Inhibitors of Cyclophilin A: Current and Anticipated Pharmaceutical Agents for Inflammatory Diseases and Cancers
Abstract
:1. Introduction
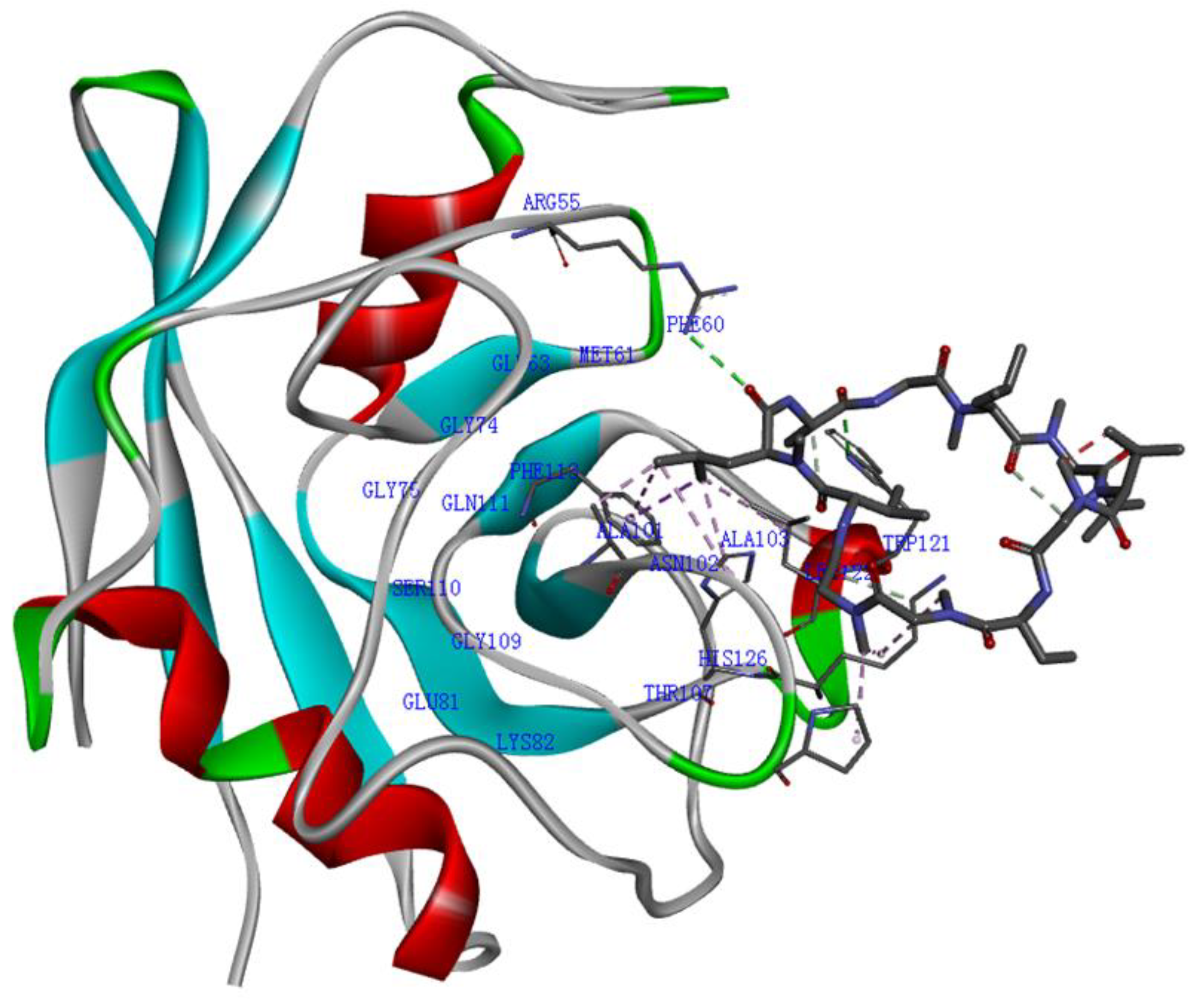
2. Cyclic Peptides as Cyclophilin A Inhibitors
2.1. Cyclosporin A (CsA)
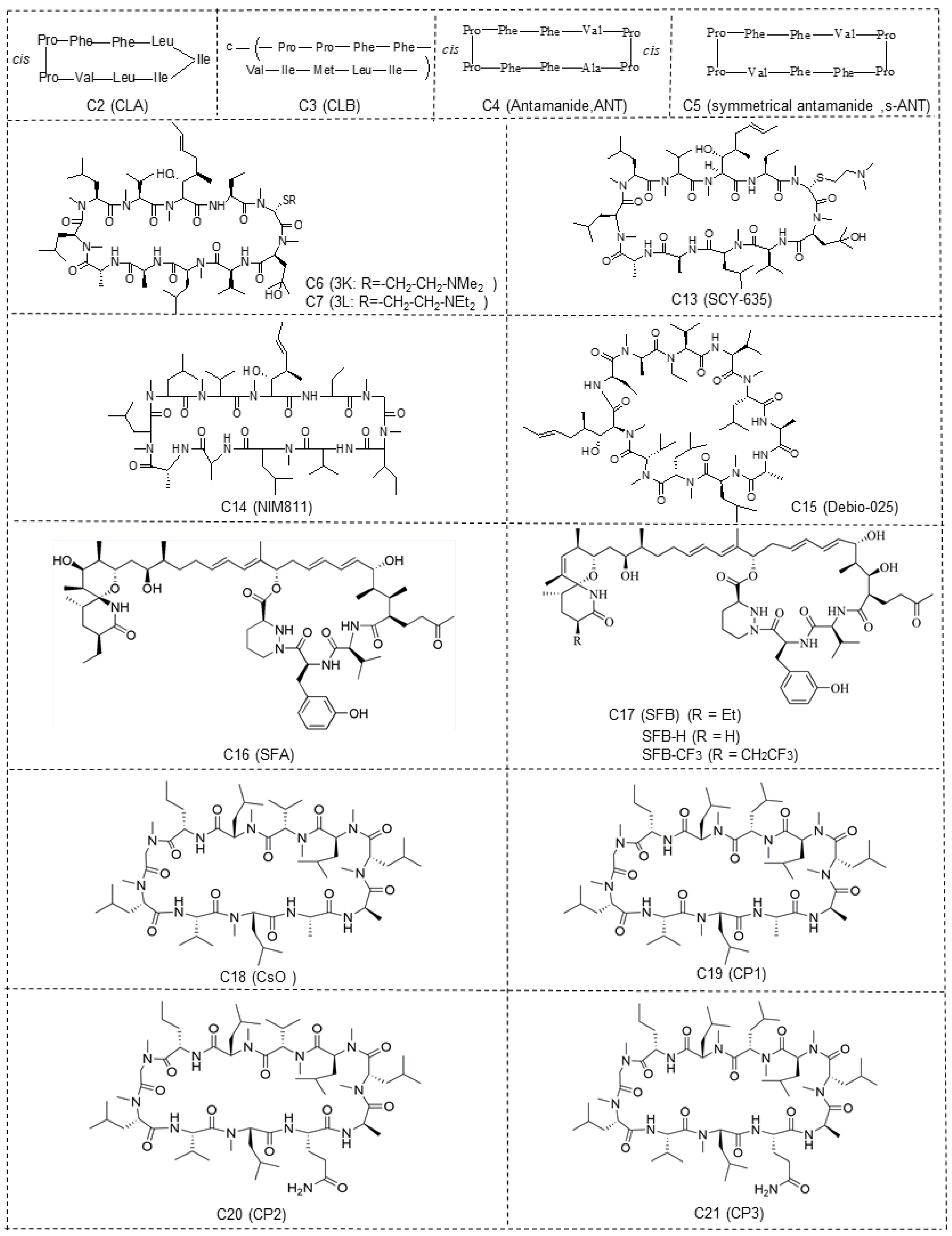
2.2. Cyclolinopeptides and the Analogues
2.3. Cyclosporin A Derivatives
2.4. Cyclosporin A Analogues
2.4.1. SCY-635
2.4.2. [Me-Ile-4]cyclosporine (NIM811)
2.4.3. Alisporivir (Debio-025)
2.5. Sanglifehrin A (SFA)—A Natural Product
2.6. Cyclosporin O (CsO)—A Natural Macrocycle
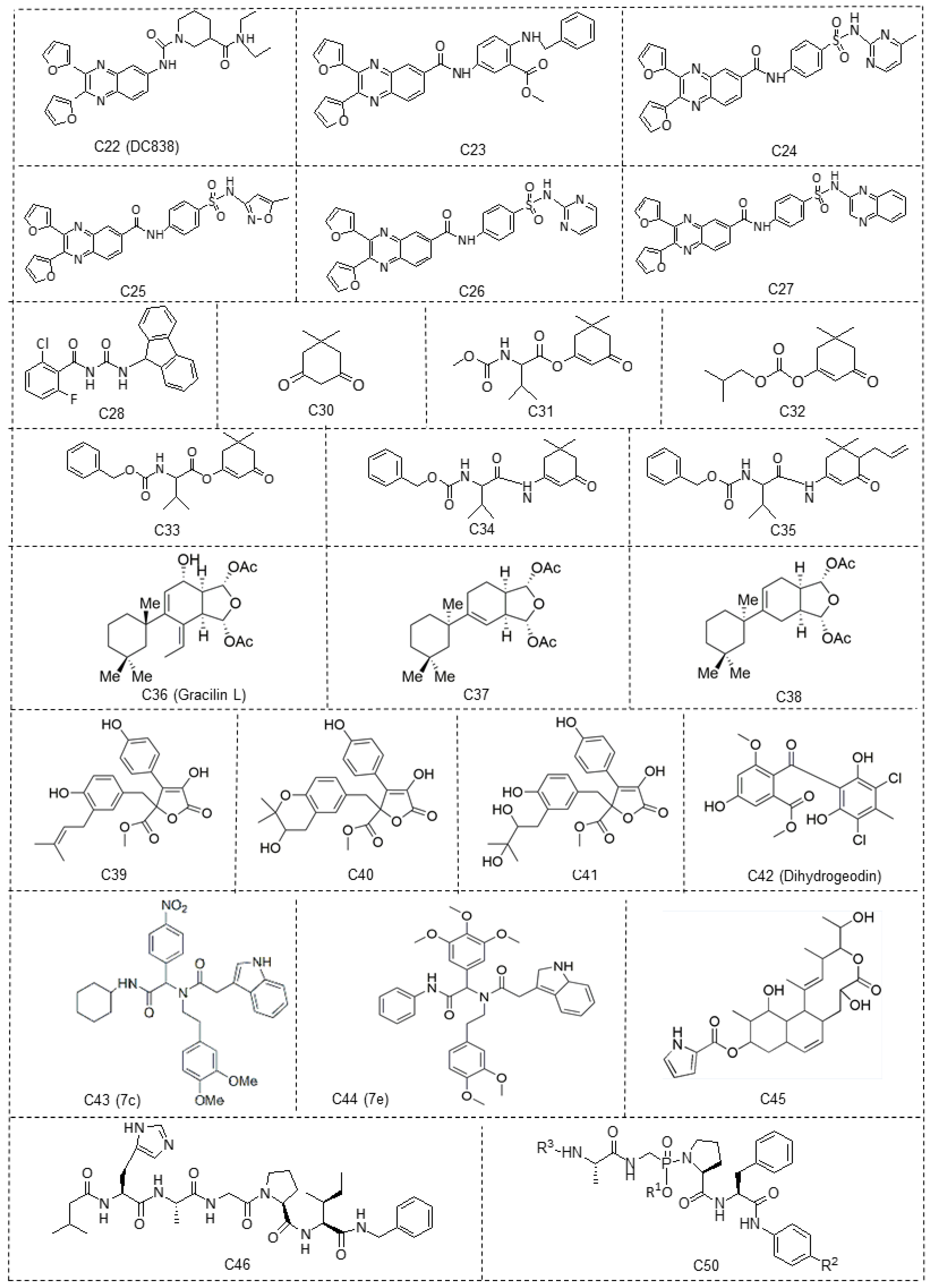
3. Small Molecular Inhibitors of Cyclophilin A
3.1. Quinoxaline Derivatives
3.2. Cyclophilin A Inhibitor 239836
3.3. Aryl 1-Indanylketones
3.4. Dimedone Analogues
3.5. Gracilins—Natural Diterpenes Derivative
3.6. Dichloro-Benzophenone Derivative—Natural Compound
3.7. Other Novel Small Molecular Inhibitors of Cyclophilin A
4. Peptide Analogues
4.1. Heptapeptides
4.2. N- or C-Terminal Modification of Gag Peptide
4.3. Trp-Gly-Pro (WGP)
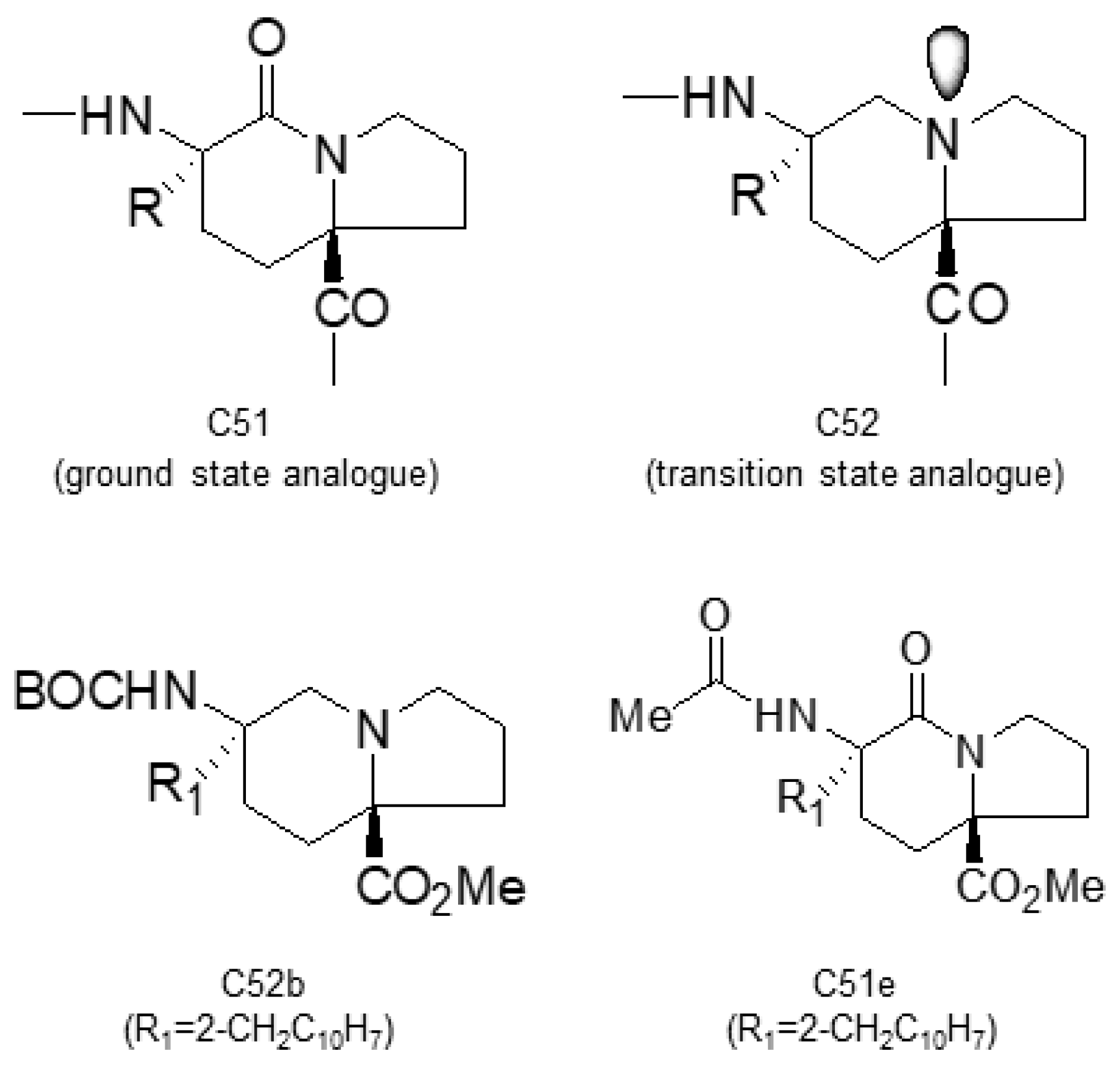
4.4. Pseudopeptides
4.5. “Self-Reproduction of Chirality” Analogues
5. Cyclophilin A Inhibitors in Clinical Trials
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alam, M.R.; Baetz, D.; Ovize, M. Cyclophilin D and myocardial ischemia–reperfusion injury: A fresh perspective. J. Mol. Cell. Cardiol. 2015, 78, 80–89. [Google Scholar] [CrossRef]
- Heitman, J.; Cullen, B.R. Cyclophilin B Escorts the Hepatitis C Virus RNA Polymerase: A Viral Achilles Heel? Mol. Cell 2005, 19, 145–146. [Google Scholar] [CrossRef] [PubMed]
- Ping, W.; Joseph, H. The cyclophilins. Genome Biol. 2005, 6, 226. [Google Scholar]
- Zhao, X.; Xia, C.; Wang, X.; Wang, H.; Xin, M.; Yu, L.; Liang, Y. Cyclophilin J PPIase Inhibitors Derived from 2,3-Quinoxaline-6 Amine Exhibit Antitumor Activity. Front. Pharmacol. 2018, 9, 126. [Google Scholar] [CrossRef] [PubMed]
- Lang, K.; Schmid, F.X.; Fischer, G. Catalysis of protein folding by prolyl isomerase. Nature 1987, 329, 268–270. [Google Scholar] [CrossRef]
- Fischer, G.; Wittmann-Liebold, B.; Lang, K.; Kiefhaber, T.; Schmid, F.X. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature 1989, 337, 476–478. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.L.; Walker, J.R.; Campagna-Slater, V.; Finerty, P.J.; Paramanathan, R.; Bernstein, G.; Mackenzie, F.; Tempel, W.; Ouyang, H.; Lee, W.H. Structural and Biochemical Characterization of the Human Cyclophilin Family of Peptidyl-Prolyl Isomerases. PLoS Biol. 2010, 8, e1000439. [Google Scholar] [CrossRef] [PubMed]
