma, E.; Tao, P.; Zhou, X.; Xing, Y.; Chen, L.; Zhang, Y.; Li, J.; Xu, K.; Wang, H.; et al. Chemical-NIR dual-powered CuS/Pt nanomotors for tumor hypoxia modulation, deep tumor penetration and augmented synergistic phototherapy. J. Mater. Sci. Technol. 2023, 148, 171–185. [Google Scholar] [CrossRef]
- Liu, W.; Wang, W.; Dong, X.; Sun, Y. Near-Infrared Light-Powered Janus Nanomotor Significantly Facilitates Inhibition of Amyloid-β Fibrillogenesis. ACS Appl. Mater. Interfaces 2020, 12, 12618–12628. [Google Scholar] [CrossRef] [PubMed]
- Maric, T.; Løvind, A.; Zhang, Z.; Geng, J.; Boisen, A. Near-Infrared Light-Driven Mesoporous SiO2/Au Nanomotors for Eradication of Pseudomonas aeruginosa Biofilm. Adv. Health Mater. 2023, 12, e2203018. [Google Scholar] [CrossRef] [PubMed]
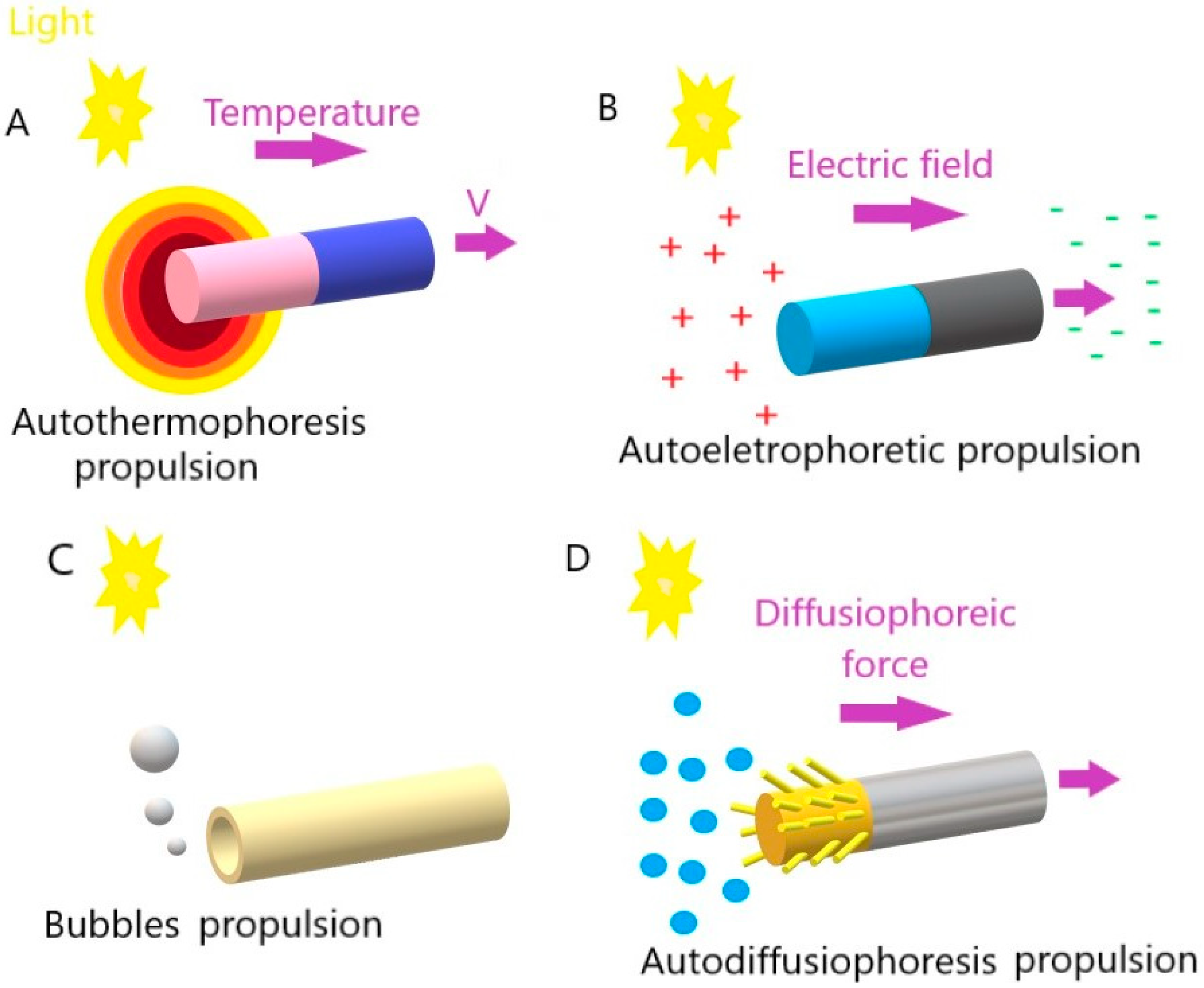
