Release Pattern of Light Aromatic Hydrocarbons during the Biomass Roasting Process
Abstract
1. Introduction
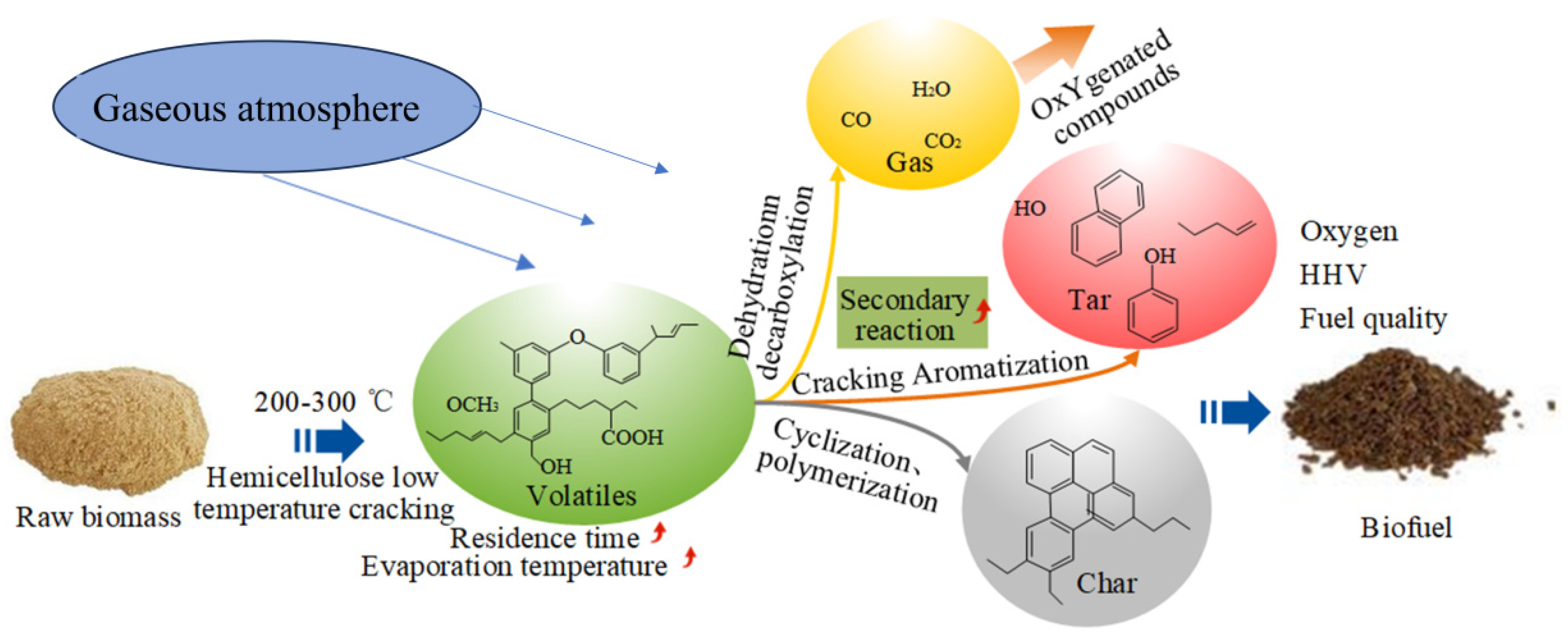
2. Macroscopic Effects of Baking Pretreatment on Biomass
3. Influence of Roasting Atmosphere on Biomass Refining Effect
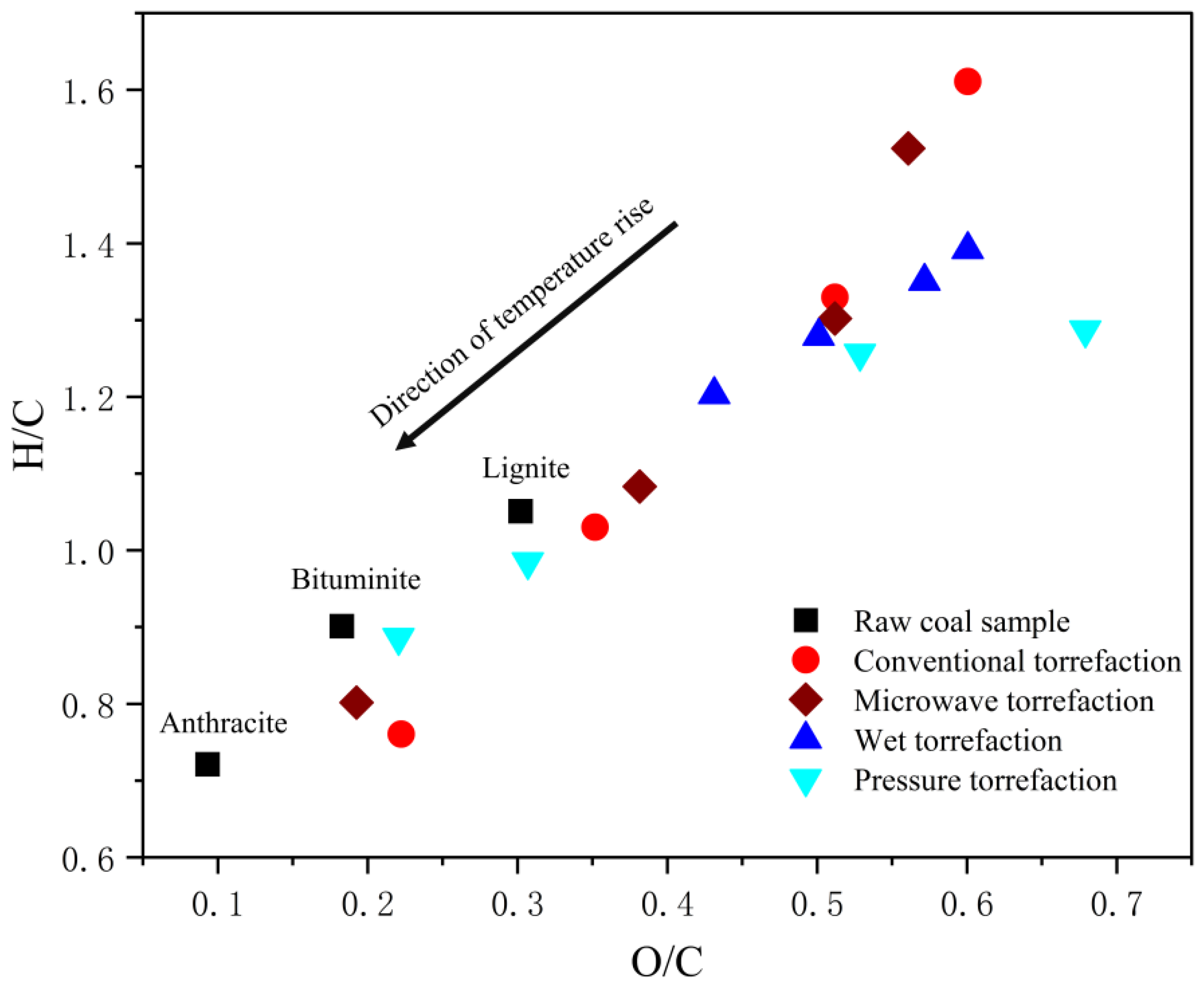
4. Influence of Roasting Method on Biomass Fuel Quality
5. Effect of Baking Temperature on the Evolution of Organic Fractions in Biomass
6. Effect of Catalyst Baking on the Evolution of Biomass Organic Fractions
7. Summary
- The baking temperature selection is crucial. The baking temperature range should be between 200 °C and 300 °C. Excessively high temperatures will make the organic components of biomass rapidly decompose from baking to pyrolysis. At a baking temperature of 270 °C, biomass generally does not undergo significant decomposition and can produce some aromatic components. Moreover, the water is completely removed, and the oxygen content is further reduced by removing CO and CO. In addition, the complex cross-linking structure of the biomass is broken, and the methoxy in the more stable lignin is effectively removed.