- Camilloni, C.; Sahakyan, A.B.; Holliday, M.J.; Isern, N.G.; Zhang, F.; Eisenmesser, E.Z.; Vendruscolo, M. Cyclophilin A catalyzes proline isomerization by an electrostatic handle mechanism. Proc. Natl. Acad. Sci. USA 2014, 111, 10203–10208. [Google Scholar] [CrossRef]
- Nigro, P.; Pompilio, G.; Capogrossi, M.C. Cyclophilin A: A key player for human disease. Cell Death Dis. 2013, 4, e888. [Google Scholar] [CrossRef]
- Handschumacher, R.E.; Harding, M.W.; Rice, J.; Drugge, R.J.; Speicher, D.W. Cyclophilin: A specific cytosolic binding protein for cyclosporin A. Science 1984, 226, 544–547. [Google Scholar] [CrossRef]
- Harding, M.W.; Handschumacher, R.E.; Speicher, D.W. Isolation and Amino Acid Sequence of Cyclophilin. J. Biol. Chem. 1986, 261, 8547–8555. [Google Scholar] [CrossRef]
- Kallen, J.; Spitzfaden, C.; Zurini, M.G.; Wider, G.; Widmer, H.; Wüthrich, K.; Walkinshaw, M.D. Structure of human cyclophilin and its binding site for cyclosporin A determined by X-ray. Nature 1991, 353, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Ke, H. Similarities and differences between human cyclophilin A and other beta-barrel structures. Structural refinement at 1.63 A resolution. J. Mol. Biol. 1992, 228, 539–550. [Google Scholar] [CrossRef]
- Ottiger, M.; Zerbe, O.; GuÈntert, P.; WuÈthrich, K. The NMR solution conformation of unligated human cyclophilin A. J. Mol. Biol. 1997, 272, 64–81. [Google Scholar] [CrossRef] [PubMed]
- Thériault, Y.; Logan, T.M.; Meadows, R.; Yu, L.; Olejniczak, E.T.; Holzman, T.F.; Simmer, R.L.; Fesik, S.W. Solution structure of the cyclosporin A/cyclophilin complex by NMR. Nature 1993, 361, 88–91. [Google Scholar] [CrossRef]
- Wiederrecht, G.; Lam, E.; Hung, S.; Martin, M.; Sigal, N. The mechanism of action of FK-506 and cyclosporin A. Ann. N. Y. Acad. Sci. 1993, 696, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Sherry, B.; Zybarth, G.; Alfano, M.; Dubrovsky, L.; Mitchell, R.; Rich, D.; Ulrich, P.; Bucala, R.; Cerami, A.; Bukrinsky, M. Role of cyclophilin A in the uptake of HIV-1 by macrophages and T lymphocytes. Proc. Natl. Acad. Sci. USA 1998, 95, 1758–1763. [Google Scholar] [CrossRef]
- Hong, F.; Lee, J.; Song, J.W.; Lee, S.J.; Ahn, H.; Cho, J.J.; Ha, J.; Kim, S.S. Cyclosporin A blocks muscle differentiation by inducing oxidative stress and inhibiting the peptidyl-prolyl-cis-trans isomerase activity of cyclophilin A: Cyclophilin A protects myoblasts from cyclosporin A-induced cytotoxicity. FASEB J. 2002, 16, 1633–1635. [Google Scholar] [CrossRef]