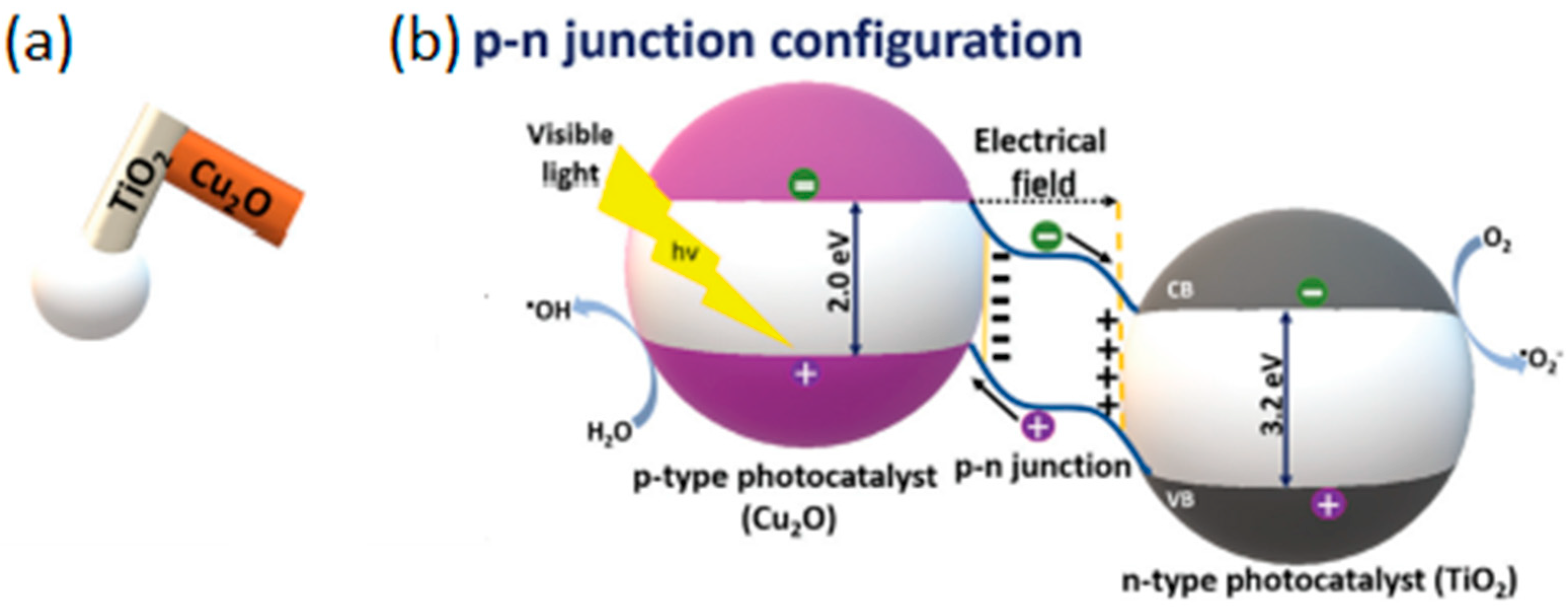


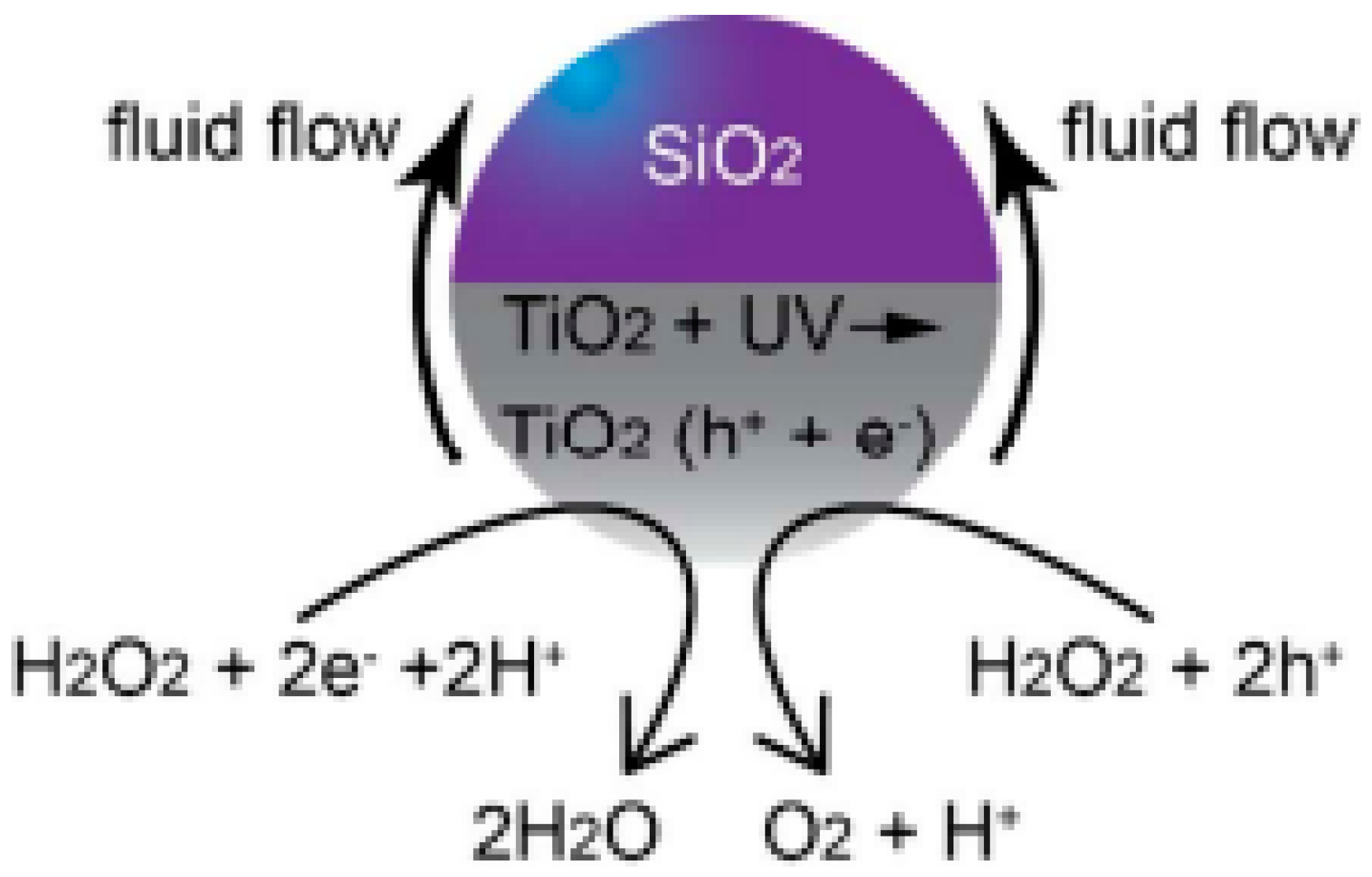
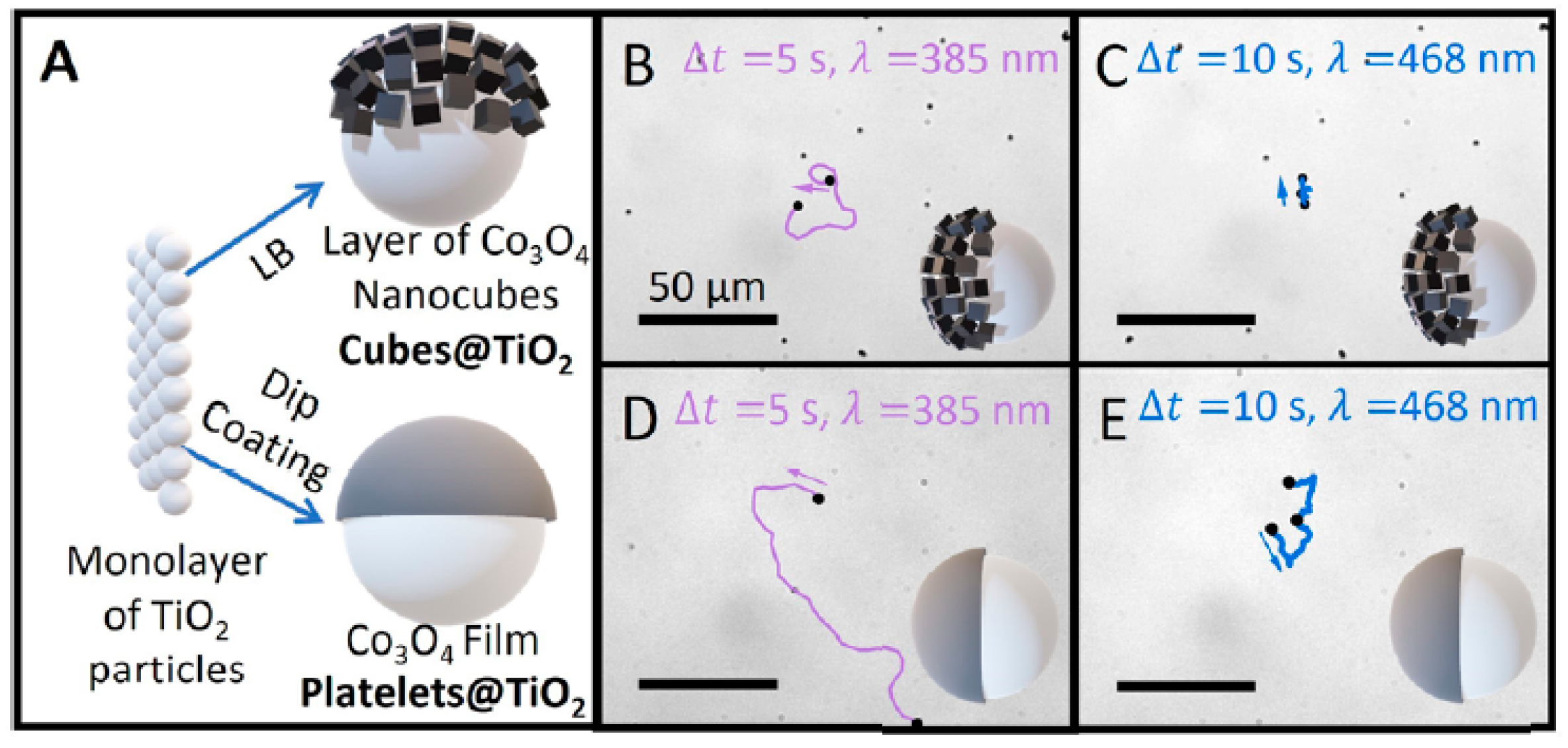

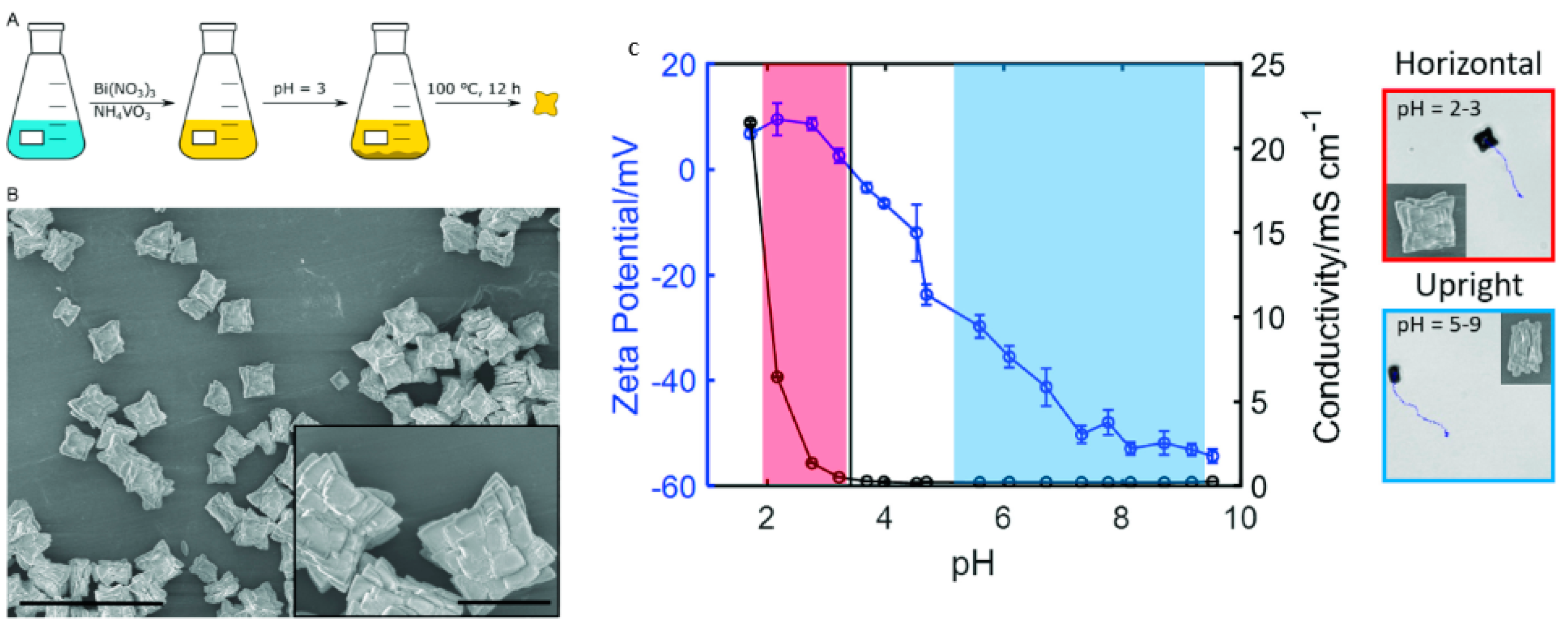


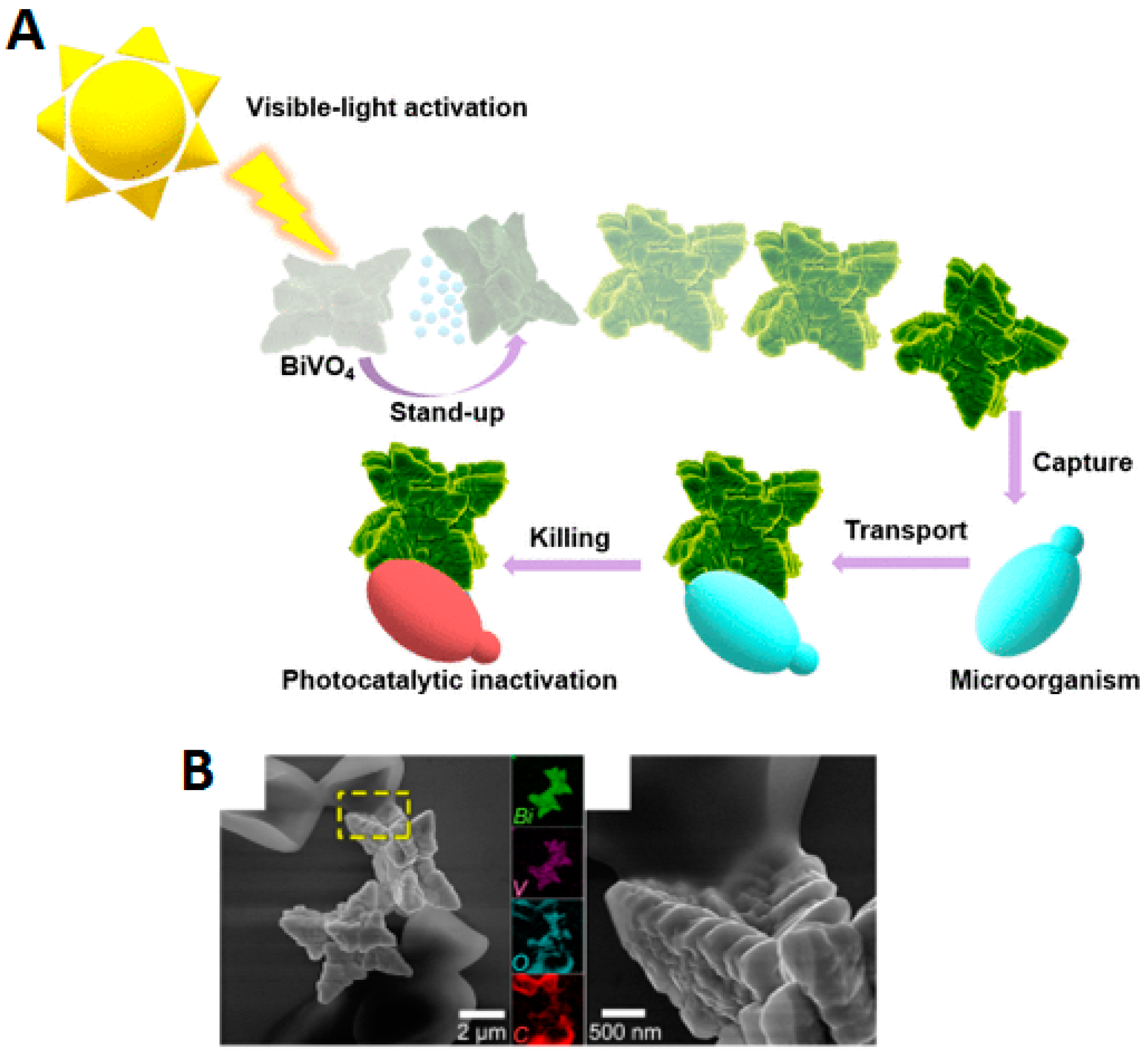
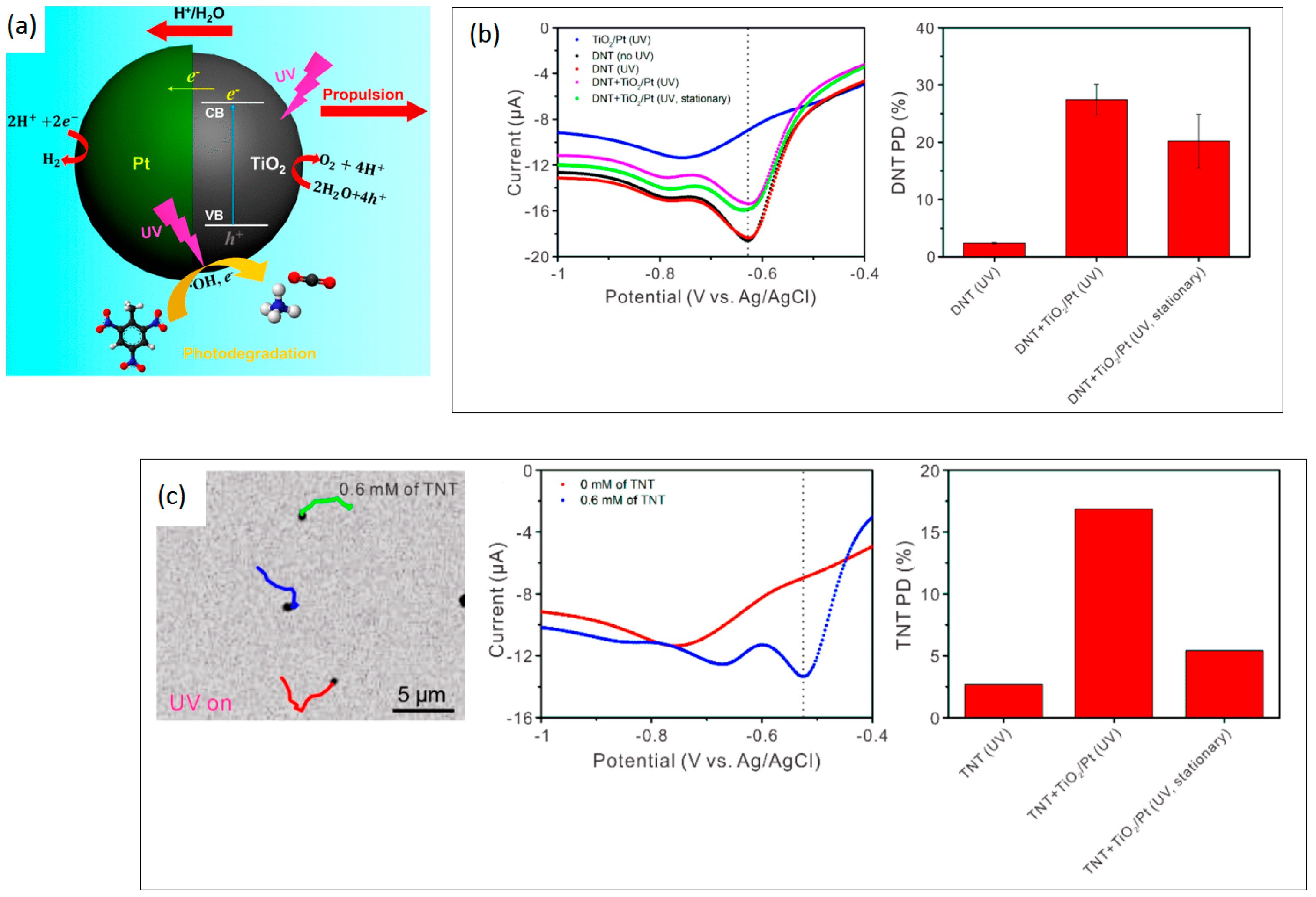
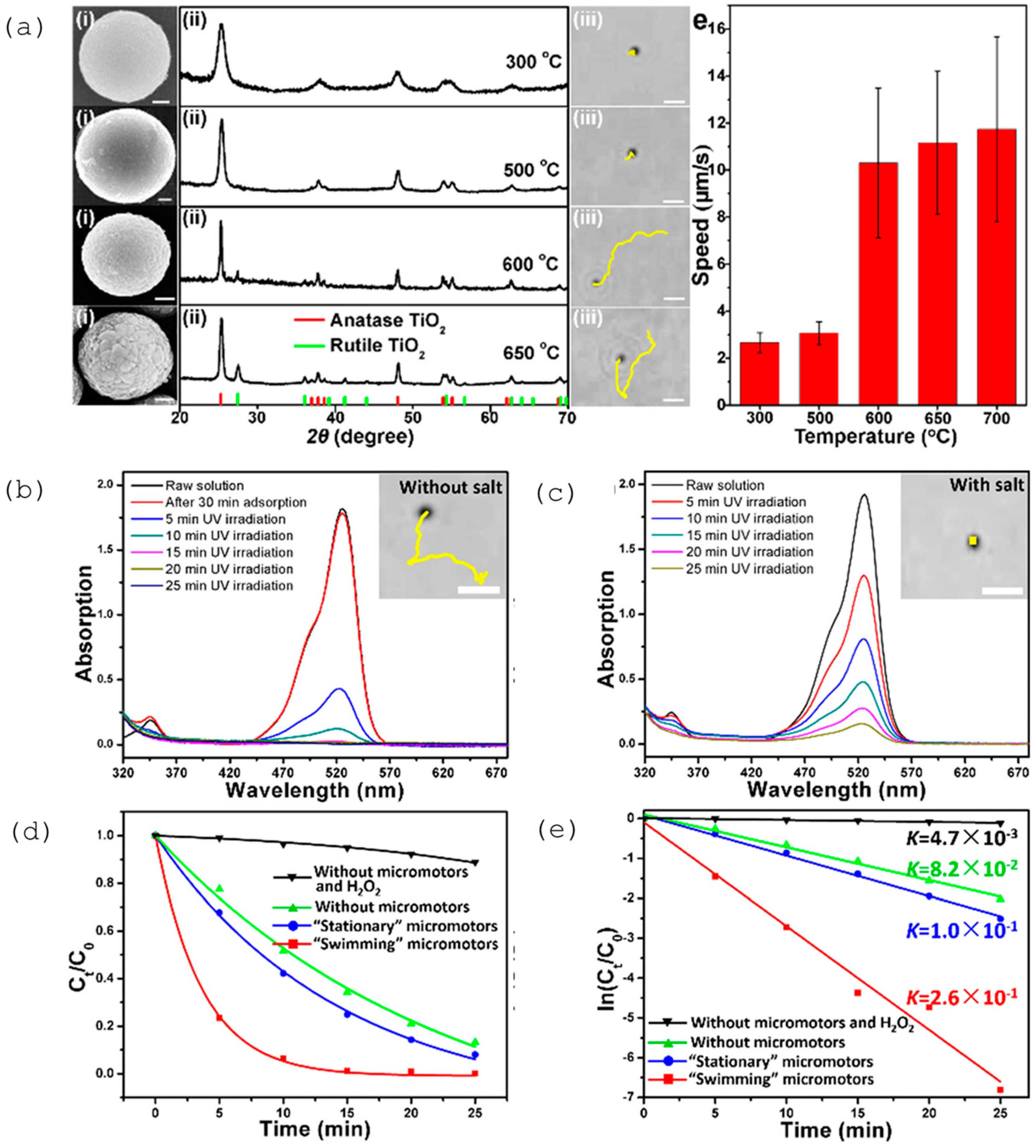


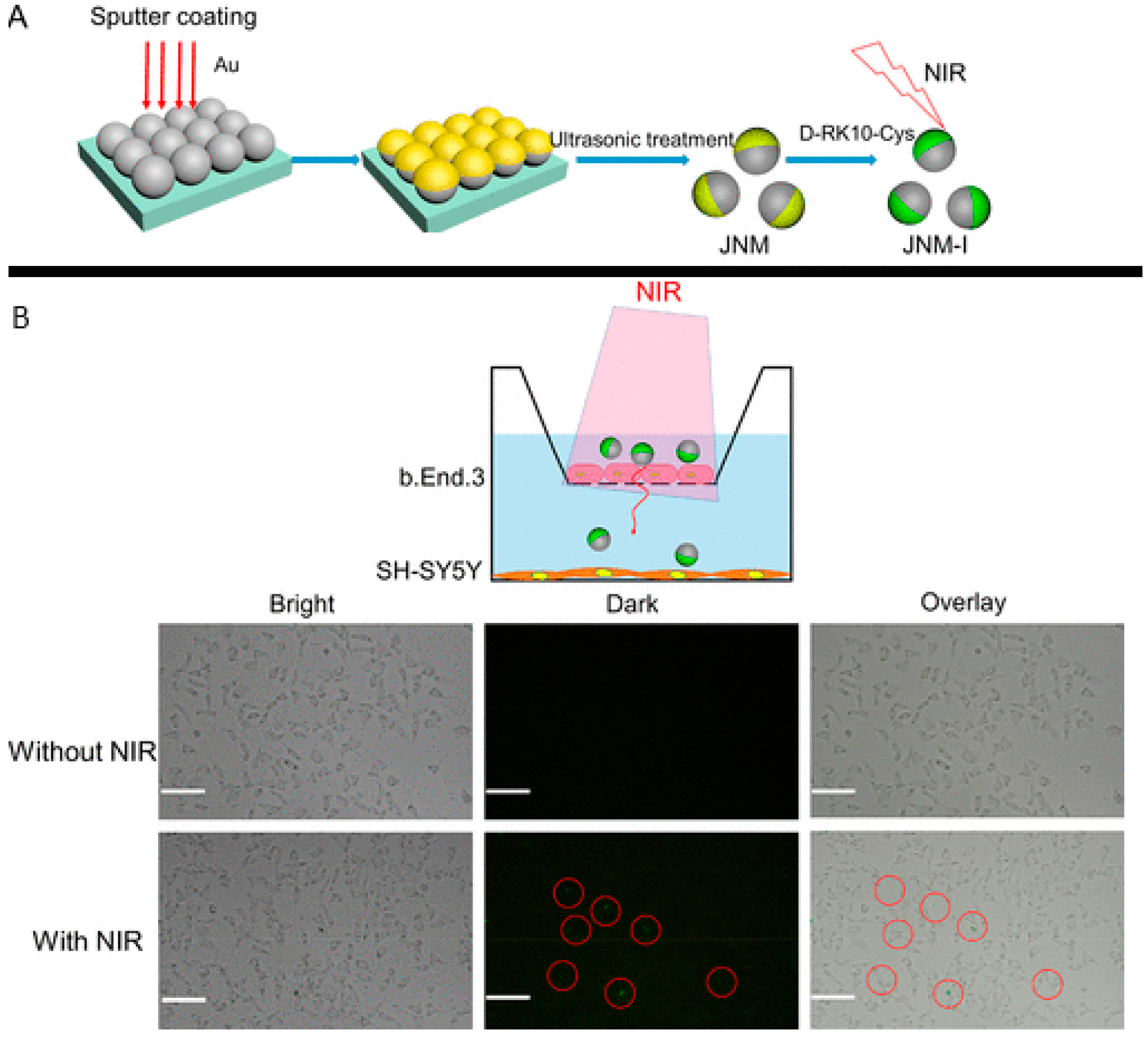
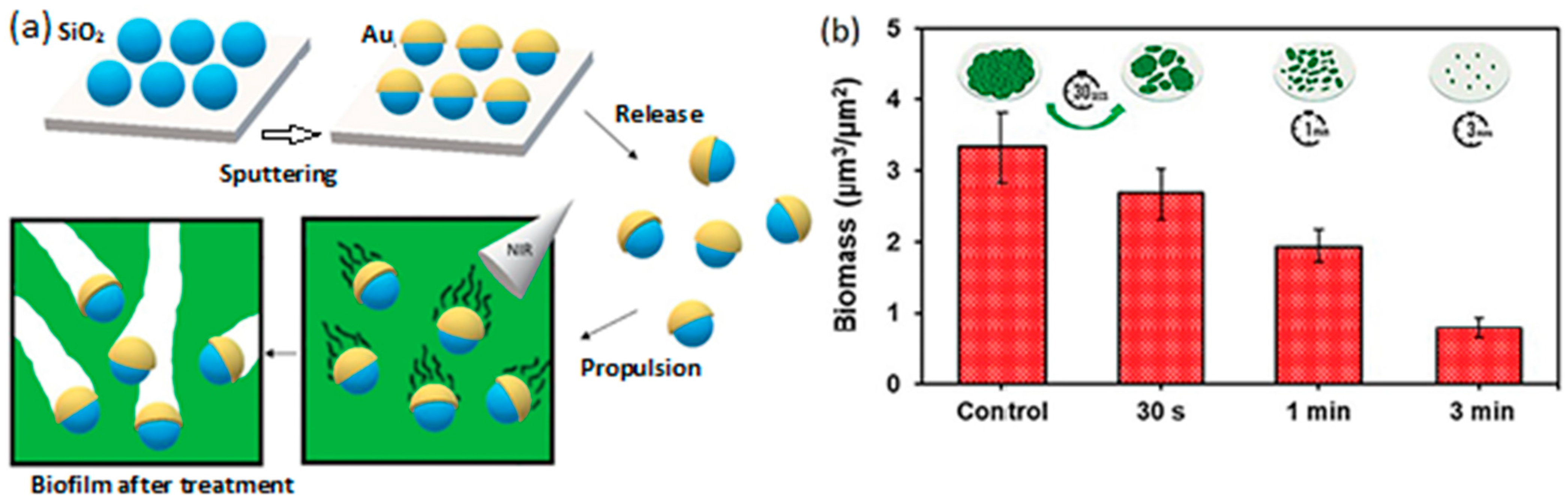
| Chemical Composition | Propulsion Mechanism | Morphology | Scale (nm) | Light Range and Maximum Intensity (mW.cm−2) | Velocity [μm.s−1] | Ref. |
|---|---|---|---|---|---|---|
| TiO2/Au motors | Self-electrophoretic | Janus hollow sphere | ~800 | UV (16) | 48.5 | [27] |
| TiO2-Fe motors | Self-electrophoresis | Janus sphere | ~2000 | UV (21) | 7.8 | [28] |
| TiO2@N-Au motors | Self-electrophoresis n | Wire | 235 ± 35 | Vis (100) | 2.5 ± 0.4 | [29] |
