Unpredictable Chemical Diversity of Essential Oils in Cinnamomum burmanni (Lauraceae) Living Collections: Beyond Maternally Inherited Phylogenetic Relationships
Abstract
:1. Introduction
2. Results
2.1. Chemical Compositions and Chemotype Identification
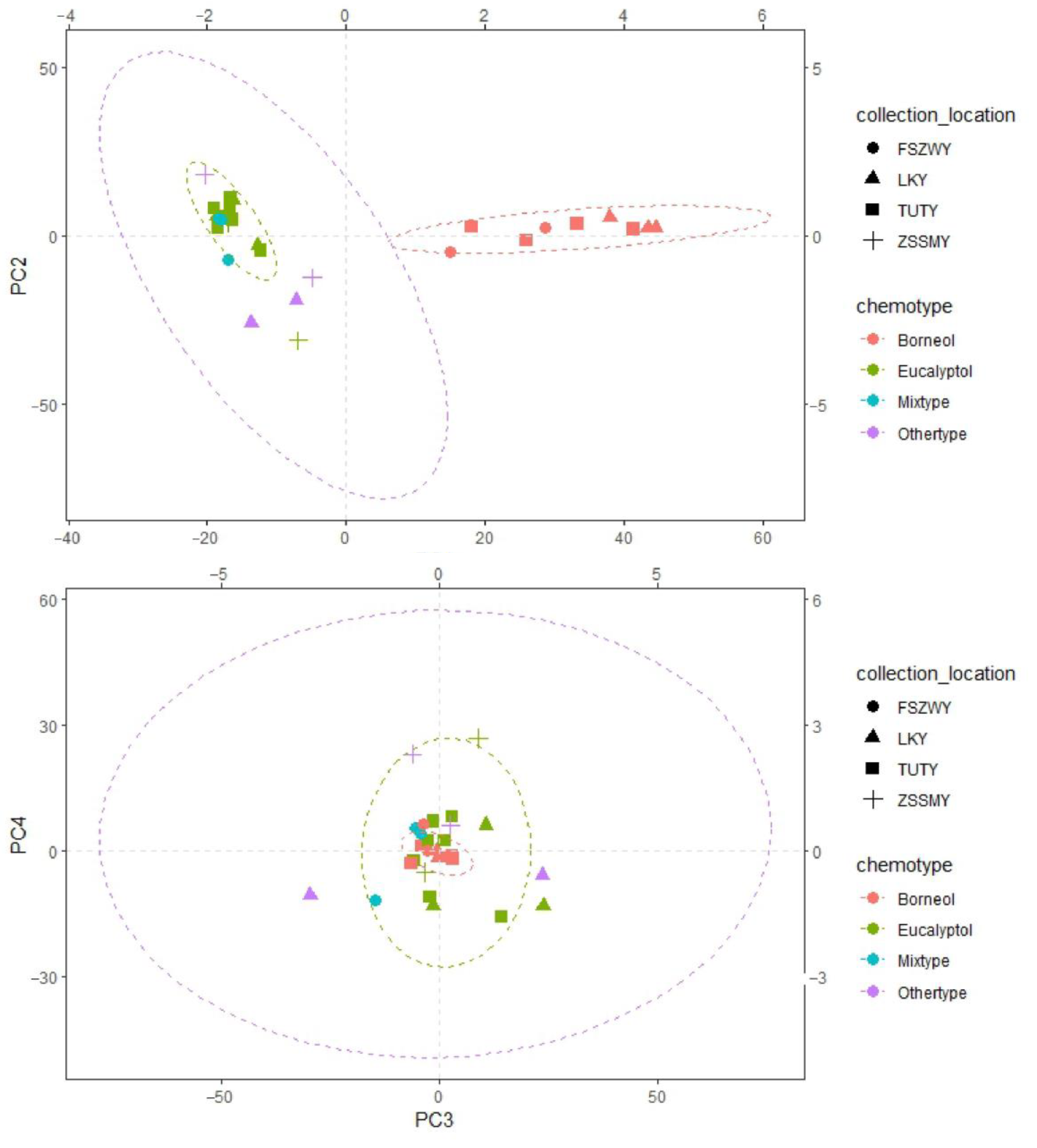
2.2. PCA and Correlation Analysis
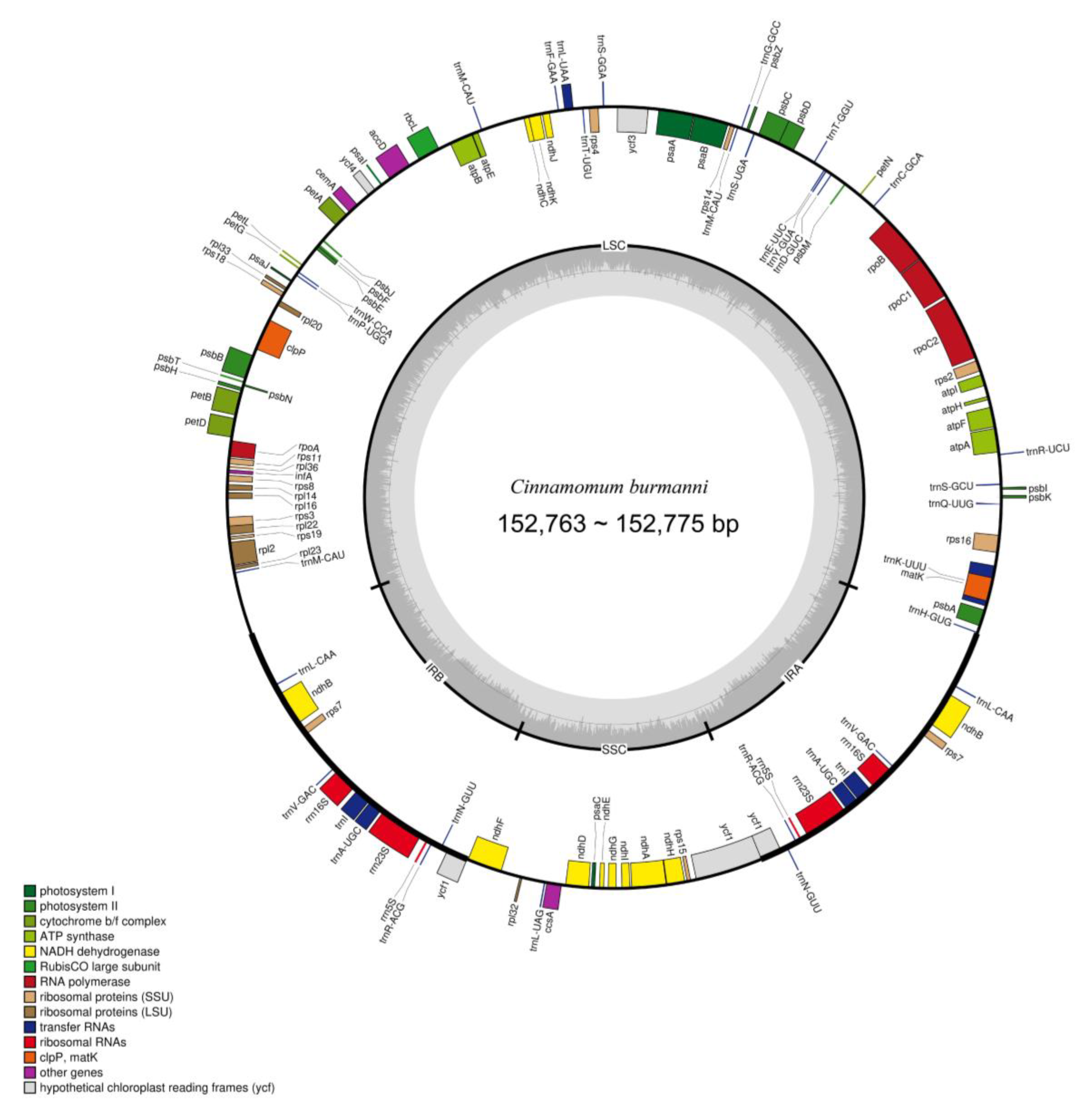
2.3. Plastid Genome De Novo Assembly and Gene Organization
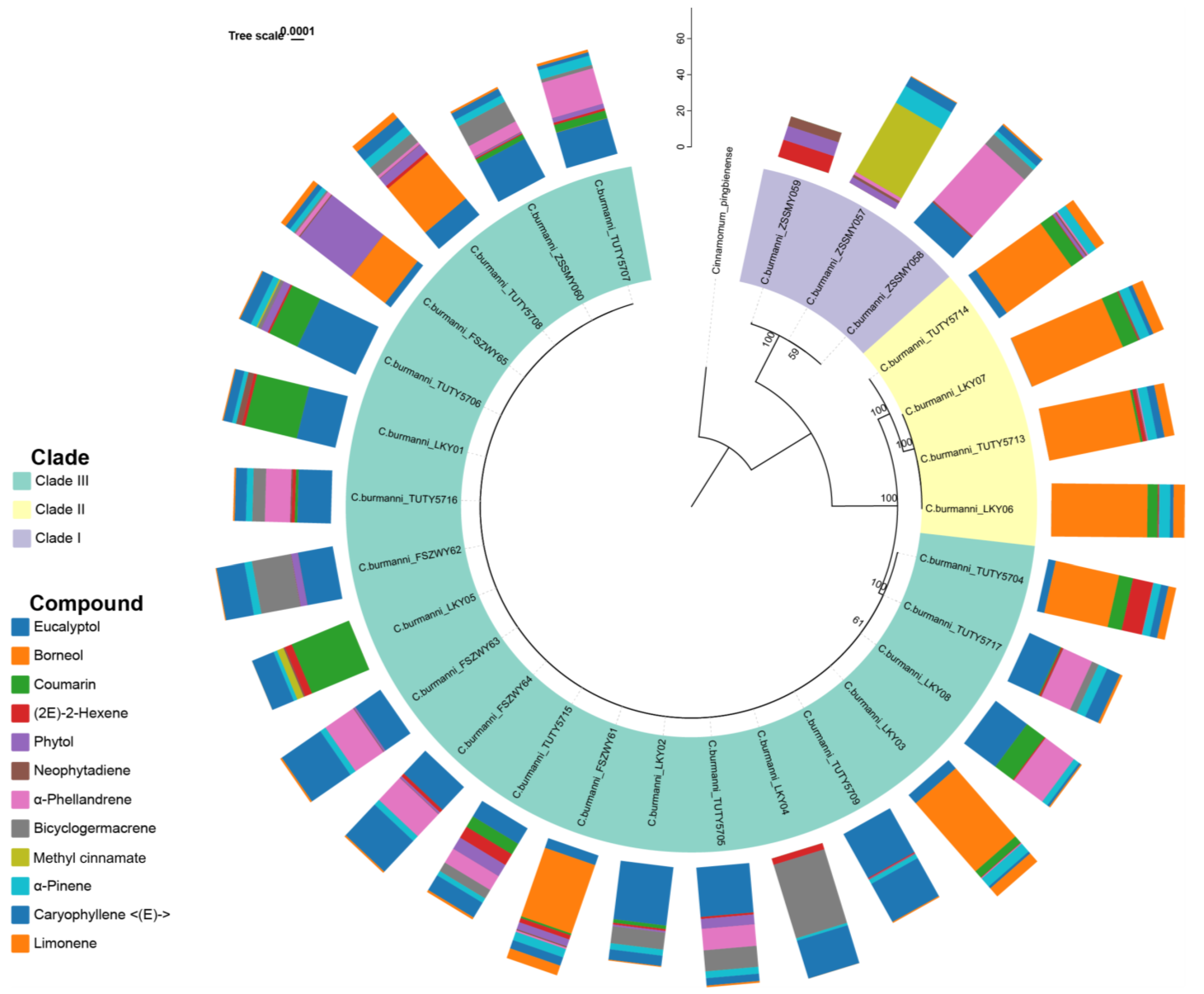
2.4. Phylogenetic Analysis
2.5. Phylogenetic Signal Test
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Essential Oils Extraction and Identification
4.3. Statistical Analysis of Essential Oils
4.4. DNA Extraction, Sequencing, Plastome Assembly, and Annotation
4.5. Phylogenetic Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Z.; Liu, B.; Yang, Y.; Ferguson, D.K. Phylogeny and taxonomy of Cinnamomum (Lauraceae). Ecol. Evol. 2022, 12, e9378. [Google Scholar] [CrossRef]
- Wu, Z.; Raven, P.H.; Hong, D. Flora of China; Volume 7: Menispermaceae through Capparaceae; Science Press: Beijing, China, 2008. [Google Scholar]
- Padalia, R.C.; Verma, R.S.; Sah, A.; Karki, N.; Chauhan, A.; Sakia, D.; Krishna, B. Study on Chemotypic variations in essential oil of Cinnamomum tamala (Buch.-Ham.) Nees et Eberm. and their antibacterial and antioxidant potential. J. Essent. Oil Bear. Plants 2012, 15, 800–808. [Google Scholar] [CrossRef]
- Bandara, T.; Uluwaduge, I.; Jansz, E.R. Bioactivity of cinnamon with special emphasis on diabetes mellitus: A review. Int. J. Food Sci. Nutr. 2012, 63, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Melgarejo-Flores, B.G.; Ortega-Ramírez, L.A.; Silva-Espinoza, B.A.; González-Aguilar, G.A.; Miranda, M.R.A.; Ayala-Zavala, J.F. Antifungal protection and antioxidant enhancement of table grapes treated with emulsions, vapors, and coatings of cinnamon leaf oil. Postharvest Biol. Technol. 2013, 86, 321–328. [Google Scholar] [CrossRef]
- Rao, P.V.; Gan, S.H. Cinnamon: A Multifaceted Medicinal Plant. Evid. Based Complement. Altern. Med. 2014, 2014, 642942. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumari, R.; Mishra, S. Pharmacological properties and their medicinal uses of Cinnamomum: A review. J. Pharm. Pharmacol. 2019, 71, 1735–1761. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Su, J.; Li, L.; Li, B.; Li, W. A new source of natural D-borneol and its characteristic. J. Med. Plants Res. 2011, 5, 7. [Google Scholar]
