The Impact of N/O-Functional Groups on the Sorption Capabilities of Activated Carbons Derived from Furfuryl Alcohol
Abstract
:1. Introduction
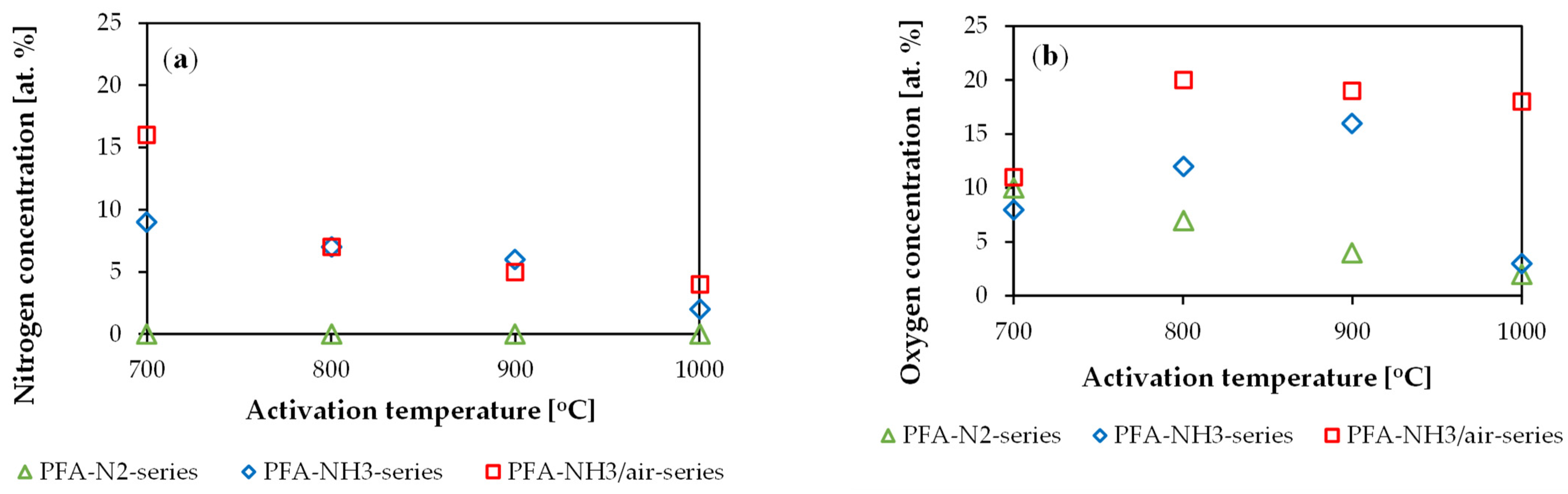
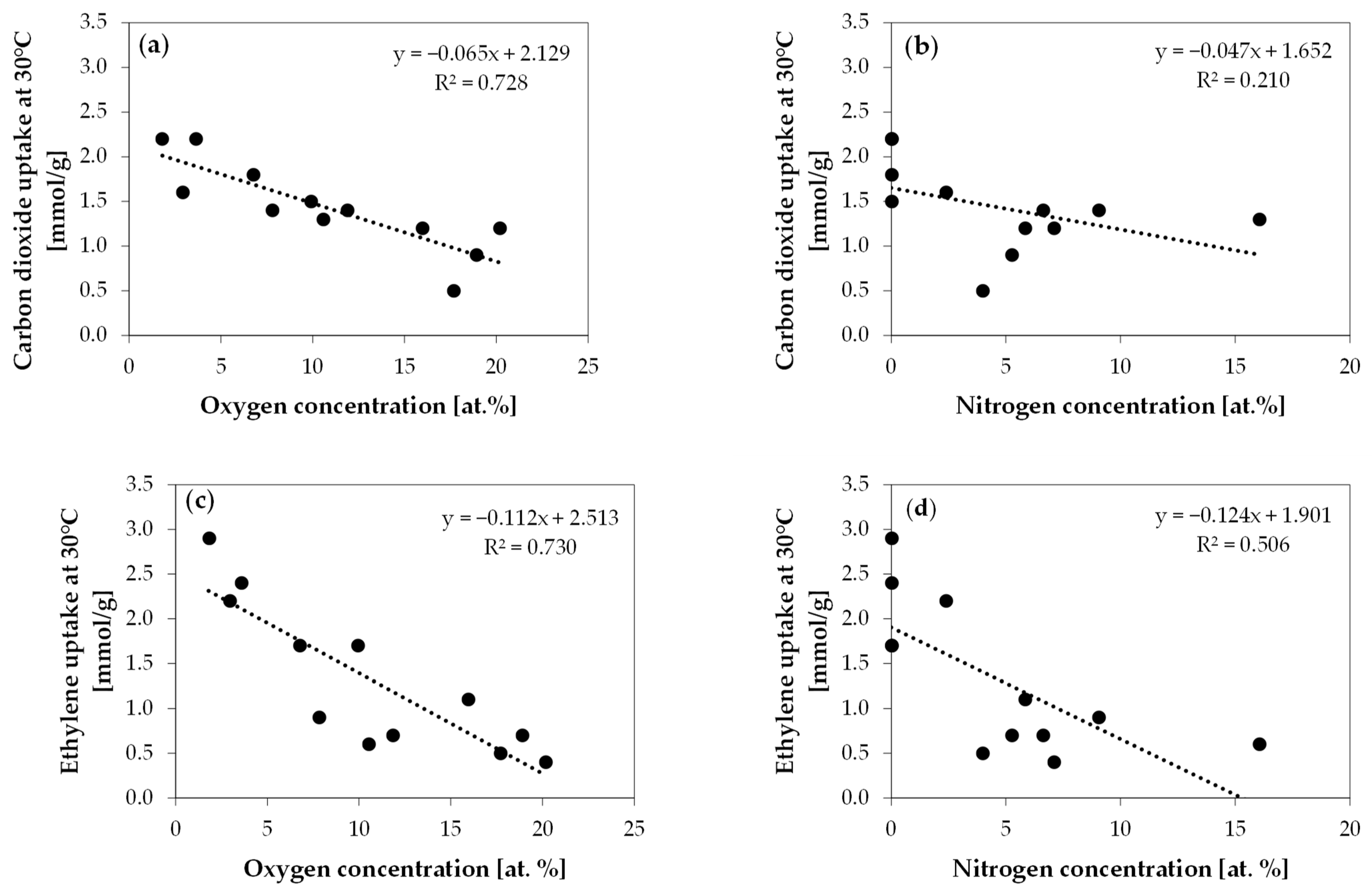
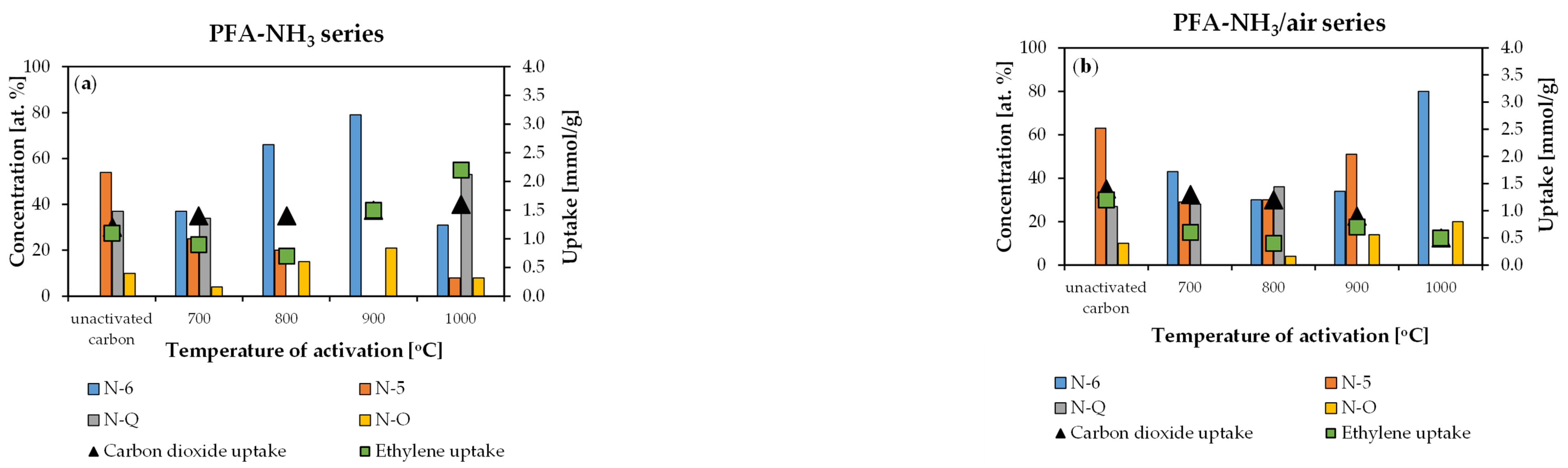
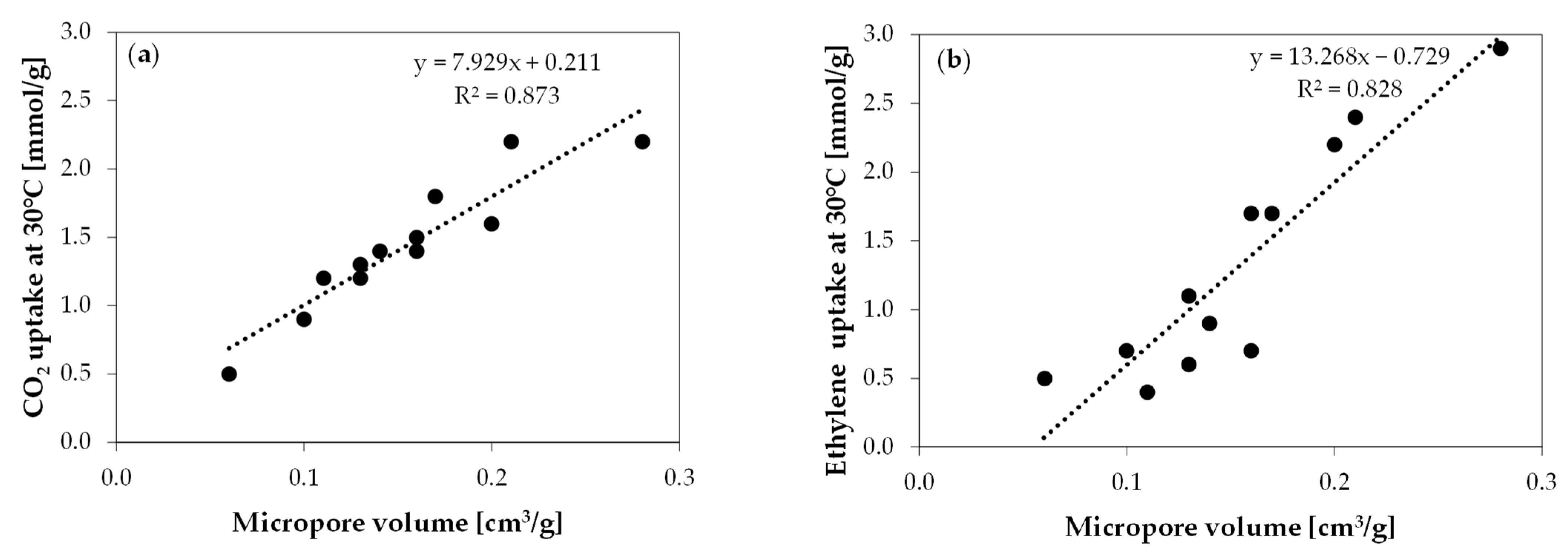
2. Results and Discussion
3. Materials and Methods
3.1. Materials
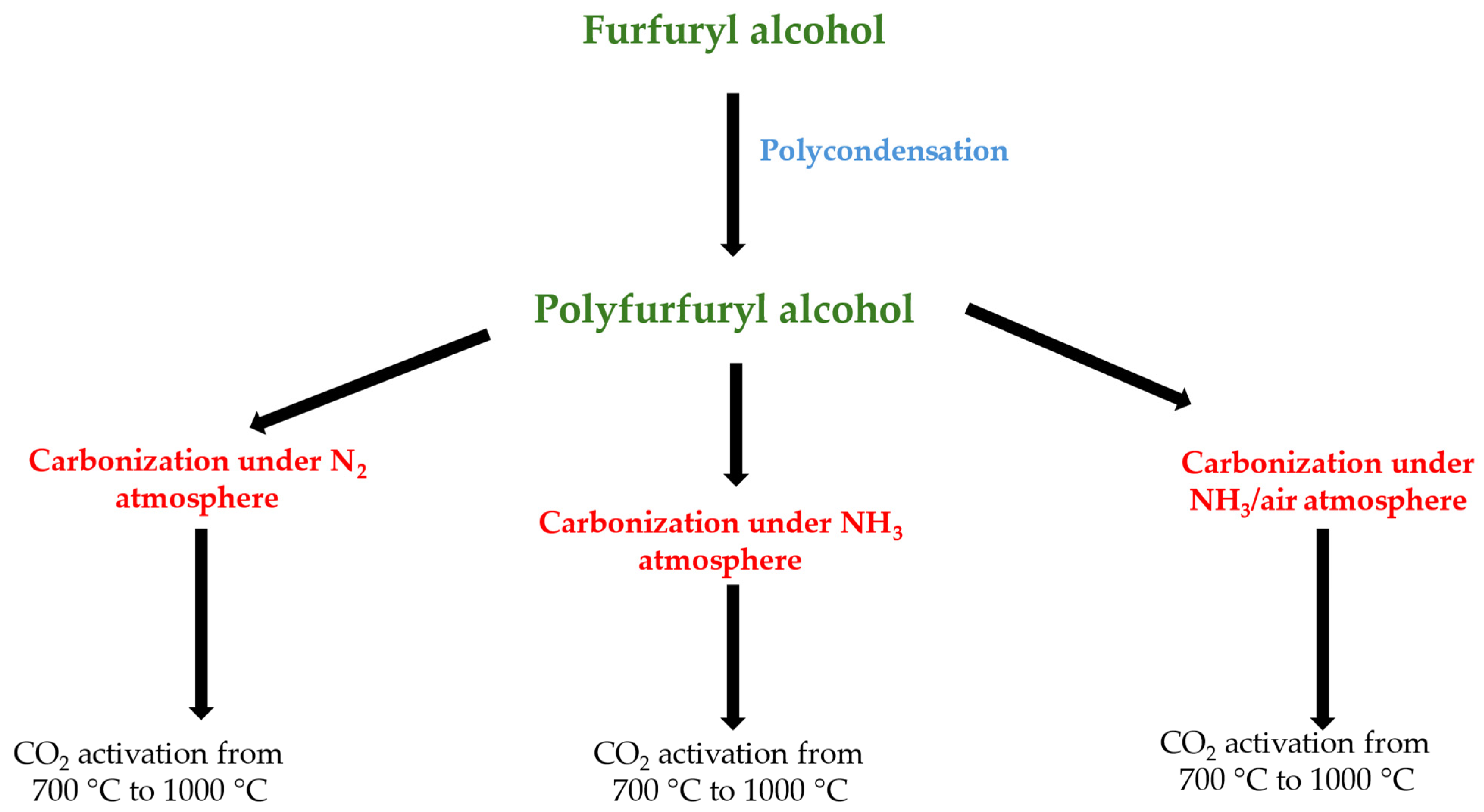
3.1.1. Preparation of Polyfurfuryl Alcohol
3.1.2. Carbonization of Polyfurfuryl Alcohol
3.1.3. Activation Process
3.2. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tlili, N.; Grevillot, G.; Vallieres, C. Carbon Dioxide Capture and Recovery by Means of TSA and/or VSA. Int. J. Greenh. Gas Control. 2009, 3, 519–527. [Google Scholar] [CrossRef]
- Rychtowski, P.; Tryba, B.; Skrzypska, A.; Felczak, P.; Sreńscek-Nazzal, J.; Wróbel, R.J.; Nishiguchi, H.; Toyoda, M. Role of the Hydroxyl Groups Coordinated to TiO2 Surface on the Photocatalytic Decomposition of Ethylene at Different Ambient Conditions. Catalysts 2022, 12, 386. [Google Scholar] [CrossRef]
- Chen, Y.; Grimplet, J.; David, K.; Castellarin, S.D.; Terol, J.; Wong, D.C.J.; Luo, Z.; Schaffer, R.; Celton, J.-M.; Talon, M.; et al. Ethylene Receptors and Related Proteins in Climacteric and Non-Climacteric Fruits. Plant Sci. 2018, 276, 63–72. [Google Scholar] [CrossRef]
- Azam, K.; Shezad, N.; Shafiq, I.; Akhter, P.; Akhtar, F.; Jamil, F.; Shafique, S.; Park, Y.-K.; Hussain, M. A Review on Activated Carbon Modifications for the Treatment of Wastewater Containing Anionic Dyes. Chemosphere 2022, 306, 135566. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, X.; Yang, D. Adsorption of Cr(VI) in Wastewater Using Magnetic Multi-Wall Carbon Nanotubes. Water Sci. Eng. 2015, 8, 226–232. [Google Scholar] [CrossRef]
- Deng, Z.; Sun, S.; Li, H.; Pan, D.; Patil, R.R.; Guo, Z.; Seok, I. Modification of Coconut Shell-Based Activated Carbon and Purification of Wastewater. Adv. Compos. Hybrid Mater. 2021, 4, 65–73. [Google Scholar] [CrossRef]
- Ouyang, J.; Zhou, L.; Liu, Z.; Heng, J.Y.Y.; Chen, W. Biomass-Derived Activated Carbons for the Removal of Pharmaceutical Mircopollutants from Wastewater: A Review. Sep. Purif. Technol. 2020, 253, 117536. [Google Scholar] [CrossRef]
- Rahman, M.A.; Amin, S.M.R.; Alam, A.M.S. Removal of Methylene Blue from Waste Water Using Activated Carbon Prepared from Rice Husk. Dhaka Univ. J. Sci. 2012, 60, 185–189. [Google Scholar] [CrossRef]
- Sajjadi, S.-A.; Mohammadzadeh, A.; Tran, H.N.; Anastopoulos, I.; Dotto, G.L.; Lopičić, Z.R.; Sivamani, S.; Rahmani-Sani, A.; Ivanets, A.; Hosseini-Bandegharaei, A. Efficient Mercury Removal from Wastewater by Pistachio Wood Wastes-Derived Activated Carbon Prepared by Chemical Activation Using a Novel Activating Agent. J. Environ. Manag. 2018, 223, 1001–1009. [Google Scholar] [CrossRef]
- Dissanayake, P.D.; You, S.; Igalavithana, A.D.; Xia, Y.; Bhatnagar, A.; Gupta, S.; Kua, H.W.; Kim, S.; Kwon, J.-H.; Tsang, D.C.W.; et al. Biochar-Based Adsorbents for Carbon Dioxide Capture: A Critical Review. Renew. Sustain. Energy Rev. 2020, 119, 109582. [Google Scholar] [CrossRef]