- Towers, G.J.; Hatziioannou, T.; Cowan, S.; Goff, S.P.; Luban, J.; Bieniasz, P.D. Cyclophilin A modulates the sensitivity of HIV-1 to host restriction factors. Nat. Med. 2003, 9, 1138–1143. [Google Scholar] [CrossRef]
- Colgan, J.; Asmal, M.; Neagu, M.; Yu, B.; Schneidkraut, J.; Lee, Y.; Sokolskaja, E.; Andreotti, A.; Luban, J. Cyclophilin A Regulates TCR Signal Strength in CD4+ T Cells via a Proline-Directed Conformational Switch in Itk. Immunity 2004, 21, 189–201. [Google Scholar] [CrossRef]
- Kim, S.H.; Lessner, S.M.; Sakurai, Y.; Galis, Z.S. Cyclophilin A as a novel biphasic mediator of endothelial activation and dysfunction. Am. J. Pathol. 2004, 164, 1567–1574. [Google Scholar] [CrossRef]
- Sokolskaja, E.; Luban, J. Cyclophilin, TRIM5, and innate immunity to HIV-1. Curr. Opin. Microbiol. 2006, 9, 404–408. [Google Scholar] [CrossRef]
- Satoh, K.; Nigro, P.; Berk, B.C. Oxidative Stress and Vascular Smooth Muscle Cell Growth: A Mechanistic Linkage by Cyclophilin A. Antioxid. Redox Signal. 2010, 12, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Campa, M.J.; Wang, M.Z.; Howard, B.; Fitzgerald, M.C.; Patz, E.F. Protein Expression Profiling Identifies Macrophage Migration Inhibitory Factor and Cyclophilin A as Potential Molecular Targets in Non-Small Cell Lung Cancer. Cancer Res. 2003, 63, 1652–1656. [Google Scholar] [PubMed]
- Obama, K.; Kato, T.; Hasegawa, S.; Satoh, S.; Nakamura, Y.; Furukawa, Y. Overexpression of peptidyl-prolyl isomerase-like 1 is associated with the growth of colon cancer cells. Clin. Cancer Res. 2006, 12, 70–76. [Google Scholar] [CrossRef]
- Galigniana, M.D.; Morishima, Y.; Gallay, P.A.; Pratt, W.B. Cyclophilin-A is bound through its peptidylprolyl isomerase domain to the cytoplasmic dynein motor protein complex. J. Biol. Chem. 2004, 279, 55754–55759. [Google Scholar] [CrossRef] [PubMed]
- Seizer, P.; Geisler, T.; Bigalke, B.; Schneider, M.; Klingel, K.; Kandolf, R.; Stellos, K.; Schreieck, J.; Gawaz, M.; May, A.E. EMMPRIN and its ligand Cyclophilin A as novel diagnostic markers in inflammatory cardiomyopathy. Int. J. Cardiol. 2013, 163, 299–304. [Google Scholar] [CrossRef]
- Bobardt, M.; Hopkins, S.; Baugh, J.; Chatterji, U.; Hernandez, F.; Hiscott, J.; Sluder, A.; Lin, K.; Gallay, P.A. HCV NS5A and IRF9 compete for CypA binding. J. Hepatol. 2013, 58, 16–23. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, Z.; Liu, W. Insights into the Roles of Cyclophilin A During Influenza Virus Infection. Viruses 2013, 5, 182–191. [Google Scholar] [CrossRef]
