Structure, Absolute Configuration, Antiproliferative and Phytotoxic Activities of Icetexane and Abietane Diterpenoids from Salvia carranzae and Chemotaxonomic Implications
Abstract
:1. Introduction
2. Results and Discussion
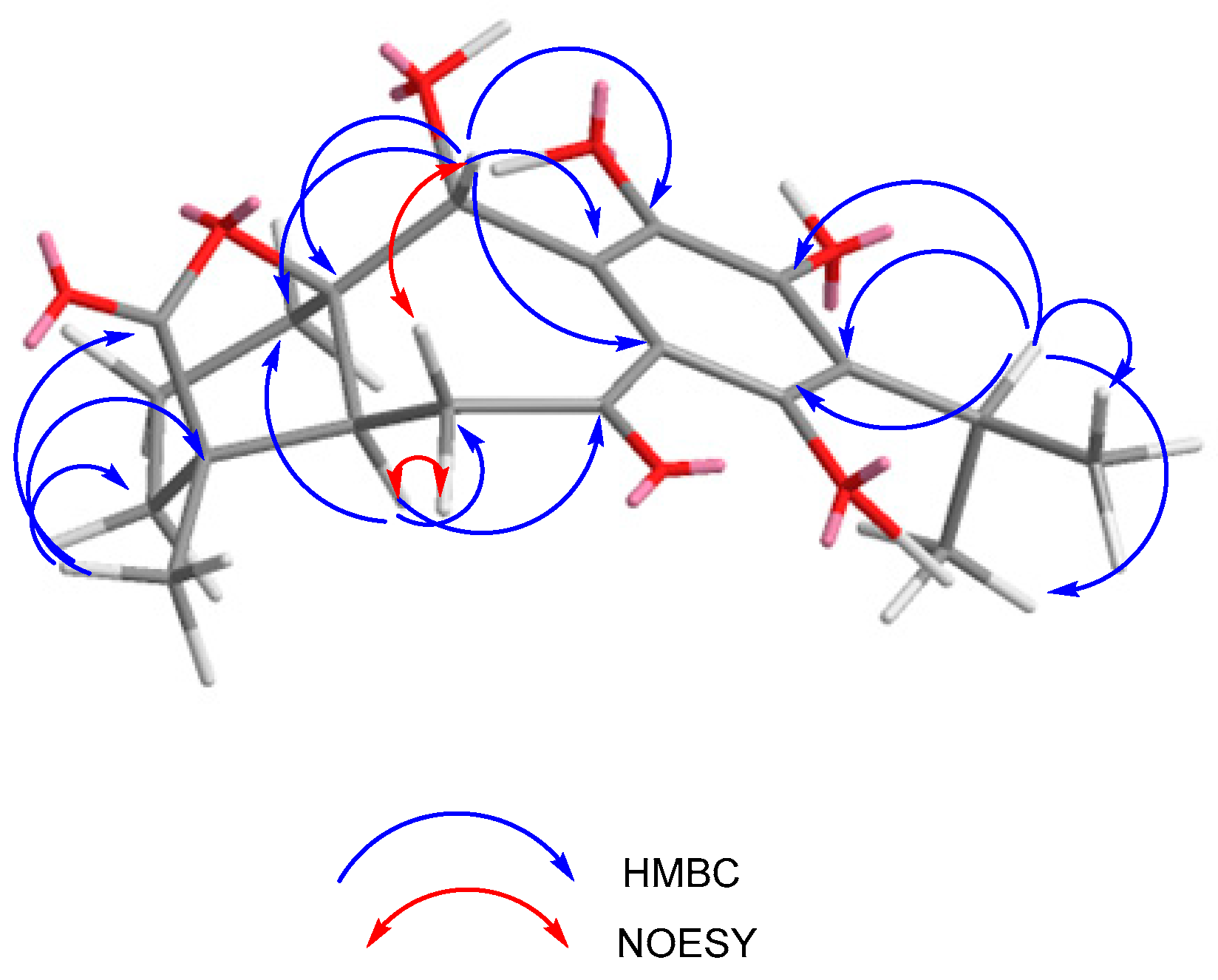
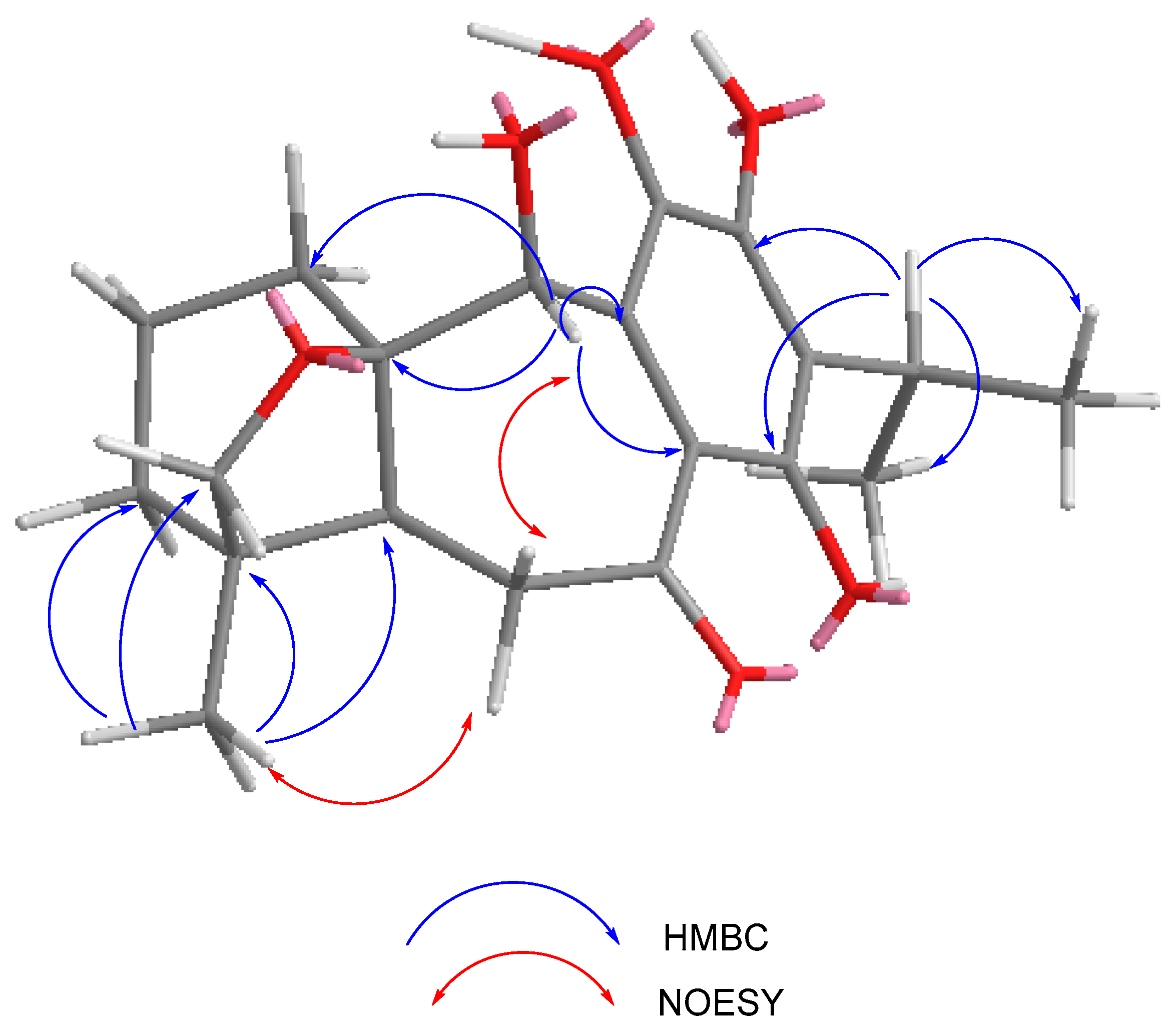
