Cationic Vitamin E-TPGS Mixed Micelles of Berberine to Neutralize Doxorubicin-Induced Cardiotoxicity via Amelioration of Mitochondrial Dysfunction and Impeding Apoptosis
Abstract
:1. Introduction
2. Results
2.1. Nanoformulation of Cationic Brb–Mixed Micelles and Physico-Chemical Characterization
2.2. In Vitro Release, Biocompatibility, and Hemocompatibility of Brb Nanoformulations
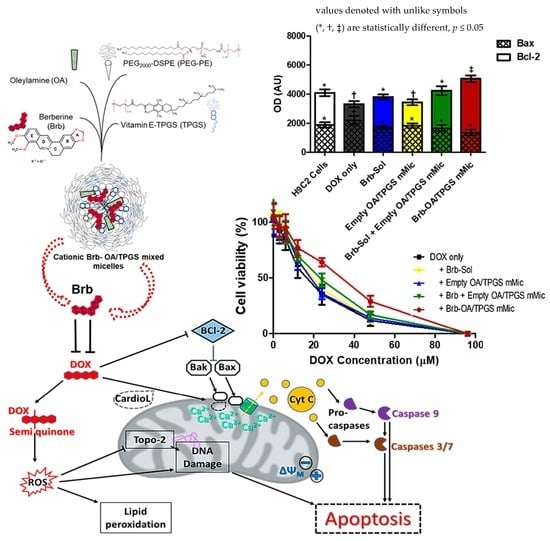
2.3. Cationic Brb–Mixed Micelles Help Protect against Anthracycline-Induced Cardiovascular Cell Death at a Cytotoxic Level of DOX
2.4. Recovery of Mitochondrial Membrane Integrity and Function by Cationic Brb–Mixed Micelles following DOX-Induced Damage
2.5. Significant Relative Reduction in Apoptotic Caspase-9 and Elevation of Antiapoptotic Bcl-2 Mediates Mitohormetic Cationic Brb–Mixed Micelles Protection against DOX Cardiotoxicity
3. Discussion
4. Materials and Methods
4.1. Materials and Cell Lines
4.2. Formulation of Berberine–Mixed Micelles
4.3. Physicochemical Characterization of Brb-Containing Cationic Mixed Micelles
4.3.1. Particle Size Analysis
4.3.2. Zeta Potential (ζ) Measurements
4.4. Encapsulation Efficiency (EE%) Determination
4.5. Physical Stability of Brb–Micelles
4.6. In Vitro Release of Brb from Micelles at “Sink” Conditions
4.7. In Vitro Hemolysis Assay
4.8. In Vitro Cell Culture
4.9. In Vitro Biocompatibility Screen
4.10. Cell Viability Assays following Doxorubicin Challenge
4.11. Apoptosis Assays
4.11.1. Flow Cytometry
4.11.2. Orange Mitochondrial Polarization Assay
4.12. Mitochondrial Apoptosis Marker Evaluation
4.12.1. Caspase 8 and 9 Activation Assays
4.12.2. Evaluation of Bax and Bcl-2 Activation by Western Blotting
4.13. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lefrak, E.A.; Pitha, J.; Rosenheim, S.; Gottlieb, J.A. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer 1973, 32, 302–314. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, S.; Dai, Y. Research progress of therapeutic drugs for doxorubicin-induced cardiomyopathy. Biomed. Pharmacother. 2022, 156, 113903. [Google Scholar] [CrossRef] [PubMed]
- Allen, A. The cardiotoxicity of chemotherapeutic drugs. Semin. Oncol. 1992, 19, 529–542. [Google Scholar]
- Doroshow, J.H. Effect of anthracycline antibiotics on oxygen radical formation in rat heart. Cancer Res. 1983, 43, 460–472. [Google Scholar]
- Xiong, C.; Wu, Y.Z.; Zhang, Y.; Wu, Z.X.; Chen, X.Y.; Jiang, P.; Guo, H.C.; Xie, K.R.; Wang, K.X.; Su, S.W. Protective effect of berberine on acute cardiomyopathy associated with doxorubicin treatment. Oncol. Lett. 2018, 15, 5721–5729. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhang, J.P. Bcl-xL is required for the protective effects of low-dose berberine against doxorubicin-induced cardiotoxicity through blocking apoptosis and activating mitophagy-mediated ROS elimination. Phytomed. Int. J. Phytother. Phytopharm. 2022, 101, 154130. [Google Scholar] [CrossRef]
- Wang, Y.; Liao, J.; Luo, Y.; Li, M.; Su, X.; Yu, B.; Teng, J.; Wang, H.; Lv, X. Berberine Alleviates Doxorubicin-Induced Myocardial Injury and Fibrosis by Eliminating Oxidative Stress and Mitochondrial Damage via Promoting Nrf-2 Pathway Activation. Int. J. Mol. Sci. 2023, 24, 3257. [Google Scholar] [CrossRef]