- Zhang, T.; Zheng, Y.; Fu, C.; Yang, H.; Liu, X.; Qiu, F.; Wang, X.; Wang, Z. Chemical Variation and Environmental Influence on Essential Oil of Cinnamomum camphora. Molecules 2023, 28, 973. [Google Scholar] [CrossRef]
- Yang, Z.; An, W.; Liu, S.; Huang, Y.; Xie, C.; Huang, S.; Zheng, X. Mining of candidate genes involved in the biosynthesis of dextrorotatory borneol in Cinnamomum burmannii by transcriptomic analysis on three chemotypes. PeerJ 2020, 8, e9311. [Google Scholar] [CrossRef]
- Chai, Y.; Yin, Z.; Fan, Q.; Zhang, Z.; Ye, K.; Xu, Y.; Xiao, W.; Chai, X.; Zhu, T.; Nie, H. Protective Effects of Angong Niuhuang Pill on Early Atherosclerosis in ApoE-/- Mice by Reducing the Inflammatory Response. Evid. Based Complement. Altern. Med. 2019, 2019, 9747212. [Google Scholar] [CrossRef]
- Chen, Z.-X.; Xu, Q.-Q.; Shan, C.-S.; Shi, Y.-H.; Wang, Y.; Chang, R.C.-C.; Zheng., G.Q. Borneol for Regulating the Permeability of the Blood-Brain Barrier in Experimental Ischemic Stroke: Preclinical Evidence and Possible Mechanism. Oxidative Med. Cell. Longev. 2019, 2019, 2936737. [Google Scholar] [CrossRef]
- Huang, J.; Tang, X.; Ye, F.; He, J.; Kong, X. Clinical Therapeutic Effects of Aspirin in Combination with Fufang Danshen Diwan, a Traditional Chinese Medicine Formula, on Coronary Heart Disease: A Systematic Review and Meta-Analysis. Cell. Physiol. Biochem. 2016, 39, 1955–1963. [Google Scholar] [CrossRef]
- Ren, L.; Wang, J.; Feng, L.; Wang, S.; Li, J. Efficacy of Suxiao Jiuxin Pill on Coronary Heart Disease: A Meta-Analysis of Randomized Controlled Trials. Evid. Based Complement. Altern. Med. 2018, 2018, 9745804. [Google Scholar] [CrossRef]
- Fu, M.; Lu, Z.; Ma, X. Enhanced extraction efficiency of natural D-borneol from Mei Pian tree leaves pretreated with deep eutectic solvents. Food Sci. Nutr. 2020, 8, 3806–3813. [Google Scholar] [CrossRef]
- Cheng, C.; Liu, X.-W.; Du, F.-F.; Li, M.-J.; Xu, F.; Wang, F.-Q.; Liu, Y.; Li, C.; Sun, Y. Sensitive assay for measurement of volatile borneol, isoborneol, and the metabolite camphor in rat pharmacokinetic study of Borneolum (Bingpian) and Borneolum syntheticum (synthetic Bingpian). Acta Pharmacol. Sin. 2013, 34, 1337–1348. [Google Scholar] [CrossRef]