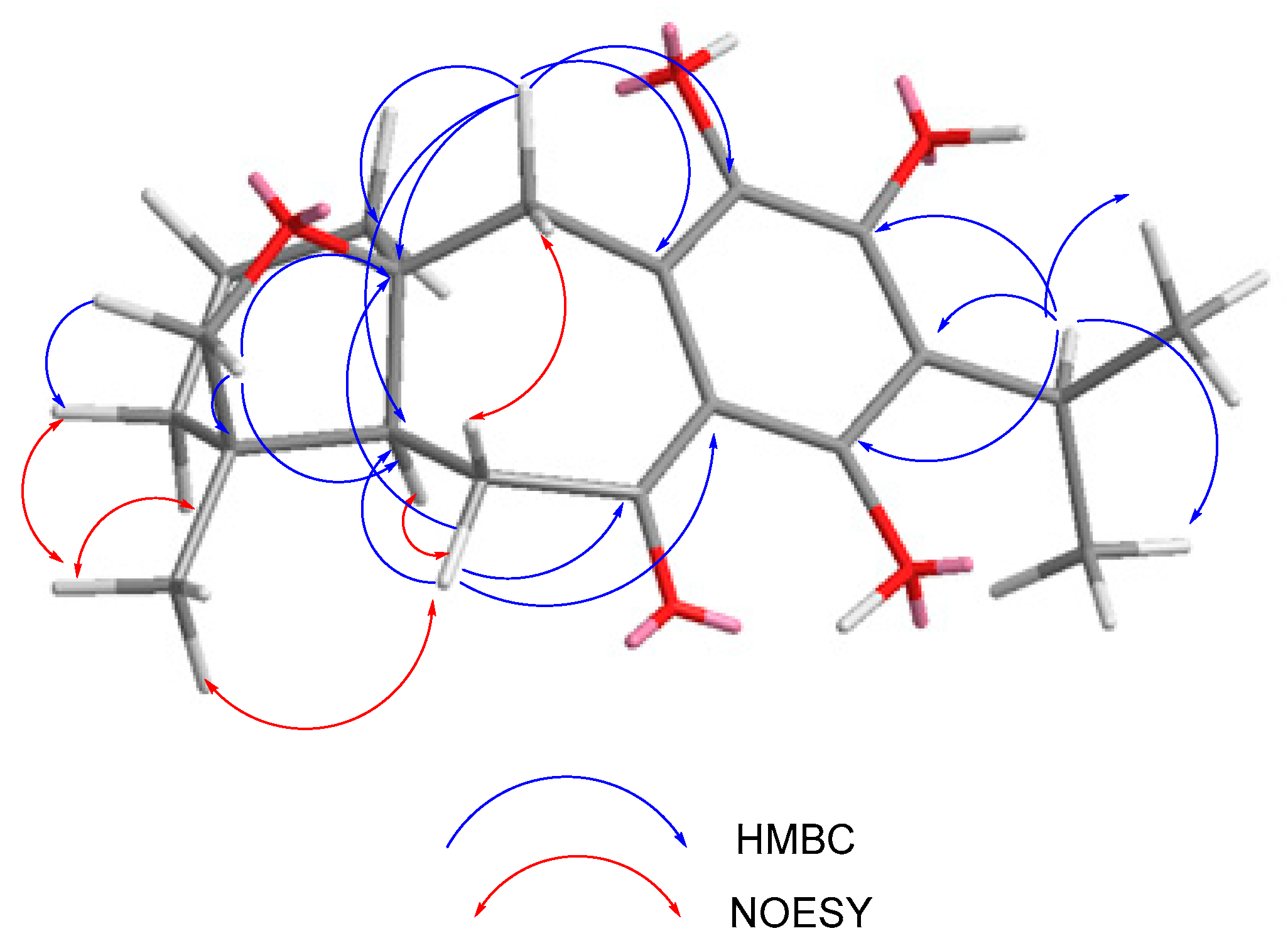
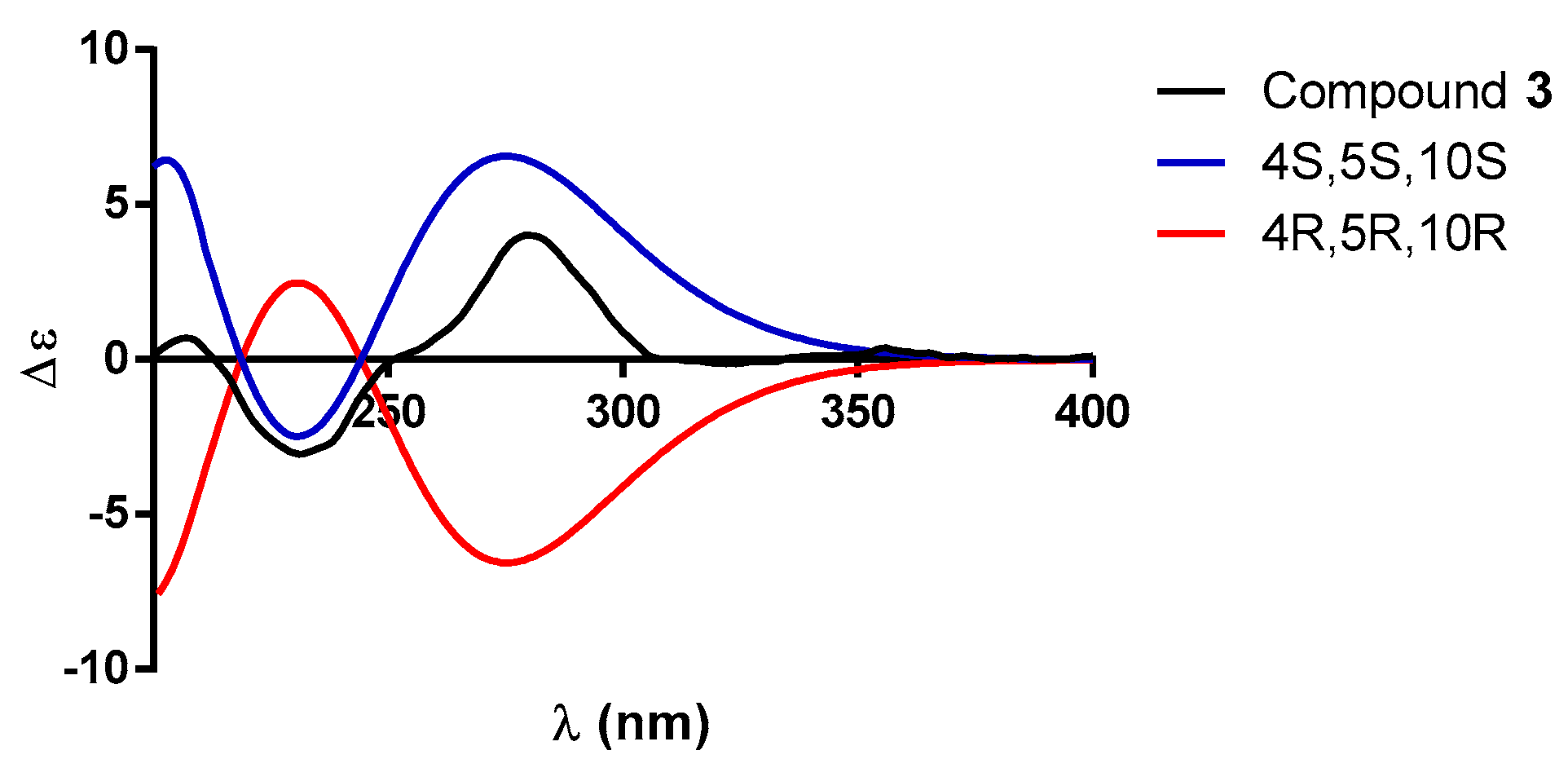
2.1. Characterization
2.2. Biological Activity
2.2.1. Antiproliferative Activity
2.2.2. Phytotoxic Activity
3. Materials and Methods
3.1. General Experimental Procedure
3.2. Plant Material
3.3. Extraction and Isolation
- Compound 1, Yellow powder; m.p. 178–180 °C; [α]D-287 (c 0.001, MeOH); UV (MeOH) λmax (log ε) 214 (3.99), 243 (3.64), 300 (3.85), 348 (3.62), 369 (3.69) nm; IR (CHCl3) νmax 3593, 3500, 3352, 2962, 2877, 1776, 1601, 1422, 1323, 1289, 1169, 1093, 935, 903 cm−1; 1H and 13C NMR (CDCl3) (see Table 1); HR-DART-MS m/z 377.1586 [M + H]+ (calculated for C20H25O7, 377.1600).