- Zhu, X.; Wei, Y.; Yang, B.; Yin, X.; Guo, X. The mitohormetic response as part of the cytoprotection mechanism of berberine: Berberine induces mitohormesis and mechanisms. Mol. Med. 2020, 26, 10. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Zhu, Z.; Liu, H.; Guo, H.; Xiong, C.; Xie, K.; Zhang, X.; Su, S. Protective effect of berberine on doxorubicin-induced acute hepatorenal toxicity in rats. Mol. Med. Rep. 2016, 13, 3953–3960. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Wu, H.; Wei, J.; Miao, R.; Zhang, Y.; Tian, J. Research progress on the pharmacological effects of berberine targeting mitochondria. Front. Endocrinol. 2022, 13, 982145. [Google Scholar] [CrossRef]
- Yan, X.J.; Yu, X.; Wang, X.P.; Jiang, J.F.; Yuan, Z.Y.; Lu, X.; Lei, F.; Xing, D.M. Mitochondria play an important role in the cell proliferation suppressing activity of berberine. Sci. Rep. 2017, 7, 41712. [Google Scholar] [CrossRef] [PubMed]
- Devarajan, N.; Jayaraman, S.; Mahendra, J.; Venkatratnam, P.; Rajagopal, P.; Palaniappan, H.; Ganesan, S.K. Berberine—A potent chemosensitizer and chemoprotector to conventional cancer therapies. Phytother. Res. PTR 2021, 35, 3059–3077. [Google Scholar] [CrossRef] [PubMed]
- Barzegar, E.; Fouladdel, S.; Movahhed, T.K.; Atashpour, S.; Ghahremani, M.H.; Ostad, S.N.; Azizi, E. Effects of berberine on proliferation, cell cycle distribution and apoptosis of human breast cancer T47D and MCF7 cell lines. Iran. J. Basic Med. Sci. 2015, 18, 334–342. [Google Scholar]
- Shen, R.; Kim, J.J.; Yao, M.; Elbayoumi, T.A. Development and evaluation of vitamin E d-alpha-tocopheryl polyethylene glycol 1000 succinate-mixed polymeric phospholipid micelles of berberine as an anticancer nanopharmaceutical. Int. J. Nanomed. 2016, 11, 1687–1700. [Google Scholar]
- Cheng, Y.; Ji, Y. Mitochondria-targeting nanomedicine self-assembled from GSH-responsive paclitaxel-ss-berberine conjugate for synergetic cancer treatment with enhanced cytotoxicity. J. Control. Release 2020, 318, 38–49. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Zhang, L.; Wu, Z.X.; Shan, T.T.; Xiong, C. Berberine Ameliorates Doxorubicin-Induced Cardiotoxicity via a SIRT1/p66Shc-Mediated Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 2150394. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim Fouad, G.; Ahmed, K.A. Neuroprotective Potential of Berberine Against Doxorubicin-Induced Toxicity in Rat’s Brain. Neurochem. Res. 2021, 46, 3247–3263. [Google Scholar] [CrossRef]
- Ibrahim Fouad, G.; Ahmed, K.A. The protective impact of berberine against doxorubicin-induced nephrotoxicity in rats. Tissue Cell 2021, 73, 101612. [Google Scholar] [CrossRef]
- Coelho, A.R.; Martins, T.R.; Couto, R.; Deus, C.; Pereira, C.V.; Simoes, R.F.; Rizvanov, A.A.; Silva, F.; Cunha-Oliveira, T.; Oliveira, P.J.; et al. Berberine-induced cardioprotection and Sirt3 modulation in doxorubicin-treated H9c2 cardiomyoblasts. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2904–2923. [Google Scholar] [CrossRef]
- Rawal, S.; Gupta, P.; Bhatnagar, P.; Yadav, H.N.; Dinda, A.K. Solid Lipid Nanoformulation of Berberine Attenuates Doxorubicin Triggered in vitro Inflammation in H9c2 Rat Cardiomyocytes. Comb. Chem. High Throughput Screen. 2022, 25, 1695–1706. [Google Scholar]