- Yang, M.-Y.; Khine, A.A.; Liu, J.-W.; Cheng, H.-C.; Hu, A.; Chen, H.-P.; Shih, T.-L. Resolution of isoborneol and its isomers by GC/MS to identify “synthetic” and “semi-synthetic” borneol products. Chirality 2018, 30, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Mathen, P.G.; Sreekrishnan, T.P.; Kumar, K.P.G.; Mohan, N. Camphor Poisoning: A Rare Cause of Acute Symptomatic Seizures in Children. J. Emergencies Trauma Shock 2018, 11, 228–229. [Google Scholar] [CrossRef] [PubMed]
- As, K.; Khc, B. Chemical composition of leaf and seed oils of Dryobalanops aromatica Gaertn. (Dipterocarpaceae). ASEAN J. Sci. Technol. Dev. 2012, 29, 105. [Google Scholar]
- Wu, H.; Zhu, L.-F.; Li, Y.-J. A Study on Infraspecific Types of Cheracter of Cinnamomum burmanni. J. Integr. Plant Biol. 1992, 34, 302–308. [Google Scholar]
- Zhu, P.L.; Hong, X.L.; Yang, C.X. Present Situation and Development of Forest Chinese Medicine in Jiangxi. Jiangxi For. Sci. Technol. 2011, 41–44. [Google Scholar] [CrossRef]
- Pereira, I.; Severino, P.; Santos, A.C.; Silva, A.M.; Souto, E.B. Linalool bioactive properties and potential applicability in drug delivery systems. Colloids and Surfaces B: Biointerfaces 2018, 171, 566–578. [Google Scholar] [CrossRef]
- Banerjee, A.; Sharkey, T.D. Methylerythritol 4-phosphate (MEP) pathway metabolic regulation. Nat. Prod. Rep. 2014, 31, 1043–1055. [Google Scholar] [CrossRef]
- Li, F.; Huang, S.; Mei, Y.; Wu, B.; Hou, Z.; Zhan, P.; Hou, Z.; Huang, W.; Zhao, J.; Wang, J. Genome assembly provided new insights into the Cinnamomum burmannii evolution and D-borneol biosynthesis differences between chemotypes. Ind. Crops Prod. 2022, 186, 115181. [Google Scholar] [CrossRef]
- Ma, Q.; Ma, R.; Su, P.; Jin, B.; Guo, J.; Tang, J.; Chen, T.; Zeng, W.; Lai, C.; Ling, F.; et al. Elucidation of the essential oil biosynthetic pathways in Cinnamomum burmannii through identification of six terpene synthases. Plant Sci. 2022, 317, 111203. [Google Scholar] [CrossRef]
- Yang, Y.; Song, Y.; Xin, P. The chloroplast genome of aromatic plants Cinnamomum burmanni (Lauraceae). Mitochondrial DNA Part B 2019, 4, 3616–3617. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-F.; Ma, H.; Ci, X.-Q.; Li, L.; Song, Y.; Liu, B.; Li, H.-W.; Wang, S.-L.; Qu, X.-J.; Hu, J.-L.; et al. Can plastid genome sequencing be used for species identification in Lauraceae? Bot. J. Linn. Soc. 2021, 197, 1–14. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, J.; Huang, H.-W. Parallel situ conservation: A new plant conservation strategy to integrate in situ and ex situ conservation of plants. Biodiv. Sci. 2023, 31, 23184. [Google Scholar] [CrossRef]