| TiO2-Au motors | Self-electrophoresis and acoustic effect | Capsule | ~2150 | UV (80) | 39.5 | [30] |
| 2D TiO2 motors | Bubble-propelled | Tubes | 150 | UV (50) | 52.0 | [17] |
| Au/TiO2 motors | Self-electrophoresis | Capsule | 175 | Vis (100) | 1.9 ± 0.5 | [31] |
| Hybrid TiO2/Au/Pt motor | Self-diffusiophoresis | Janus sphere | ~1000 | UV (10) | 13.0 | [18] |
| Cubes Co3O4 @TiO2 and Platelets Co3O4@TiO2 | Brownian diffusion and self-electrophoresis | Janus sphere | 900 | UV (20) Vis (16) | Cubes—5.0 and 10.0 Platelets—13.9 and 19.3 for Vis and UV light | [32] |
| Au/TiO2 motors | Self-diffusiophoresis | Pillars | 1500 | UV (320) | 4.0 | [19] |
| Hydrogel-based motors | Bubble-propelled | Spheres | 200–370 | UV (300) | 100.0 to 135.0 | [33] |
| Hybrid TiO2@SiO2 motors | Self-diffusiophoresis | Janus spheres elongated | 3200 | UV (20) | 5.0 ± 2.0 | [34] |