- The depolymerization of cellulose and hemicellulose occurs during biomass roasting. Adding catalysts such as ZSM-5 and zeolite during the roasting process enables the timely dehydroxylation and carboxylation of oxygenated intermediates in the produced bio-oil, thus converting the oxygenated intermediates to aromatics.
- The baking method affects the degree of depolymerization of the organic components in the biomass. It has a lesser effect on the aromatic yield and a greater effect on the physical structure of the biomass and the biochar yield.
- Other conditions have a minimal effect on the precipitation of aromatic components in the biomass baking product, which is negligible compared with the effects of the reaction temperature and catalyst.
- Optimizing the baking temperature can improve the aromatic yield in the biomass baking stage. Moreover, using suitable catalysts can promote the conversion of oxygenated intermediates in the bio-oil to aromatics, and reasonable control of the baking temperature and catalyst type can effectively improve the yield of aromatic components.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nemitallah, M.A.; Abdelhafez, A.A.; Ali, A.; Mansir, I.; Habib, M.A. Frontiers in Combustion Techniques and Burner Designs for Emissions Control and CO2 Capture: A Review. Int. J. Energy Res. 2019, 43, 7790–7822. [Google Scholar]
- Jiang, D.; Sun, K.; Wei, Q.; Hui, Z.; Xun, W.; Qiang, L.; Zhou, J.; Ge, Y. Development of biomass power generation industry and suggestions in China. Renew. Energy Resour. 2014, 32, 542–546. [Google Scholar]
- Wang, X.; Jian, L.; Huang, Y.; Song, X. Technologies for Recovering Low-temperature Heat in Delayed Coking Units. Sino-Glob. Energy 2015, 20, 94–98. [Google Scholar]
- Javanmard, A.; Abdul Patah, M.F.; Zulhelmi, A. A comprehensive overview of the continuous torrefaction method: Operational characteristics, applications, and challenges. J. Energy Inst. 2023, 108, 101199. [Google Scholar] [CrossRef]
- Che, Q.-F.; Yang, M.-J.; Wang, H.-H.; Chen, X.; Chen, W.; Yang, Q.; Yang, H.-P.; Chen, H.-P. Aromatics production with metal oxides and ZSM-5 as catalysts in catalytic pyrolysis of wood sawdust. Fuel Process. Technol. 2019, 188, 146–152. [Google Scholar] [CrossRef]
- Klaas, M.; Greenhalf, C.; Ouadi, M.; Jahangiri, H.; Hornung, A.; Briens, C.; Berruti, F. the effect of torrefaction pre treatment on the pyrolysis of corn cobs. Results Eng. 2020, 7, 100165. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, Z.; Zhao, Y.Y.; Xu, L.; Feng, S.; Wang, Z.Z.; Zhang, L.; Shen, B.X.; Fu, J. Effects of torrefaction pretreatment on fuel quality and combustion characteristics of biomass: A review. Fuel 2024, 358, 130314. [Google Scholar] [CrossRef]
- Wang, H.-C.; Yu, K.-L.; Chen, W.-H.; Pillejera, M.K.; Pillejera, M.K.; Bi, X.; Tran, K.Q.; Pétrissans, A.; Pétrissans, M. Variation of lignocellulosic biomass structure from torrefaction: A critical review. Renew. Sustain. Energy Rev. 2021, 152, 111698. [Google Scholar]
