Application of Copper–Sulfur Compound Electrode Materials in Supercapacitors
Abstract
:1. Introduction
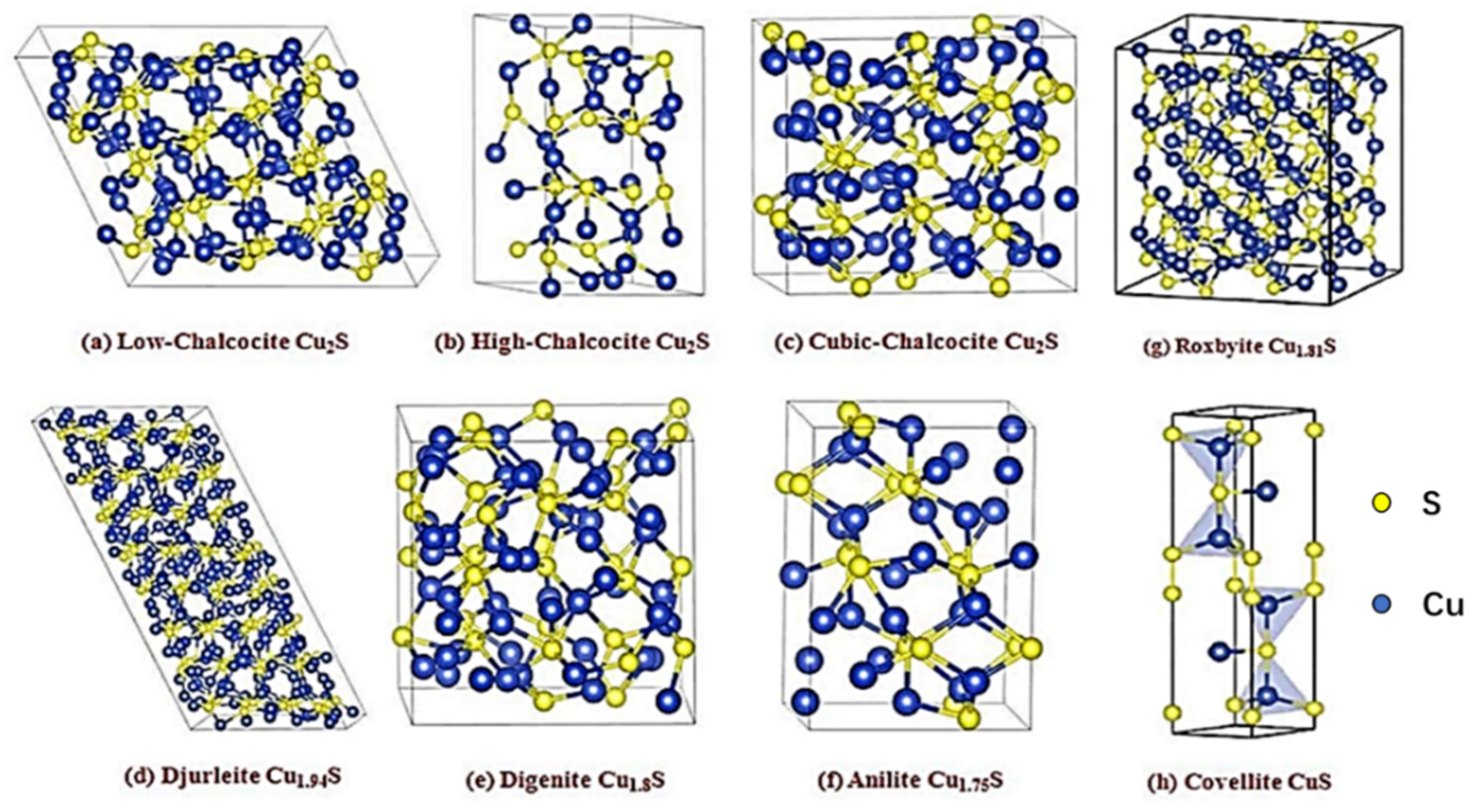
2. Crystal Structure and Properties of Copper–Sulfur Compounds
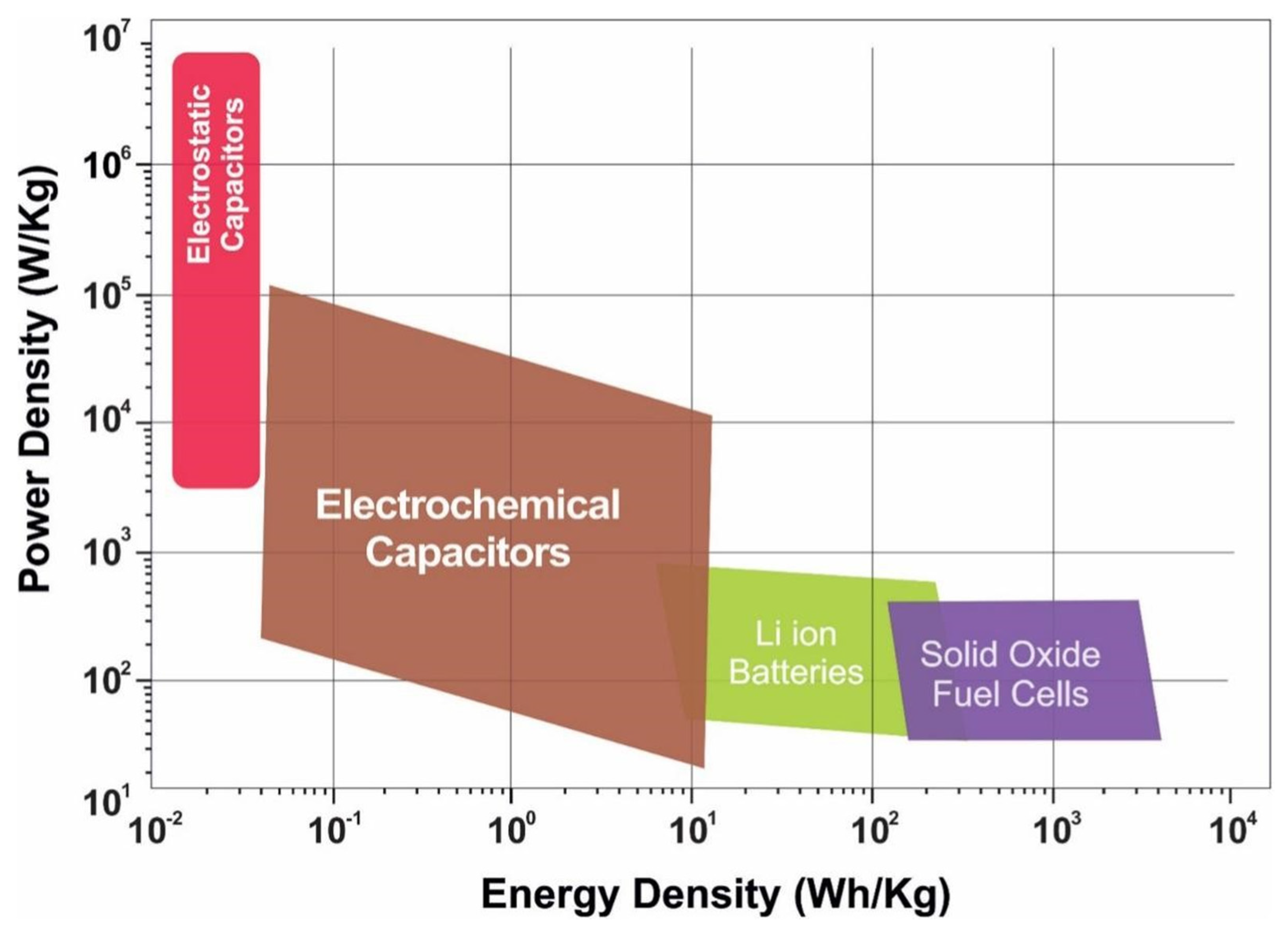
3. Copper–Sulfur Compounds with Different Stoichiometric Ratios in SC
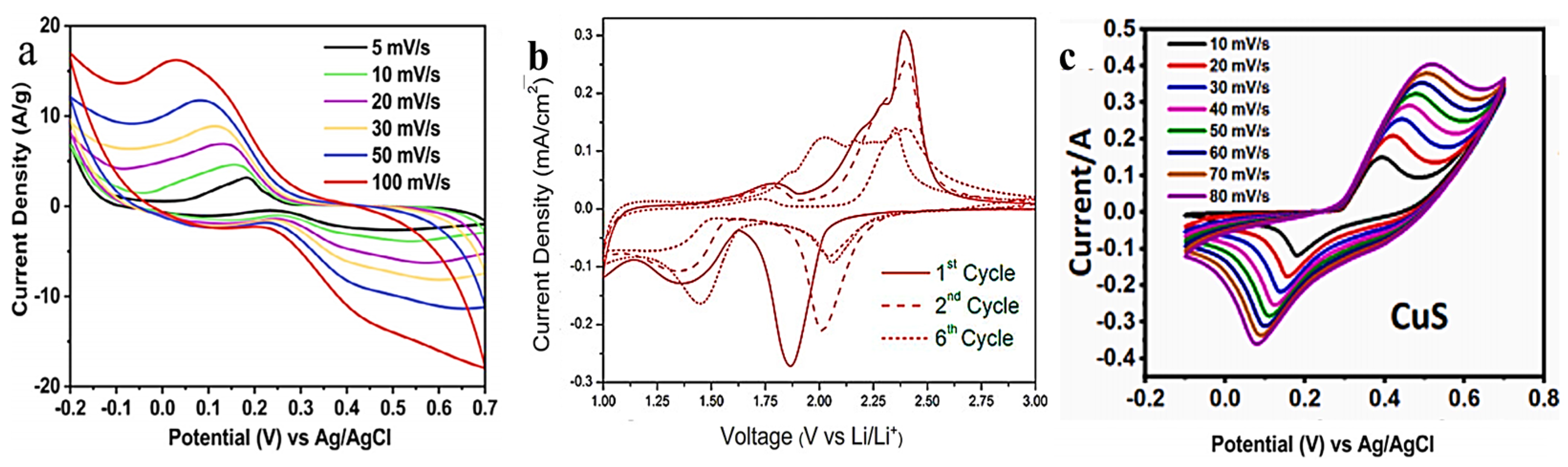
4. Preparation of Copper–Sulfur Compounds for SC Applications
5. Different Conductive Substrates for the Application of Copper–Sulfur Compounds in SC
6. Copper–Sulfur Composite with Other Materials in SC
6.1. Forming Polymetallic Sulfides with Other Metals
6.2. Complexes with Other Metal Compounds
6.3. Copper–Sulfur Composite with Carbon-Based Materials for SC Applications
6.3.1. Copper–Sulfur Composite with Graphene for SC Applications
6.3.2. Copper–Sulfur Composite with Carbon Nanotubes for SC Applications
6.3.3. Copper Sulfide Composite with Activated Carbon in SC
6.3.4. Copper–Sulfur Compounds Compounded with CC in SC
6.3.5. Copper–Sulfur Composite with Acetylene Black in SC
6.3.6. MOF-Derived Copper–Sulfur Compound/Carbon-Based Nanocomposites for SC Applications
6.4. Copper–Sulfur Compounds Compounded with Conductive Polymers for SC Applications
7. Summary and Expectation
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhong, M.; Zhang, M.; Li, X. Carbon Nanomaterials and Their Composites for Supercapacitors. Carbon Energy 2022, 4, 950–985. [Google Scholar] [CrossRef]
- Han, Z.; Fang, R.; Chu, D.; Wang, D.-W.; Ostrikov, K. Introduction to Supercapacitors. Nanoscale Adv. 2023, 5, 4015–4017. [Google Scholar] [CrossRef]
- Winter, M.; Brodd, R.J. What Are Batteries, Fuel Cells, and Supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef]
- Vinchhi, P.; Khandla, M.; Chaudhary, K.; Pati, R. Recent Advances on Electrolyte Materials for SOFC: A Review. Inorg. Chem. Commun. 2023, 152, 110724. [Google Scholar] [CrossRef]
- Samdhyan, K.; Chand, P.; Anand, H.; Saini, S. Development of Carbon-Based Copper Sulfide Nanocomposites for High Energy Supercapacitor Applications: A Comprehensive Review. J. Energy Storage 2022, 46, 103886. [Google Scholar] [CrossRef]
- Volfkovich, Y.M. Self-Discharge of Supercapacitors: A Review. Russ. J. Electrochem. 2023, 59, 24–36. [Google Scholar] [CrossRef]
- Anjana, P.M.; Sarath Kumar, S.R.; Rakhi, R.B. Direct Growth of Mn(OH)2/Co(OH)2 Nanocomposite on Carbon Cloth for Flexible Supercapacitor Electrodes. J. Energy Storage 2021, 33, 102151. [Google Scholar] [CrossRef]
- Wang, F.; Wu, X.; Yuan, X.; Liu, Z.; Zhang, Y.; Fu, L.; Zhu, Y.; Zhou, Q.; Wu, Y.; Huang, W. Latest Advances in Supercapacitors: From New Electrode Materials to Novel Device Designs. Chem. Soc. Rev. 2017, 46, 6816–6854. [Google Scholar] [CrossRef] [PubMed]
- Iro, Z.S.; Subramani, C.; Dash, S.S. A Brief Review on Electrode Materials for Supercapacitor. Int. J. Electrochem. Sci. 2016, 11, 10628–10643. [Google Scholar] [CrossRef]
- Xie, J.; Yang, P.; Wang, Y.; Qi, T.; Lei, Y.; Li, C.M. Puzzles and Confusions in Supercapacitor and Battery: Theory and Solutions. J. Power Sources 2018, 401, 213–223. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and Mechanisms of Asymmetric Supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef]
- Akinwolemiwa, B.; Wei, C.; Chen, G.Z. Mechanisms and Designs of Asymmetrical Electrochemical Capacitors. Electrochim. Acta 2017, 247, 344–357. [Google Scholar] [CrossRef]
- Nagaraju, G.; Cha, S.M.; Sekhar, S.C.; Yu, J.S. Metallic Layered Polyester Fabric Enabled Nickel Selenide Nanostructures as Highly Conductive and Binderless Electrode with Superior Energy Storage Performance. Adv. Energy Mater. 2017, 7, 1601362. [Google Scholar] [CrossRef]
- Shaikh, S.; Rabinal, M.K. Rapid Ambient Growth of Copper Sulfide Microstructures: Binder Free Electrodes for Supercapacitor. J. Energy Storage 2020, 28, 101288. [Google Scholar] [CrossRef]
- Stevic, Z. Supercapacitors Based on Copper Sulfides. Ph.D. Thesis, University of Belgrade, Belgrade, Serbia, 2004. [Google Scholar]
- Wang, L.-W. High Chalcocite Cu2S: A Solid-Liquid Hybrid Phase. Phys. Rev. Lett. 2012, 108, 085703. [Google Scholar] [CrossRef]
- Majumdar, D. Recent Progress in Copper Sulfide Based Nanomaterials for High Energy Supercapacitor Applications. J. Electroanal. Chem. 2021, 880, 114825. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, R. An Elegant Room Temperature Procedure for the Precise Control of Composition in the Cu–S System. Inorg. Chem. 2011, 50, 9204–9206. [Google Scholar] [CrossRef]
- Gu, Y.; Li, T.; Guo, B.; Jiang, Y.; Wen, W.; Wu, J.; Zhao, L. Copper Sulfide Nanostructures and Their Sodium Storage Properties. CrystEngComm 2020, 22, 7082–7089. [Google Scholar] [CrossRef]
- Sun, S.; Li, P.; Liang, S.; Yang, Z. Diversified Copper Sulfide (Cu2−xS) Micro-/Nanostructures: A Comprehensive Review on Synthesis, Modifications and Applications. Nanoscale 2017, 9, 11357–11404. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Davoisne, C.; Tarascon, J.-M.; Guéry, C. Growth of Single-Crystal Copper Sulfide Thin Films via Electrodeposition in Ionic Liquid Media for Lithium Ion Batteries. J. Mater. Chem. 2012, 22, 5295. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.; Han, Y.; Qi, X.; Li, X.; Fan, H.; Meng, L. Metal-Organic Framework-Derived Carbon Coated Copper Sulfide Nanocomposites as a Battery-Type Electrode for Electrochemical Capacitors. Mater. Lett. 2019, 236, 131–134. [Google Scholar] [CrossRef]
- Foley, S.; Geaney, H.; Bree, G.; Stokes, K.; Connolly, S.; Zaworotko, M.J.; Ryan, K.M. Copper Sulfide (CuxS) Nanowire-in-Carbon Composites Formed from Direct Sulfurization of the Metal-Organic Framework HKUST-1 and Their Use as Li-Ion Battery Cathodes. Adv. Funct. Mater. 2018, 28, 1800587. [Google Scholar] [CrossRef]
- Zhang, Z.; An, Y.; Feng, J.; Ci, L.; Duan, B.; Huang, W.; Dong, C.; Xiong, S. Carbon Coated Copper Sulfides Nanosheets Synthesized via Directly Sulfurizing Metal-Organic Frameworks for Lithium Batteries. Mater. Lett. 2016, 181, 340–344. [Google Scholar] [CrossRef]