| Hybrid light/acoustic-powered motors | Self-diffusiophoresis | Janus spheres elongated | 1500 | UV (50) | 27.0 ± 9.0 | [35] |
| Cu@TiO2 and Au@TiO2 motors | Self-electrophoresis and diffusiophoresis | Janus spheres | 700 | UV (315) | Au = 23.0 Cu = 38.0 | [36] |
| Au@TiO2-SiO2 motors | Self-electrophoresis | Janus spheres elongated | 250 | NIR (10) | 0.7 ± 0.1 | [37] |
| TiO2/Pt; SiO2/Pt ZnO/Pt motors | Self-electrophoresis | Janus spheres | ~3000 | NIR (10) | 21.0 to 30.0 | [38] |
| Magnetic CoO–TiO2 motors | Self-electrophoresis | Janus sphere | ~4000 | UV (100) Vis (6) | Vis = 11.5 ± 0.7 UV = 6.2 ± 0.2 | [39] |
| TiO2/MnO2 motors | Bubble-propelled | Janus sphere | 240 | UV (20) | 48.1 | [16] |
| Metal/TiO2 motors ((Pt, Au, Ag, Fe, Cu) | Self-electrophoresis | Janus sphere | 2200 | UV (40) | Pt = 8.9; Au = 2.1 Ag = 3.6; Fe = 4.3 Cu = 5.1 | [40] |