- Chen, Y.-Q.; Liu, B.; Yang, H.-P.; Yang, Q.; Chen, H.-P. Evolution of functional groups and pore structure during cotton and corn stalks torrefaction and its correlation with hydrophobicity. Fuel 2014, 137, 41–49. [Google Scholar] [CrossRef]
- Cahyanti, M.N.; Krishna, T.R.; Doddapaneni, C.; Kikas, T. Biomass torrefaction: An overview on process parameters, economic and environmental aspects and recent advancements. Bioresour. Technol. 2020, 301, 122737. [Google Scholar] [CrossRef]
- Shen, D.-K.; Zhang, L.-Q.; Xue, J.-T.; Guan, S.-P.; Liu, Q.; Xiao, R. Thermal degradation of xylan-based hemicellulose under oxidative atmosphere. Carbohydr. Polym. 2015, 127, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.-Y.; Cen, K.-H.; Cao, X.-B.; Li, Y.-J.; Zhang, Y.-M.; Ma, H.-H. Restudy on torrefaction of corn stalk from the point of view of deoxygenation and decarbonization. J. Anal. Appl. Pyrolysis 2018, 135, 85–93. [Google Scholar] [CrossRef]
- Wang, C.-W.; Peng, J.-H.; Li, H.; Bi, X.T.; Legros, R.; Lim, C.J.; Sokhansanj, S. Oxidative torrefaction of biomass residues and densification of torrefied sawdust to pellets. Bioresour. Technol. 2013, 127, 318–325. [Google Scholar] [CrossRef]
- Brachi, P.; Chirone, R.; Miccio, M.; Ruoppolo, G. Fluidized bed torrefaction of commercial wood pellets: Process performance and solid product quality. Energy Fuels 2018, 32, 9459–9469. [Google Scholar] [CrossRef]
- Li, M.-F.; Chen, C.-Z.; Li, X.; Shen, Y.; Bian, J.; Sun, R.-C. Torrefaction of bamboo under nitrogen atmosphere: Influence of temperature and time on the structure and properties of the solid product. Fuel 2015, 161, 193–196. [Google Scholar] [CrossRef]
- Medic, D.; Darr, M.; Shah, A.; Potter, B.; Zimmerman, J. Effects of torrefaction process parameters on biomass feedstock upgrading. Fuel 2012, 91, 147–154. [Google Scholar] [CrossRef]
- Wang, M.-J.; Huang, Y.-F.; Chiueh, P.-T.; Kuan, W.-H.; Lo, S.-L. Microwave-induced torrefaction of rice husk and sugarcane residues. Energy 2012, 37, 177–184. [Google Scholar] [CrossRef]
- Zhang, S.-P.; Chen, T.; Xiong, Y.-Q.; Dong, Q. Effects of wet torrefaction on the physicochemical properties and pyrolysis product properties of rice husk. Energy Convers. Manag. 2017, 141, 403–409. [Google Scholar] [CrossRef]
- Xue, Y.; Zhou, S.-Y.; Leng, E.W.; Cui, C.-H.; Zhou, Z.-Y.; Peng, Y.-F. Comprehensive utilization of agricultural wastes by combined wet torrefaction and pyrolysis. J. Anal. Appl. Pyrolysis 2021, 160, 105358. [Google Scholar] [CrossRef]
- Gao, P.; Guo, D.-Z.; Liang, C.; Liu, G.-Y.; Yang, S.-X. Nitrogen conversion during the rapid pyrolysis of raw/torrefied wheat straw. Fuel 2020, 259, 116227. [Google Scholar] [CrossRef]