- Compound 2, Yellow powder; m.p. 140–142 °C; [α]D-160 (c 0.0006, MeOH); UV (MeOH) λmax (log ε) 215 (4.12), 244 (3.71), 302 (3.83), 367 (3.63) nm; IR (CHCl3) νmax 3591, 3501, 3319, 2960, 1936, 2874, 1775, 1711, 1599, 1423, 1327, 1289, 1168, 1118, 1019, 919, 193 cm−1; 1H and 13C NMR (CDCl3) (see Table 1); HR-DART-MS m/z 363.1808 [M + H]+ (calculated for C20H27O6, 363.1804).
- Compound 3, Yellow powder; m.p. 285–290 °C; [α]D-89 (c 0.0021, MeOH); UV (MeOH) λmax (log ε) 208 (3.78), 242 (3.12), 295 (3.63), 352 (2.94) nm; IR (ATR) νmax 3317, 3083, 2933, 2870, 1597, 1570, 1462, 1379, 1319, 1245, 1162, 1146, 1119, 1013, 994, 969, 901, 807, 189, 698, 641, 596, 567, 533, 498, 422 cm−1; 1H and 13C NMR (MeOD) (see Table 1); HR-DART-MS m/z 347.1853 [M + H]+ (calculated for C20H27O5, 347.1858).
- Compound 5, Yellow powder; m.p. 275–276 °C; [α]D +352 (c 0.0013, MeOH); UV (MeOH) λmax (log ε) 207 (4.07), 277 (3.91), 313 (3.92) nm; IR (CHCl3) νmax 3383, 2965, 2938, 2879, 1775, 1670, 1642, 1614, 1397, 1382, 1336, 1276, 1193, 1117, 1018, 922 cm−1; 1H NMR (CD2Cl2, 700 MHz) δ 2.11 (1H, dd, J = 13.2, 5.9, H-1a), 1.50 (1H, m, H-1b), 1.89 (1H, dt, J = 11.7, 5.8, H-2a), 1.78 (1H, m, H-2b), 1.73 (1H, m, H-3a), 1.54 (1H, m, H-3b), 2.08 (1H, d, J = 14.0, H-5), 2.67 (1H, dd, J = 14.0, 9.5, H-6a), 2.02 (1H, dt, J = 14.0, 5.2, H-6b), 7.35 (1H, dd, J = 9.5, 5.2, H-7), 3.51 (1H, hep, J = 7.1, H-15), 1.25 (3H, d, J = 7.2, H-16), 1.24 (3H, d, J = 7.2, H-17), 1.21 (3H, s, H-18), 7.24 (1H, s, H-20), 7.58 (1H, s, 12-OH; 13C NMR (CD2Cl2, 700 MHz) δ 34.8 (CH2-1), 20.1 (CH2-2), 35.8 (CH2-3), 48.4 (C-4), 57.7 (CH-5), 25.8 (CH2-6), 143.2 (CH-7), 129.0 (C-8), 132.9 (C-9), 86.0 (C-10), 182.7 (C-11), 155.1 (C-12), 132.7 (C-13), 184.1 (C-14), 25.7 (CH-15), 19.6 (CH3-16), 19.8 (CH3-17), 17.6 (CH3-18), 178.0 (C-19), 140.6 (CH-20). HR-DART-MS m/z 343.1548 [M + H]+ (calculated for C20H23O5, 343.1545).
3.4. CG-MS Analysis
3.5. Computational Methods
3.6. Cytotoxicity Assay
3.7. Phytotoxic Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drew, B.T. Evolution, Pollination Biology, and Species Richness of Salvia (Lamiaceae). Int. J. Plant Sci. 2020, 181, 767–769. [Google Scholar] [CrossRef]
- Bentham, G. Labiatae. In Genera Plantarum; Bentham, G., Hooker, J.D., Eds.; Reeve and Co: London, UK, 1876; Volume 2, pp. 1160–1196. [Google Scholar]
- Esquivel, B.; Sánchez, A.A.; Aranda, E. Natural Products of Agrochemical Interest from Mexican Labiatae. In Phytochemicals and Phytopharmaceuticals; Shahidi, F., Ho, C., Eds.; AOCS Press: Champaign, IL, USA, 2000; pp. 371–385. [Google Scholar]
- Harley, R.M.; Heywood, C. Chromosome Numbers in Tropical American Labiatae. In Advances in Labiatae Science; Harley, R.M., Reynolds, T., Eds.; Royal Botanic Gardens: London, UK, 1992; pp. 211–246. [Google Scholar]
- Kriebel, R.; Drew, B.T.; Drummond, C.P.; González-Gallegos, J.G.; Celep, F.; Mahdjoub, M.M.; Rose, J.P.; Xiang, C.L.; Hu, G.X.; Walker, J.B.; et al. Tracking Temporal Shifts in Area, Biomes, and Pollinators in the Radiation of Salvia (Sages) across Continents: Leveraging Anchored Hybrid Enrichment and Targeted Sequence Data. Am. J. Bot. 2019, 106, 573–597. [Google Scholar] [CrossRef]
- Drew, B.T.; González-Gallegos, J.G.; Xiang, C.L.; Kriebel, R.; Drummond, C.P.; Walker, J.B.; Sytsma, K.J. Salvia United: The Greatest Good for the Greatest Number. Taxon 2017, 66, 133–145. [Google Scholar] [CrossRef]