| TiO2–Pt motors | Self-electrophoresis | Janus sphere | 1200 | UV (123) | 35.0 | [41] |
| TiO2 motors | Self-electrophoresis | Hollow spheres | ~1000 | UV–LED (590) | 6.5 to 9.5 | [42] |
| Au/TiO2/Cu2O hybrid motors | Self-electrophoresis and diffusiophoresis | Spheres elongated | ~5000 | UV–Vis (600) | Value not calculated | [43] |
| dielectric SiO2/TiO2 motors | Self-electrophoresis | Spheres | ~1000 | UV (35) | 8.6 | [15] |
| MOFs funcionalizado | Self-electrophoresis | Spheres elongated | 2600 | UV–Vis (14) | 40.0 | [44] |
| Au-ZnO motors | Self-electrophoresis | Rods | 90 | UV (250) | 24.2 | [45] |
| ZnO/Pt motors | Self-electrophoresis | Janus spheres | ~2000 | UV (135) | 32.0 | [46] |
| Sb2Se3/ZnO motors | Self-electrophoresis | Wire | 100 | UV (200) | 3.9 | [47] |
| ZnO/ZnO2/Pt motor | Self- diffusiophoresis and bubble-propelled | Janus spheres | 5000 | Vis (20) | 352.0 | [48] |
| Au/ Fe3O4 motors | Self-electrophoresis and diffusiophoresis | Rods | 200 | Vis (33) | 37.2 | [49] |
| ZnO/Pt motors | Self-electrophoresis diffusiophoresis | Sheets | ~1000 | UV (400) | 2.5 | [50] |
| Graphene Aerogel motors | Self-electrophoresis | Janus spheres | 100–1000 | NIR (9) | 17.6 | [51] |
| GQDs/PtNPs motors | Self-diffusiophoretic | Tubs | nano | Vis (N/A) | 440.0 | [52] |
| Cu2O@GO motors | Self-electrophoresis/ diffusiophoretic and thermophoresis | Spheres | ~2000 | Vis (32) NIR (2.5) | 16.6 | [13] |
| Cu2+1O motors | Self-diffusiophoretic | Spheres | ~1000 | Vis (84) | 107.0 | [53] |
| Cu2O@CdSe motors | Self-diffusiophoretic | Octahedral structure | ~1000 | UV–Vis (180) | 55.1 | [54] |
