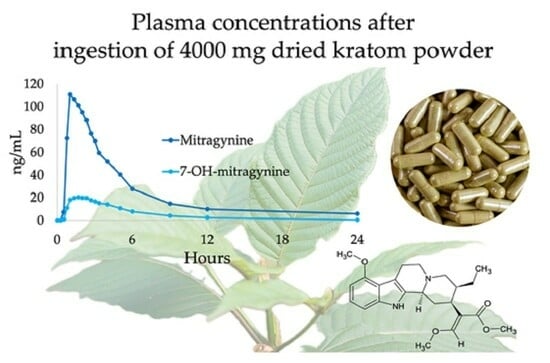
Human Mitragynine and 7-Hydroxymitragynine Pharmacokinetics after Single and Multiple Daily Doses of Oral Encapsulated Dried Kratom Leaf Powder
Abstract
:1. Introduction
2. Results
2.1. Participants
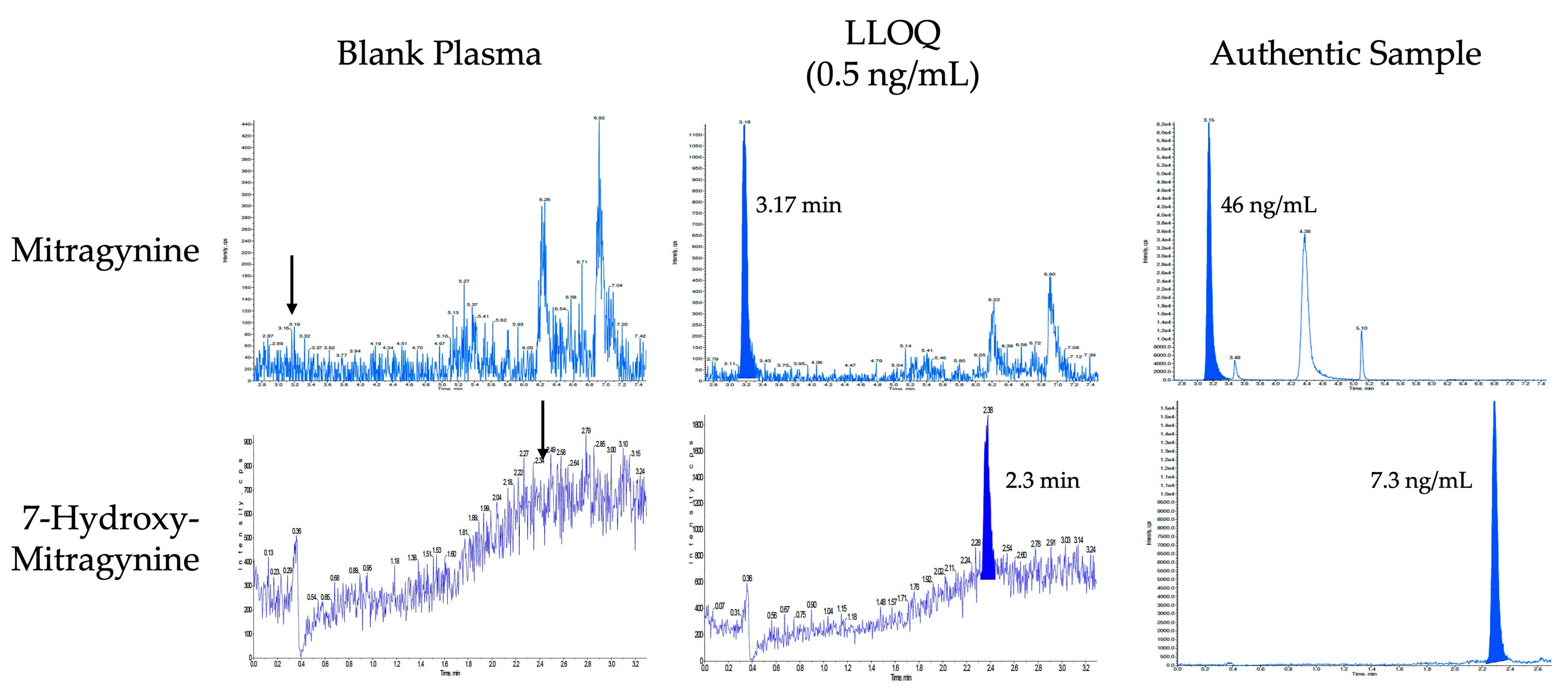
2.2. Bioanalysis
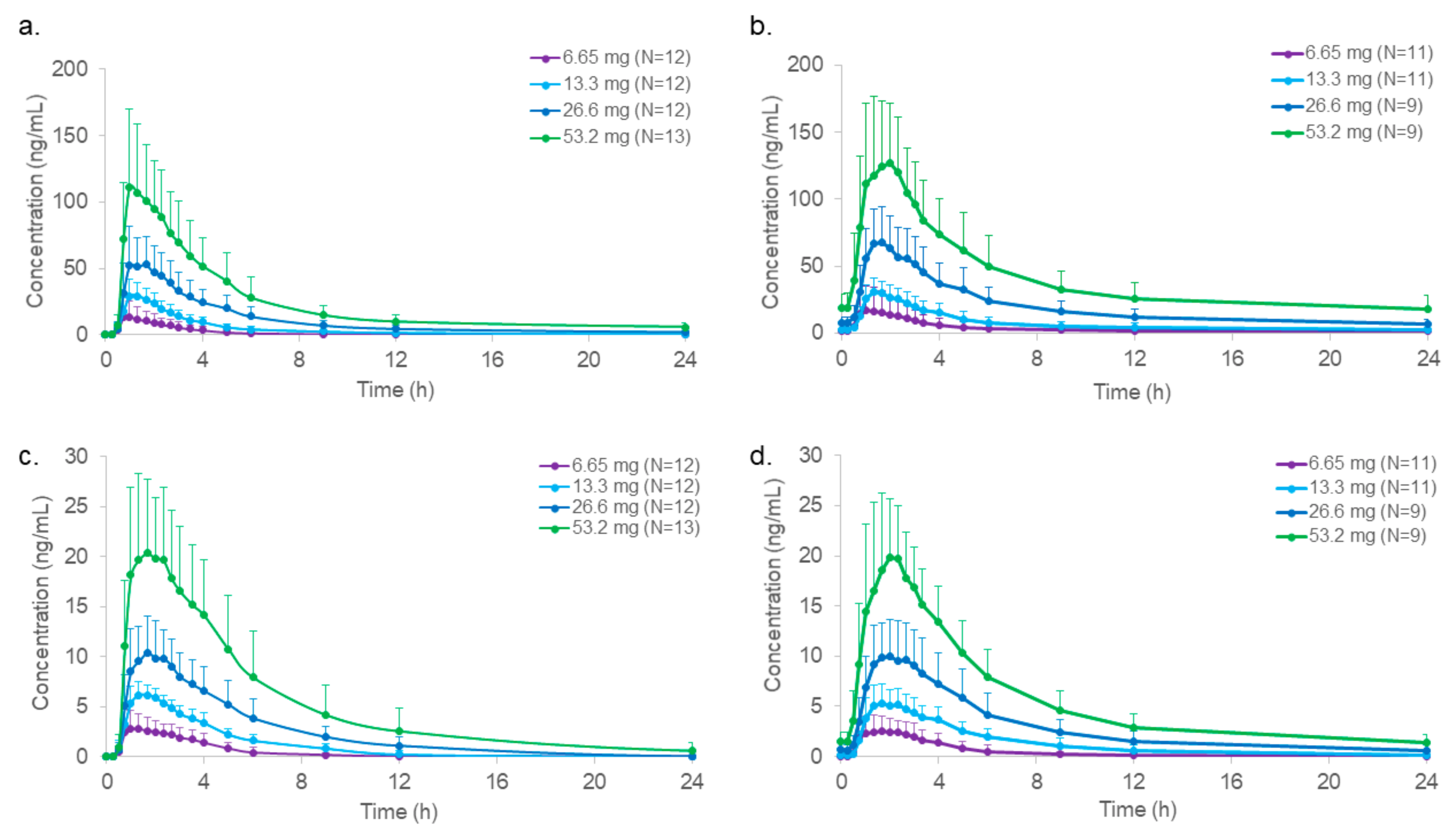
2.3. Pharmacokinetics
2.4. Ratios of 7-Hydroxymitragynine/Mitragynine Plasma Concentrations
2.5. Accumulation of Mitragynine and 7-Hydroxymitragynine during MD
2.6. Time to Reach Steady State for Mitragynine and 7-Hydroxymitragynine during Multiple Dosing
3. Discussion
4. Materials and Methods
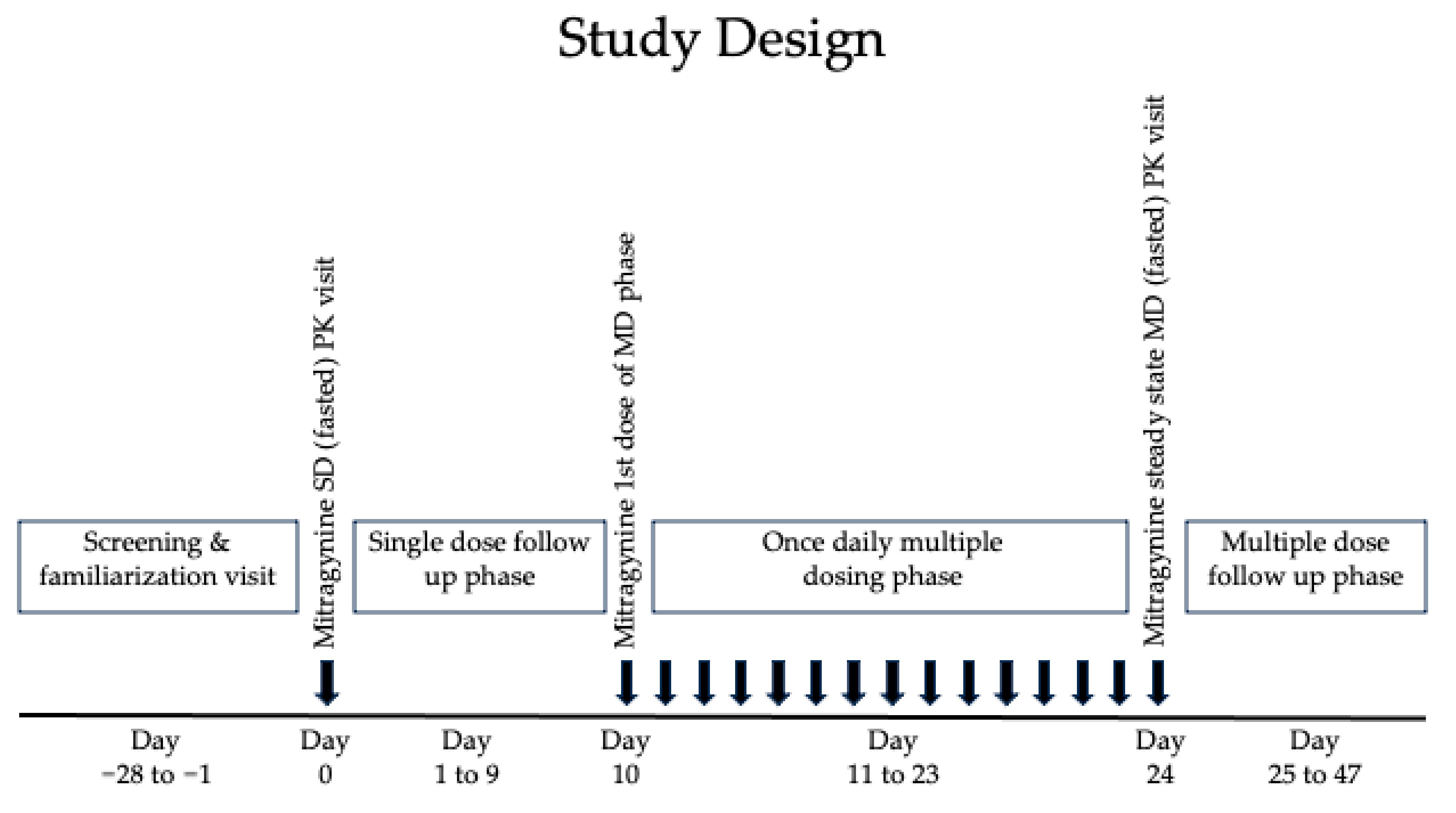
4.1. Study Design
4.2. Study Population
4.3. Dosing
4.4. Blood Collection Timeline for Single and Multiple Dose Pharmacokinetic Study
4.5. Pharmacokinetics
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, P.N.; Lund, J.A.; Murch, S.J. A botanical, phytochemical and ethnomedicinal review of the genus Mitragyna korth: Implications for products sold as kratom. J. Ethnopharmacol. 2017, 202, 302–325. [Google Scholar] [CrossRef]
- Grundmann, O. Patterns of Kratom use and health impact in the US-Results from an online survey. Drug Alcohol Depend. 2017, 176, 63–70. [Google Scholar] [CrossRef]
- Covvey, J.R.; Vogel, S.M.; Peckham, A.M.; Evoy, K.E. Prevalence and characteristics of self-reported kratom use in a representative US general population sample. J. Addict. Dis. 2020, 38, 506–513. [Google Scholar] [CrossRef]
- Williams, R.S.; Nikitin, D. The internet market for Kratom, an opioid alternative and variably legal recreational drug. Int. J. Drug Policy 2020, 78, 102715. [Google Scholar] [CrossRef]