- Madzaki, H.; KarimGhani, W.A.W.A.B.; NurZalikhaRebitanim; AzilBahariAlias. Carbon Dioxide Adsorption on Sawdust Biochar. Procedia Eng. 2016, 148, 718–725. [Google Scholar] [CrossRef]
- Gopinath, A.; Kadirvelu, K. Strategies to Design Modified Activated Carbon Fibers for the Decontamination of Water and Air. Environ. Chem. Lett. 2018, 16, 1137–1168. [Google Scholar] [CrossRef]
- Abd, A.A.; Othman, M.R.; Kim, J. A Review on Application of Activated Carbons for Carbon Dioxide Capture: Present Performance, Preparation, and Surface Modification for Further Improvement. Environ. Sci. Pollut. Res. 2021, 28, 43329–43364. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Santos, Á.A.; Castel, C.; Favre, E. A Review of Gas Separation Technologies within Emission Reduction Programs in the Iron and Steel Sector: Current Application and Development Perspectives. Sep. Purif. Technol. 2018, 194, 425–442. [Google Scholar] [CrossRef]
- Saha, D.; Orkoulas, G.; Chen, J.; Hensley, D.K. Adsorptive Separation of CO2 in Sulfur-Doped Nanoporous Carbons: Selectivity and Breakthrough Simulation. Microporous Mesoporous Mater. 2017, 241, 226–237. [Google Scholar] [CrossRef]
- Tsivadze, A.Y.; Gur’yanov, V.V.; Petukhova, G.A. Preparation of Spherical Activated Carbon from Furfural, Its Properties and Prospective Applications in Medicine and the National Economy. Prot. Met. Phys. Chem. Surf. 2011, 47, 612–620. [Google Scholar] [CrossRef]
- Howell, C.A.; Sandeman, S.R.; Zheng, Y.; Mikhalovsky, S.V.; Nikolaev, V.G.; Sakhno, L.A.; Snezhkova, E.A. New Dextran Coated Activated Carbons for Medical Use. Carbon 2016, 97, 134–146. [Google Scholar] [CrossRef]
- Prasad, R.D.; Desai, C.B.; Shrivastav, O.P.; Charmode, N.; Prasad, S.R.; Samant, A.; Mirajkar, R.; Banga, S.; Shaikh, V.S.; Padvi, M.N.; et al. A Critical Review on Design and Development of Carbon Nanotubes for Veterinary Medicine. ES Food Agrofor. 2022, 9, 15–38. [Google Scholar] [CrossRef]
- Bagheri, M.; Jafari, S.M.; Eikani, M.H. Development of Ternary Nanoadsorbent Composites of Graphene Oxide, Activated Carbon, and Zero-valent Iron Nanoparticles for Food Applications. Food Sci. Nutr. 2019, 7, 2827–2835. [Google Scholar] [CrossRef]
- Abbas, Q.; Raza, R.; Shabbir, I.; Olabi, A.G. Heteroatom Doped High Porosity Carbon Nanomaterials as Electrodes for Energy Storage in Electrochemical Capacitors: A Review. J. Sci. Adv. Mater. Devices 2019, 4, 341–352. [Google Scholar] [CrossRef]
- Barsukov, I.V.; Johnson, C.S.; Doninger, J.E.; Barsukov, V.Z. (Eds.) New Carbon Based Materials for Electrochemical Energy Storage Systems: Batteries, Supercapacitors and Fuel Cells; NATO Science Series II: Mathematics, Physics and Chemistry; Springer: Dordrecht, The Netherlands, 2006; Volume 229, ISBN 978-1-4020-4810-4. [Google Scholar]
- Oglou, R.C.; Gokce, Y.; Yagmur, E.; Ghobadi, T.G.U.; Aktas, Z. Highly Stable Megalopolis Lignite Based N and S Self-Doped Hierarchically Porous Activated Carbons for High Performance Supercapacitors and Ash Content Effects on Performance. J. Energy Storage 2022, 46, 103817. [Google Scholar] [CrossRef]
- Gao, F.; Shao, G.; Qu, J.; Lv, S.; Li, Y.; Wu, M. Tailoring of Porous and Nitrogen-Rich Carbons Derived from Hydrochar for High-Performance Supercapacitor Electrodes. Electrochim. Acta 2015, 155, 201–208. [Google Scholar] [CrossRef]