- Ren, H.; He, T.; Wen, S.-F.; Dong, H. The main features of the world-class national botanical garden with Chinese characteristics. Biodiv. Sci. 2023, 31, 23192. [Google Scholar] [CrossRef]
- Chang, H.-T.; Lin, C.-Y.; Hsu, L.-S.; Chang, S.-T. Thermal degradation of linalool-chemotype Cinnamomum osmophloeum leaf essential oil and its stabilization by microencapsulation with β-cyclodextrin. Molecules 2021, 26, 409. [Google Scholar] [CrossRef]
- Qiu, F.; Yang, H.; Zhang, T.; Wang, X.; Wen, S.; Su, X. Chemical Composition of Leaf Essential Oil of Cinnamomum porrectum (Roxb.) Kosterm. J. Essent. Oil Bear. Plants 2019, 22, 1313–1321. [Google Scholar] [CrossRef]
- Yang, H.-K.; Zhang, T.; Wang, X.-D.; Wen, S.-F.; Guo, Y.; Jiang, X.-M. A Study on the Chemical Comoponents in Essential Oil from Leavesof Cinnamomum kanehirae and Chemotype Divisions. Acta Agric. Univ. Jiangxiensis 2016, 38, 668–673. [Google Scholar] [CrossRef]
- Frizzo, C.D.; Santos, A.C.; Paroul, N.; Serafini, L.A.; Dellacassa, E.; Lorenzo, D.; Moyna, P. Essential oils of camphor tree (Cinnamomum camphora Nees & Eberm) cultivated in Southern Brazil. Braz. Arch. Biol. Technol. 2000, 43, 313–316. [Google Scholar]
- Nath, S.C.; Sarma Baruah, A.K. Eugenol as the Major Component of the Leaf Oils of Cinnamomum impressinervium Meissn. J. Essent. Oil Res. 1994, 6, 211–212. [Google Scholar] [CrossRef]
- Patel, K.; Ali, S.; Sotheeswaran, S.; Dufour, J.-P. Composition of the Leaf Essential Oil of Cinnamomum verum (Lauraceae) from Fiji Islands. J. Essent. Oil Bear. Plants 2007, 10, 374–377. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, C.; Xiao, Z.; Zhang, H.; Cao, M.; Liu, Y.; Jin, Z. Chemical Constituents and Chemotypes of Fresh Leaf Essential Oil of Wild Species Belonging to Sect. Camphor (Trew.) Meissn. in Southeastern China. J. Essent. Oil Bear. Plants 2019, 22, 1115–1122. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Yeh, T.-F.; Hsu, F.-L.; Lin, C.-Y.; Chang, S.-T.; Chang, H.-T. Xanthine oxidase inhibitory activity and thermostability of cinnamaldehyde-chemotype leaf oil of Cinnamomum osmophloeum microencapsulated with β-cyclodextrin. Molecules 2018, 23, 1107. [Google Scholar] [CrossRef]
- Murray, R.D.H. The Naturally Occurring Coumarins. In Fortschritte der Chemie organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products; Murray, R.D.H., Herz, W., Falk, H., Kirby, G.W., Moore, R.E., Eds.; Springer: Vienna, Austria, 2002; pp. 1–619. [Google Scholar]