- Will, M.; Claßen-Bockhoff, R. Time to Split Salvia s.l. (Lamiaceae)—New Insights from Old World Salvia Phylogeny. Mol. Phylogenet. Evol. 2017, 109, 33–58. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.X.; Takano, A.; Drew, B.T.; Liu, E.D.; Soltis, D.E.; Soltis, P.S.; Peng, H.; Xiang, C.L. Phylogeny and Staminal Evolution of Salvia (Lamiaceae, Nepetoideae) in East Asia. Ann. Bot. 2018, 122, 649–668. [Google Scholar] [CrossRef] [PubMed]
- Villaseñor, J.L. Catálogo de Las Plantas Vasculares Nativas de México. Rev. Mex. Biodivers. 2016, 87, 559–902. [Google Scholar] [CrossRef]
- Martínez-Gordillo, M.; Bedolla-García, B.; Cornejo-Tenorio, G.; Fragoso-Martínez, I.; García-Peña, M.D.R.; González-Gallegos, J.G.; Lara-Cabrera, S.I.; Zamudio, S. Lamiaceae de México. Bot. Sci. 2017, 95, 780–806. [Google Scholar] [CrossRef]
- Ramamoorthy, T.P. Salvia L. In Flora fanerogámica del Valle de México; Calderón de Rzedowski, G., Rzedowski, J., Eds.; Instituto de Ecología, A.C./Comisión Nacional para el Cono cimiento y Uso de la Biodiversidad: Tlalpan, Mexico, 2005; pp. 632–644. ISBN 9786077607366. [Google Scholar]
- González-Gallegos, J.G.; Castro-Castro, A.; Quintero-Fuentes, V.; Mendoza-López, M.E.; De Castro-Arce, E. Revisión Taxonómica de Lamiceae Del Occidente de México. Ibugana 2016, 7, 3–545. [Google Scholar]
- González-Gallegos, J.G.; Bedolla-García, B.Y.; Cornejo-Tenorio, G.; Fernández-Alonso, J.L.; Fragoso-Martínez, I.; García-Peña, M.D.R.; Harley, R.M.; Klitgaard, B.; Martínez-Gordillo, M.J.; Wood, J.R.I.; et al. Richness and Distribution of Salvia Subg. Calosphace (Lamiaceae). Int. J. Plant Sci. 2020, 181, 831–856. [Google Scholar] [CrossRef]
- Bedolla-García, B.Y.; Zamudio, S.; Castillo-Gómez, H.A. Salvia huastecana (Lamiaceae), a New Species from San Luis Potosí, Mexico. Phytotaxa 2020, 433, 1–8. [Google Scholar] [CrossRef]
- González-Gallegos, J.G.; Castro-Castro, A.; Ávila-González, H. Salvia rhizomatosa (Lamiaceae) a New Species from Sierra Madre Occidental in Durango, Mexico, with a Synopsis of Salvia Sect. Brandegeia. Phytotaxa 2020, 434, 255–269. [Google Scholar] [CrossRef]
- Fragoso-Martínez, I.; Martínez-Gordillo, M.; Salas, S. Salvia fimbriaticalyx, a New Species of Salvia (Lamiaceae) from Oaxaca, Mexico. Phytotaxa 2021, 518, 241–250. [Google Scholar] [CrossRef]
- González-Gallegos, J.G.; Pío-León, J.F.; Castro-Castro, A. Salvia beltraniorum (Lamiaceae), a New Species in Savannoid Vegetation from Cosalá, Sinaloa, Mexico. Phytotaxa 2021, 529, 160–170. [Google Scholar] [CrossRef]
- González-Gallegos, J.G.; Bedolla-García, B.Y.; Uría, R. Salvia gomezpompae (Lamiaceae), a New Species from Veracruz, Mexico. Bot. Sci. 2021, 1, 976–990. [Google Scholar] [CrossRef]
- Bedolla-García, B.Y.; Zamudio, S. Four New Species of Salvia (Lamiaceae) from Central Mexico. Phytotaxa 2015, 217, 35–52. [Google Scholar] [CrossRef]
- Epling, C. A Revision of Salvia Subgenus Calosphace. Feddes Repert. Specierum Nov. Regni Veg. 1939, 110, 1–383. [Google Scholar]
- Esquivel, B.; Cardenas, J.; Toscano, A.; Soriano-García, M.; Rodríguez-Hahn, L. Structure of Salvigenolide, a Novel Diterpenoid with a Rearranged Neo-Clerodane Skeleton from Salvia fulgens. Tetrahedron 1985, 41, 3213–3217. [Google Scholar] [CrossRef]