| Cu2O motors | Self-diffusiophoretic | Octahedral structure | 864 | UV–Vis (180) | 10.8 | [55] |
| GO/BiVO4 motors | Self-diffusiophoretic | Spheres | micro | Vis (180) | 25 | [56] |
| Single-component BiVO4 motors | Self-diffusiophoretic | Janus star | 4000–8000 | UV–Vis (5) | 5 ± 1 | [57] |
| TiO2/Pt motors | Self-electrophoresis | Janus sphere | ~1 μm | UV (40) | 9.7 ± 1.98 | [58] |
| Au-Cu motors | Bubble-propelled | Tubes | ~1 μm | NIR (6.4) | 2.2 ± 0.3 | [59] |
| TiO2 crystalline motors | Self-electrophoresis/ diffusiophoretic | Janus sphere | 500 nm | UV (80) | 11 | [60] |
| WO3 motors | Self-diffusiophoretic | Spheres | 1-2 μm | UV (160) | 2.0 ± 0.1 | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, V.R.A.; Azenha, M.A. Recent Advances in Light-Driven Semiconductor-Based Micro/Nanomotors: Optimization Strategies and Emerging Applications. Molecules 2024, 29, 1154. https://doi.org/10.3390/molecules29051154
Ferreira VRA, Azenha MA. Recent Advances in Light-Driven Semiconductor-Based Micro/Nanomotors: Optimization Strategies and Emerging Applications. Molecules. 2024; 29(5):1154. https://doi.org/10.3390/molecules29051154
Chicago/Turabian StyleFerreira, Vanessa R. A., and Manuel A. Azenha. 2024. "Recent Advances in Light-Driven Semiconductor-Based Micro/Nanomotors: Optimization Strategies and Emerging Applications" Molecules 29, no. 5: 1154. https://doi.org/10.3390/molecules29051154
APA StyleFerreira, V. R. A., & Azenha, M. A. (2024). Recent Advances in Light-Driven Semiconductor-Based Micro/Nanomotors: Optimization Strategies and Emerging Applications. Molecules, 29(5), 1154. https://doi.org/10.3390/molecules29051154