- Schimmel, J.; Amioka, E.; Rockhill, K.; Haynes, C.M.; Black, J.C.; Dart, R.C.; Iwanicki, J.L. Prevalence and description of kratom (Mitragyna speciosa) use in the United States: A cross-sectional study. Addiction 2021, 116, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Laforest, L.C.; Kuntz, M.A.; Kanumuri, S.R.R.; Mukhopadhyay, S.; Sharma, A.; O’Connor, S.E.; McCurdy, C.R.; Nadakuduti, S.S. Metabolite and Molecular Characterization of Mitragyna speciosa Identifies Developmental and Genotypic Effects on Monoterpene Indole and Oxindole Alkaloid Composition. J. Nat. Prod. 2023, 86, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- Shellard, E.J. The alkaloids of Mitragyna with special reference to those of Mitragyna speciosa, Korth. Bull. Narc. 1974, 26, 41–55. [Google Scholar] [PubMed]
- Eisenman, S.W. The Botany of Mitragyna speciosa (Korth.) Havil. and Related Species. In Kratom and Other Mitragynines: The Chemistry and Pharmacology of Opioids from a Non-Opium Source, 1st ed.; Raffa, R.B., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 57–76. [Google Scholar]
- Leksungnoen, N.; Andriyas, T.; Ngernsaengsaruay, C.; Uthairatsamee, S.; Racharak, P.; Sonjaroon, W.; Kjelgren, R.; Pearson, B.J.; McCurdy, C.R.; Sharma, A. Variations in mitragynine content in the naturally growing Kratom (Mitragyna speciosa) population of Thailand. Front. Plant Sci. 2022, 13, 1028547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Sharma, A.; León, F.; Avery, B.; Kjelgren, R.; McCurdy, C.R.; Pearson, B.J. Effects of Nutrient Fertility on Growth and Alkaloidal Content in Mitragyna speciosa (Kratom). Front. Plant Sci. 2020, 11, 597696. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Sharma, A.; León, F.; Avery, B.; Kjelgren, R.; McCurdy, C.R.; Pearson, B.J. Plant growth and phytoactive alkaloid synthesis in kratom [Mitragyna speciosa (Korth.)] in response to varying radiance. PLoS ONE 2022, 17, e0259326. [Google Scholar] [CrossRef] [PubMed]