- Zhang, T.T.; Zhang, J.F.; Ge, H. Functions of cyclophilin A in atherosclerosis. Exp. Clin. Cardiol. 2013, 18, e118–e124. [Google Scholar]
- Gerhard, H. Cyclophilin A as a Target of Cisplatin Chemosensitizers. Curr. Cancer Drug Targets 2014, 14, 46–58. [Google Scholar]
- Dawar, F.U.; Wu, J.; Zhao, L.; Khattak, M.N.K.; Mei, J.; Lin, L. Updates in understanding the role of cyclophilin A in leukocyte chemotaxis. J. Leukoc. Biol. 2017, 101, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Seizer, P.; Gawaz, M.; May, A.E. Cyclophilin A and EMMPRIN (CD147) in cardiovascular diseases. Cardiovasc. Res. 2014, 102, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.F.; Hsieh, C.C.; Wu, M.J.; Chen, C.H.; Lin, T.H.; Hsieh, M. Novel findings of secreted cyclophilin A in diabetic nephropathy and its association with renal protection of dipeptidyl peptidase 4 inhibitor. Clin. Chim. Acta 2016, 463, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Vinitha, A.; Kartha, C.C. Cyclophilin A enhances macrophage differentiation and lipid uptake in high glucose conditions: A cellular mechanism for accelerated macro vascular disease in diabetes mellitus. Cardiovasc. Diabetol. 2016, 15, 152. [Google Scholar] [CrossRef]
- Lahaye, X.; Satoh, T.; Gentili, M.; Cerboni, S.; Silvin, A.; Conrad, C.; Ahmed-Belkacem, A.; Rodriguez, E.; Guichou, J.-F.; Bosquet, N. Nuclear Envelope Protein SUN2 Promotes Cyclophilin-A-Dependent Steps of HIV Replication. Cell Rep. 2016, 15, 879–892. [Google Scholar] [CrossRef]
- Ren, Y.X.; Wang, S.J.; Fan, J.H.; Sun, S.J.; Li, X.; Padhiar, A.A.; Zhang, J.N. CD147 stimulates hepatoma cells escaping from immune surveillance of T cells by interaction with Cyclophilin A. Biomed. Pharmacother. 2016, 80, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Schiene-Fischer, C.; Fischer, G.; Braun, M. Non-Immunosuppressive Cyclophilin Inhibitors. Angew. Chem. Int. Ed. 2022, 61, e202201597. [Google Scholar] [CrossRef]
- Saleh, T.; Jankowski, W.; Sriram, G.; Rossi, P.; Shah, S.; Lee, K.B.; Cruz, L.A.; Rodriguez, A.J.; Birge, R.B.; Kalodimos, C.G. Cyclophilin A promotes cell migration via the Abl-Crk signaling pathway. Nat. Chem. Biol. 2016, 12, 117–123. [Google Scholar] [CrossRef]
- Kim, H.; Oh, Y.; Kim, K.; Jeong, S.; Chon, S.; Kim, D.; Jung, M.H.; Pak, Y.K.; Ha, J.; Kang, I.; et al. Cyclophilin A regulates JNK/p38-MAPK signaling through its physical interaction with ASK1. Biochem. Biophys. Res. Commun. 2015, 464, 112–117. [Google Scholar] [CrossRef]