- Shi, K.; Ren, M.; Zhitomirsky, I. Activated Carbon-Coated Carbon Nanotubes for Energy Storage in Supercapacitors and Capacitive Water Purification. ACS Sustain. Chem. Eng. 2014, 2, 1289–1298. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, Z. The Rational Design of Biomass-Derived Carbon Materials towards next-Generation Energy Storage: A Review. Renew. Sustain. Energy Rev. 2020, 134, 110308. [Google Scholar] [CrossRef]
- Iwanow, M.; Gärtner, T.; Sieber, V.; König, B. Activated Carbon as Catalyst Support: Precursors, Preparation, Modification and Characterization. Beilstein J. Org. Chem. 2020, 16, 1188–1202. [Google Scholar] [CrossRef]
- Samikannu, A.; Konwar, L.J.; Mäki-Arvela, P.; Mikkola, J.-P. Renewable N-Doped Active Carbons as Efficient Catalysts for Direct Synthesis of Cyclic Carbonates from Epoxides and CO2. Appl. Catal. B Environ. 2019, 241, 41–51. [Google Scholar] [CrossRef]
- Liew, R.K.; Chong, M.Y.; Osazuwa, O.U.; Nam, W.L.; Phang, X.Y.; Su, M.H.; Cheng, C.K.; Chong, C.T.; Lam, S.S. Production of Activated Carbon as Catalyst Support by Microwave Pyrolysis of Palm Kernel Shell: A Comparative Study of Chemical versus Physical Activation. Res. Chem. Intermed. 2018, 44, 3849–3865. [Google Scholar] [CrossRef]
- Tangsathitkulchai, C.; Ngernyen, Y.; Tangsathitkulchai, M. Surface Modification and Adsorption of Eucalyptus Wood-Based Activated Carbons: Effects of Oxidation Treatment, Carbon Porous Structure and Activation Method. Korean J. Chem. Eng. 2009, 26, 1341–1352. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Bautista-Toledo, I.; Ferro-García, M.A.; Moreno-Castilla, C. Activated Carbon Surface Modifications by Adsorption of Bacteria and Their Effect on Aqueous Lead Adsorption: Adsorption of E Coli on Activated Carbons. J. Chem. Technol. Biotechnol. 2001, 76, 1209–1215. [Google Scholar] [CrossRef]
- Sun, Y.; Li, K.; Zhao, J.; Wang, J.; Tang, N.; Zhang, D.; Guan, T.; Jin, Z. Nitrogen and Sulfur Co-Doped Microporous Activated Carbon Macro-Spheres for CO2 Capture. J. Colloid Interface Sci. 2018, 526, 174–183. [Google Scholar] [CrossRef]
- Walton, K.S.; Abney, M.B.; Douglas LeVan, M. CO2 Adsorption in Y and X Zeolites Modified by Alkali Metal Cation Exchange. Microporous Mesoporous Mater. 2006, 91, 78–84. [Google Scholar] [CrossRef]
- Plaza, M.G.; González, A.S.; Pevida, C.; Rubiera, F. Green Coffee Based CO2 Adsorbent with High Performance in Postcombustion Conditions. Fuel 2015, 140, 633–648. [Google Scholar] [CrossRef]
- Sivadas, D.L.; Vijayan, S.; Rajeev, R.; Ninan, K.N.; Prabhakaran, K. Nitrogen-Enriched Microporous Carbon Derived from Sucrose and Urea with Superior CO2 Capture Performance. Carbon 2016, 109, 7–18. [Google Scholar] [CrossRef]
- Sun, N.; Zhu, B.; Cai, X.; Yu, L.; Yuan, X.; Zhang, Y. Enhanced Interfacial Properties of Carbon Fiber/Polyamide Composites by In-Situ Synthesis of Polyamide 6 on Carbon Fiber Surface. Appl. Surf. Sci. 2022, 599, 153889. [Google Scholar] [CrossRef]
- Li, Y.; Liang, Y.; Hu, H.; Dong, H.; Zheng, M.; Xiao, Y.; Liu, Y. KNO3-Mediated Synthesis of High-Surface-Area Polyacrylonitrile-Based Carbon Material for Exceptional Supercapacitors. Carbon 2019, 152, 120–127. [Google Scholar] [CrossRef]