- Wang, Y.-H.; Avula, B.; Nanayakkara, N.P.D.; Zhao, J.; Khan, I.A. Cassia Cinnamon as a Source of Coumarin in Cinnamon-Flavored Food and Food Supplements in the United States. J. Agric. Food Chem. 2013, 61, 4470–4476. [Google Scholar] [CrossRef] [PubMed]
- Code of Federal Regulations. Title 21, Part 189- Substances prohibited from use in human food: Sec. 189.130 Coumarin. 2011. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-189 (accessed on 5 March 2024).
- Lake, B.G. Coumarin Metabolism, Toxicity and Carcinogenicity: Relevance for Human Risk Assessment. Food Chem. Toxicol. 1999, 37, 423–453. [Google Scholar] [CrossRef] [PubMed]
- Willis, K. State of the World’s Plants 2017; Royal Botanics Gardens Kew: Richmond, UK, 2017. [Google Scholar]
- Ihaka, R.; Gentleman, R. R: A Language for Data Analysis and Graphics. J. Comput. Graph. Stat. 1996, 5, 299–314. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2. WIREs Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Pedersen, T.L.; Pedersen, M.T.L.; LazyData, T.; Rcpp, I.; Rcpp, L. Package “Ggforce”. Accelerating “Ggplot2”. R Package Version 0.3 2020, 1, 477. [Google Scholar]
- Wei, T.; Simko, V.; Levy, M.; Xie, Y.; Jin, Y.; Zemla, J. Package ‘corrplot’. Statistician 2017, 56, e24. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Paradis, E.; Blomberg, S.; Bolker, B.; Brown, J.; Claude, J.; Cuong, H.S.; Desper, R.; Didier, G. Package ‘ape’. Anal. Phylogenetics Evol. Version 2019, 13, 47. [Google Scholar]
- Harmon, L.; Weir, J.; Brock, C.; Glor, R.; Challenger, W.; Hunt, G.; FitzJohn, R.; Pennell, M.; Slater, G.; Brown, J.; et al. Package ‘geiger’. R Package Version 2015, 2, 3. [Google Scholar]
- Revell, L.J. Phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 2012, 2, 217–223. [Google Scholar] [CrossRef]
- Orme, D.; Freckleton, R.; Thomas, G.; Petzoldt, T.; Fritz, S.; Isaac, N.; Pearse, W. The caper package: Comparative analysis of phylogenetics and evolution in R. R Package Version 2013, 5, 1–36. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Jin, J.-J.; Yu, W.-B.; Yang, J.-B.; Song, Y.; dePamphilis, C.W.; Yi, T.-S.; Li, D.Z. GetOrganelle: A fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 2020, 21, 241. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Wick, R.R.; Schultz, M.B.; Zobel, J.; Holt, K.E. Bandage: Interactive visualization of de novo genome assemblies. Bioinformatics 2015, 31, 3350–3352. [Google Scholar] [CrossRef] [PubMed]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Xie, J.; Chen, Y.; Cai, G.; Cai, R.; Hu, Z.; Wang, H. Tree Visualization by One Table (tvBOT): A web application for visualizing, modifying and annotating phylogenetic trees. Nucleic Acids Res. 2023, 51, W587–W592. [Google Scholar] [CrossRef]
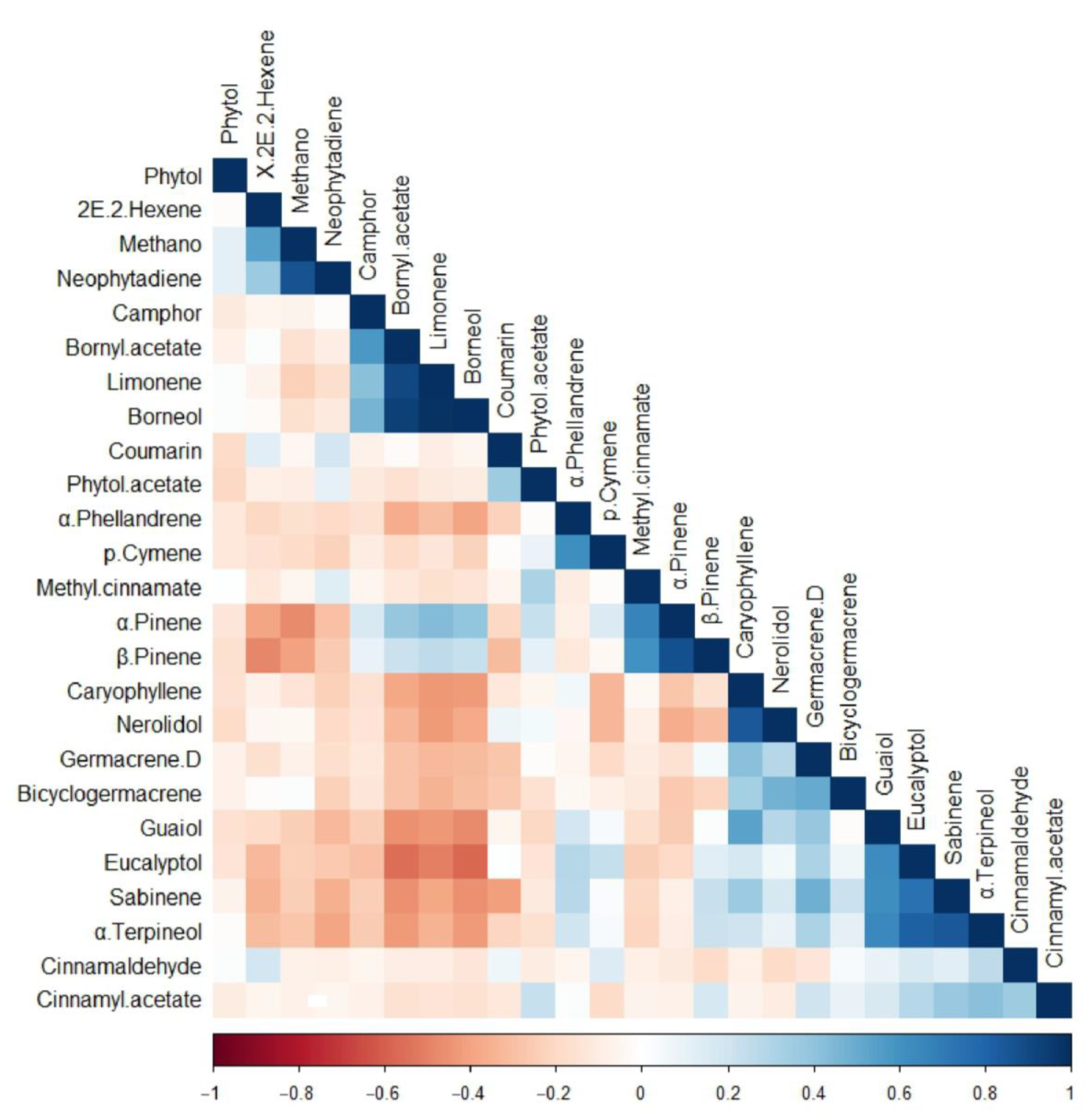
| Compounds | RI | LKY03 | LKY05 | TUTY5706 | TUTY5713 | ZSSMY057 | ZSSMY060 | FSZWY61 | FSZWY63 |
|---|---|---|---|---|---|---|---|---|---|
| (2E)-2-Hexene | 850 | 0.32 | 4.12 | 1.07 | 1.88 | 0.67 | 2.45 | ||
| α-Pinene | 933 | 5.78 | 2.1 | 3.47 | 5.06 | 10.69 | 4.04 | 4.8 | 3.43 |
| Sabinene | 972 | 1.92 | 2.1 | 6.92 | 1.48 | 6.21 | |||
| β-Pinene | 978 | 2.15 | 1.97 | 1.81 | 4.44 | 1.75 | 1.87 | 1.41 | |
| α-Phellandrene | 1007 | 0.55 | 0.65 | 1.89 | 6.52 | 0.93 | 18.69 | ||
| p-Cymene | 1025 | 2.34 | 0.89 | 0.53 | 2.3 | 6.58 | 0.41 | ||
| Limonene | 1030 | 5.88 | 0.59 | 5.48 | 0.42 | 1.1 | 5.6 | 0.94 | |
| Eucalyptol | 1032 | 6.05 | 35.85 | 24.01 | 5.65 | 16.8 | |||
| Camphor | 1149 | 4.13 | |||||||
| Borneol | 1173 | 50.15 | 51.49 | 40.15 | |||||
| α-Terpineol | 1199 | 2.26 | 0.9 | 7.37 | 6.26 | 1.53 | 3.92 | ||
| (E)-Cinnamaldehyde | 1273 | 0.03 | 1.08 | ||||||
| Bornyl acetate | 1285 | 2.81 | 6.89 | 2.82 | |||||
| Methyl cinnamate | 1384 | 3.8 | 0.82 | 44.25 | |||||
| (E)-Caryophyllene | 1424 | 1.33 | 12.86 | 6.64 | 4.22 | 5.87 | 4.01 | 4.85 | 25.9 |
| Coumarin | 1438 | 4.47 | 34.31 | 17.06 | 1.24 | 2.69 | 1.01 | ||
| (E)-Cinnamyl acetate | 1448 | 0.93 | |||||||
| Bicyclogermacrene | 1497 | 1.9 | 12.41 | ||||||
| (E)-Nerolidol | 1561 | 2.51 | 1.29 | 3.2 | |||||
| Germacrene D | 1480 | ||||||||
| Guaiol | 1603 | 2.3 | 2.21 | 2.81 | 1.44 | 2.05 | |||
| Neophytadiene | 1836 | 0.86 | 0.16 | 0.6 | 1.46 | 0.26 | 0.71 | 0.39 | |
| Phytol | 2106 | 4.1 | 3.54 | 0.75 | 4.05 | 1.05 | |||
| Phytol acetate | 2212 | 4.66 | 6.77 | 4.26 |
| Compounds | PC1 | PC2 | PC3 | PC4 |
|---|---|---|---|---|
| (2E)-2-Hexene | 0.007137 | −0.07748 | 0.025247 | −0.01351 |
| α-Pinene | 0.03166 | −0.01109 | 0.013891 | 0.079372 |
| Sabinene | −0.0747 | 0.120587 | −0.10141 | −0.06655 |
| β-Pinene | 0.007353 | 0.004175 | 0.001428 | 0.020175 |
| α-Phellandrene | −0.19855 | 0.507336 | −0.10965 | 0.618629 |