- Emmel, E.A.; Verweij, C.L.; Durand, D.B.; Higgins, K.M.; Lacy, E.; Crabtree, G.R. Cyclosporin A specifically inhibits function of nuclear proteins involved in T cell activation. Science 1989, 246, 1617–1620. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, W.M.; Corthésy, B.; Bram, R.J.; Crabtree, G.R. Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature 1991, 352, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Albers, M.W.; Wandless, T.J.; Luan, S.; Alberg, D.G.; Belshaw, P.J.; Cohen, P.; MacKintosh, C.; Klee, C.B.; Schreiber, S.L. Inhibition of T cell signaling by immunophilin-ligand complexes correlates with loss of calcineurin phosphatase activity. Biochemistry 1992, 31, 3896–3901. [Google Scholar] [CrossRef]
- Pua, K.H.; Stiles, D.T.; Sowa, M.E.; Verdine, G.L. IMPDH2 Is an Intracellular Target of the Cyclophilin A and Sanglifehrin A Complex. Cell Rep. 2017, 18, 432–442. [Google Scholar] [CrossRef]
- Dawar, F.U.; Tu, J.; Khattak, M.N.; Mei, J.; Lin, L. Cyclophilin A: A Key Factor in Virus Replication and Potential Target for Anti-viral Therapy. Curr. Issues Mol. Biol. 2017, 21, 1–20. [Google Scholar] [CrossRef]
- Liu, C.; Perilla, J.R.; Ning, J.; Lu, M.; Hou, G.; Ramalho, R.; Himes, B.A.; Zhao, G.; Bedwell, G.J.; Byeon, I.J.; et al. Cyclophilin A stabilizes the HIV-1 capsid through a novel non-canonical binding site. Nat. Commun. 2016, 7, 10714. [Google Scholar] [CrossRef]
- Lu, M.; Hou, G.; Zhang, H.; Suiter, C.L.; Ahn, J.; Byeon, I.J.; Perilla, J.R. Dynamic allostery governs cyclophilin A-HIV capsid interplay. Proc. Natl. Acad. Sci. USA 2015, 112, 14617–14622. [Google Scholar] [CrossRef] [PubMed]
- Daito, T.; Watashi, K.; Sluder, A.; Ohashi, H.; Nakajima, S.; Borroto-Esoda, K.; Fujita, T.; Wakita, T. Cyclophilin inhibitors reduce phosphorylation of RNA-dependent protein kinase to restore expression of IFN-stimulated genes in HCV-infected cells. Gastroenterology 2014, 147, 463–472. [Google Scholar] [CrossRef]
- Han, J.; Lee, M.K.; Jang, Y.; Cho, W.J.; Kim, M. Repurposing of cyclophilin A inhibitors as broad-spectrum antiviral agents. Drug Discov. Today 2022, 27, 1895–1912. [Google Scholar] [CrossRef]
- de Wilde, A.H.; Pham, U.; Posthuma, C.C.; Snijder, E.J. Cyclophilins and cyclophilin inhibitors in nidovirus replication. Virology 2018, 522, 46–55. [Google Scholar] [CrossRef]