- Singh, J.; Basu, S.; Bhunia, H. Dynamic CO2 Adsorption on Activated Carbon Adsorbents Synthesized from Polyacrylonitrile (PAN): Kinetic and Isotherm Studies. Microporous Mesoporous Mater. 2019, 280, 357–366. [Google Scholar] [CrossRef]
- Ra, E.J.; Raymundo-Piñero, E.; Lee, Y.H.; Béguin, F. High Power Supercapacitors Using Polyacrylonitrile-Based Carbon Nanofiber Paper. Carbon 2009, 47, 2984–2992. [Google Scholar] [CrossRef]
- Deng, S.; Wei, H.; Chen, T.; Wang, B.; Huang, J.; Yu, G. Superior CO2 Adsorption on Pine Nut Shell-Derived Activated Carbons and the Effective Micropores at Different Temperatures. Chem. Eng. J. 2014, 253, 46–54. [Google Scholar] [CrossRef]
- Lewicka, K. Activated Carbons Prepared from Hazelnut Shells, Walnut Shells and Peanut Shells for High CO2 Adsorption. Pol. J. Chem. Technol. 2017, 19, 38–43. [Google Scholar] [CrossRef]
- Şahin, Ö.; Saka, C. Preparation and Characterization of Activated Carbon from Acorn Shell by Physical Activation with H2O–CO2 in Two-Step Pretreatment. Bioresour. Technol. 2013, 136, 163–168. [Google Scholar] [CrossRef]
- Yang, M.; Guo, L.; Hu, G.; Hu, X.; Xu, L.; Chen, J.; Dai, W.; Fan, M. Highly Cost-Effective Nitrogen-Doped Porous Coconut Shell-Based CO2 Sorbent Synthesized by Combining Ammoxidation with KOH Activation. Environ. Sci. Technol. 2015, 49, 7063–7070. [Google Scholar] [CrossRef]
- Drage, T.C.; Arenillas, A.; Smith, K.M.; Pevida, C.; Piippo, S.; Snape, C.E. Preparation of Carbon Dioxide Adsorbents from the Chemical Activation of Urea–Formaldehyde and Melamine–Formaldehyde Resins. Fuel 2007, 86, 22–31. [Google Scholar] [CrossRef]
- Beckert, M.; Menzel, M.; Tölle, F.J.; Bruchmann, B.; Mülhaupt, R. Nitrogenated Graphene and Carbon Nanomaterials by Carbonization of Polyfurfuryl Alcohol in the Presence of Urea and Dicyandiamide. Green Chem. 2015, 17, 1032–1037. [Google Scholar] [CrossRef]
- Zgrzebnicki, M.; Nair, V.; Mitra, S.; Kałamaga, A.; Przepiórski, J.; Wrobel, R.J. N-Doped Activated Carbon Derived from Furfuryl Alcohol—Development of Porosity, Properties, and Adsorption of Carbon Dioxide and Ethene. Chem. Eng. J. 2022, 427, 131709. [Google Scholar] [CrossRef]
- Feng, Y.; Cao, M.; Yang, L.; Zhang, X.-F.; Wang, Y.; Yu, D.; Gu, X.; Yao, J. Bilayer N-Doped Carbon Derived from Furfuryl Alcohol-Wrapped Melamine Sponge as High-Performance Supercapacitor. J. Electroanal. Chem. 2018, 823, 633–637. [Google Scholar] [CrossRef]
- Nur, M.M.; Saleh, T.A. Melamine-Modified Polyacrylic Grafted on Activated Carbon and Its Efficiency for Shale Inhibition. Upstream Oil Gas Technol. 2022, 8, 100065. [Google Scholar] [CrossRef]
- Tiwari, D.; Goel, C.; Bhunia, H.; Bajpai, P.K. Melamine-Formaldehyde Derived Porous Carbons for Adsorption of CO2 Capture. J. Environ. Manag. 2017, 197, 415–427. [Google Scholar] [CrossRef]
- Kong, Q.; Zhang, X.; Ma, K.; Gong, Y.; Peng, H.; Qi, W. PEI-Modified Chitosan/Activated Carbon Composites for Cu(II) Removal from Simulated Pyrophosphate Plating Rinsing Wastewater. Int. J. Biol. Macromol. 2023, 251, 126429. [Google Scholar] [CrossRef]