- Tuo, J.; Xie, Y.; Song, J.; Chen, Y.; Guo, Q.; Liu, X.; Ni, X.; Xu, D.; Huang, H.; Yin, S.; et al. Development of a novel berberine-mediated mitochondria-targeting nano-platform for drug-resistant cancer therapy. J. Mater. Chem. B 2016, 4, 6856–6864. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, Y.; Wang, X.; Gao, X.; Liu, Y.; Zhang, X.; He, Z.; Wang, D.; Wang, Y. Ratiometric delivery of doxorubicin and berberine by liposome enables superior therapeutic index than Doxil®. Asian J. Pharm. Sci. 2020, 15, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, N.; Song, H.; Xi, X.; Wang, J.; Hao, A.; Li, T. Preparation of an anhydrous reverse micelle delivery system to enhance oral bioavailability and anti-diabetic efficacy of berberine. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2011, 44, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Sawant, R.R.; Torchilin, V.P. Polymeric micelles: Polyethylene glycol-phosphatidylethanolamine (PEG-PE)-based micelles as an example. Methods Mol. Biol. 2010, 624, 131–149. [Google Scholar] [PubMed]
- Mu, L.; Elbayoumi, T.A.; Torchilin, V.P. Mixed micelles made of poly(ethylene glycol)-phosphatidylethanolamine conjugate and d-alpha-tocopheryl polyethylene glycol 1000 succinate as pharmaceutical nanocarriers for camptothecin. Int. J. Pharm. 2005, 306, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Brownlow, B.; Nagaraj, V.J.; Nayel, A.; Joshi, M.; Elbayoumi, T. Development and In Vitro Evaluation of Vitamin E-Enriched Nanoemulsion Vehicles Loaded with Genistein for Chemoprevention Against UVB-Induced Skin Damage. J. Pharm. Sci. 2015, 104, 3510–3523. [Google Scholar] [CrossRef] [PubMed]
- Ashton, J.C. Drug combination studies and their synergy quantification using the Chou-Talalay method–letter. Cancer Res. 2015, 75, 2400. [Google Scholar] [CrossRef]
- Sawant, R.R.; Torchilin, V.P. Multifunctionality of lipid-core micelles for drug delivery and tumour targeting. Mol. Membr. Biol. 2010, 27, 232–246. [Google Scholar] [CrossRef]
- Yao, M.; Elbayoumi, T. Anionic and Cationic Vitamin E-TPGS Mixed Polymeric Phospholipid Micellar Vehicles. Methods Mol. Biol. 2019, 2000, 31–41. [Google Scholar]
- Sawant, R.R.; Sawant, R.M.; Torchilin, V.P. Mixed PEG-PE/vitamin E tumor-targeted immunomicelles as carriers for poorly soluble anti-cancer drugs: Improved drug solubilization and enhanced in vitro cytotoxicity. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Pharm. Verfahrenstechnik e.V 2008, 70, 51–57. [Google Scholar] [CrossRef]
- Guan, Y.; Wang, L.Y.; Wang, B.; Ding, M.H.; Bao, Y.L.; Tan, S.W. Recent Advances of D-alpha-tocopherol Polyethylene Glycol 1000 Succinate Based Stimuli-responsive Nanomedicine for Cancer Treatment. Curr. Med. Sci. 2020, 40, 218–231. [Google Scholar] [CrossRef]
- Cabral, H.; Matsumoto, Y.; Mizuno, K.; Chen, Q.; Murakami, M.; Kimura, M.; Terada, Y.; Kano, M.R.; Miyazono, K.; Uesaka, M.; et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 2011, 6, 815–823. [Google Scholar] [CrossRef]
- Bose, A.; Roy Burman, D.; Sikdar, B.; Patra, P. Nanomicelles: Types, properties and applications in drug delivery. IET Nanobiotechnol. 2021, 15, 19–27. [Google Scholar] [CrossRef]
- Vuddanda, P.R.; Rajamanickam, V.M.; Yaspal, M.; Singh, S. Investigations on agglomeration and haemocompatibility of vitamin E TPGS surface modified berberine chloride nanoparticles. BioMed Res. Int. 2014, 2014, 951942. [Google Scholar] [CrossRef]