| p-Cymene | −0.0315 | 0.110504 | 0.037194 | 0.122443 |
| Limonene | 0.089563 | 0.047327 | −0.01443 | 0.002037 |
| Eucalyptol | −0.31716 | 0.54983 | 0.097892 | −0.39991 |
| Camphor | 0.015634 | 0.004143 | −0.00539 | 0.001487 |
| Borneol | 0.889358 | 0.249417 | −0.12253 | −0.0748 |
| α-Terpineol | −0.05399 | 0.104253 | −0.03002 | −0.07722 |
| (E)-Cinnamaldehyde | −0.01036 | 0.007017 | 0.013644 | −0.02766 |
| Bornyl acetate | 0.100396 | 0.026362 | −0.01025 | −0.00878 |
| Methyl Cinnamate | −0.02336 | −0.45327 | 0.17169 | 0.442389 |
| (E)-Caryophyllene | −0.14359 | −0.15773 | −0.25804 | −0.16853 |
| Coumarin | −0.00125 | −0.12113 | 0.673163 | −0.315 |
| Methano | −0.00269 | −0.02349 | 0.002636 | 0.00352 |
| (E)-Cinnamyl acetate | −0.0114 | 0.019374 | −0.00923 | −0.01975 |
| Germacrene D | −0.02679 | −0.01251 | −0.07591 | −0.05683 |
| Bicyclogermacrene | −0.12734 | −0.26582 | −0.61817 | −0.27916 |
| (E)-Nerolidol | −0.01728 | −0.03422 | −0.03042 | −0.03499 |
| Guaiol | −0.03271 | 0.036051 | −0.00172 | −0.03787 |
| Neophytadiene | −0.00028 | −0.03547 | 0.037002 | 0.014107 |
| Phytol acetate | −0.00544 | −0.05115 | 0.084152 | 0.034344 |
| Phytol | 0.018862 | −0.09068 | −0.05362 | 0.098565 |
| Compounds | Pagel’s λ | p-Value |
|---|---|---|
| Eucalyptol | 0.52 | 0.15 |
| Borneol | 0.66 | 0.19 |
| Coumarin | 0.28 | 0.44 |
| (2E)-2-Hexene | 7.33 × 10−5 | 1 |
| Phytol | 7.33 × 10−5 | 1 |
| Neophytadiene | 7.33 × 10−5 | 1 |
| α-Phellandrene | 7.33 × 10−5 | 1 |
| Bicyclogermacrene | 7.33 × 10−5 | 1 |
| Methyl cinnamate | 7.33 × 10−5 | 1 |
| α-Pinene | 0.19 | 7.33 × 10−15 |
| Caryophyllene | 0.19 | 1.02 × 10−10 |
| Limonene | 7.33 × 10−5 | 1 |
| Voucher Number | Collecting Location | Collecting Date | Collector | Tree Height (m) | Diameter at Breast Height (cm) |
|---|---|---|---|---|---|
| LKY01 | GA | 2022.08.03 | PW Xie, JL Chen, JC Zhan | 4.6 | 12.5 |
| LKY02 | GA | 2022.08.03 | PW Xie, JL Chen, JC Zhan | 3.8 | 10.5 |
| LKY03 | GA | 2022.08.03 | PW Xie, JL Chen, JC Zhan | 7.5 | 17.8 |
| LKY04 | GA | 2022.08.03 | PW Xie, JL Chen, JC Zhan | 3.3 | 10.2 |
| LKY05 | GA | 2022.08.03 | PW Xie, JL Chen, JC Zhan | 3.8 | 10.6 |
| LKY06 | GA | 2022.08.03 | PW Xie, JL Chen, JC Zhan | 6.8 | 16.5 |
| LKY07 | FBG | 2022.08.03 | PW Xie, JL Chen, JC Zhan | 7.2 | 14.2 |
| LKY08 | FBG | 2022.08.03 | PW Xie, JL Chen, JC Zhan | 7.8 | 21.8 |
| FSZWY61 | FBG | 2022.08.07 | TY Tu, PW Xie | 8.8 | 31.8 |
| FSZWY62 | FBG | 2022.08.07 | TY Tu, PW Xie | 8.5 | 33.5 |