- Zhang, S.; Shi, X.; Wróbel, R.; Chen, X.; Mijowska, E. Low-Cost Nitrogen-Doped Activated Carbon Prepared by Polyethylenimine (PEI) with a Convenient Method for Supercapacitor Application. Electrochim. Acta 2019, 294, 183–191. [Google Scholar] [CrossRef]
- Nian, Y.-R.; Teng, H. Nitric Acid Modification of Activated Carbon Electrodes for Improvement of Electrochemical Capacitance. J. Electrochem. Soc. 2002, 149, A1008. [Google Scholar] [CrossRef]
- Yao, S.; Zhang, J.; Shen, D.; Xiao, R.; Gu, S.; Zhao, M.; Liang, J. Removal of Pb(II) from Water by the Activated Carbon Modified by Nitric Acid under Microwave Heating. J. Colloid Interface Sci. 2016, 463, 118–127. [Google Scholar] [CrossRef]
- Long, X.; Duan, B.; Cao, H.; Jia, M.; Wu, L. Removal of NO with the Hexamminecobalt(II) Solution Catalyzed by the Activated Carbon Treated with Acetic Acid. J. Ind. Eng. Chem. 2018, 62, 217–224. [Google Scholar] [CrossRef]
- Kolanowski, Ł.; Graś-Ligocka, M.; Krawczyk, P.; Buchwald, T.; Lota, K.; Lota, G. Ozonation with Ammoxidation as a Method of Obtaining O, N-Doped Carbon Electrode Material to Electrochemical Capacitors. Electrochim. Acta 2022, 413, 140130. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, S.; Yang, Y.; Fu, X.; Jiang, T.; Yang, J. Ammonium Nitrate-Assisted Synthesis of Nitrogen/Sulfur-Codoped Hierarchically Porous Carbons Derived from Ginkgo Leaf for Supercapacitors. ACS Omega 2019, 4, 5904–5914. [Google Scholar] [CrossRef] [PubMed]
- Othman, F.E.C.; Yusof, N.; Samitsu, S.; Abdullah, N.; Hamid, M.F.; Nagai, K.; Abidin, M.N.Z.; Azali, M.A.; Ismail, A.F.; Jaafar, J.; et al. Activated Carbon Nanofibers Incorporated Metal Oxides for CO2 Adsorption: Effects of Different Type of Metal Oxides. J. CO2 Util. 2021, 45, 101434. [Google Scholar] [CrossRef]
- Bayrak, Y.; Topallar, H.; Karagöz, B.; Kılıç, İ. Kinetics and Thermodynamics of Cr(VI), Cu(II), and Ni(II) Adsorption on Activated Carbon Prepared from Rice Hulls. J. Dispers. Sci. Technol. 2013, 34, 1248–1256. [Google Scholar] [CrossRef]
- Ma, X.-Q.; Zhang, B.; Xu, Z.-X.; Tan, Y.; Li, B.; Zhang, Y.-C.; Ni, G.-S.; Zhou, W.-Y.; Luque, R.; Zhang, H.-Y. N-Rich and O-Poor Doped Carbon Prepared via Facile Ammonium Nitrate Assisted Hydrothermal Carbonization for Robust Supercapacitors. J. Clean. Prod. 2022, 373, 133903. [Google Scholar] [CrossRef]
- Petrovic, B.; Gorbounov, M.; Soltani, S.M. Impact of Surface Functional Groups and Their Introduction Methods on the Mechanisms of CO2 Adsorption on Porous Carbonaceous Adsorbents. Carbon Capture Sci. Technol. 2022, 3, 100045. [Google Scholar] [CrossRef]
- Sun, F.; Liu, X.; Gao, J.; Pi, X.; Wang, L.; Qu, Z. Highlighting the Role of Nitrogen Doping in Enhancing CO2 Uptake onto Carbon Surfaces: A Combined Experimantal and Computional Analysis. J. Mater. Chem. A 2016, 47, 18248–18252. [Google Scholar] [CrossRef]
- Ma, X.; Li, L.; Chen, R.; Wang, C.; Zhou, K.; Li, H. Doping of Alkali Metals in Carbon Frameworks for Enhancing CO2 Capture: A Theoretical Study. Fuel 2019, 236, 942–948. [Google Scholar] [CrossRef]
- Chen, C.-C. A Literature Study on Business Culture and Management in Taiwan and Mainland China. Procedia Eng. 2012, 29, 360–364. [Google Scholar] [CrossRef]
- Liu, Y.; Wilcox, J. Effects of Surface Heterogeneity on the Adsorption of CO2 in Microporous Carbons. Environ. Sci. Technol. 2012, 46, 1940–1947. [Google Scholar] [CrossRef]