- Shin, M.; Awasthi, G.P.; Sharma, K.P.; Pandey, P.; Park, M.; Ojha, G.P.; Yu, C. Nanoarchitectonics of Three-Dimensional Carbon Nanofiber-Supported Hollow Copper Sulfide Spheres for Asymmetric Supercapacitor Applications. Int. J. Mol. Sci. 2023, 24, 9685. [Google Scholar] [CrossRef]
- Liu, J.; Pu, L.; Zhang, Q.; Cheng, Z.; Zheng, Y.; Wang, Y.; Liu, W.; Li, S.; Zhang, J. Tracking Surface Ionic Movement of Ni3S2@CuS Electrode Materials with High Electrochemical Performance. Chem. Eng. J. 2023, 461, 141910. [Google Scholar] [CrossRef]
- Barqi, J.; Masoudpanah, S.M.; Bafghi, M.S. Facile Synthesis of Plate-like Copper Sulfide Powder as an Electrode Material for High-Performance Supercapacitors. J. Mater. Sci. Mater. Electron. 2020, 31, 17614–17623. [Google Scholar] [CrossRef]
- Shah, M.Z.U.; Sajjad, M.; Hou, H.; Rahman, S.U.; Shah, A. Copper Sulfide Nanoparticles on Titanium Dioxide (TiO2) Nanoflakes: A New Hybrid Asymmetrical Faradaic Supercapacitors with High Energy Density and Superior Lifespan. J. Energy Storage 2022, 55, 105651. [Google Scholar] [CrossRef]
- Huang, K.-J.; Zhang, J.-Z.; Fan, Y. One-Step Solvothermal Synthesis of Different Morphologies CuS Nanosheets Compared as Supercapacitor Electrode Materials. J. Alloys Compd. 2015, 625, 158–163. [Google Scholar] [CrossRef]
- Qian, L.; Tian, X.; Yang, L.; Mao, J.; Yuan, H.; Xiao, D. High Specific Capacitance of CuS Nanotubes in Redox Active Polysulfide Electrolyte. RSC Adv. 2013, 3, 1703–1708. [Google Scholar] [CrossRef]
- Pilyté, S.; Valiulien, G.; Žielien, A.; Vinkevičius, J. Study of Interaction of ZnS Coatings with Cu(I) and Cu(II) by Cyclic Voltammetry. J. Electroanal. Chem. 1997, 436, 127–132. [Google Scholar] [CrossRef]
- Kim, W.J.; Cho, S.; Hong, J.; Hong, J.P. Hierarchically Nanostructured 1D-2D Flowerlike Copper Sulfide Electrode for High-Performance Supercapacitor Application by One-Pot Synthetic Procedure. Appl. Surf. Sci. 2022, 578, 152086. [Google Scholar] [CrossRef]
- Heydari, H.; Moosavifard, S.E.; Shahraki, M.; Elyasi, S. Facile Synthesis of Nanoporous CuS Nanospheres for High-Performance Supercapacitor Electrodes. J. Energy Chem. 2017, 26, 762–767. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, X.; Sun, Y.; Zhang, X.; Tang, L.; Zhang, X. Double-Shell CuS Nanocages as Advanced Supercapacitor Electrode Materials. J. Power Sources 2017, 355, 31–35. [Google Scholar] [CrossRef]
- Justin Raj, C.; Kim, B.C.; Cho, W.-J.; Lee, W.-G.; Seo, Y.; Yu, K.-H. Electrochemical Capacitor Behavior of Copper Sulfide (CuS) Nanoplatelets. J. Alloys Compd. 2014, 586, 191–196. [Google Scholar] [CrossRef]
- Zhou, W.; Miao, J.; Yan, X.; Li, Y.; Zhu, Y.; Zhang, W.; Zhang, M.; Zhu, W.; Javed, M.S.; Pan, J.; et al. Boosted Electrochemical Performance of CuS Anchored on Carbon Cloth as an Integrated Electrode for Quasi-Solid-State Flexible Supercapacitor. J. Electroanal. Chem. 2021, 897, 115610. [Google Scholar] [CrossRef]
- Hsu, Y.-K.; Chen, Y.-C.; Lin, Y.-G. Synthesis of Copper Sulfide Nanowire Arrays for High-Performance Supercapacitors. Electrochim. Acta 2014, 139, 401–407. [Google Scholar] [CrossRef]
- Peng, H.; Ma, G.; Mu, J.; Sun, K.; Lei, Z. Controllable Synthesis of CuS with Hierarchical Structures via a Surfactant-Free Method for High-Performance Supercapacitors. Mater. Lett. 2014, 122, 25–28. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Kumar Veerasubramani, G.; Rao, A.N.; Jae Kim, S. One-Pot Hydrothermal Synthesis, Characterization and Electrochemical Properties of CuS Nanoparticles towards Supercapacitor Applications. Mater. Res. Express 2014, 1, 035006. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, H.; Yang, J.; Qin, Q.; Fan, H.; Wei, C.; Zheng, W. Solvothermal Synthesis of 3D Hierarchical CuS Microspheres from a Cu-Based Ionic Liquid Precursor for High-Performance Asymmetric Supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 21735–21744. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Zheng, Y.; Hu, X.; Shang, Y.; Zhang, Y. Interconnected CuS Nanowalls with Rough Surfaces Grown on Nickel Foam as High-Performance Electrodes for Supercapacitors. RSC Adv. 2016, 6, 59976–59983. [Google Scholar] [CrossRef]
- Xu, W.; Liang, Y.; Su, Y.; Zhu, S.; Cui, Z.; Yang, X.; Inoue, A.; Wei, Q.; Liang, C. Synthesis and Properties of Morphology Controllable Copper Sulphide Nanosheets for Supercapacitor Application. Electrochim. Acta 2016, 211, 891–899. [Google Scholar] [CrossRef]
- Fu, W.; Han, W.; Zha, H.; Mei, J.; Li, Y.; Zhang, Z.; Xie, E. Nanostructured CuS Networks Composed of Interconnected Nanoparticles for Asymmetric Supercapacitors. Phys. Chem. Chem. Phys. 2016, 18, 24471–24476. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S.; Kim, H.-Y.; Kim, B.-S. Expeditious and Eco-Friendly Fabrication of Highly Uniform Microflower Superstructures and Their Applications in Highly Durable Methanol Oxidation and High-Performance Supercapacitors. J. Mater. Chem. A 2016, 4, 12253–12262. [Google Scholar] [CrossRef]
- De, B.; Balamurugan, J.; Kim, N.H.; Lee, J.H. Enhanced Electrochemical and Photocatalytic Performance of Core–Shell CuS@Carbon Quantum Dots@Carbon Hollow Nanospheres. ACS Appl. Mater. Interfaces 2017, 9, 2459–2468. [Google Scholar] [CrossRef] [PubMed]
- Ikkurthi, K.D.; Srinivasa Rao, S.; Jagadeesh, M.; Reddy, A.E.; Anitha, T.; Kim, H.-J. Synthesis of Nanostructured Metal Sulfides via a Hydrothermal Method and Their Use as an Electrode Material for Supercapacitors. New J. Chem. 2018, 42, 19183–19192. [Google Scholar] [CrossRef]
- Sonai Muthu, N.; Samikannu, S.D.; Gopalan, M. Influence of Thiourea Concentration on the CuS Nanostructures and Identification of the Most Suited Electrolyte for High Energy Density Supercapacitor. Ionics 2019, 25, 4409–4423. [Google Scholar] [CrossRef]