- Kamble, S.H.; Berthold, E.C.; Kanumuri, S.R.R.; King, T.I.; Kuntz, M.A.; Leon, F.; Mottinelli, M.; McMahon, L.R.; McCurdy, C.R.; Sharma, A. Metabolism of Speciociliatine, an Overlooked Kratom Alkaloid for its Potential Pharmacological Effects. AAPS J. 2022, 24, 86. [Google Scholar] [CrossRef]
- Ismail, I.; Wahab, S.; Sidi, H.; Das, S.; Lin, L.J.; Razali, R. Kratom and Future Treatment for the Opioid Addiction and Chronic Pain: Periculo Beneficium? Curr. Drug Targets 2019, 20, 166–172. [Google Scholar] [CrossRef]
- Macko, E.; Weisbach, J.A.; Douglas, B. Some observations on the pharmacology of mitragynine. Arch. Int. Pharmacodyn. Ther. 1972, 198, 145–161. [Google Scholar]
- Prozialeck, W.C.; Avery, B.A.; Boyer, E.W.; Grundmann, O.; Henningfield, J.E.; Kruegel, A.C.; McMahon, L.R.; McCurdy, C.R.; Swogger, M.T.; Veltri, C.A.; et al. Kratom policy: The challenge of balancing therapeutic potential with public safety. Int. J. Drug Policy 2019, 70, 70–77. [Google Scholar] [CrossRef]
- Matsumoto, K.; Hatori, Y.; Murayama, T.; Tashima, K.; Wongseripipatana, S.; Misawa, K.; Kitajima, M.; Takayama, H.; Horie, S. Involvement of mu-opioid receptors in antinociception and inhibition of gastrointestinal transit induced by 7-hydroxymitragynine, isolated from Thai herbal medicine Mitragyna speciosa. Eur. J. Pharmacol. 2006, 549, 63–70. [Google Scholar] [CrossRef]
- Kruegel, A.C.; Gassaway, M.M.; Kapoor, A.; Váradi, A.; Majumdar, S.; Filizola, M.; Javitch, J.A.; Sames, D. Synthetic and Receptor Signaling Explorations of the Mitragyna Alkaloids: Mitragynine as an Atypical Molecular Framework for Opioid Receptor Modulators. J. Am. Chem. Soc. 2016, 138, 6754–6764. [Google Scholar] [CrossRef]