- Esquivel, B.; Cardenas, J.; Rodriguez-Hahn, L.; Ramamoorthy, T.P. The Diterpenoid Constituents of Salvia fulgens and Salvia microphylla. J. Nat. Prod. 1987, 50, 738–740. [Google Scholar] [CrossRef]
- Narukawa, Y.; Fukui, M.; Hatano, K.; Takeda, T. Four New Diterpenoids from Salvia fulgens Cav. J. Nat. Med. 2006, 60, 58–63. [Google Scholar] [CrossRef]
- Esquivel, B.; Cardenas, J.; Ramamoorthy, T.P.; Rodriguez-Hahn, L. Clerodane Diterpenoids of Salvia lineata. Phytochemistry 1986, 25, 2381–2384. [Google Scholar] [CrossRef]
- Esquivel, B.; Martínez, N.D.S.; Cárdenas, J.; Ramamoorthy, T.P.; Rodríguez-Hahn, L. The Pimarane-Type Diterpenoids of Salvia microphylla Var. Neurepia. Planta Med. 1989, 55, 62–63. [Google Scholar] [CrossRef]
- Bautista, E.; Toscano, R.A.; Ortega, A. Microphyllandiolide, a New Diterpene with an Unprecedented Skeleton from Salvia microphylla. Org. Lett. 2013, 15, 3210–3213. [Google Scholar] [CrossRef]
- Bautista, E.; Toscano, R.A.; Ortega, A. 5,10- Seco—Neo -Clerodanes and Neo -Clerodanes from Salvia microphylla. J. Nat. Prod. 2014, 77, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, B.; Bustos-Brito, C.; Sánchez-Castellanos, M.; Nieto-Camacho, A.; Ramírez-Apan, T.; Joseph-Nathan, P.; Quijano, L. Structure, Absolute Configuration, & Antiproliferative Activity of Abietane & Icetexane Diterpenoids from Salvia ballotiflora. Molecules 2017, 22, 1690. [Google Scholar] [CrossRef] [PubMed]
- Cezarotto, C.S.; Dorneles, A.; Baldissera, F.G.; da Silva, M.B.; Markoski, M.M.; Júnior, L.C.R.; Peres, A.; Fazolo, T.; Bordignon, S.A.L.; Apel, M.A.; et al. Leishmanicidal and Antichemotactic Activities of Icetexanes from Salvia uliginosa Benth. Phytomedicine 2019, 58, 152748. [Google Scholar] [CrossRef] [PubMed]
- Campos-Xolalpa, N.; Alonso-Castro, Á.J.; Sánchez-Mendoza, E.; Zavala-Sánchez, M.Á.; Pérez-Gutiérrez, S. Cytotoxic Activity of the Chloroform Extract and Four Diterpenes Isolated from Salvia ballotiflora. Rev. Bras. Farmacogn. 2017, 27, 302–305. [Google Scholar] [CrossRef]
- Esquivel, B.; Burgueño-Tapia, E.; Bustos-Brito, C.; Pérez-Hernández, N.; Quijano, L.; Joseph-Nathan, P. Absolute Configuration of the Diterpenoids Icetexone and Conacytone from Salvia ballotaeflora. Chirality 2018, 30, 177–188. [Google Scholar] [CrossRef]
- Brandt, C.W.; Neubauer, L.G. 221. Miro Resin. Part I. Ferruginol. J. Chem. Soc. 1939, 1031–1037. [Google Scholar] [CrossRef]
- González, M.A. Aromatic Abietane Diterpenoids: Their Biological Activity and Synthesis. Nat. Prod. Rep. 2015, 32, 684–704. [Google Scholar] [CrossRef]
- Gómez-Rivera, A.; González-Cortazar, M.; Herrera-Ruíz, M.; Zamilpa, A.; Rodríguez-López, V. Sessein and Isosessein with Anti-Inflammatory, Antibacterial and Antioxidant Activity Isolated from Salvia sessei Benth. J. Ethnopharmacol. 2018, 217, 212–219. [Google Scholar] [CrossRef]
- Jimenez, E.M.; Portugal, M.E.; Lira-Rocha, A.; Soriano-Garcia, M.; Toscano, R.A. A New Royleanone-Type Diterpene from Salvia sessei. J. Nat. Prod. 1988, 51, 243–248. [Google Scholar] [CrossRef]
- Hernández, M.; Esquivel, B.; Cárdenas, J.; Rodríguez-Hahn, L.; Ramamoorthy, T.P. Diterpenoid Abietane Quinones Isolated from Salvia regla. Phytochemistry 1987, 26, 3297–3299. [Google Scholar] [CrossRef]
- Ortega, A.; Cárdenas, J.; Gage, D.A.; Maldonado, E. Abietane and Clerodane Diterpenes from Salvia regla. Phytochemistry 1995, 39, 931–933. [Google Scholar] [CrossRef]