- Miura, K.; Nakagawa, H.; Ashida, R.; Ashida, R.; Sakuramoto, Y.; Nakagawa, K. Carbonization of cellulose under mechanical pressure as a means for increasing char yield. Am. Chem. Soc. 2005, 230, 1685–1686. [Google Scholar]
- Sun, Y.-M.; Tong, S.; Li, X.; Hu, Z.-Z.; Sun, M.-Y.; Li, G.; Liu, H.; Hu, H.-Y.; Luo, G.-Q.; Yao, H. Gas-pressurized torrefaction of biomass wastes: Self-promoted deoxygenation of rice straw at low temperature. Fuel 2022, 308, 122029. [Google Scholar] [CrossRef]
- Tong, S.; Sun, Y.-M.; Li, X.; Hu, Z.-Z.; Dacres Omar, D.; Worasuwannarak, N.; Luo, G.-Q.; Liu, H.; Hu, H.-Y.; Yao, H. Gas-pressurized torrefaction of biomass wastes: Roles of pressure and secondary reactions. Bioresour. Technol. 2020, 313, 123640. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Zhao, Z.-H.; Liu, Y.-T.; Guo, D.-Z.; Yang, S.-X. Effect of gas-pressurized torrefaction on the upgrading and pyrolysis characteristics of corn stalk. J. Fuel Chem. Technol. 2022, 50, 735–747. [Google Scholar] [CrossRef]
- Zhong, S.; Zhang, B.; Liu, C.; Mwenya, S.; Zhang, H. A minireview on catalytic fast co-pyrolysis of lignocellulosic biomass for bio-oil upgrading via enhancing monocyclic aromatics. J. Anal. Appl. Pyrolysis 2022, 164, 105544. [Google Scholar] [CrossRef]
- Niu, Y.; Lv, Y.; Lei, Y.; Liu, S.; Hui, S. Biomass torrefaction: Properties, applications, challenges, and economy. Renew. Sustain. Energy Rev. 2019, 115, 109395. [Google Scholar] [CrossRef]
- Zheng, A.-Q.; Zhao, Z.-L.; Chang, S.; Huang, Z.; Wang, X.-B.; He, F.; Li, H.-B. Effect of torrefaction on structure and fast pyrolysis behavior of corncobs. Bioresour. Technol. 2013, 128, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Wannapeera, J.; Fungtammasan, B.; Worasuwannarak, N. Effects of temperature and holding time during torrefaction on the pyrolysis behaviors of woody biomass. J. Anal. Appl. Pyrolysis 2011, 92, 99–105. [Google Scholar] [CrossRef]
- Cao, X.-B.; Luo, Q.-L.; Fang, Y.; Liu, G.-R.; Chen, S.-Y.; Li, Y.-J.; Li, X.; Lu, Y. Effects of oxidative torrefaction on the physicochemical properties and pyrolysis products of hemicellulose in bamboo processing residues. Ind. Crops Prod. 2023, 191, 115986. [Google Scholar] [CrossRef]
- Simonic, M.; Goricanec, D.; Urbancl, D. Impact of torrefaction on biomass properties depending on temperature and operation time. Sci. Total Environ. 2020, 740, 140086. [Google Scholar] [CrossRef]
- Chen, D.-Y.; Li, J.; Zhang, T.; Li, S.; Wang, J.; Niu, W.-S.; Liu, Y.-Y.; Zheng, A.-Q.; Zhao, Z.-L. Advancing biomass pyrolysis by torrefaction pretreatment. Linking the productions of bio-oil and oxygenated chemicals to torrefaction severity. Fuel 2022, 330, 125514. [Google Scholar] [CrossRef]
- Lê, T.K.; Commandré, J.M.; Valette, J.; Volle, G.; Meyer, M. Detailed identification and quantification of the condensable species released during the torrefaction of lignocellulosic biomasses. Fuel Process. Technol. 2015, 139, 226–235. [Google Scholar]