- Stolt, A.C.; Schröder, H.; Neurath, H.; Grecksch, G.; Höllt, V.; Meyer, M.R.; Maurer, H.H.; Ziebolz, N.; Havemann-Reinecke, U.; Becker, A. Behavioral and neurochemical characterization of kratom (Mitragyna speciosa) extract. Psychopharmacology 2014, 231, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Váradi, A.; Marrone, G.F.; Palmer, T.C.; Narayan, A.; Szabó, M.R.; Le Rouzic, V.; Grinnell, S.G.; Subrath, J.J.; Warner, E.; Kalra, S.; et al. Mitragynine/Corynantheidine Pseudoindoxyls As Opioid Analgesics with Mu Agonism and Delta Antagonism, Which Do Not Recruit beta-Arrestin-2. J. Med. Chem. 2016, 59, 8381–8397. [Google Scholar] [CrossRef] [PubMed]
- Eastlack, S.C.; Cornett, E.M.; Kaye, A.D. Kratom-Pharmacology, Clinical Implications, and Outlook: A Comprehensive Review. Pain Ther. 2020, 9, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Prozialeck, W.C.; Jivan, J.K.; Andurkar, S.V. Pharmacology of kratom: An emerging botanical agent with stimulant, analgesic and opioid-like effects. J. Am. Osteopath. Assoc. 2012, 112, 792–799. [Google Scholar] [PubMed]
- Obeng, S.; Kamble, S.H.; Reeves, M.E.; Restrepo, L.F.; Patel, A.; Behnke, M.; Chear, N.J.; Ramanathan, S.; Sharma, A.; Leon, F.; et al. Investigation of the Adrenergic and Opioid Binding Affinities, Metabolic Stability, Plasma Protein Binding Properties, and Functional Effects of Selected Indole-Based Kratom Alkaloids. J. Med. Chem. 2020, 63, 433–439. [Google Scholar] [CrossRef]
- Henningfield, J.E.; Rodricks, J.V.; Magnuson, A.M.; Huestis, M.A. Respiratory effects of oral mitragynine and oxycodone in a rodent model. Psychopharmacology 2022, 239, 3793–3804. [Google Scholar] [CrossRef] [PubMed]
- National Institute on Drug Abuse. Kratom. Available online: https://nida.nih.gov/research-topics/kratom (accessed on 5 October 2023).