- Kadam, S.L.; Bulakhe, R.N.; Kadam, R.A.; Yewale, M.A. Electrochemical Synthesis of CuS Thin Film for Supercapacitor Application. Macromol. Symp. 2020, 392, 1900209. [Google Scholar] [CrossRef]
- Kadam, S.L.; Kadam, R.A.; Yewale, M.A. New arts commerce and science college parner ahmednagar Preparation and Performance of CuS Thin Films in Non-Aqueous Medium as Supercapacitor Electrode Materials. Int. J. Eng. Res. 2020, V9, IJERTV9IS020341. [Google Scholar] [CrossRef]
- Raghavendra, K.V.G.; Gopi, C.V.V.M.; Vinodh, R.; Durga, I.K.; Kim, H.-J. One-Step Facile Synthesis of Dense Cloud-like Tiny Bundled Nanoparticles of CuS Nanostructures as an Efficient Electrode Material for High-Performance Supercapacitors. J. Energy Storage 2020, 27, 101148. [Google Scholar] [CrossRef]
- Quan, Y.; Wang, G.; Lu, L.; Wang, Z.; Xu, H.; Liu, S.; Wang, D. High-Performance Pseudo-Capacitor Energy Storage Device Based on a Hollow-Structured Copper Sulfide Nanoflower and Carbon Quantum Dot Nanocomposite. Electrochim. Acta 2020, 353, 136606. [Google Scholar] [CrossRef]
- Attia, S.Y.; Mohamed, S.G. Detergent-Free Micelle-Assisted Synthesis of Carbon-Containing Hexagonal CuS Nanostructures for Efficient Supercapacitor Electrode Materials. Electrochim. Acta 2022, 407, 139918. [Google Scholar] [CrossRef]
- Barqi, J.; Masoudpanah, S.M.; Liu, X.; Bafghi, M.S.; Ong, C.K. Fabrication of Porous Cu2S Nanosheets for High Performance Hybrid Supercapacitor. J. Energy Storage 2022, 45, 103781. [Google Scholar] [CrossRef]
- Zhao, T.; Peng, X.; Zhao, X.; Hu, J.; Yang, W.; Li, T.; Ahmad, I. Facile Preparation and High Capacitance Performance of Copper Sulfide Microspheres as Supercapacitor Electrode Material. Compos. Part B Eng. 2019, 163, 26–35. [Google Scholar] [CrossRef]
- Peng, Q.; Zhang, S.; Yang, H.; Sheng, B.; Xu, R.; Wang, Q.; Yu, Y. Boosting Potassium Storage Performance of the Cu2S Anode via Morphology Engineering and Electrolyte Chemistry. ACS Nano 2020, 14, 6024–6033. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, F.; Ji, Y.; Yang, M.; Liu, W.; Wang, W.; Sun, Q.; Zhang, Z.; Zhao, X.; Liu, X. Controllable Synthesis of Various Kinds of Copper Sulfides (CuS, Cu7S4, Cu9S5) for High-Performance Supercapacitors. Dalton Trans. 2015, 44, 10431–10437. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.S.; Dai, S.; Wang, M.; Xi, Y.; Lang, Q.; Guo, D.; Hu, C. Faradic Redox Active Material of Cu7S4 Nanowires with a High Conductance for Flexible Solid State Supercapacitors. Nanoscale 2015, 7, 13610–13618. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Liang, J.; Liu, J.; Sun, P.; Bu, J.; Zhang, W.; Chen, G. Synthesis of Porous Cu7.2S4 Sub-Microspheres by an Ion Exchange Method for High-Performance Supercapacitors. RSC Adv. 2016, 6, 16832–16837. [Google Scholar] [CrossRef]
- Muddassir, Y.; Tahir, S.; Ali, A.; Mahmood, K.; Ur Rehman, U.; Ashfaq, A.; Manzoor, A.; Ikram, S. Morphology-Dependent Thermoelectric Properties of Mixed Phases of Copper Sulfide (Cu2−xS) Nanostructures Synthesized by Hydrothermal Method. Appl. Phys. A 2021, 127, 457. [Google Scholar] [CrossRef]
- Zhai, S.; Jin, K.; Zhou, M.; Fan, Z.; Zhao, H.; Zhao, Y.; Li, X.; Cai, Z. In-Situ Growth of Flower-like CuS Microsphere on Carbonized Cotton for High-Performance Flexible Supercapacitor. Colloids Surf. Physicochem. Eng. Asp. 2019, 575, 75–83. [Google Scholar] [CrossRef]
- Ding, Y.; Lin, R.; Xiong, S.; Zhu, Y.; Yu, M.; Duan, X. The Effect of Copper Sulfide Stoichiometric Coefficient and Morphology on Electrochemical Performance. Molecules 2023, 28, 2487. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Gong, L.; Zhao, X.; Wang, C.; Liang, Q.; Zhang, W.; Wang, L.; Yu, K.; Dai, Y.; Zhou, B. Copper Sulfide Nanoparticles with Potential Bifunctional Properties: Supercapacitor and Photocatalysis. CrystEngComm 2021, 23, 3870–3879. [Google Scholar] [CrossRef]
- Ranjith Kumar, D.; Kesavan, S.; Baynosa, M.L.; Shim, J.-J. Flower-like Cu1.8S Nanostructures for High-Performance Flexible Solid-State Supercapacitors. Appl. Surf. Sci. 2018, 448, 547–558. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Z.; Zhang, S.; Luo, W.; Zhang, G. Controllable Synthesis of CuS Hollow Microflowers Hierarchical Structures for Asymmetric Supercapacitors. Appl. Surf. Sci. 2018, 442, 711–719. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, J. Definitions of Pseudocapacitive Materials: A Brief Review. Energy Environ. Mater. 2019, 2, 30–37. [Google Scholar] [CrossRef]
- Patil, A.M.; Lokhande, A.C.; Chodankar, N.R.; Shinde, P.A.; Kim, J.H.; Lokhande, C.D. Interior Design Engineering of CuS Architecture Alteration with Rise in Reaction Bath Temperature for High Performance Symmetric Flexible Solid State Supercapacitor. J. Ind. Eng. Chem. 2017, 46, 91–102. [Google Scholar] [CrossRef]
- Samdhyan, K.; Chand, P.; Anand, H. Effect of Different Precursors on the Electrochemical Behavior of CuS Nanostructures as Electrode Material for Supercapacitor Applications. Mater. Today Proc. 2023, 76, 56–62. [Google Scholar] [CrossRef]
- Patil, A.M.; Lokhande, A.C.; Shinde, P.A.; Lokhande, C.D. Flexible Asymmetric Solid-State Supercapacitors by Highly Efficient 3D Nanostructured α-MnO2 and h-CuS Electrodes. ACS Appl. Mater. Interfaces 2018, 10, 16636–16649. [Google Scholar] [CrossRef]
- Ying, Z.; Zhao, S.; Yue, J.; Ju, T.; Zhang, Y.; Xie, J.; Wang, Q. 3D Hierarchical CuS Microflowers Constructed on Copper Powders-Filled Nickel Foam as Advanced Binder-Free Electrodes. J. Alloys Compd. 2020, 821, 153437. [Google Scholar] [CrossRef]
- He, X.; Hu, Y.; Tian, H.; Li, Z.; Huang, P.; Jiang, J.; Wang, C. In-Situ Growth of Flexible 3D Hollow Tubular Cu2S Nanorods on Cu Foam for High Electrochemical Performance Supercapacitor. J. Mater. 2020, 6, 192–199. [Google Scholar] [CrossRef]