- Chen, W.; Miao, Y.Q.; Fan, D.J.; Yang, S.S.; Lin, X.; Meng, L.K.; Tang, X. Bioavailability study of berberine and the enhancing effects of TPGS on intestinal absorption in rats. AAPS PharmSciTech 2011, 12, 705–711. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Deng, J.; Jia, X.; Zhou, J.; Lv, H. Solid dispersion of berberine-phospholipid complex/TPGS 1000/SiO2: Preparation, characterization and in vivo studies. Int. J. Pharm. 2014, 465, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Pham, J.; Brownlow, B.; Elbayoumi, T. Mitochondria-specific pro-apoptotic activity of genistein lipidic nanocarriers. Mol. Pharm. 2013, 10, 3789–3800. [Google Scholar] [CrossRef] [PubMed]
- Alonso Villela, S.M.; Kraiem, H.; Bouhaouala-Zahar, B.; Bideaux, C.; Aceves Lara, C.A.; Fillaudeau, L. A protocol for recombinant protein quantification by densitometry. MicrobiologyOpen 2020, 9, 1175–1182. [Google Scholar] [CrossRef] [PubMed]






| Formulation (Lipid Phase, M) | Brb Loading (mg/mL) | Particle Size (nm), D0 | PDI | Zeta Potential, ζ (mV) | Particle Size (nm), D90 | Brb Encapsulation Efficiency, D90 (EE %) |
|---|---|---|---|---|---|---|
| 3:1 PEG-DSPE:TPGS | Empty | 21.7 ± 1.6 * | 0.263 | −27.3 ± 1.5 * | 26.8 ± 1.4 * | N/A |
| 1.89 ± 0.2 * | 25.4 ± 1.5 † | 0.247 | −25.9 ± 1.7 * | 27.4 ± 2.1 † | 96.4 ± 5.7 * | |
| 5%OA/3:1 PEG-DSPE:TPGS | Empty | 22.9 ± 2.1 * | 0.274 | +16.8 ± 2.3 † | 23.2 ± 1.9 † | N/A |
| 1.94 ± 0.14 * | 27.3 ± 1.6 ‡ | 0.255 | +18.2 ± 1.1 † | 26.9 ± 1.8 ‡ | 94.9 ± 6.8 * |
| Treatment | IC50 in H9C2 (µM) | IC50 in A10 (µM) |
|---|---|---|
| DOX only | 19.6 ± 3.5 * | 18.2 ± 2.9 * |
| DOX + Brb-Sol | 23.8 ± 2.8 † | 21.5 ± 3.4 † |
| DOX + Empty OA/TPGS mMic | 21.2 ± 2.5 ‡ | 19.4 ± 3.1 * |
| DOX + Brb + Empty OA/TPGS mMic | 22.4 ± 3.7 † | 20.6 ± 3.8 † |
| DOX + Brb-OA/TPGS mMic | 31.8 ± 5.1 # | 29.7 ± 4.8 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metwally, A.A.; Ganguly, S.; Biomi, N.; Yao, M.; Elbayoumi, T. Cationic Vitamin E-TPGS Mixed Micelles of Berberine to Neutralize Doxorubicin-Induced Cardiotoxicity via Amelioration of Mitochondrial Dysfunction and Impeding Apoptosis. Molecules 2024, 29, 1155. https://doi.org/10.3390/molecules29051155
Metwally AA, Ganguly S, Biomi N, Yao M, Elbayoumi T. Cationic Vitamin E-TPGS Mixed Micelles of Berberine to Neutralize Doxorubicin-Induced Cardiotoxicity via Amelioration of Mitochondrial Dysfunction and Impeding Apoptosis. Molecules. 2024; 29(5):1155. https://doi.org/10.3390/molecules29051155
Chicago/Turabian StyleMetwally, Abdelkader A., Samayita Ganguly, Nora Biomi, Mingyi Yao, and Tamer Elbayoumi. 2024. "Cationic Vitamin E-TPGS Mixed Micelles of Berberine to Neutralize Doxorubicin-Induced Cardiotoxicity via Amelioration of Mitochondrial Dysfunction and Impeding Apoptosis" Molecules 29, no. 5: 1155. https://doi.org/10.3390/molecules29051155
APA StyleMetwally, A. A., Ganguly, S., Biomi, N., Yao, M., & Elbayoumi, T. (2024). Cationic Vitamin E-TPGS Mixed Micelles of Berberine to Neutralize Doxorubicin-Induced Cardiotoxicity via Amelioration of Mitochondrial Dysfunction and Impeding Apoptosis. Molecules, 29(5), 1155. https://doi.org/10.3390/molecules29051155