- Potnuri, R.; Dadi, V.S.; Chinta, S.R.; Veluru, S.; Abhishankar, K.; Manan, S. The Effect of Torrefaction Temperature and Catalyst Loading in Microwave-Assisted in-situ Catalytic Co-Pyrolysis of Torrefied Biomass and Plastic Wastes. Bioresour. Technol. 2022, 364, 128099. [Google Scholar] [CrossRef]
- Valizadeh, S.; Oh, D.; Jae, J.; Pyo, S.; Jang, H.; Yim, H.; Rhee, G.H.; Khan, M.A.; Jeon, B.H.; Lin, K.Y.A.; et al. Effect of torrefaction and fractional condensation on the quality of bio-oil from biomass pyrolysis for fuel applications. Fuel 2022, 312, 122959. [Google Scholar] [CrossRef]
- Zhao, X.-Q.; Zhou, X.; Wang, G.-X.; Zhou, P.; Wang, W.-L.; Song, Z.-L. Evaluating the effect of torrefaction on the pyrolysis of biomass and the biochar catalytic performance on dry reforming of methane. Renew. Energy 2022, 192, 313–325. [Google Scholar] [CrossRef]
- Wen, L.; Wang, J.-F.; Han, H.-C. Effect of Torrefaction Conditions on the Torrefaction Characteristics of Biomass. Adv. New Renew. Energy 2019, 7, 116–122. [Google Scholar]
- MaKela, M.; Benavente, V.; Fullana, A. Hydrothermal carbonization of lignocellulosic biomass: Effect of process conditions on hydrochar properties. Appl. Energy 2015, 155, 576–584. [Google Scholar] [CrossRef]
- Agar, D.; Demartini, N.; Hupa, M. Influence of Elevated Pressure on the Torrefaction of Wood. Energy Fuels 2016, 30, 2127–2136. [Google Scholar] [CrossRef]
- Chen, X.; Che, Q.-F.; Li, S.-J.; Liu, Z.-H.; Yang, H.-P.; Chen, Y.-Q.; Wang, X.-H.; Shao, J.-A.; Chen, H.-P. Recent developments in lignocellulosic biomass catalytic fast pyrolysis: Strategies for the optimization of bio-oil quality and yield. Fuel Process. Technol. 2019, 196, 106180. [Google Scholar] [CrossRef]
- Dai, L.-L.; Wang, Y.-P.; Liu, Y.-H.; Ruan, R.; He, C.; Yu, Z.-T.; Jiang, L.; Zeng, Z.-H.; Tian, X.-J. Integrated process of lignocellulosic biomass torrefaction and pyrolysis for upgrading bio-oil production: A state-of-the-art review. Renew. Sustain. Energy Rev. 2019, 107, 20–36. [Google Scholar] [CrossRef]
- Zheng, Y.-W.; Wang, F.; Yang, X.-Q.; Huang, Y.-B.; Liu, C.; Zheng, Z.-F.; Gu, J.-Y. Study on aromatics production via the catalytic pyrolysis vapor upgrading of biomass using metal-loaded modified H-ZSM-5. J. Anal. Appl. Pyrolysis 2017, 126, 169–179. [Google Scholar] [CrossRef]
- Li, X.; Su, L.; Wang, Y.; Yu, Y.; Wang, C.; Li, X.; Wang, Z. Catalytic fast pyrolysis of Kraft lignin with HZSM-5 zeolite for producing aromatic hydrocarbons. Front. Environ. Sci. Eng. 2012, 6, 295–303. [Google Scholar] [CrossRef]
- Liang, J.; Shan, G.-C.; Sun, Y.-F. Catalytic fast pyrolysis of lignocellulosic biomass: Critical role of zeolite catalysts. Renew. Sustain. Energy Rev. 2021, 139, 110707. [Google Scholar] [CrossRef]