- Lee, Y.-W.; Kim, B.-S.; Hong, J.; Lee, J.; Pak, S.; Jang, H.-S.; Whang, D.; Cha, S.; Sohn, J.I.; Kim, J.M. A Pseudo-Capacitive Chalcogenide-Based Electrode with Dense 1-Dimensional Nanoarrays for Enhanced Energy Density in Asymmetric Supercapacitors. J. Mater. Chem. A 2016, 4, 10084–10090. [Google Scholar] [CrossRef]
- Dose, W.M.; Piernas-Muñoz, M.J.; Maroni, V.A.; Trask, S.E.; Bloom, I.; Johnson, C.S. Capacity Fade in High Energy Silicon-Graphite Electrodes for Lithium-Ion Batteries. Chem. Commun. 2018, 54, 3586–3589. [Google Scholar] [CrossRef]
- Grozdanov, I.; Najdoski, M. Optical and Electrical Properties of Copper Sulfide Films of Variable Composition. J. Solid State Chem. 1995, 114, 469–475. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Niu, H.; Chen, H.; Yang, L.; Bai, L.; Liang, Y.; Wei, D.; Yang, H. Construction of CNT/CuS/FeOOH Hierarchical Composites on Carbon Cloth for High-Performance Solid-State Flexible Supercapacitors. Electrochim. Acta 2023, 469, 143256. [Google Scholar] [CrossRef]
- Xiao, P.; Sk, M.A.; Thia, L.; Ge, X.; Lim, R.J.; Wang, J.-Y.; Lim, K.H.; Wang, X. Molybdenum Phosphide as an Efficient Electrocatalyst for the Hydrogen Evolution Reaction. Energy Environ. Sci. 2014, 7, 2624–2629. [Google Scholar] [CrossRef]
- Liu, W.; Niu, H.; Yang, J.; Cheng, K.; Ye, K.; Zhu, K.; Wang, G.; Cao, D.; Yan, J. Ternary Transition Metal Sulfides Embedded in Graphene Nanosheets as Both the Anode and Cathode for High-Performance Asymmetric Supercapacitors. Chem. Mater. 2018, 30, 1055–1068. [Google Scholar] [CrossRef]
- Zequine, C.; Bhoyate, S.; Wang, F.; Li, X.; Siam, K.; Kahol, P.K.; Gupta, R.K. Effect of Solvent for Tailoring the Nanomorphology of Multinary CuCo2S4 for Overall Water Splitting and Energy Storage. J. Alloys Compd. 2019, 784, 1–7. [Google Scholar] [CrossRef]
- Pawar, S.M.; Pawar, B.S.; Babar, P.T.; Ahmed, A.T.A.; Chavan, H.S.; Jo, Y.; Cho, S.; Kim, J.; Hou, B.; Inamdar, A.I.; et al. Nanoporous CuCo2O4 Nanosheets as a Highly Efficient Bifunctional Electrode for Supercapacitors and Water Oxidation Catalysis. Appl. Surf. Sci. 2019, 470, 360–367. [Google Scholar] [CrossRef]
- Shan, X.; Charles, D.S.; Xu, W.; Feygenson, M.; Su, D.; Teng, X. Biphase Cobalt–Manganese Oxide with High Capacity and Rate Performance for Aqueous Sodium-Ion Electrochemical Energy Storage. Adv. Funct. Mater. 2018, 28, 1703266. [Google Scholar] [CrossRef]
- Chavan, G.T.; Yadav, A.; Fugare, B.Y.; Shinde, N.M.; Tamboli, M.S.; Kamble, S.S.; Sikora, A.; Warycha, J.; Lokhande, B.J.; Kang, S.-W.; et al. Three Dimensional Hierarchical Flower-like CoCuS/Co1-xCuS Electrodes for Electrochemical Supercapacitors. J. Alloys Compd. 2022, 901, 162822. [Google Scholar] [CrossRef]
- Wang, J.; Quan, Y.; Wang, G.; Wang, D.; Xiao, J.; Gao, S.; Xu, H.; Liu, S.; Cui, L. 3D Hollow Cage Copper Cobalt Sulfide Derived from Metal–Organic Frameworks for High-Performance Asymmetric Supercapacitors. CrystEngComm 2021, 23, 7385–7396. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, C.; He, Y.; Wu, X.; Ge, R.; Zu, X.; Li, S.; Qiao, L. High-Performance Asymmetric Supercapacitors Realized by Copper Cobalt Sulfide Crumpled Nanoflower and N, F Co-Doped Hierarchical Nanoporous Carbon Polyhedron. J. Power Sources 2020, 456, 228023. [Google Scholar] [CrossRef]
- Choi, J.; Morey, K.; Kumar, A.; Neupane, D.; Mishra, S.R.; Perez, F.; Gupta, R.K. Self-Assembled Cotton-like Copper–Molybdenum Sulfide and Phosphide as a Bifunctional Electrode for Green Energy Storage and Production. Mater. Today Chem. 2022, 24, 100848. [Google Scholar] [CrossRef]
- Yu, F.; Tiong, V.T.; Pang, L.; Zhou, R.; Wang, X.; Waclawik, E.R.; Ostrikov, K.; Wang, H. Flower-like Cu5Sn2S7/ZnS Nanocomposite for High Performance Supercapacitor. Chin. Chem. Lett. 2019, 30, 1115–1120. [Google Scholar] [CrossRef]
- Liu, L.; Annamalai, K.P.; Tao, Y. A Hierarchically Porous CuCo2S4/Graphene Composite as an Electrode Material for Supercapacitors. Carbon 2016, 110, 520. [Google Scholar] [CrossRef]
- Shen, J.; Tang, J.; Dong, P.; Zhang, Z.; Ji, J.; Baines, R.; Ye, M. Construction of Three-Dimensional CuCo2S4 /CNT/Graphene Nanocomposite for High Performance Supercapacitors. RSC Adv. 2016, 6, 13456–13460. [Google Scholar] [CrossRef]
- Moosavifard, S.E.; Fani, S.; Rahmanian, M. Hierarchical CuCo2S4 Hollow Nanoneedle Arrays as Novel Binder-Free Electrodes for High-Performance Asymmetric Supercapacitors. Chem. Commun. 2016, 52, 4517–4520. [Google Scholar] [CrossRef]
- Ahmed, A.T.A.; Chavan, H.S.; Jo, Y.; Cho, S.; Kim, J.; Pawar, S.M.; Gunjakar, J.L.; Inamdar, A.I.; Kim, H.; Im, H. One-Step Facile Route to Copper Cobalt Sulfide Electrodes for Supercapacitors with High-Rate Long-Cycle Life Performance. J. Alloys Compd. 2017, 724, 744–751. [Google Scholar] [CrossRef]
- Xu, W.; Lu, J.; Huo, W.; Li, J.; Wang, X.; Zhang, C.; Gu, X.; Hu, C. Direct Growth of CuCo2S4 Nanosheets on Carbon Fiber Textile with Enhanced Electrochemical Pseudocapacitive Properties and Electrocatalytic Properties towards Glucose Oxidation. Nanoscale 2018, 10, 14304–14313. [Google Scholar] [CrossRef]
- Chen, L.; Lin, R.; Yan, C. Nitrogen-Doped Double-Layer Graphite Supported CuCo2S4 Electrode for High-Performance Asymmetric Supercapacitors. Mater. Lett. 2019, 235, 6–10. [Google Scholar] [CrossRef]
- Li, M.-L.; Xiao, K.; Su, H.; Li, N.; Cai, Y.-P.; Liu, Z.-Q. CuCo2 S4 Nanosheets Coupled With Carbon Nanotube Heterostructures for Highly Efficient Capacitive Energy Storage. ChemElectroChem 2018, 5, 2496–2502. [Google Scholar] [CrossRef]
- Sahoo, S.; Krishnamoorthy, K.; Pazhamalai, P.; Mariappan, V.K.; Kim, S.-J. Copper Molybdenum Sulfide: A Novel Pseudocapacitive Electrode Material for Electrochemical Energy Storage Device. Int. J. Hydrog. Energy 2018, 43, 12222–12232. [Google Scholar] [CrossRef]
- Du, J.; Yan, Q.; Li, Y.; Cheng, K.; Ye, K.; Zhu, K.; Yan, J.; Cao, D.; Zhang, X.; Wang, G. Hierarchical Copper Cobalt Sulfides Nanowire Arrays for High-Performance Asymmetric Supercapacitors. Appl. Surf. Sci. 2019, 487, 198–205. [Google Scholar] [CrossRef]