- Yang, H.; Jian, C.; Yang, J.; Qiao, S.; Zhao, S.; Long, Y. Cyclophilin A is upregulated in small cell lung cancer and activates ERK1/2 signal. Biochem. Biophys. Res. Commun. 2007, 361, 763–767. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, B.; Yu, L. In silico comparison of gene expression levels in ten human tumor types reveals candidate genes associated with carcinogenesis. Cytogenet. Genome Res. 2006, 112, 53–59. [Google Scholar] [CrossRef]
- Lee, J. Role of cyclophilin a during oncogenesis. Arch. Pharmacal Res. 2010, 33, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, X.; Bai, S.; Wang, Z.; Chen, L.; Wei, Y.; Huang, C. Proteomics identification of cyclophilin a as a potential prognostic factor and therapeutic target in endometrial carcinoma. Mol. Cell. Proteom. 2008, 7, 1810–1823. [Google Scholar] [CrossRef] [PubMed]
- Obchoei, S.; Wongkhan, S.; Wongkham, C.; Li, M.; Yao, Q.; Chen, C. Cyclophilin A: Potential functions and therapeutic target for human cancer. Med. Sci. Monit. 2009, 15, Ra221–Ra232. [Google Scholar] [PubMed]
- Zhu, D.; Wang, Z.; Zhao, J.J.; Calimeri, T.; Meng, J.; Hideshima, T.; Fulciniti, M.; Kang, Y.; Ficarro, S.B.; Tai, Y.T.; et al. The Cyclophilin A-CD147 complex promotes the proliferation and homing of multiple myeloma cells. Nat. Med. 2015, 21, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Naoumov, N.V. Cyclophilin inhibition as potential therapy for liver diseases. J. Hepatol. 2014, 61, 1166–1174. [Google Scholar] [CrossRef]
- Grigoryeva, E.S.; Cherdyntseva, N.V.; Karbyshev, M.S.; Volkomorov, V.V.; Stepanov, I.V.; Zavyalova, M.V.; Perelmuter, V.M.; Buldakov, M.A.; Afanasjev, S.G.; Tuzikov, S.A.; et al. Expression of cyclophilin A in gastric adenocarcinoma patients and its inverse association with local relapses and distant metastasis. Pathol. Oncol. Res. 2014, 20, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Min, W.; Gou, J. Knockdown of cyclophilin A reverses paclitaxel resistance in human endometrial cancer cells via suppression of MAPK kinase pathways. Cancer Chemother. Pharmacol. 2013, 72, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Bonfils, C.; Bec, N.; Larroque, C.; Del Rio, M.; Gongora, C.; Pugnière, M.; Martineau, P. Cyclophilin A as negative regulator of apoptosis by sequestering cytochrome c. Biochem. Biophys. Res. Commun. 2010, 393, 325–330. [Google Scholar] [CrossRef]
- Brazin, K.N.; Mallis, R.J.; Fulton, D.B.; Andreotti, A.H. Regulation of the tyrosine kinase Itk by the peptidyl-prolyl isomerase cyclophilin A. Proc. Natl. Acad. Sci. USA 2002, 99, 1899–1904. [Google Scholar] [CrossRef] [PubMed]