- Esquivel, B.; Calderon, J.S.; Flores, E.; Chavez, C.; Juarez, M. Abietane and Icetexane Diterpenoids from Salvia pubescens. Nat. Prod. Lett. 1997, 10, 87–93. [Google Scholar] [CrossRef]
- Fragoso-Martínez, I.; Martínez-Gordillo, M.; Salazar, G.A.; Sazatornil, F.; Jenks, A.A.; García-Peña, M.D.R.; Barrera-Aveleida, G.; Benitez-Vieyra, S.; Magallón, S.; Cornejo-Tenorio, G.; et al. Phylogeny of the Neotropical Sages (Salvia Subg. Calosphace; Lamiaceae) and Insights into Pollinator and Area Shifts. Plant Syst. Evol. 2018, 304, 43–55. [Google Scholar] [CrossRef]
- Jenks, A.A.; Walker, J.B.; Kim, S.C. Phylogeny of New World Salvia Subgenus Calosphace (Lamiaceae) Based on CpDNA (PsbA-TrnH) and NrDNA (ITS) Sequence Data. J. Plant Res. 2013, 126, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Forzato, C.; Nitti, P. New Diterpenes with Potential Antitumoral Activity Isolated from Plants in the Years 2017–2022. Plants 2022, 11, 2240. [Google Scholar] [CrossRef] [PubMed]
- Bisio, A.; Fraternale, D.; Giacomini, M.; Giacomelli, E.; Pivetti, S.; Russo, E.; Caviglioli, G.; Romussi, G.; Ricci, D.; De Tommasi, N. Phytotoxicity of Salvia spp. Exudates. Crop Prot. 2010, 29, 1434–1446. [Google Scholar] [CrossRef]
- Gerhard, B.T.A.A.H.Y.B. SpecDis: Quantifying the Comparison of Claculated and Experimental Electronic Circular Dichroism Spectra. Chirality 2013, 25, 243–249. [Google Scholar] [CrossRef]
- Bustos-Brito, C.; Pérez-Juanchi, D.; Rivera-Chávez, J.; Hernández-Herrera, A.D.; Bedolla-García, B.Y.; Zamudio, S.; Ramírez-Apan, T.; Quijano, L.; Esquivel, B. Clerodane and 5 10-Seco-Clerodane-Type Diterpenoids from Salvia involucrata. J. Mol. Struct. 2021, 1237, 130367. [Google Scholar] [CrossRef]
- Monks, A.; Dominic, S.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A.; et al. Feasibility of a High-Flux Anticancer Drug Screen Using a Diverse Panel of Cultured Human Tumor Cell Lines. JNCI J. Natl. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Macías-Rubalcava, M.L.; Ruiz-Velasco Sobrino, M.E.; Meléndez-González, C.; Hernández-Ortega, S. Naphthoquinone Spiroketals and Organic Extracts from the Endophytic Fungus Edenia gomezpompae as Potential Herbicides. J. Agric. Food Chem. 2014, 62, 3553–3562. [Google Scholar] [CrossRef] [PubMed]
- García-Méndez, M.C.; Macías-Ruvalcaba, N.A.; Lappe-Oliveras, P.; Hernández-Ortega, S.; Macías-Rubalcava, M.L. Phytotoxic Potential of Secondary Metabolites and Semisynthetic Compounds from Endophytic Fungus Xylaria feejeensis Strain SM3e-1b Isolated from Sapium macrocarpum. J. Agric. Food Chem. 2016, 64, 4255–4263. [Google Scholar] [CrossRef] [PubMed]



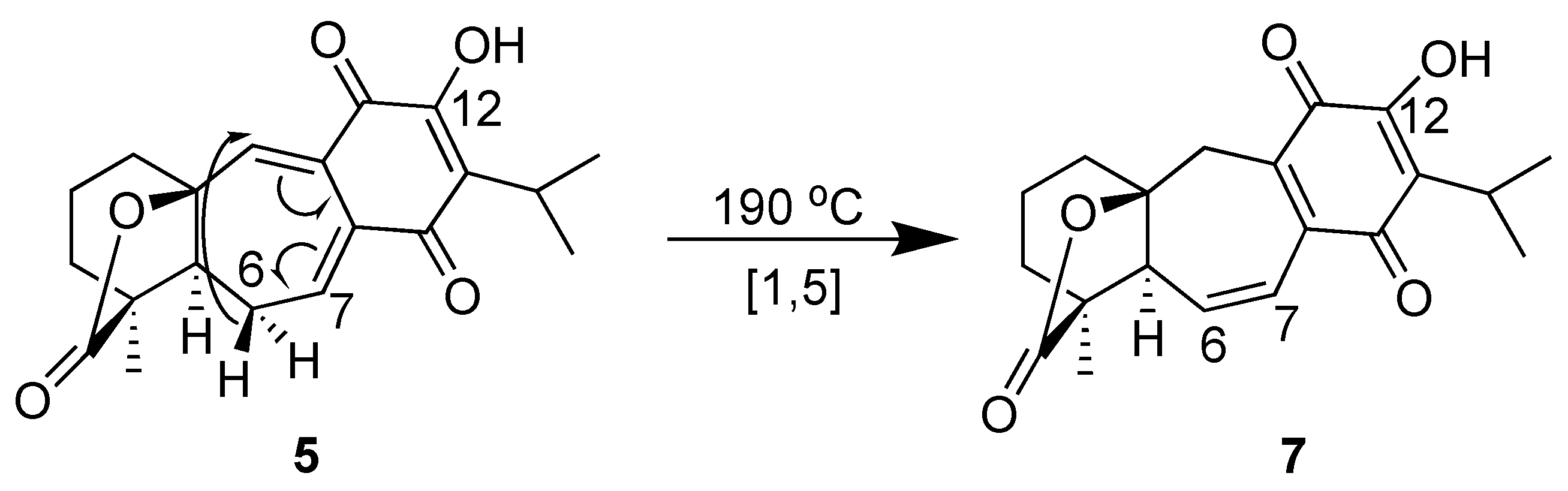
| 1 | 2 | 3 * | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Position | δC | Type | δH (J in Hz) | δC | Type | δH (J in Hz) | δC | Type | δH (J in Hz) |
| 1a | 25.0 | CH2 | 2.54, dd (13.5, 6.1) | 28.6 | CH2 | 2.28, dd (13.6, 6.2) | 37.6 | CH2 | 1.81, dd (13.4, 6.4) |
| 1b | 1.64, m | CH2 | 1.52, dd (13.6, 6.0) | 1.60, td (12.9, 6.1) | |||||
| 2a | 19.1 | CH2 | 1.97, dt (13.5, 6.1) | 19.6 | CH2 | 1.60, m | 21.1 | CH2 | 1.90, dh (19.6, 6.3) |
| 2b | 1.75, m | 1.49, m | 1.66, m | ||||||
| 3a | 36.2 | CH2 | 1.81, m | 39.7 | CH2 | 1.80, m | 40.3 | CH2 | 1.53, dd (13.3, 6.4) |
| 3b | 1.65, m | 1.84, m | 1.45, tdd (13.3, 5.8, 2.2) | ||||||
| 4 | 47.6 | C | 44.1 | C | 44.9 | C | |||
| 5 | 50.1 | CH | 2.11, dd (12.4, 1.5) | 51.5 | CH | 1.77, dd (10.8, 3.6) | 53.4 | CH | 1.65, m |