- Plaza, M.G.; Thurecht, K.J.; Pevida, C.; Rubiera, F.; Pis, J.J.; Snape, C.E. Influence of Oxidation upon the CO2 Capture Performance of a Phenolic-Resin-Derived Carbon. Fuel Process. Technol. 2013, 110, 53–60. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, Y.; Xie, M.; Du, C.; Chen, J.; Wan, L.; Zhang, Y. Clean Production of N, O-Doped Activated Carbon by Water Vapor Carbonization/Activation of Expired Coffee for High-Volumetric Supercapacitor. Appl. Surf. Sci. 2022, 589, 153011. [Google Scholar] [CrossRef]
- Zapata-Benabithe, Z.; Carrasco-Marín, F.; Moreno-Castilla, C. Preparation, Surface Characteristics, and Electrochemical Double-Layer Capacitance of KOH-Activated Carbon Aerogels and Their O- and N-Doped Derivatives. J. Power Sources 2012, 219, 80–88. [Google Scholar] [CrossRef]
- Luo, L.; Chen, T.; Li, Z.; Zhang, Z.; Zhao, W.; Fan, M. Heteroatom Self-Doped Activated Biocarbons from Fir Bark and Their Excellent Performance for Carbon Dioxide Adsorption. J. CO2 Util. 2018, 25, 89–98. [Google Scholar] [CrossRef]
- Plaza, M.G.; Rubiera, F.; Pis, J.J.; Pevida, C. Ammoxidation of Carbon Materials for CO2 Capture. Appl. Surf. Sci. 2010, 256, 6843–6849. [Google Scholar] [CrossRef]
- Wang, S.-H.; Hwang, Y.-K.; Choi, S.W.; Yuan, X.; Lee, K.B.; Chang, F.-C. Developing Self-Activated Lignosulfonate-Based Porous Carbon Material for Ethylene Adsorption. J. Taiwan Inst. Chem. Eng. 2020, 115, 315–320. [Google Scholar] [CrossRef]
- Kałamaga, A.; Román-Martínez, M.C.; Lillo-Ródenas, M.A.; Wróbel, R.J. The Influence of NH4NO3 and NH4ClO4 on Porous Structure Development of Activated Carbons Produced from Furfuryl Alcohol. Molecules 2022, 27, 7860. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Huang, J.; Yu, Q.; Demir, M.; Kilic, M.; Altay, B.N.; Hu, X.; Wang, L. N-Doped Porous Carbon Derived from Macadamia Nut Shell for CO2 Adsorption. Fuel Process. Technol. 2023, 249, 107854. [Google Scholar] [CrossRef]
- Bai, J.; Huang, J.; Yu, Q.; Demir, M.; Akgul, E.; Altay, B.N.; Hu, X.; Wang, L. Fabrication of Coconut Shell-Derived Porous Carbons for CO2 Adsorption Application. Front. Chem. Sci. Eng. 2023, 17, 1122–1130. [Google Scholar] [CrossRef]
- Han, J.; Zhang, L.; Zhao, B.; Qin, L.; Wang, Y.; Xing, F. The N-Doped Activated Carbon Derived from Sugarcane Bagasse for CO2 Adsorption. Ind. Crops Prod. 2019, 128, 290–297. [Google Scholar] [CrossRef]
- Yue, L.; Xia, Q.; Wang, L.; Wang, L.; DaCosta, H.; Yang, J.; Hu, X. CO2 Adsorption at Nitrogen-Doped Carbons Prepared by K2CO3 Activation of Urea-Modified Coconut Shell. J. Colloid Interface Sci. 2018, 511, 259–267. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, G.; Gao, M.; Huang, L.; Li, L.; Liu, S.; Xie, C.; Zhang, Y.; Yu, S. N-Doped Porous Carbon from Different Nitrogen Sources for High-Performance Supercapacitors and CO2 Adsorption. J. Alloys Compd. 2019, 786, 826–838. [Google Scholar] [CrossRef]
- Meng, L.-Y.; Park, S.-J. One-Pot Synthetic Method to Prepare Highly N-Doped Nanoporous Carbons for CO2 Adsorption. Mater. Chem. Phys. 2014, 143, 1158–1163. [Google Scholar] [CrossRef]