- Sherry, B.; Yarlett, N.; Strupp, A.; Cerami, A. Identification of cyclophilin as a proinflammatory secretory product of lipopolysaccharide-activated macrophages. Proc. Natl. Acad. Sci. USA 1992, 89, 3511–3515. [Google Scholar] [CrossRef]
- Jin, Z.G.; Melaragno, M.G.; Liao, D.F.; Yan, C.; Berk, B.C. Cyclophilin A Is a Secreted Growth Factor Induced by Oxidative Stress. Circ. Res. 2000, 87, 789–796. [Google Scholar] [CrossRef]
- Andersen, H.; Jensen, O.N.; Eriksen, E.F. A proteome study of secreted prostatic factors affecting osteoblastic activity: Identification and characterisation of cyclophilin A. Eur. J. Cancer 2003, 39, 989–995. [Google Scholar] [CrossRef]
- Suzuki, J.; Jin, Z.G.; Meoli, D.F.; Matoba, T.; Berk, B.C. Cyclophilin A Is Secreted by a Vesicular Pathway in Vascular Smooth Muscle Cells. Circ. Res. 2006, 98, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Matoba, T.; Suzuki, J.; O’Dell, M.R.; Nigro, P.; Cui, Z.; Mohan, A.; Pan, S.; Li, L.; Jin, Z.G.; et al. Cyclophilin A mediates vascular remodeling by promoting inflammation and vascular smooth muscle cell proliferation. Circulation 2008, 117, 3088–3098. [Google Scholar] [CrossRef] [PubMed]
- Arora, K.; Gwinn, W.M.; Bower, M.A.; Watson, A.; Okwumabua, I.; MacDonald, H.R.; Bukrinsky, M.I.; Constant, S.L. Extracellular cyclophilins contribute to the regulation of inflammatory responses. J. Immunol. 2005, 175, 517–522. [Google Scholar] [CrossRef]
- Billich, A.; Winkler, G.; Aschauer, H.; Rot, A.; Peichl, P. Presence of cyclophilin A in synovial fluids of patients with rheumatoid arthritis. J. Exp. Med. 1997, 185, 975–980. [Google Scholar] [CrossRef]
- Jin, Z.G.; Lungu, A.O.; Xie, L.; Wang, M.; Wong, C.; Berk, B.C. Cyclophilin A is a proinflammatory cytokine that activates endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1186–1191. [Google Scholar] [CrossRef]
- Liu, L.; Li, C.; Xiang, J.; Dong, W.; Cao, Z. Over-expression and potential role of cyclophilin A in human periodontitis. J. Periodontal Res. 2013, 48, 615–622. [Google Scholar] [CrossRef]
- Yurchenko, V.; Constant, S.; Bukrinsky, M. Dealing with the family: CD147 interactions with cyclophilins. Immunology 2006, 117, 301–309. [Google Scholar] [CrossRef]
- Landskron, J.; Taskén, K. CD147 in regulatory T cells. Cell. Immunol. 2013, 282, 17–20. [Google Scholar] [CrossRef]
- Sànchez-Tilló, E.; Wojciechowska, M.; Comalada, M.; Farrera, C.; Lloberas, J.; Celada, A. Cyclophilin A is required for M-CSF-dependent macrophage proliferation. Eur. J. Immunol. 2006, 36, 2515–2524. [Google Scholar] [CrossRef]