- Mullen, C.A.; Tarves, P.C.; Raymundo, L.M.; Schultz, E.L.; Boateng, A.A.; Trierweiler, J.O. Fluidized Bed Catalytic Pyrolysis of Eucalyptus over HZSM-5: Effect of Acid Density and Gallium Modification on Catalyst Deactivation. Energy Fuels 2018, 32, 1771–1778. [Google Scholar] [CrossRef]
- Cheng, Y.-T.; Jae, J.; Shi, J.; Fan, W.; Huber, G.W. Production of renewable aromatic compounds by catalytic fast pyrolysis of lignocellulosic biomass with bifunctional Ga/ZSM-5 catalysts. Angew. Chem. 2012, 51, 1387–1390. [Google Scholar] [CrossRef] [PubMed]
- Veses, A.; Puértolas, B.; López, J.M.; Callén, M.S.; Solsona, B.; García, T. Promoting Deoxygenation of Bio-Oil by Metal-Loaded Hierarchical ZSM-5 Zeolites. ACS Sustain. Chem. Eng. 2016, 4, 1653–1660. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, H.; Zhou, F.; Chen, K.-Q.; Qiao, K.; Lu, X.-Y.; Ouyang, P.-K.; Fu, J. Catalytic fast pyrolysis of rice straw to aromatic compounds over hierarchical HZSM-5 produced by alkali treatment and metal-modification. J. Anal. Appl. Pyrolysis 2018, 131, 76–84. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, J.; Rui, X. Catalytic Pyrolysis of Willow Wood with Me/ZSM-5 (Me = Mg, K, Fe, Ga, Ni) to Produce Aromatics and Olefins. Bioresources 2013, 8, 5612–5621. [Google Scholar] [CrossRef]
- Zheng, Y.-W.; Wang, J.-D.; Wang, D.-C.; Zheng, Z.-F. Advanced catalytic upgrading of biomass pyrolysis vapor to bio-aromatics hydrocarbon: A review. Appl. Energy Combust. Sci. 2022, 10, 100061. [Google Scholar] [CrossRef]
- Mayer, F.M.; Teixeira, C.M.; Pacheco, J.G.A.; de Souza, C.T.; de Vila Bauer, D.; Caramão, E.B.; da Silveira Espíndola, J.; Trierweiler, J.O.; Lopez, O.W.P.; Zini, C.A. Characterization of analytical fast pyrolysis vapors of medium-density fiberboard (mdf) using metal-modified HZSM-5. J. Anal. Appl. Pyrol 2018, 136, 87–95. [Google Scholar] [CrossRef]
- Sun, P.; Gao, G.; Zhao, Z.; Xia, C.; Li, F. Acidity-regulation for enhancing the stability of Ni/HZSM-5 catalyst for valeric biofuel production. Appl. Catal. B Environ. 2016, 189, 19–25. [Google Scholar] [CrossRef]
- Uslamin, E.A.; Kosinov, N.A.; Pidko, E.A.; Hensen, E.J.M. Catalytic conversion of furanic compounds over Ga-modified ZSM-5 zeolites as a route to biomass-derived aromatics. Green Chem. 2018, 20, 3818–3827. [Google Scholar] [CrossRef]
- Iliopoulou, E.F.; Stefanidis, S.D.; Kalogiannis, K.G.; Delimitis, A.; Lappas, A.A.; Triantafyllidis, K.S. Catalytic upgrading of biomass pyrolysis vapors using transition metal-modified ZSM-5 zeolite. Appl. Catal. B Environ. 2012, 127, 281–290. [Google Scholar] [CrossRef]

| Catalyst | Feedstock | Ratio of Biomass to Catalyst | T/°C | Aromatic Content/% |
|---|---|---|---|---|
| HZSM-5 | Prairie cordgrass | 10:1 | 600 | 24.5 |
| Co/HZSM-5 | Prairie cordgrass | 10:1 | 600 | 30.3 |
| Mo/HZSM-5 | Prairie cordgrass | 10:1 | 600 | 26.7 |
| MoO3/HZSM-5 | Torrefied switchgrass | 1:10 | 700 | 21.9 |
| Mo2C/HZSM-5 | Torrefied switchgrass | 1:10 | 700 | 25.0 |
| Fe/HZSM-5 | switchgrass | 1:10 | 550 | 17.0 |