- He, S.; Chen, G.; Xiao, H.; Shi, G.; Ruan, C.; Ma, Y.; Dai, H.; Yuan, B.; Chen, X.; Yang, X. Facile Preparation of N-Doped Activated Carbon Produced from Rice Husk for CO2 Capture. J. Colloid Interface Sci. 2021, 582, 90–101. [Google Scholar] [CrossRef]
- Ye, P.; Fang, Z.; Su, B.; Xing, H.; Yang, Y.; Su, Y.; Ren, Q. Adsorption of Propylene and Ethylene on 15 Activated Carbons. J. Chem. Eng. Data 2010, 55, 5669–5672. [Google Scholar] [CrossRef]
- Al-Muhtaseb, S.A. Adsorption and Desorption Equilibria of Nitrogen, Methane, Ethane, and Ethylene on Date-Pit Activated Carbon. J. Chem. Eng. Data 2010, 55, 313–319. [Google Scholar] [CrossRef]
- Liang, W.; Zhang, Y.; Wang, X.; Wu, Y.; Zhou, X.; Xiao, J.; Li, Y.; Wang, H.; Li, Z. Asphalt-Derived High Surface Area Activated Porous Carbons for the Effective Adsorption Separation of Ethane and Ethylene. Chem. Eng. Sci. 2017, 162, 192–202. [Google Scholar] [CrossRef]
- Ma, X.; Ouyang, F. Adsorption Properties of Biomass-Based Activated Carbon Prepared with Spent Coffee Grounds and Pomelo Skin by Phosphoric Acid Activation. Appl. Surf. Sci. 2013, 268, 566–570. [Google Scholar] [CrossRef]
- Lozano-Castelló, D.; Cazorla-Amorós, D.; Linares-Solano, A. Usefulness of CO2 Adsorption at 273 K for the Characterization of Porous Carbons. Carbon 2004, 42, 1233–1242. [Google Scholar] [CrossRef]
- Gęsikiewicz-Puchalska, A.; Zgrzebnicki, M.; Michalkiewicz, B.; Narkiewicz, U.; Morawski, A.W.; Wrobel, R.J. Improvement of CO2 Uptake of Activated Carbons by Treatment with Mineral Acids. Chem. Eng. J. 2017, 309, 159–171. [Google Scholar] [CrossRef]
- Lange, N.A. Handbook of Chemistry, 10th ed.; McGraw-Hill Book Co.: New York, NY, USA, 1967. [Google Scholar]


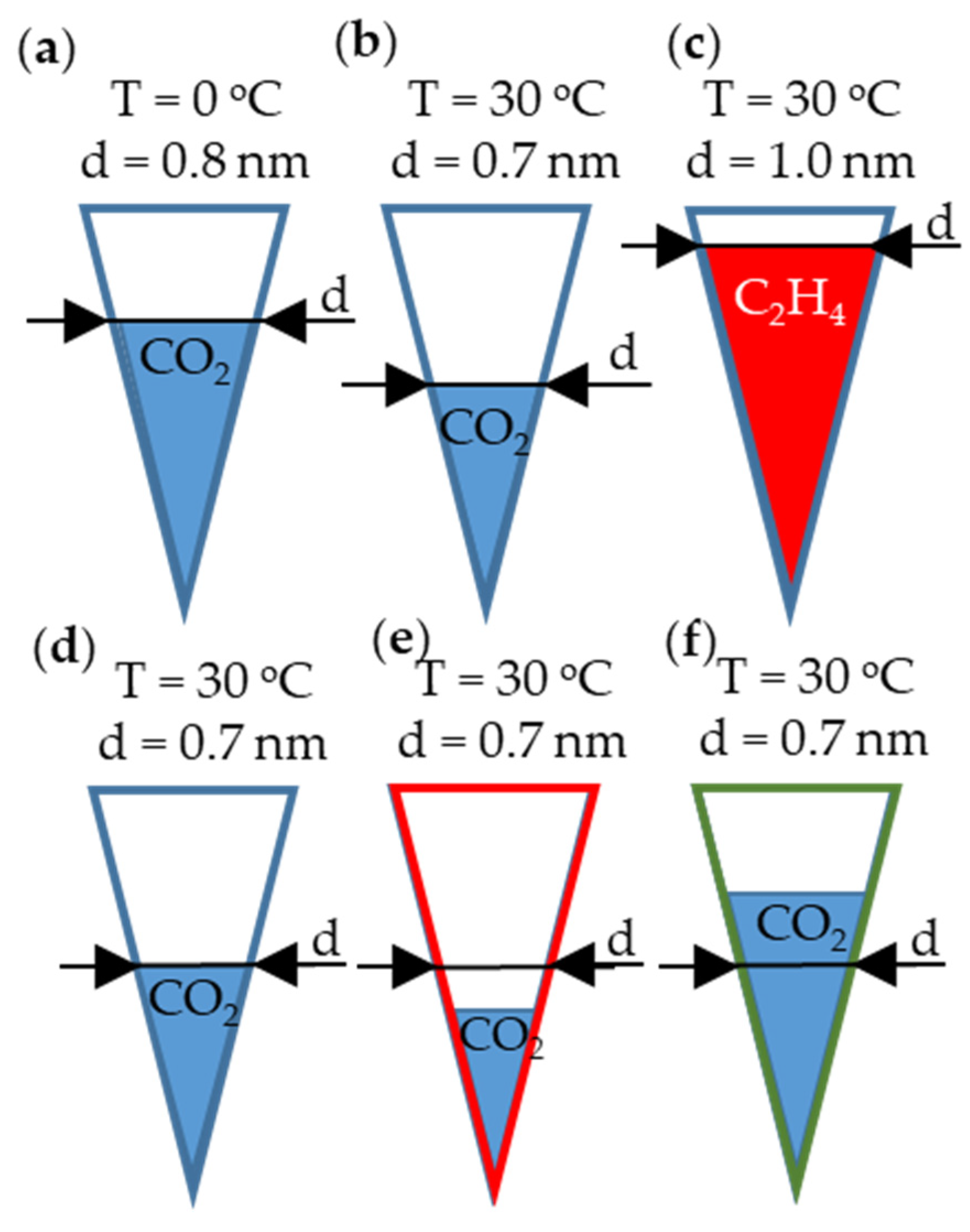
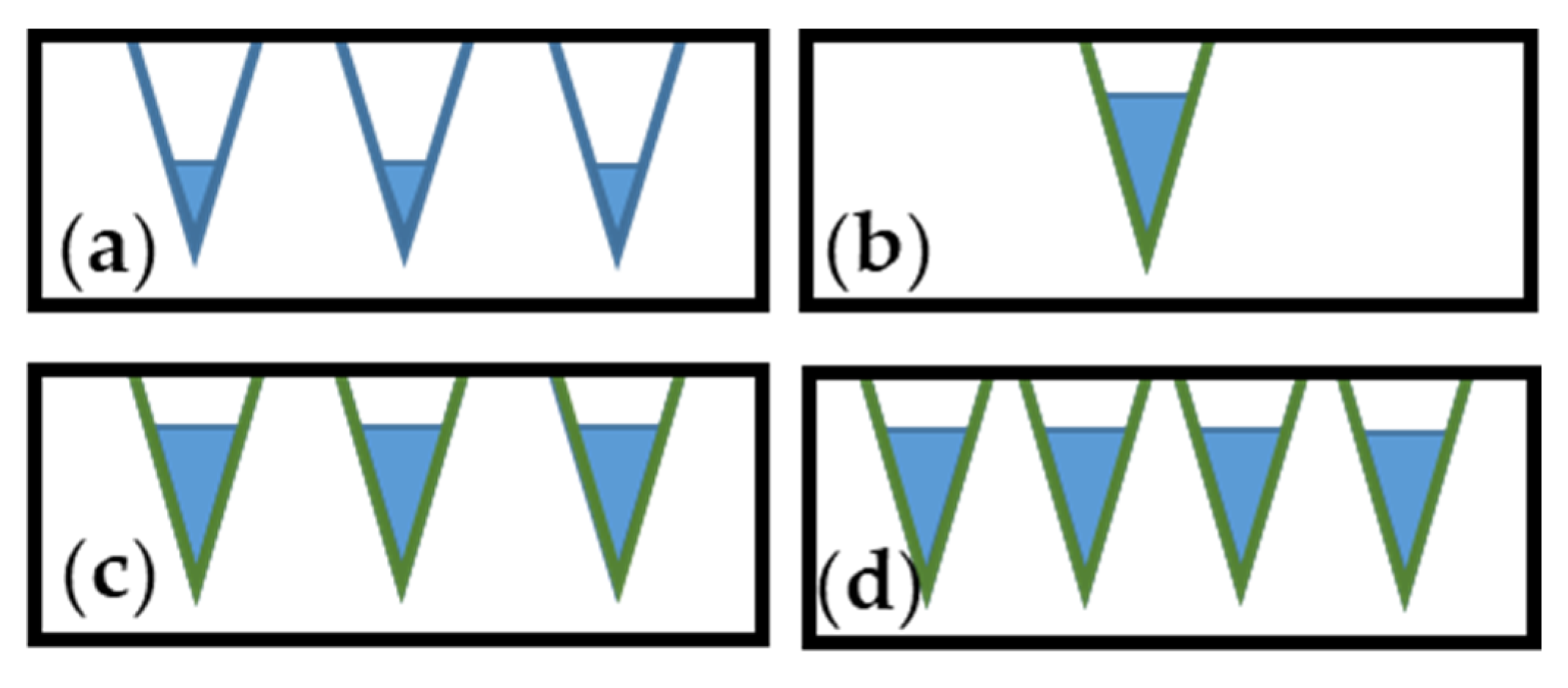
| Atmosphere of Carbonization | Yield of Carbonization [%] |
|---|---|
| Nitrogen | 55 |
| Ammonia | 63 |
| Ammonia/air | 69 |
| Sample | Concentration [at.%] | ||
|---|---|---|---|
| C | O | N | |
| PFA | 76 | 24 | 0 |
| PFA-N2 | 95 | 5 | 0 |
| PFA-NH3 | 91 | 3 | 6 |
| PFA-NH3/air | 65 | 10 | 25 |
| Temperature of Activation [°C] | Yield of Activation Process [%] | ||
|---|---|---|---|
| PFA-N2 Series | PFA-NH3 Series | PFA-NH3/Air Series | |
| 700 | 95 | 97 | 96 |
| 800 | 94 | 94 | 94 |
| 900 | 83 | 79 | 79 |
| 1000 | 70 | 62 | 55 |
| Raw Material | Nitrogen Source | CO2 Uptake [mmol/g] | Method of Activation | Reference |
|---|---|---|---|---|
| Macadamia nutshell | Melamine | 4.4 at 0 °C | KOH activation | [71] |
| Coconut shell | - | 4.2 at 25 °C | KOH activation | [72] |
| Sugarcane bagasse | Urea | 4.8 at 25 °C | KOH activation | [73] |
| Coconut shell | Urea | 3.7 at 25 °C | K2CO3 activation | [74] |
| Chitosan and glucose | Ammonia, chitosan | 5.5 at 25 °C | Autoclave | [75] |
| PPy | - | 3.7 at 25 °C | NaOH activation | [76] |
| Rice husk | Chitosan | 3.7 at 25 °C | KOH activation | [77] |
| PFA | Ammonia nitrate | 2.1 at 30 °C | CO2 activation | [70] |
| PFA | - | 2.2 at 30 °C | CO2 activation | This study |
| Ammonia | 1.6 at 30 °C | |||
| Ammonia/air | 0.5 at 30 °C |
| Raw Material | Nitrogen Source | C2H4 Uptake [mmol/g] | Reference |
|---|---|---|---|
| Commercial carbon | - | 3.1 at 40 °C | [78] |