- Wang, T.; Liu, M.; Ma, H. Facile Synthesis of Flower-Like Copper-Cobalt Sulfide as Binder-Free Faradaic Electrodes for Supercapacitors with Improved Electrochemical Properties. Nanomaterials 2017, 7, 140. [Google Scholar] [CrossRef]
- Tang, J.; Ge, Y.; Shen, J.; Ye, M. Facile Synthesis of CuCo2S4as a Novel Electrode Material for Ultrahigh Supercapacitor Performance. Chem. Commun. 2016, 52, 1509–1512. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Hu, R.; Zhu, D.; Qi, J.; He, Y.; Meng, Q.; Wei, F.; Ren, Y.; Sui, Y. Controlled Synthesis and Formation Mechanism of Flower-like CuS/NiS Microspheres for Supercapacitors. Surf. Interfaces 2021, 22, 100871. [Google Scholar] [CrossRef]
- Dai, J.; Luo, L.; Tang, Z.; Lv, Y.; Xie, H.; Zuo, H.; Yang, C.; Wang, X.; Fan, M.; Xu, Y.; et al. Strategy for Constructing Highly Stable Supercapacitors: Channeling of Thin-Layer Polyaniline to Enhance Pseudo-Capacitance of the CuS/polyaniline@MoS2 Composites. Compos. Sci. Technol. 2022, 219, 109240. [Google Scholar] [CrossRef]
- You, Y.; Qu, K.; Shi, C.; Sun, Z.; Huang, Z.; Li, J.; Dong, M.; Guo, Z. Binder-Free CuS/ZnS/Sodium Alginate/rGO Nanocomposite Hydrogel Electrodes for Enhanced Performance Supercapacitors. Int. J. Biol. Macromol. 2020, 162, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhao, S.; Yu, X.; Li, Y.; Chen, H.; Han, L. Metal–Organic Framework Templated Fabrication of Cu7S4 @Ni(OH)2 Core–Shell Nanoarrays for High-Performance Supercapacitors. Inorg. Chem. Front. 2020, 7, 427–436. [Google Scholar] [CrossRef]
- Sun, M.; Li, Z.; Fang, Q.; Han, S.; Cai, C.; Li, H.; Shen, W.; Liu, X.; Fu, Y. Room-Temperature Synthesized Porous Cu(OH)2/Cu7S4 Hybrid Nanowires as a High-Performance Electrode Material for Asymmetric Supercapacitors. J. Mater. Chem. A 2020, 8, 724–734. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, X.; Zheng, Y.; Zhang, Y.; Xu, J. Synthesis and Electrochemical Performance of CuO/CuxSy Octahedral Core-Shell Structure. J. Mater. Eng. 2020, 48, 98–105. [Google Scholar]
- Tian, Y.; Su, Z.; Zhao, Z.; Cong, B.; Wang, M. Spherical NiCo2O4/CuS Composites for Supercapacitor Electrodes. Mater. Lett. 2020, 264, 127400. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, C.; Zhang, Z.; Min, S.; Qian, X. A Facile One-Step Route to Synthesize Three-Layer Nanostructure of CuS/RGO/Ni3S2 and Its High Electrochemical Performance. RSC Adv. 2016, 6, 16963–16971. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, J.; Yang, F.; Wang, M.; Zhang, T.; Hao, Y.; Yang, W. Sandwich-like Porous MXene/Ni3S4/CuS Derived from MOFs as Superior Supercapacitor Electrode. J. Alloys Compd. 2022, 906, 163863. [Google Scholar] [CrossRef]
- Raju, T.D.; Gopalakrishnan, A.; Badhulika, S. Facile Synthesis of 3D/2D Cu2Se Cauliflower/CuS Nanosheets Composite as a Binder-Free Electrode for High-Performance Asymmetric Solid-State Supercapacitors. J. Alloys Compd. 2020, 845, 156241. [Google Scholar] [CrossRef]
- Duan, X.; Ding, Y.; Yu, M. Research on CuxSy/CoSz/Graphene Supercapacitor Electrode Materials. Mater. Express 2020, 10, 1725–1731. [Google Scholar] [CrossRef]
- He, Y.; Zhang, X.; Wang, S.; Meng, J.; Sui, Y.; Wei, F.; Qi, J.; Meng, Q.; Ren, Y.; Zhuang, D. Rubik’s Cube-like Ni3S4/CuS2 Nanocomposite for High-Performance Supercapacitors. J. Alloys Compd. 2020, 847, 156312. [Google Scholar] [CrossRef]
- Brown, J.W.; Geetha, D.; Ramesh, P.S.; Podili, S. Hydrothermal Synthesis and Electrochemical Characterization of HexagonalZr-CuS Nanocomposite and Its Charge Storage Capacity. Asian J. Chem. 2020, 32, 1635–1641. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-Based Composite Materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Zhou, W.; Feng, T.; Zhang, Y.; Liu, H.; Yu, H.; Tian, L.; Pu, Y. One-Pot Solvothermal Synthesis of Flower-like Copper Sulfide/Reduced Graphene Oxide Composite Superstructures as High-Performance Supercapacitor Electrode Materials. J. Mater. Sci. Mater. Electron. 2017, 28, 5931–5940. [Google Scholar] [CrossRef]
- Balu, R.; Dakshanamoorthy, A. Synthesis of Wool Ball-like Copper Sulfide Nanospheres Embedded Graphene Nanocomposite as Electrode for High-performance Symmetric Supercapacitor Device. Int. J. Energy Res. 2022, 46, 6730–6744. [Google Scholar] [CrossRef]
- El-Hout, S.I.; Mohamed, S.G.; Gaber, A.; Attia, S.Y.; Shawky, A.; El-Sheikh, S.M. High Electrochemical Performance of rGO Anchored CuS Nanospheres for Supercapacitor Applications. J. Energy Storage 2021, 34, 102001. [Google Scholar] [CrossRef]
- BoopathiRaja, R.; Parthibavarman, M.; Prabhu, S.; Ramesh, R. A Facile One Step Hydrothermal Induced Hexagonal Shaped CuS/rGO Nanocomposites for Asymmetric Supercapacitors. Mater. Today Proc. 2020, 26, 3507–3513. [Google Scholar] [CrossRef]
- Bulakhe, R.N.; Alfantazi, A.; Rok Lee, Y.; Lee, M.; Shim, J.-J. Chemically Synthesized Copper Sulfide Nanoflakes on Reduced Graphene Oxide for Asymmetric Supercapacitors. J. Ind. Eng. Chem. 2021, 101, 423–429. [Google Scholar] [CrossRef]
- Malavekar, D.B.; Lokhande, V.C.; Mane, V.J.; Kale, S.B.; Bulakhe, R.N.; Patil, U.M.; In, I.; Lokhande, C.D. Facile Synthesis of Layered Reduced Graphene Oxide–Copper Sulfide (rGO-CuS) Hybrid Electrode for All Solid-State Symmetric Supercapacitor. J. Solid State Electrochem. 2020, 24, 2963–2974. [Google Scholar] [CrossRef]
- Huang, K.-J.; Zhang, J.-Z.; Liu, Y.; Liu, Y.-M. Synthesis of Reduced Graphene Oxide Wrapped-Copper Sulfide Hollow Spheres as Electrode Material for Supercapacitor. Int. J. Hydrog. Energy 2015, 40, 10158–10167. [Google Scholar] [CrossRef]
- De, B.; Kuila, T.; Kim, N.H.; Lee, J.H. Carbon Dot Stabilized Copper Sulphide Nanoparticles Decorated Graphene Oxide Hydrogel for High Performance Asymmetric Supercapacitor. Carbon 2017, 122, 247–257. [Google Scholar] [CrossRef]