| 6a | 39.9 | CH2 | 2.72, dd (17.5, 12.4) | 40.4 | CH2 | 2.78, m | 41.9 | CH2 | 2.95, dd (16.8, 12.4) |
| 6b | 2.80, dd (17.5, 1.5) | 2.75, d (16.8) | |||||||
| 7a | 202.7 | C | 204.6 | C | 208.1 | C | |||
| 7b | 126.3 | C | |||||||
| 8 | 109.8 | C | 110.1 | C | 113.6 | C | |||
| 9 | 114.1 | C | 117.0 | C | 88.0 | C | |||
| 10 | 87.5 | C | 88.8 | C | 136.4 | C | |||
| 11 | 136.9 | C | 136.3 | C | 154.9 | C | |||
| 12 | 151.9 | C | 151.4 | C | 120.3 | C | |||
| 13 | 121.2 | C | 120.4 | C | 160.5 | C | |||
| 14 | 158.8 | C | 158.6 | C | 25.7 | CH | 3.51, hept (7.1) | ||
| 15 | 24.5 | CH | 3.57, hept (7.1) | 24.5 | CH | 3.55, hept (7.1) | 20.5 | CH3 | 1.31, d (7.1) |
| 16 | 20.1 | CH3 | 1.34, d (7.1) | 20.17 | CH3 | 1.32, d (7.1) | 20.6 | CH3 | 1.30, d (7.1) |
| 17 | 19.8 | CH3 | 1.35, d (7.1) | 20.22 | CH3 | 1.33, d (7.1) | 19.2 | CH3 | 1.02, s |
| 18 | 16.9 | CH3 | 1.18, s | 28.5 | CH3 | 1.00, s | 78.2 | CH2 | 3.86, d (7.7) |
| 19a | 178.8 | C | 77.9 | CH2 | 3.90, d (7.9) | 3.72, dd (7.7, 2.2) | |||
| 19b | 3.72, dd (7.9, 2.1) | 35.9 | CH2 | 3.44, d (13.6) | |||||
| 20a | 76.4 | CH | 5.44, s | 78.5 | CH | 5.32, s | 2.84, d (13.6) | ||
| 11-OH | 9.30, s | 9.20, brs | |||||||
| 12-OH | 6.69, s | 6.57, brs | |||||||
| 14-OH | 12.76, s | 12.92, s | |||||||
| Compound | IC50 (μM) (SI) | ||||
|---|---|---|---|---|---|
| U251 | K562 | HCT-15 | SKLU-1 | COS-7 | |
| 5 | 0.43 ± 0.01 (2.8) | 0.45 ± 0.01 (2.7) | 0.84 ± 0.07 (1.4) | 0.73 ± 0.06 (1.7) | 1.21 ± 0.1 |
| 6 | 1.34 ± 0.04 (0.7) | 1.29 ± 0.06 (0.7) | 1.03 ± 0.10 (0.9) | 0.95 ± 0.09 (1.0) | 0.91 ± 0.05 |
| Adriamycin | 0.08 ± 0.003 (3.1) | 0.20 ± 0.02 (12.5) | 0.16 ± 0.01 (1.6) | 0.20 ± 0.02 (1.3) | 0.25 ± 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bustos-Brito, C.; Torres-Medicis, J.P.; Bedolla-García, B.Y.; Zamudio, S.; Ramírez-Apan, T.; Macías-Rubalcava, M.L.; Quijano, L.; Esquivel, B. Structure, Absolute Configuration, Antiproliferative and Phytotoxic Activities of Icetexane and Abietane Diterpenoids from Salvia carranzae and Chemotaxonomic Implications. Molecules 2024, 29, 1226. https://doi.org/10.3390/molecules29061226
Bustos-Brito C, Torres-Medicis JP, Bedolla-García BY, Zamudio S, Ramírez-Apan T, Macías-Rubalcava ML, Quijano L, Esquivel B. Structure, Absolute Configuration, Antiproliferative and Phytotoxic Activities of Icetexane and Abietane Diterpenoids from Salvia carranzae and Chemotaxonomic Implications. Molecules. 2024; 29(6):1226. https://doi.org/10.3390/molecules29061226
Chicago/Turabian StyleBustos-Brito, Celia, Juan Pablo Torres-Medicis, Brenda Y. Bedolla-García, Sergio Zamudio, Teresa Ramírez-Apan, Martha Lydia Macías-Rubalcava, Leovigildo Quijano, and Baldomero Esquivel. 2024. "Structure, Absolute Configuration, Antiproliferative and Phytotoxic Activities of Icetexane and Abietane Diterpenoids from Salvia carranzae and Chemotaxonomic Implications" Molecules 29, no. 6: 1226. https://doi.org/10.3390/molecules29061226
APA StyleBustos-Brito, C., Torres-Medicis, J. P., Bedolla-García, B. Y., Zamudio, S., Ramírez-Apan, T., Macías-Rubalcava, M. L., Quijano, L., & Esquivel, B. (2024). Structure, Absolute Configuration, Antiproliferative and Phytotoxic Activities of Icetexane and Abietane Diterpenoids from Salvia carranzae and Chemotaxonomic Implications. Molecules, 29(6), 1226. https://doi.org/10.3390/molecules29061226