| Date seeds | - | 2.9 at 30 °C | [79] |
| Asphalt | - | 7.2 at 25 °C | [80] |
| Hardwood lignosulfonate powder | - | 2.2 at 25 °C | [69] |
| Coffee beans | - | 2.3 at 35 °C | [81] |
| PFA | Ammonium nitrate | 3.4 at 30 °C | [70] |
| PFA | - | 2.9 at 30 °C | This study |
| Ammonia | 2.2 at 30 °C | ||
| Ammonia/air | 0.5 at 30 °C |
| Sample | SSA 1 [m2/g] | Vtotal 2 [cm3/g] | Vmicro 3 [cm3/g] | Vmeso 4 [cm3/g] |
|---|---|---|---|---|
| PFA-N2-700 | 75 | 0.04 | 0.02 | 0.01 |
| PFA-N2-800 | 105 | 0.05 | 0.04 | 0.01 |
| PFA-N2-900 | 539 | 0.19 | 0.15 | 0.03 |
| PFA-N2-1000 | 746 | 0.30 | 0.27 | 0.00 |
| PFA-NH3-700 | 14 | 0.01 | 0.00 | 0.01 |
| PFA-NH3-800 | 53 | 0.03 | 0.02 | 0.01 |
| PFA-NH3-900 | 188 | 0.08 | 0.07 | 0.00 |
| PFA-NH3-1000 | 768 | 0.31 | 0.29 | 0.00 |
| PFA-NH3/air-700 | 16 | 0.01 | 0.01 | 0.00 |
| PFA-NH3/air-800 | 32 | 0.02 | 0.01 | 0.00 |
| PFA-NH3/air-900 | 116 | 0.05 | 0.04 | 0.00 |
| PFA-NH3/air-1000 | 125 | 0.05 | 0.05 | 0.00 |
| Sample | CO2 Uptake at 0 °C [mmol/g] | SCO2 [m2/g] | Vtotal [cm3/g] | V0.7nm [cm3/g] | V0.8nm [cm3/g] | V1.0nm [cm3/g] |
|---|---|---|---|---|---|---|
| PFA-N2-700 | 2.4 | 520 | 0.16 | 0.11 | 0.11 | 0.14 |
| PFA-N2-800 | 2.6 | 556 | 0.17 | 0.12 | 0.12 | 0.15 |
| PFA-N2-900 | 3.2 | 689 | 0.21 | 0.15 | 0.16 | 0.18 |
| PFA-N2-1000 | 3.9 | 817 | 0.28 | 0.18 | 0.19 | 0.23 |
| PFA-NH3-700 | 1.9 | 401 | 0.14 | 0.09 | 0.10 | 0.11 |
| PFA-NH3-800 | 2.0 | 425 | 0.16 | 0.09 | 0.09 | 0.13 |
| PFA-NH3-900 | 1.6 | 348 | 0.13 | 0.07 | 0.07 | 0.10 |
| PFA-NH3-1000 | 2.6 | 544 | 0.20 | 0.11 | 0.12 | 0.16 |
| PFA-NH3/air-700 | 1.9 | 407 | 0.13 | 0.09 | 0.10 | 0.11 |
| PFA-NH3/air-800 | 1.6 | 316 | 0.11 | 0.05 | 0.09 | 0.10 |
| PFA-NH3/air-900 | 1.3 | 262 | 0.10 | 0.05 | 0.06 | 0.08 |
| PFA-NH3/air-1000 | 0.8 | 169 | 0.06 | 0.03 | 0.04 | 0.05 |
| Sample | Oxygen Concentration [at.%] | Nitrogen Concentration [at.%] | CO2 Uptake, mmol/cm3 (d = 0.7 nm) | CO2 Uptake, mmol/cm3 (d = 0.8 nm) | C2H4 Uptake, mmol/cm3 (d = 1.0 nm) |
|---|---|---|---|---|---|
| PFA-N2-700 | 10 | 0 | 14 | 22 | 12 |
| PFA-N2-800 | 7 | 0 | 15 | 22 | 11 |
| PFA-N2-900 | 4 | 0 | 14 | 20 | 13 |
| PFA-N2-1000 | 2 | 0 | 12 | 21 | 13 |
| PFA-NH3-700 | 8 | 9 | 14 | 19 | 8 |
| PFA-NH3-800 | 12 | 7 | 16 | 22 | 5 |
| PFA-NH3-900 | 16 | 6 | 17 | 23 | 11 |
| PFA-NH3-1000 | 3 | 2 | 13 | 22 | 14 |
| PFA-NH3/air-700 | 11 | 16 | 13 | 19 | 5 |
| PFA-NH3/air-800 | 20 | 7 | 13 | 18 | 4 |
| PFA-NH3/air-900 | 19 | 5 | 15 | 22 | 9 |
| PFA-NH3/air-1000 | 18 | 4 | 13 | 20 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kałamaga, A.; Wróbel, R.J. The Impact of N/O-Functional Groups on the Sorption Capabilities of Activated Carbons Derived from Furfuryl Alcohol. Molecules 2024, 29, 987. https://doi.org/10.3390/molecules29050987
Kałamaga A, Wróbel RJ. The Impact of N/O-Functional Groups on the Sorption Capabilities of Activated Carbons Derived from Furfuryl Alcohol. Molecules. 2024; 29(5):987. https://doi.org/10.3390/molecules29050987
Chicago/Turabian StyleKałamaga, Agnieszka, and Rafał J. Wróbel. 2024. "The Impact of N/O-Functional Groups on the Sorption Capabilities of Activated Carbons Derived from Furfuryl Alcohol" Molecules 29, no. 5: 987. https://doi.org/10.3390/molecules29050987