- Tian, Z.; Dou, H.; Zhang, B.; Fan, W.; Wang, X. Three-Dimensional Graphene Combined with Hierarchical CuS for the Design of Flexible Solid-State Supercapacitors. Electrochim. Acta 2017, 237, 109–118. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, J.; Li, G.; Sun, Y.; Zhang, G.; Zheng, W. Ionic Liquid-Assisted Synthesis of rGO Wrapped Three-Dimensional CuS Ordered Nanoerythrocytes with Enhanced Performance for Asymmetric Supercapacitors. Chem. Eng. J. 2017, 325, 424–432. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Q.; Ma, T.; Fan, W. Synthesis and Electrochemical Properties of Nitrogen-Doped Graphene/Copper Sulphide Nanocomposite for Supercapacitor. J. Nanosci. Nanotechnol. 2017, 17, 2811–2816. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, K.; Zhou, J.; Shen, J.; Ye, M. CuS Nanoplatelets Arrays Grown on Graphene Nanosheets as Advanced Electrode Materials for Supercapacitor Applications. J. Mater. Sci. Technol. 2018, 34, 2342–2349. [Google Scholar] [CrossRef]
- Zhao, T.; Yang, W.; Zhao, X.; Peng, X.; Hu, J.; Tang, C.; Li, T. Facile Preparation of Reduced Graphene Oxide/Copper Sulfide Composite as Electrode Materials for Supercapacitors with High Energy Density. Compos. Part B Eng. 2018, 150, 60–67. [Google Scholar] [CrossRef]
- Zhu, W.; Ou, X.; Lu, Z.; Chen, K.; Ling, Y.; Zhang, H. Enhanced Performance of Hierarchical CuS Clusters Applying TRGO as Conductive Carrier for Supercapacitors. J. Mater. Sci. Mater. Electron. 2019, 30, 5760–5770. [Google Scholar] [CrossRef]
- Singhal, R.; Thorne, D.; LeMaire, P.K.; Martinez, X.; Zhao, C.; Gupta, R.K.; Uhl, D.; Scanley, E.; Broadbridge, C.C.; Sharma, R.K. Synthesis and Characterization of CuS, CuS/Graphene Oxide Nanocomposite for Supercapacitor Applications. AIP Adv. 2020, 10, 035307. [Google Scholar] [CrossRef]
- Ghosh, K.; Srivastava, S.K. Enhanced Supercapacitor Performance and Electromagnetic Interference Shielding Effectiveness of CuS Quantum Dots Grown on Reduced Graphene Oxide Sheets. ACS Omega 2021, 6, 4582–4596. [Google Scholar] [CrossRef]
- Zhuang, G.; Sun, Y.; Chen, X. CuS Cluster Microspheres Anchored on Reduced Graphene Oxide as Electrode Material for Asymmetric Supercapacitors with Outstanding Performance. J. Mater. Sci. Mater. Electron. 2021, 32, 4805–4814. [Google Scholar] [CrossRef]
- Zhao, T.; Peng, X.; Zhao, X.; Hu, J.; Jiang, T.; Lu, X.; Zhang, H.; Li, T.; Ahmad, I. Preparation and Performance of Carbon Dot Decorated Copper Sulphide/Carbon Nanotubes Hybrid Composite as Supercapacitor Electrode Materials. J. Alloys Compd. 2020, 817, 153057. [Google Scholar] [CrossRef]
- Quan, Y.; Zhang, M.; Wang, G.; Lu, L.; Wang, Z.; Xu, H.; Liu, S.; Min, Q. 3D Hierarchical Porous CuS Flower-Dispersed CNT Arrays on Nickel Foam as a Binder-Free Electrode for Supercapacitors. New J. Chem. 2019, 43, 10906–10914. [Google Scholar] [CrossRef]
- Zhu, T.; Xia, B.; Zhou, L.; Lou, X.W.D. Arrays of Ultrafine CuS Nanoneedles Supported on a CNT Backbone for Application in Supercapacitors. J. Mater. Chem. 2012, 22, 7851. [Google Scholar] [CrossRef]
- Huang, K.-J.; Zhang, J.-Z.; Xing, K. One-Step Synthesis of Layered CuS/Multi-Walled Carbon Nanotube Nanocomposites for Supercapacitor Electrode Material with Ultrahigh Specific Capacitance. Electrochim. Acta 2014, 149, 28–33. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, X.; Wang, W.; Cheng, J.; Yan, H.; Tang, C.; Kim, J.-K.; Luo, Y. Hierarchical, Porous CuS Microspheres Integrated with Carbon Nanotubes for High-Performance Supercapacitors. Sci. Rep. 2015, 5, 16584. [Google Scholar] [CrossRef] [PubMed]
- Ravi, S.; Gopi, C.V.V.M.; Kim, H.J. Enhanced Electrochemical Capacitance of Polyimidazole Coated Covellite CuS Dispersed CNT Composite Materials for Application in Supercapacitors. Dalton Trans. 2016, 45, 12362–12371. [Google Scholar] [CrossRef]
- Hou, X.; Liu, X.; Lu, Y.; Cheng, J.; Luo, R.; Yu, Q.; Wei, X.; Yan, H.; Ji, X.; Kim, J.-K.; et al. Copper Sulfide Nanoneedles on CNT Backbone Composite Electrodes for High-Performance Supercapacitors and Li-S Batteries. J. Solid State Electrochem. 2017, 21, 349–359. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on Supercapacitors: Technologies and Materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Li, C.; He, P.; Jia, L.; Zhang, X.; Zhang, T.; Dong, F.; He, M.; Wang, S.; Zhou, L.; Yang, T.; et al. Facile Synthesis of 3D CuS Micro-Flowers Grown on Porous Activated Carbon Derived from Pomelo Peel as Electrode for High-Performance Supercapacitors. Electrochim. Acta 2019, 299, 253–261. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, M.; Lu, L.; Xu, H.; Xiao, Z.; Liu, S.; Gao, S.; Yu, Z. One-Pot Synthesis of CuS Nanoflower-Decorated Active Carbon Layer for High-Performance Asymmetric Supercapacitors. ChemNanoMat 2018, 4, 964–971. [Google Scholar] [CrossRef]
- Gong, S.-G.; Shi, Y.-H.; Su, Y.; Qi, F.; Song, Y.-H.; Yang, G.-D.; Li, B.; Wu, X.-L.; Zhang, J.-P.; Tong, C.-Y.; et al. Introduction of S-S Bond to Flexible Supercapacitors for High Mass Specific Capacity and Stability. J. Alloys Compd. 2022, 911, 165080. [Google Scholar] [CrossRef]
- Jin, K.; Zhou, M.; Zhao, H.; Zhai, S.; Ge, F.; Zhao, Y.; Cai, Z. Electrodeposited CuS Nanosheets on Carbonized Cotton Fabric as Flexible Supercapacitor Electrode for High Energy Storage. Electrochim. Acta 2019, 295, 668–676. [Google Scholar] [CrossRef]
- Ahmad, W. P-Type NiO Nanoparticles Enhanced Acetylene Black as Efficient Counter Electrode for Dye-Sensitized Solar Cells. Mater. Res. Bull. 2015, 67, 185–190. [Google Scholar] [CrossRef]
- Hou, H.; Yang, Y.; Zhu, Y.; Jing, M.; Pan, C.; Fang, L.; Song, W.; Yang, X.; Ji, X. An Electrochemical Study of Sb/Acetylene Black Composite as Anode for Sodium-Ion Batteries. Electrochim. Acta 2014, 146, 328–334. [Google Scholar] [CrossRef]
- Sun, Y. Synthesis of a Ternary Polyaniline@acetylene Black-Sulfur Material by Continuous Two-Step Liquid Phase for Lithium Sulfur Batteries. Electrochim. Acta 2015, 158, 143–151. [Google Scholar] [CrossRef]