- Trakulsrichai, S.; Sathirakul, K.; Auparakkitanon, S.; Krongvorakul, J.; Sueajai, J.; Noumjad, N.; Sukasem, C.; Wananukul, W. Pharmacokinetics of mitragynine in man. Drug Des. Devel. Ther. 2015, 9, 2421–2429. [Google Scholar] [CrossRef]
- Tanna, R.S.; Nguyen, J.T.; Hadi, D.L.; Manwill, P.K.; Flores-Bocanegra, L.; Layton, M.E.; White, J.R.; Cech, N.B.; Oberlies, N.H.; Rettie, A.E.; et al. Clinical Pharmacokinetic Assessment of Kratom (Mitragyna speciosa), a Botanical Product with Opioid-like Effects, in Healthy Adult Participants. Pharmaceutics 2022, 14, 620. [Google Scholar] [CrossRef] [PubMed]
- Tanna, R.S.; Cech, N.B.; Oberlies, N.H.; Rettie, A.E.; Thummel, K.E.; Paine, M.F. Translating Kratom-Drug Interactions: From Bedside to Bench and Back. Drug Metab. Dispos. 2023, 51, 923–935. [Google Scholar] [CrossRef]
- Sahin, S.; Benet, L.Z. The operational multiple dosing half-life: A key to defining drug accumulation in patients and to designing extended release dosage forms. Pharm. Res. 2008, 25, 2869–2877. [Google Scholar] [CrossRef]
- Kamble, S.H.; Sharma, A.; King, T.I.; León, F.; McCurdy, C.R.; Avery, B.A. Metabolite profiling and identification of enzymes responsible for the metabolism of mitragynine, the major alkaloid of Mitragyna speciosa (kratom). Xenobiotica 2019, 49, 1279–1288. [Google Scholar] [CrossRef]
- Court, M.H. Canine cytochrome P-450 pharmacogenetics. Vet. Clin. N. Am. Small Anim. Pract. 2013, 43, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, E.A.; King, T.I.; Kamble, S.H.; Raju, K.S.R.; Berthold, E.C.; Leon, F.; Avery, B.A.; McMahon, L.R.; McCurdy, C.R.; Sharma, A. Pharmacokinetics and Safety of Mitragynine in Beagle Dogs. Planta Med. 2020, 86, 1278–1285. [Google Scholar] [CrossRef] [PubMed]
- Center for Drug Evaluation and Research. FDA Bioavailability Guideline: Bioavailability Studies Submitted in NDAs or INDs—General Considerations Guidance for Industry. Available online: https://www.fda.gov/media/121311/download (accessed on 16 November 2023).