| FSZWY63 | FBG | 2022.08.07 | TY Tu, PW Xie | 8.5 | 30.5 |
| FSZWY64 | FBG | 2022.08.07 | TY Tu, PW Xie | 7.8 | 31.2 |
| FSZWY65 | FBG | 2022.08.07 | TY Tu, PW Xie | 7.5 | 28.8 |
| TUTY5704 | FBG | 2022.08.07 | TY Tu, PW Xie | 3.2 | 10.5 |
| TUTY5705 | FBG | 2022.08.07 | TY Tu, PW Xie | 2.5 | 8.2 |
| TUTY5706 | FBG | 2022.08.07 | TY Tu, PW Xie | 2.2 | 7.8 |
| TUTY5707 | FBG | 2022.08.07 | TY Tu, PW Xie | 7.0 | 20.6 |
| TUTY5708 | FBG | 2022.08.07 | TY Tu, PW Xie | 7.5 | 22.4 |
| TUTY5709 | FBG | 2022.08.07 | TY Tu, PW Xie | 7.2 | 21.5 |
| TUTY5713 | SCNBG | 2022.08.17 | TY Tu | 7.6 | 20.2 |
| TUTY5714 | SCNBG | 2022.08.17 | TY Tu | 2.1 | 7.5 |
| TUTY5715 | SCNBG | 2022.08.17 | TY Tu | 5.8 | 15.8 |
| TUTY5716 | SCNBG | 2022.08.17 | TY Tu | 4.5 | 12.6 |
| TUTY5717 | SCNBG | 2022.08.17 | TY Tu | 7.5 | 22.6 |
| ZSSMY057 | ZA | 2022.08.15 | HM Lian, YL Zhong, PW Xie | 5.2 | 15.6 |
| ZSSMY058 | ZA | 2022.08.15 | HM Lian, YL Zhong, PW Xie | 5.7 | 15.8 |
| ZSSMY059 | ZA | 2022.08.15 | HM Lian, YL Zhong, PW Xie | 5.7 | 16.2 |
| ZSSMY059 | ZA | 2022.08.15 | HM Lian, YL Zhong, PW Xie | 4.5 | 16.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, P.; Yang, Q.; Chen, J.; Tu, T.; Lian, H.; He, B.; Cai, Y. Unpredictable Chemical Diversity of Essential Oils in Cinnamomum burmanni (Lauraceae) Living Collections: Beyond Maternally Inherited Phylogenetic Relationships. Molecules 2024, 29, 1206. https://doi.org/10.3390/molecules29061206
Xie P, Yang Q, Chen J, Tu T, Lian H, He B, Cai Y. Unpredictable Chemical Diversity of Essential Oils in Cinnamomum burmanni (Lauraceae) Living Collections: Beyond Maternally Inherited Phylogenetic Relationships. Molecules. 2024; 29(6):1206. https://doi.org/10.3390/molecules29061206
Chicago/Turabian StyleXie, Peiwu, Qiyi Yang, Jielian Chen, Tieyao Tu, Huiming Lian, Boxiang He, and Yanling Cai. 2024. "Unpredictable Chemical Diversity of Essential Oils in Cinnamomum burmanni (Lauraceae) Living Collections: Beyond Maternally Inherited Phylogenetic Relationships" Molecules 29, no. 6: 1206. https://doi.org/10.3390/molecules29061206
APA StyleXie, P., Yang, Q., Chen, J., Tu, T., Lian, H., He, B., & Cai, Y. (2024). Unpredictable Chemical Diversity of Essential Oils in Cinnamomum burmanni (Lauraceae) Living Collections: Beyond Maternally Inherited Phylogenetic Relationships. Molecules, 29(6), 1206. https://doi.org/10.3390/molecules29061206