- Huang, K.-J.; Zhang, J.-Z.; Jia, Y.-L.; Xing, K.; Liu, Y.-M. Acetylene Black Incorporated Layered Copper Sulfide Nanosheets for High-Performance Supercapacitor. J. Alloys Compd. 2015, 641, 119–126. [Google Scholar] [CrossRef]
- Wu, R.; Wang, D.P.; Kumar, V.; Zhou, K.; Law, A.W.K.; Lee, P.S.; Lou, J.; Chen, Z. MOFs-Derived Copper Sulfides Embedded within Porous Carbon Octahedra for Electrochemical Capacitor Applications. Chem. Commun. 2015, 51, 3109–3112. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Liu, Y.; Mao, B.; Xin, N.; Jia, H.; Shi, W. In-Situ Embedding MOFs-Derived Copper Sulfide Polyhedrons in Carbon Nanotube Networks for Hybrid Supercapacitor with Superior Energy Density. Electrochim. Acta 2020, 329, 135130. [Google Scholar] [CrossRef]
- Liu, Y.-P.; Qi, X.-H.; Li, L.; Zhang, S.-H.; Bi, T. MOF-Derived PPy/Carbon-Coated Copper Sulfide Ceramic Nanocomposite as High-Performance Electrode for Supercapacitor. Ceram. Int. 2019, 45, 17216–17223. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, Q.; Pei, H.; Pan, W.; Liu, Y.; Xu, S.; Cao, S. Synchronous Dual Roles of Copper Sulfide on the Insulating PET Fabric for High-Performance Portable Flexible Supercapacitors. Energy Fuels 2021, 35, 6880–6891. [Google Scholar] [CrossRef]
- Peng, H.; Ma, G.; Sun, K.; Mu, J.; Wang, H.; Lei, Z. High-Performance Supercapacitor Based on Multi-Structural CuS@polypyrrole Composites Prepared by in Situ Oxidative Polymerization. J. Mater. Chem. A 2014, 2, 3303. [Google Scholar] [CrossRef]
- Peng, S.; Fan, L.; Wei, C.; Liu, X.; Zhang, H.; Xu, W.; Xu, J. Flexible Polypyrrole/Copper Sulfide/Bacterial Cellulose Nanofibrous Composite Membranes as Supercapacitor Electrodes. Carbohydr. Polym. 2017, 157, 344–352. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, S.; Xu, Y. Two-Step Synthesis of CuS/C@PANI Nanocomposite as Advanced Electrode Materials for Supercapacitor Applications. Nanomaterials 2020, 10, 1034. [Google Scholar] [CrossRef]


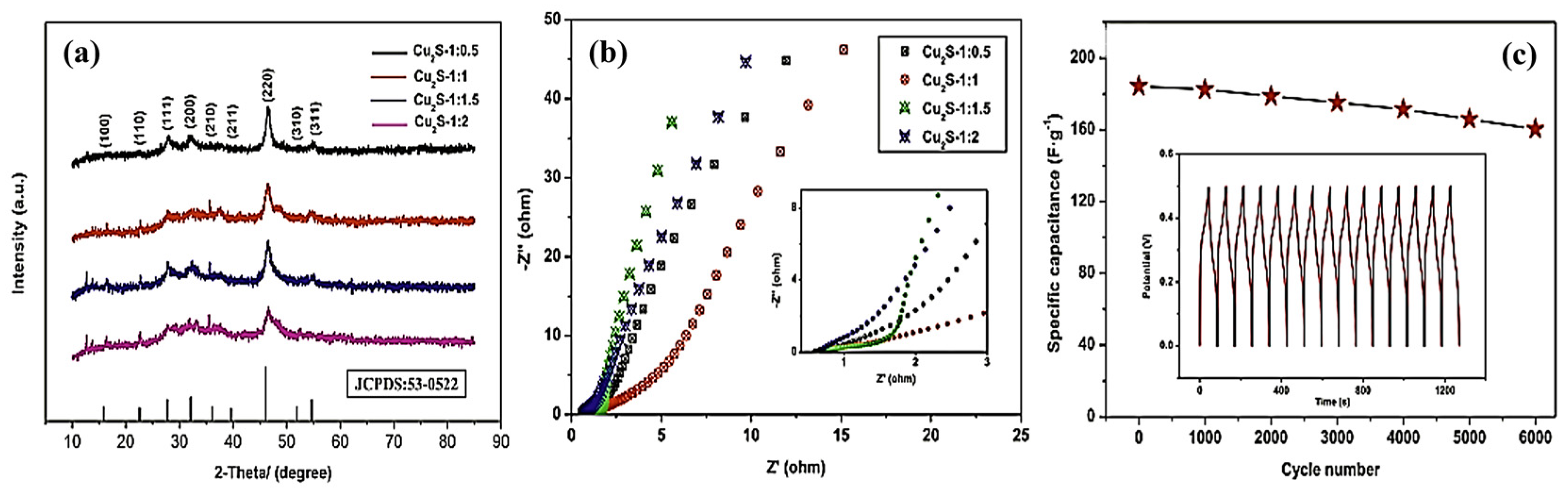
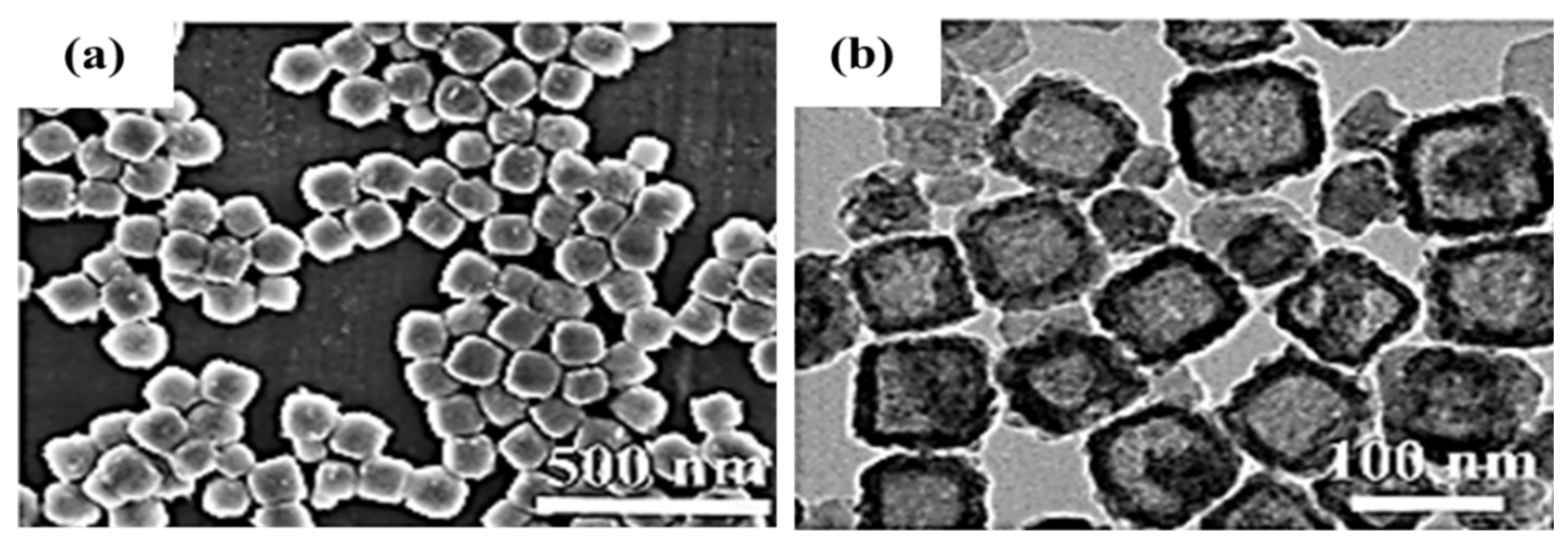
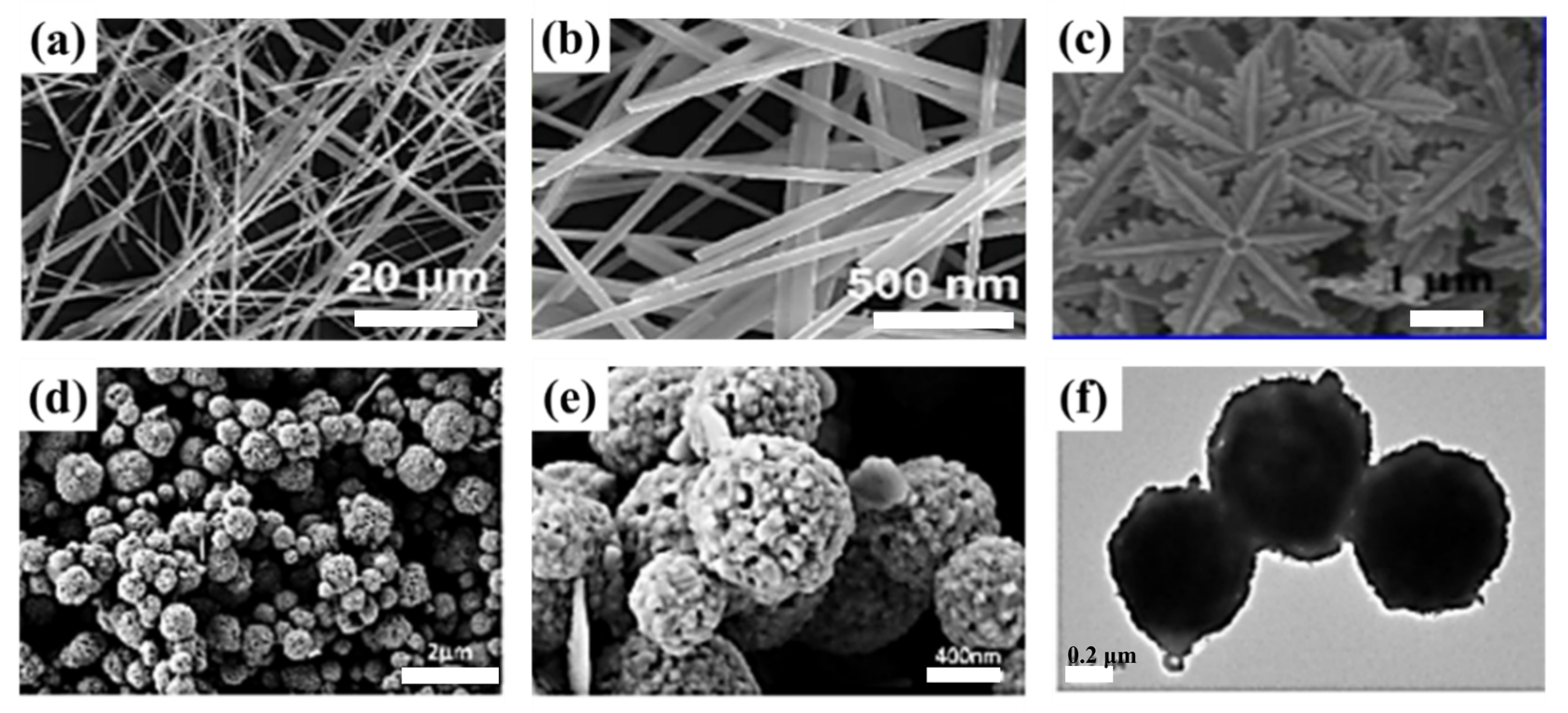
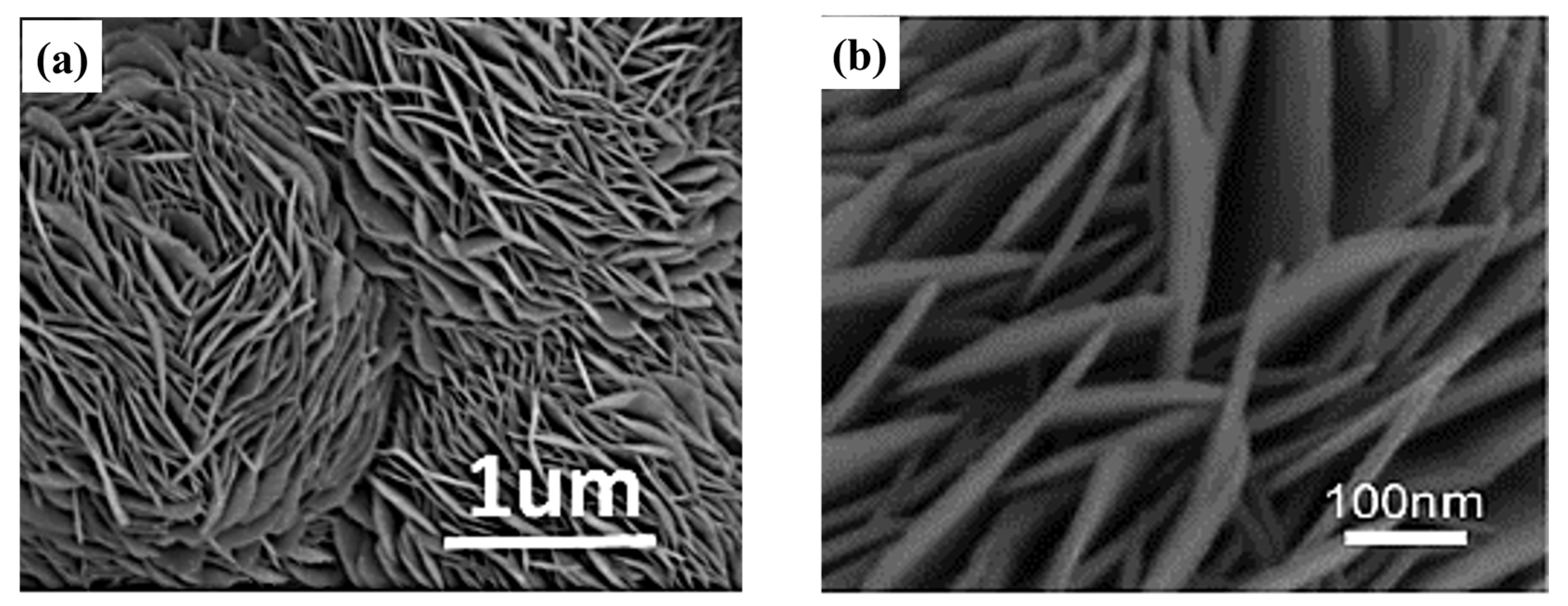
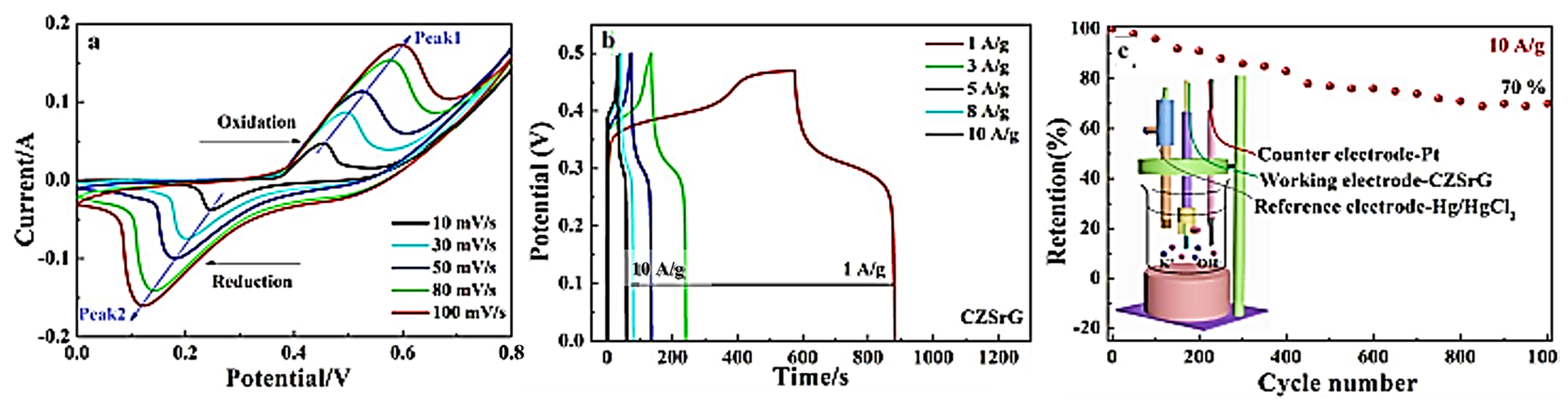
| NO. | Electrode Material | Measurement Type | Operating Window (V) | Electrolyte | Energy Storage Performance | Retention Rate | Refs |
|---|---|---|---|---|---|---|---|
| 1 | CuS nanoplatelets | Three-electrode | −0.40~0.30 | 1 M LiClO4 | 72.85 F g−1 (3 A g−1) | - | [35] |
| 2 | CuS nanoflakes | Three-electrode | −0.90~0.20 | 3 M KOH | 436.5 mF cm−2 (10 mA cm−2) | 75.1% after 5000 cycles | [36] |
| 3 | CuS nanowire array | Three-electrode | 0.00~0.50 | 1 M NaOH | 305 F g−1 (0.6 mA cm−2) | 87% after 5000 cycles | [37] |
| 4 | Flower-like CuS | Three-electrode | −1.10~0.40 | 2 M KOH | 597 F g−1 (1 A g−1) | 80% after 1000 cycles | [38] |
| 5 | CuS nanoparticles | Three-electrode | −0.20~0.70 | 1 M NaOH | 101.34 F g−1 (1.5 mA cm−2) | 81% after 1000 cycles | [39] |
| 6 | CuS | Three-electrode | 0.00~0.50 | 2 M KOH | 237 F g−1 (0.5 A g−1) | 88% after 4000 cycles | [40] |
| 7 | CuS nanosheets | Three-electrode | −0.40~0.60 | 6 M KOH | 833.3 F g−1 (1 A g−1) | 75.4% after 500 cycles | [29] |
| 8 | Three-dimensional CuS nanowalls | Three-electrode | −0.30~0.60 | 2 M KOH | 1124 F g−1 (15 mA cm−2) | 90.7% after 2000 cycles | [41] |
| 9 | CuS spherical clusters | Three-electrode | 0.00~0.50 | 3 M KOH | 713 F g−1 (1 A g−1) | 73% after 1000 cycles | [42] |
| 10 | Nanostructured CuS networks | Three-electrode | 0.00~0.50 | 2 M KOH | 49.8 mAh g−1 (1 A g−1) | 80.5% after 1500 cycles | [43] |
| 11 | Three-dimensional CuS microflower | Three-electrode | 0.00~0.50 | 2 M NaOH | 438.0 F g−1 (3 mA cm−2) | 87% after 2000 cycles | [44] |
| 12 | core–shell CuS@CQDs | Three-electrode | −0.10~0.50 | 6 M KOH | 618 F g−1 (1 A g−1) | 95% after 4000 cycles | [45] |
| 13 | CuS | Three-electrode | 0.00~0.45 | 2 M KOH | 718.48 F g−1 (2 A g−1) | 89.2% after 3000 cycles | [46] |
| 14 | CuS | Three-electrode | −0.20~0.50 | 2 M KOH | 298 F g−1 (2 A g−1) | 100% after 2000 cycles | [47] |
| 15 | CuS thin films | Three-electrode | −0.40~0.80 | 1 M NaOH | 132 F g−1 (50 mA cm−2) | - | [48] |
| 16 | CuS thin films | Two-electrode | −1.50~1.00 | 1 M NaOH | 102 F g−1 (10 mV s−1) | - | [49] |
| 17 | CuS nanoparticles | Three-electrode | −0.20~0.60 | 3 M KOH | 164.053 mAh g−1 (1 A g−1) | 97.12% after 4000 cycles | [50] |
| 18 | CuS@CQDs | Three-electrode | −0.10~0.50 | 6 M KOH | 920.5 F g−1 (0.5 A g−1) | 92.8% after 10,000 cycles | [51] |
| 19 | Hexagonal CuS | Three-electrode | −0.10~0.40 | 6 M KOH | 1123 F g−1 (1 A g−1) | 87% after 4000 cycles | [52] |
| NO. | Electrode Material | Measurement Type | Operating Window (V) | Electrolyte | Energy Storage Performance | Retention Rate | Refs. |
|---|---|---|---|---|---|---|---|
| 1 | CuCo2S4/ rGO | Three-electrode | 0.01~0.60 | 3 M KOH | 525 F g−1 (1 A g−1) | 83% after 1000 cycles | [85] |
| 2 | CuCo2S4/ CNT/GO | Three-electrode | 0.00~0.40 | 6 M KOH | 504 F g−1 (10 A g−1) | 92.3% after 2000 cycles | [86] |
| 3 | CuCo2S4 hollow nanoneedle arrays | Three-electrode | −0.10~0.50 | 3 M KOH | 2163 F g−1 (6 mA cm−2) | 98.7% after 6000 cycles | [87] |
| 4 | CuCo2S4 | Three-electrode | 0.00~0.45 | 4 M KOH | 516 F g−1 (10 A g−1) | 66% after 10,000 cycles | [88] |
| 5 | CuCo2S4 | Three-electrode | 0.00~0.50 | 3 M KOH | 3321.6 F g−1 (5 A g−1) | 87.1% after 3000 cycles | [89] |
| 6 | CuCo2S4 | Three-electrode | 0.00~0.45 | 6 M KOH | 1839 F g−1 (5 A g−1) | 85.3% after 2000 cycles | [90] |
| 7 | CuCo2S4/ CNT | Three-electrode | 0.00~0.45 | 1 M KOH | 1690.3 F g−1 (1 A g−1) | 95.5% after 10,000 cycles | [91] |
| 8 | Cu2MoS4 | Three-electrode | −0.80~0.20 | 1 M Na2SO4 | 127 F g−1 (1.5 mA cm−2) | 91.78% after 3000 cycles | [92] |
| 9 | CuCo2S4 | Three-electrode | 0.00~0.45 | 6 M KOH | 2446.6 F g−1 (1 A g−1) | 82% after 10,000 cycles | [93] |
| 10 | CuCo2S4 | Three-electrode | 0.00~0.45 | 1 M KOH | 592 F g−1 (1 A g−1) | 90.4% after 3000 cycles | [94] |