| Mitragynine | 6.65 mg | 13.3 mg | 26.6 mg | 53.2 mg | ||||
|---|---|---|---|---|---|---|---|---|
| Dried Kratom Leaf Powder | 500 mg | 1000 mg | 2000 mg | 4000 mg | ||||
| SD | MD | SD | MD | SD | MD | SD | MD | |
| # Subjects | 12 | 11 | 12 | 8 | 12 | 9 | 13 | 9 |
| Gender | 6 F 6 M | 6 F 5 M | 4 F 8 M | 3 F 5 M | 4 F 8 M | 3 F 6 M | 7 F 6 M | 4 F 5 M |
| Race | ||||||||
| AI | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 |
| Asian | 2 | 2 | 5 | 3 | 1 | 0 | 0 | 0 |
| Black | 0 | 0 | 0 | 0 | 4 | 2 | 1 | 1 |
| Unknown | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| White | 10 | 9 | 7 | 5 | 7 | 7 | 9 | 5 |
| Ethnicity | 2 H 10 NH | 2 H 9 NH | 1 H 11 NH | 1 H 7 NH | 0 H 12 NH | 0 H 9 NH | 3 H 10 NH | 3 H 6 NH |
| Mean age (STD) years | 30.8 (9.3) | 31.3 (9.5) | 32.9 (9.4) | 33.1 (9.8) | 35.8 (11.4) | 38.4 (12.2) | 33.2 (9.6) | 32.4 (7.8) |
| BMI Kg/m2 | 23.1 (2.7) | 22.8 (2.6) | 25.3 (2.6) | 25.3 (2.7) | 26.2 (3.4) | 25.7 (3.8) | 23.4 (2.9) | 23.9 (3.1) |
| Validation Parameter | Mitragynine | 7-Hydroxymitragynine |
|---|---|---|
| Linearity | 0.5–500 ng/mL | 0.5–100 ng/mL |
| Lower limit of quantification | 0.5 ng/mL | 0.5 ng/mL |
| Quality control (QC) concentrations | 0.5, 1.5, 250, and 375 ng/mL | 0.5, 1.5, 50, and 75 ng/mL |
| Within-run accuracy (% bias) | −11.7–11.2% | −9.7–13.6% |
| Within-run precision (%CV) | 1.4–10% | 1.2–7.4% |
| Between-run accuracy (% bias) | −0.6–2.2% | 2.4–10.4% |
| Between-run precision (%CV) | 3.9–9.3% | 4.2–10% |
| Internal standard normalized matrix factors for low and high QC | 1.00–1.02 (CV 0.7–2.2%) | 0.995–1.04 (CV 1.0–2.0%) |
| 10-fold dilution integrity %bias (%CV) | −1.7% (3.0%) | −3.0 (2.8%) |
| Plasma stability for low and high QC | ||
| 22 °C for 23 h (% bias) | −0.6–4.4% | 4.4–5.8% |
| −20 °C for 404 days (% bias) | 6.7–9.1% | −1.5–−3.1% |
| −80 °C for 404 days (% bias) | 8.4–8.9% | 9.2–11.6% |
| 4 freeze-thaw cycles (% bias) | 0.4–5.3% | 4.6–10.3% |
| Autosampler stability 4 °C 241 h (% bias) | −2.9–0.1% | −2.9–−3.8% |
| Cohort 1 6.65 mg | Cohort 2 13.3 mg | Cohort 3 26.6 mg | Cohort 4 53.2 mg | ||
|---|---|---|---|---|---|
| Single dose | n = 12 | n = 12 | n = 12 | n = 13 | |
| Cmax (ng/mL) | mean (STD) | 17.1 (15.2) | 32.1 (12.2) | 57.0 (25.1) | 125 (51.8) |
| median | 12.1 | 29.0 | 57.4 | 130 | |
| (range) | (1.8–61.5) | (16.0–58.1) | (20.4–90.0) | (34.2–204) | |
| Tmax (h) | median | 1.0 | 1.3 | 1.3 | 1.3 |
| (range) | (0.8–2.7) | (0.8–1.7) | (0.8–5.0) | (0.8–2.0) | |
| Tlast (h) | median | 12.0 | 23.4 | 71.6 | 120 |
| (range) | (4.0–71.0) | (12.0–72.2) | (0.8–120) | (23.7–169) | |
| AUC0-24 (h*ng/mL) | mean (STD) | 41.4 (32.4) | 108 (40.6) | 244 (121) | 558 (250) |
| median | 30.6 | 101 | 280 | 648 | |
| (range) | (3.6–111) | (52.8–183) | (3.3–375) | (155–970) | |
| AUC0-Tlast (h*ng/mL) | mean (STD) | 45.8 (42.2) | 120 (57.7) | 305 (164) | 837 (466) |
| median | 30.6 | 101 | 357 | 839 | |
| (range) | (3.6–137) | (52.8–237) | (3.3–480) | (179–1746) | |
| AUC0-inf (h*ng/mL) | mean (STD) | 52.8 (51.1) | 136 (72.3) | 364 1 (154) | 908 (520) |
| median | 34.8 | 108 | 417 1 | 903 | |
| (range) | (4.7–162) | (58.1–288) | (98.4–517) | (191–1951) | |
| AUC0-Tlast/ | median | 0.91 | 0.92 | 0.92 | 0.93 |