| P/HZSM-5 | Rape straw | 1:10 | 550 | 48.4 |
| Zn/HZSM-5 | Rape straw | 1:10 | 550 | 44.7 |
| Ti/HZSM-5 | Rape straw | 1:10 | 550 | 50.1 |
| Co/HZSM-5 | Jatropha residues | 1:1 | 500 | 24.6 |
| Ni/HZSM-5 | Jatropha residues | 1:1 | 500 | 20.9 |
| Mo/HZSM-5 | Jatropha residues | 1:1 | 500 | 26.9 |
| Ga/HZSM-5 | Jatropha residues | 1:1 | 500 | 27.6 |
| Pd/HZSM-5 | Jatropha residues | 1:1 | 500 | 27.2 |
| La/HZSM-5 | Rape straw | - | 500 | 49.9 |
| Mg/HZSM-5 | Woody | 1:10 | 450 | 29.0 |
| Cu/HZSM-5 | Woody | 1:10 | 450 | 31.0 |
| Sn/HZSM-5 | Woody | 1:10 | 450 | 33.0 |
| Catalyst | Feedstock | Ratio of Biomass to Catalyst | T/°C | Aromatic Content/% |
|---|---|---|---|---|
| Mo-Co/HZSM-5 | Prairie cordgrass | 10:1 | 600 | 41.1 |
| Mo-Zn/HZSM-5 | Torrefied switchgrass | 1:10 | 700 | 39.3 |
| Mo-Ag/HZSM-5 | Torrefied switchgrass | 1:10 | 700 | 23.8 |
| Ga-Ni/HZSM-5 | Debarked eucalyptus trunks | 1:10 | 600 | 16.1 |
| Zn-p/ZSM-5 | Rape straw | 1:10 | 500 | 74.6 |
| Mo2N/HZSM-5 | Pine wood | 1:5 | 750 | 73.7 |
| W2N/HZSM-5 | Pine wood | 1:5 | 750 | 43.4 |
| Mo-P/HZSM-5 | Pine wood | 1:5 | 750 | 39.1 |
| W-P/HZSM-5 | Pine wood | 1:5 | 750 | 60.6 |
| Ce-Mo2N/HZSM-5 | Pine wood | 1:5 | 750 | 71.4 |
| La-Mo2N/HZSM-5 | Pine wood | 1:5 | 750 | 17.6 |
| Cr-Mo2N/HZSM-5 | Pine wood | 1:5 | 750 | 71.6 |
| Cu-Mo2N/HZSM-5 | Pine wood | 1:5 | 750 | 61.1 |
| Fe-Mo2N/HZSM-5 | Pine wood | 1:5 | 750 | 65.6 |
| Mo-Co/HZSM-5 | Lignite | 1:1 | 600 | 80.7 |
| Ni-Co/HZSM-5 | Pine sawdust | 1:1 | 600 | 75.2 |
| Mo-Cu/HZSM-5 | Sugarcane bagasse | 1:10 | 550 | 36.5 |
| Ni-Ce/HZSM-5 | Pine wood | 1:1 | 500 | 13.8 |
| Zn-Ni/ZSM-5 | Pine wood | 1:2 | 500 | 38.5 |
| Zn-Ga/ZSM-5 | Pine wood | 1:2 | 500 | 39.3 |
| Zn-Co/ZSM-5 | Pine wood | 1:2 | 500 | 79.1 |
| Ni-Cu/HZSM-5 | Pine wood | 1:2 | 450 | 46.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Yan, Y.; Jiang, Y.; Cao, Y.; Wang, Z.; Li, J.; Yan, C.; Wang, D.; Yuan, L.; Zhao, G. Release Pattern of Light Aromatic Hydrocarbons during the Biomass Roasting Process. Molecules 2024, 29, 1188. https://doi.org/10.3390/molecules29061188
Zhao Y, Yan Y, Jiang Y, Cao Y, Wang Z, Li J, Yan C, Wang D, Yuan L, Zhao G. Release Pattern of Light Aromatic Hydrocarbons during the Biomass Roasting Process. Molecules. 2024; 29(6):1188. https://doi.org/10.3390/molecules29061188
Chicago/Turabian StyleZhao, Yaying, Yuqing Yan, Yuhang Jiang, Yang Cao, Zhuozhi Wang, Jiapeng Li, Chenshuai Yan, Danya Wang, Lu Yuan, and Guangbo Zhao. 2024. "Release Pattern of Light Aromatic Hydrocarbons during the Biomass Roasting Process" Molecules 29, no. 6: 1188. https://doi.org/10.3390/molecules29061188
APA StyleZhao, Y., Yan, Y., Jiang, Y., Cao, Y., Wang, Z., Li, J., Yan, C., Wang, D., Yuan, L., & Zhao, G. (2024). Release Pattern of Light Aromatic Hydrocarbons during the Biomass Roasting Process. Molecules, 29(6), 1188. https://doi.org/10.3390/molecules29061188