| 11 | CuCo2S4 | Three-electrode | −0.25~0.40 | Polysulfide electrolyte | 5030 F g−1 (20 A g−1) | 79.5% after 2000 cycles | [95] |
| NO. | Electrode Material | Measurement Type | Operating Window (V) | Electrolyte | Energy Storage Performance | Retention Rate | Refs |
|---|---|---|---|---|---|---|---|
| 1 | CuS/rGO/ Ni3S2 | Three-electrode | −0.10~0.45 | 6 M KOH | 1692.7 F g−1 (6.5 A g−1) | 91.5% after 4000 cycles | [103] |
| 2 | Ni3S4/CuS | Three-electrode | 0.00~0.45 | 6 M KOH | 1917 F g−1 (1 A g−1) | 91.2% after 3000 cycles | [104] |
| 3 | 3D/2D Cu2Se /CuS | Three-electrode | 0.00~0.40 | 6 M KOH | 2727 F g−1 (2.5 mA cm−2) | 70.2% after 8000 cycles | [105] |
| 4 | CuxSy/CoSz/GO | Three-electrode | 0.00~0.55 | 2 M KOH | 324 F g−1 (1 A g−1) | - | [106] |
| 5 | Ni3S4/CuS | Three-electrode | 0.00~0.60 | 2 M KOH | 888 F g−1 (1 A g−1) | 83.33% after 2000 cycles | [107] |
| 6 | CuS/ZrO2- | Three-electrode | 0.00~0.50 | 2 M KOH | 949.47 F g−1 (5 mV s−1) | - | [108] |
| NO. | Electrode Material | Measurement Type | Operating Window (V) | Electrolyte | Energy Storage Performance | Retention Rate | Refs |
|---|---|---|---|---|---|---|---|
| 1 | CuS/rGO | Three-electrode | −0.90~0.10 | 2 M KOH | 368.3 F g−1 (1 A g−1) | 88.4% after 1000 cycles | [110] |
| 2 | CuS/GO | Two-electrode | 0.00~1.00 | 3 M KOH | 197.45 F g−1 (5 mV s−1) | 90.35% after 1000 cycles | [111] |
| 3 | CuS/rGO | Three-electrode | 0.00~0.40 | 6 M KOH | 587.5 F g−1 (1 A g−1) | 95% after 2000 cycles | [112] |
| 4 | CuS/rGO | Three-electrode | 0.00~0.50 | 3 M KOH | 1604 F g−1 (2 A g−1) | 97% after 5000 cycles | [113] |
| 5 | Cu2S/rGO | Three-electrode | −1.00~0.00 | 1 M KOH | 1293 F g−1 (1 A g−1) | 94% after 10,000 cycles | [114] |
| 6 | CuS/rGO | Three-electrode | −1.10~−0.20 | 1 M LiClO4 | 1201.8 F g−1 (5 mV s−1) | 98% after 3000 cycles | [115] |
| 7 | CuS/rGO | Three-electrode | −0.20~0.40 | 6 M KOH | 2317.8 F g−1 (1 A g−1) | 96.2% after 1200 cycles | [116] |
| 8 | CuS@CQDs-GOH | Three-electrode | −0.10~0.50 | 6 M KOH | 920 F g−1 (1 A g−1) | 90% after 5000 cycles | [117] |
| 9 | CuS/GO | Three-electrode | 0.00~0.58 | 3 M KOH | 249 F g−1 (4 A g−1) | 95% after 5000 cycles | [118] |
| 10 | CuS/rGO | Three-electrode | 0.00~0.55 | 3 M KOH | 203 F g−1 (0.5 A g−1) | 90.8% after 10,000 cycles | [119] |
| 11 | CuS/CN | Three-electrode | −0.80~1.00 | 0.1 M Li2SO4 | 379 F g−1 (1 A g−1) | 72.46% after 500 cycles | [120] |
| 12 | CuS/GO | Three-electrode | −0.80~−0.15 | 6 M KOH | 497.8 F g−1 (0.2 A g−1) | 91.2% after 2000 cycles | [121] |
| 13 | CuS/rGO | Two-electrode | 0.00~1.00 | 6 M KOH | 906 F g−1 (1 A g−1) | 89% after 5000 cycles | [122] |
| 14 | CuS/rGO | Three-electrode | −0.20~0.60 | 2 M KOH | 1222.5 F g−1 (1 A g−1) | 91.2% after 2000 cycles | [123] |
| 15 | CuS/GO | Three-electrode | 0.00~0.60 | 3 M KOH | 250 F g−1 (0.5 A g−1) | 70% after 5000 cycles | [124] |
| 16 | CuS/rGO | Three-electrode | −1.00~0.00 | 2 M KOH | 3058 F g−1 (1 A g−1) | 60.3% after 1000 cycles | [125] |
| 17 | Cu2S/rGO | Three-electrode | −0.20~−0.45 | 3 M KOH | 1918.6 F g−1 (1 A g−1) | 95.4% after 5000 cycles | [126] |
| NO. | Electrode Material | Measurement Type | Operating Window (V) | Electrolyte | Energy Storage Performance | Retention Rate | Refs |
|---|---|---|---|---|---|---|---|
| 1 | CuS/CNTs | Three-electrode | 0.00~0.50 | 3 M KOH | 736.1 F g−1 (1 A g−l) | 92% after 5000 cycles | [127] |
| 2 | CuS/CNTs | Three-electrode | 0.00~0.60 | 6 M KOH | 467.02 F g−1 (0.5 A g−1) | 86% after 5000 cycles | [128] |
| 3 | CuS/CNT | Three-electrode | 0.00~0.50 | 2 M KOH | 122 F g−1 (1.2 A g−1) | 100% after 1000 cycles | [129] |
| 4 | CuS/CNTs | Three-electrode | −0.40~0.60 | 6 M KOH | 2831 F g−1 (1 A g−1) | 90% after 600 cycles | [130] |
| 5 | 3D-CuS/CNTs | Two-electrode | 0.00~0.60 | 2 M KOH | 2204 F g−1 (10 mA cm−2) | 89% after 10,000 cycles | [131] |
| 6 | CuS@CNT | Three-electrode | 0.00~1.00 | 2 M KOH | 1.51 F cm−2 (1.2 A g−1) | 92% after 1000 cycles | [132] |
| 7 | CuS/CNTs | Three-electrode | −0.20~0.60 | 2 M KOH | 566.4 F g−1 (1 A g−l) | 94.5% after 5000 cycles | [133] |
| NO. | Electrode Material | Measurement Type | Operating Window (V) | Electrolyte | Energy Storage Performance | Retention Rate | Refs |
|---|---|---|---|---|---|---|---|
| 1 | Cu1.96S/C | Two-electrode | 0.00~0.90 | 1 M KOH | 200 F g−1 (0.5 A g−1) | 80% after 3000 cycles | [143] |
| 2 | CuS/CNTs | Three-electrode | 0.00~0.50 | 6 M KOH | 606.7 F g−1 (1 A g−1) | 87.0% after 6000 cycles | [144] |
| 3 | Cu1.8S/C | Two-electrode | 1.00~3.00 | 1 M LiPF6 | 740 mAh g−1 (50 mA g−1) | 78% after 200 cycles | [23] |
| 4 | Carbon-coated Cu7S4 | Three-electrode | −0.20~0.70 | 1 M H2SO4 | 321.9 F g−1 (0.5 A g−1) | 78.1% after 3000 cycles | [22] |
| 5 | Cu9S8@C-CC@PPy | Three-electrode | −0.40~0.50 | 1 M KCl | 270.72 F g−1 (10 mV s−1) | 83.36% after 3000 cycles | [145] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, J.; Jiang, H.; Guo, P.; Li, J.; Zhu, H.; Fan, X.; Huang, L.; Sun, J.; Wang, Y. Application of Copper–Sulfur Compound Electrode Materials in Supercapacitors. Molecules 2024, 29, 977. https://doi.org/10.3390/molecules29050977
Lu J, Jiang H, Guo P, Li J, Zhu H, Fan X, Huang L, Sun J, Wang Y. Application of Copper–Sulfur Compound Electrode Materials in Supercapacitors. Molecules. 2024; 29(5):977. https://doi.org/10.3390/molecules29050977
Chicago/Turabian StyleLu, Junhua, Hedong Jiang, Pingchun Guo, Jiake Li, Hua Zhu, Xueyun Fan, Liqun Huang, Jian Sun, and Yanxiang Wang. 2024. "Application of Copper–Sulfur Compound Electrode Materials in Supercapacitors" Molecules 29, no. 5: 977. https://doi.org/10.3390/molecules29050977
APA StyleLu, J., Jiang, H., Guo, P., Li, J., Zhu, H., Fan, X., Huang, L., Sun, J., & Wang, Y. (2024). Application of Copper–Sulfur Compound Electrode Materials in Supercapacitors. Molecules, 29(5), 977. https://doi.org/10.3390/molecules29050977