| AUC0-inf | (range) | (0.74–0.96) | (0.76–0.95) | (0.87–0.95) | (0.88–0.96) |
| T1/2 (h) | mean (STD) | 8.5 (11.8) | 15.8 (16.3) | 29.3 1 (17.5) | 43.4 (23.3) |
| median | 3.7 | 10.5 | 28.2 1 | 42.9 | |
| (range) | (1.5–35.0) | (2.4–53.9) | (4.4–55.2) | (8.3–84.5) | |
| CL/F (L/h) | mean (STD) | 278 (369) | 123 (58.1) | 102 1 (79.8) | 94.0 (84.1) |
| median | 191 | 123 | 63.7 1 | 58.9 | |
| (range) | (40.9–1424) | (46.2–229) | (51.4–270) | (27.3–278) | |
| Vz/F (L) | mean (STD) | 1349 (808) | 1892 (1013) | 2875 (871) | 3788 (1287) |
| median | 1017 | 1786 | 2544 | 3314 | |
| (range) | (549–2998) | (615–4438) | (1733–4084) | (2104–6173) | |
| Multiple doses | n = 11 | n = 8 | n = 9 | (n = 9) | |
| Cmax,ss (ng/mL) | mean (STD) | 21.4 (18.9) | 33.5 (7.43) | 74.6 (22.8) | 143 (50.4) |
| median | 16.2 | 35.1 | 73.0 | 155 | |
| (range) | (2.8–60.1) | (21.8–43.1) | (42.4–109) | (64.3–215) | |
| Tmax,ss (h) | median | 1.0 | 1.7 | 1.7 | 1.7 |
| (range) | (0.8–2.3) | (1.0–4.0) | (1.0–3.0) | (1.0–3.0) | |
| Tlast,ss (h) | median | 23.9 | 95.4 | 168 | 169 |
| (range) | (4.0–240) | (23.5–239) | (71.5–240) | (72.4–383) | |
| AUC0-tau,ss (h*ng/mL) | mean (STD) | 85.1 (84.1) | 175 (79.8) | 457 (191) | 958 (393) |
| median | 60.1 | 163 | 554 | 1021 | |
| (range) | (5.5–263) | (93.4–354) | (144–655) | (227–1444) | |
| T1/2,ss (h) | mean (STD) | 25.7 2 (24.0) | 44.1 (24.2) | 67.9 3 (20.6) | 51.1 (15.9) |
| median | 14.6 2 | 40.6 | 61.2 3 | 48.6 | |
| (range) | (2.4–64.4) | (8.9–78.5) | (46.6–99.6) | (31.3–83.8) | |
| CL/F (L/h) | mean (STD) | 215 (333) | 87.4 (32.0) | 74.6 (48.0) | 74.7 (61.7) |
| median | 111 | 81.5 | 48.0 | 52.1 | |
| (range) | (25.3–1200) | (37.6–142) | (40.6–185) | (36.8–234) | |
| Vz/F (L) | mean (STD) | 2980 (1606) | 4755 (2072) | 6020 3 (3552) | 4855 (2392) |
| median | 2843 | 4033 | 5139 | 4595 | |
| (range) | (738–5482) | (1825–7872) | (2945–14,135) | (2311–10,634) | |
| Cav,ss (ng/mL) | mean (STD) | 3.6 (3.5) | 7.4 (3.3) | 19.3 (8.1) | 39.9 (16.6) |
| median | 2.5 | 6.9 | 23.4 | 42.9 | |
| (range) | (0.2–11.0) | (4.0–14.8) | (6.2–27.9) | (9.3–60.9) | |
| Ctrough (ng/mL) | mean (STD) | 1.40 (1.79) | 2.74 (1.61) | 7.70 (3.71) | 20.5 (12.2) |
| median | 0.9 | 2.5 | 8.8 | 21.3 | |
| (range) | (0–5.3) | (0.7–5.8) | (2.3–12.3) | (3.4–39.7) | |
| Fluctuation | mean (STD) | 6.54 (2.28) | 4.66 (1.44) | 3.94 (1.41) | 3.53 (1.37) |
| median | 5.6 | 5.0 | 3.4 | 3.3 | |
| (range) | (4.5–11.8) | (2.5–6.8) | (2.2–6.8) | (2.0–6.7) | |
| Cohort 1 6.65 mg | Cohort 2 13.3 mg | Cohort 3 26.6 mg | Cohort 4 53.2 mg | ||
|---|---|---|---|---|---|
| Single dose | n = 12 | n = 12 | n = 12 | n = 13 | |
| Cmax (ng/mL) | mean (STD) | 3.6 (1.9) | 6.6 (1.1) | 10.8 (3.7) | 22.7 (7.7) |
| median | 3.6 | 7.0 | 11.0 | 21.7 | |
| (range) | (1.5–8.6) | (4.8–7.8) | (4.3–16.0) | (12.5–38.6) | |
| Tmax (h) | median | 1.2 | 1.5 | 1.8 | 1.7 |
| (range) | (0.8–2.7) | (1.0–2.3) | (0.8–5.0) | (1.0–2.3) | |
| Tlast (h) | median | 5.0 | 9.0 | 12.0 | 23.3 |
| (range) | (3.5–9.0) | (6.0–12.1) | (0.75–12.0) | (9.0–71.6) | |
| AUC0-24 (h*ng/mL) | mean (STD) | 9.7 (5.4) | 25.8 (6.7) | 47.7 (22.4) | 125 (69.7) |
| median | 9.0 | 25.3 | 54.3 | 115 | |
| (range) | (2.9–19.3) | (14.7–37.6) | (0.7–78.8) | (40.2–319) | |
| AUC0-Tlast (h*ng/mL) | mean (STD) | 9.7 (5.4) | 25.8 (6.7) | 47.7 (22.4) | 130 (84.2) |
| median | 9.0 | 25.3 | 54.3 | 115 | |
| (range) | (2.9–19.3) | (14.7–37.6) | (0.7–78.8) | (40.2–380) | |
| AUC0-inf (h*ng/mL) | mean (STD) | 11.2 (5.6) | 28.8 (7.6) | 58.2 1 (20.4) | 136 (86.6) |
| median | 10.5 | 28.0 | 64.5 1 | 123 | |
| (range) | (4.1–21.0) | (16.4–41.8) | (27.6–85.7) | (41.6–391) | |
| AUCTlast/ | median | 0.85 | 0.90 | 0.91 | 0.96 |
| AUC0-inf | (range) | (0.71–0.92) | (0.87–0.92) | (0.81–0.95) | (0.93–0.97) |
| T1/2 (h) | mean (STD) | 1.7 (0.5) | 2.9 (0.9) | 3.2 1 (0.8) | 4.7 (2.7) |
| median | 1.7 | 2.7 | 3.4 | 4.0 | |
| (range) | (1.0–2.4) | (1.5–4.5) | (1.9–4.5) | (1.7–11.4) | |
| Multiple doses | n = 11 | n = 8 | n = 9 | n = 9 | |
| Cmax,ss (ng/mL) | mean (STD) | 3.2 (1.5) | 5.9 (1.5) | 11.3 (3.5) | 21.8 (6.1) |
| median | 3.3 | 5.6 | 12.0 | 20.9 | |
| (range) | (1.4–5.6) | (4.3–8.1) | (6.9–17.4) | (13.3–31.7) | |
| Tmax,ss (h) | median | 1.3 | 1.7 | 1.7 | 2.00 |
| (range) | (0.8–3.0) | (1.0–4.0) | (1.3–3.0) | (1.3–3.5) | |
| Tlast,ss (h) | median | 5.0 | 12.0 | 23.5 | 48.3 |
| (range) | (3.5–12.0) | (6.0–23.9) | (9.0–47.6) | (9.0–121) | |
| AUC0-tau,ss (h*ng/mL) | mean (STD) | 10.1 (6.8) | 28.0 (13.7) | 67.8 (31.3) | 133 (45.0) |
| median | 7.2 | 27.3 | 67.7 | 128 | |
| (range) | (3.2–22.9) | (13.5–55.7) | (25.0–119) | (105–161) | |
| T1/2,ss (h) | mean (STD) | 2.4 (1.3) | 3.8 (2.3) | 8.3 (5.0) | 24.7 (24.7) |
| median | 1.9 | 3.2 | 9.0 | 9.1 | |
| (range) | (1.1–5.3) | (1.5–8.4) | (2.1–16.7) | (2.2–71.6) | |
| Cave,ss (ng/mL) | mean (STD) | 0.4 (0.3) | 1.2 (0.6) | 2.9 (1.3) | 5.5 (1.9) |
| median | 0.3 | 1.1 | 2.9 | 5.4 | |
| (range) | (0.1–1.0) | (0.6–2.3) | (1.0–5.0) | (1.6–8.2) | |
| Ctrough (ng/mL) | mean (STD) | 0.0 (0.0) | 0.1 (0.3) | 0.6 (0.6) | 1.7 (1.0) |
| median | 0 | 0 | 0.6 | 1.6 | |
| (range) | (0–0) | (0–0.7) | (0–1.3) | (0–3.2) | |
| Fluctuation | mean (STD) | 8.8 (2.2) | 5.6 (2.1) | 4.3 (1.8) | 4.1 (1.7) |
| median | 9.8 | 5.3 | 3.4 | 3.7 | |
| (range) | (5.9–11.3) | (2.7–8.9) | (2.7–6.9) | (2.3–8.2) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huestis, M.A.; Brett, M.A.; Bothmer, J.; Atallah, R. Human Mitragynine and 7-Hydroxymitragynine Pharmacokinetics after Single and Multiple Daily Doses of Oral Encapsulated Dried Kratom Leaf Powder. Molecules 2024, 29, 984. https://doi.org/10.3390/molecules29050984
Huestis MA, Brett MA, Bothmer J, Atallah R. Human Mitragynine and 7-Hydroxymitragynine Pharmacokinetics after Single and Multiple Daily Doses of Oral Encapsulated Dried Kratom Leaf Powder. Molecules. 2024; 29(5):984. https://doi.org/10.3390/molecules29050984
Chicago/Turabian StyleHuestis, Marilyn A., Martin A. Brett, John Bothmer, and Ramsey Atallah. 2024. "Human Mitragynine and 7-Hydroxymitragynine Pharmacokinetics after Single and Multiple Daily Doses of Oral Encapsulated Dried Kratom Leaf Powder" Molecules 29, no. 5: 984. https://doi.org/10.3390/molecules29050984
APA StyleHuestis, M. A., Brett, M. A., Bothmer, J., & Atallah, R. (2024). Human Mitragynine and 7-Hydroxymitragynine Pharmacokinetics after Single and Multiple Daily Doses of Oral Encapsulated Dried Kratom Leaf Powder. Molecules, 29(5), 984. https://doi.org/10.3390/molecules29050984