- Satoh, K.; Nigro, P.; Matoba, T.; O’Dell, M.R.; Cui, Z.; Shi, X.; Mohan, A.; Yan, C.; Abe, J.; Illig, K.A.; et al. Cyclophilin A enhances vascular oxidative stress and the development of angiotensin I-induced aortic aneurysms. Nat. Med. 2009, 15, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Soe, N.N.; Sowden, M.; Baskaran, P.; Smolock, E.M.; Kim, Y.; Nigro, P.; Berk, B.C. Cyclophilin A Is Required for Angiotensin II-Induced p47phox Translocation to Caveolae in Vascular Smooth Muscle Cells. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2147–2153. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.M. Cyclosporine: A Historical Perspective on Its Role in the Treatment of Noninfectious Uveitis. J. Ocul. Pharmacol. Ther. 2017, 33, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Rüegger, A.; Kuhn, M.; Lichti, H.; Loosli, H.R.; Huguenin, R.; Quiquerez, C.; von Wartburg, A. Cyclosporin A, a Peptide Metabolite from Trichoderma polysporum (Link ex Pers.) Rifai, with a remarkable immunosuppressive activity. Helv. Chim. Acta 1976, 59, 1075–1092. [Google Scholar] [CrossRef]
- Chen, T.L.; Estey, E.H.; Othus, M.; Gardner, K.M.; Markle, L.J.; Walter, R.B. Cyclosporine modulation of multidrug resistance in combination with pravastatin, mitoxantrone and etoposide for adult patients with relapsed/refractory acute myeloid leukemia: A phase 1/2 study. Leuk. Lymphoma 2013, 54, 2534–2536. [Google Scholar] [CrossRef] [PubMed]
- Chester, J.D.; Joel, S.P.; Cheeseman, S.L.; Hall, G.D.; Braun, M.S.; Perry, J.; Davis, T.; Button, C.J.; Seymour, M.T. Phase I and pharmacokinetic study of intravenous irinotecan plus oral ciclosporin in patients with fuorouracil-refractory metastatic colon cancer. J. Clin. Oncol. 2003, 21, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, J.W.; Fung, J. A new class of cyclosporin analogues for the treatment of asthma. Expert. Opin. Investig. Drugs 2003, 12, 647–653. [Google Scholar] [CrossRef]
- Sun, C.; Li, S.; Wang, K.; Feng, H.; Tian, C.; Liu, X.; Li, X.; Yin, X.; Wang, Y.; Wei, J.; et al. Cyclosporin A as a Source for a Novel Insecticidal Product for Controlling Spodoptera frugiperda. Toxins 2022, 14, 721. [Google Scholar] [CrossRef]
- Landras, A.; Reger de Moura, C.; Jouenne, F.; Lebbe, C.; Menashi, S.; Mourah, S. CD147 Is a Promising Target of Tumor Progression and a Prognostic Biomarker. Cancers 2019, 11, 1803. [Google Scholar] [CrossRef] [PubMed]
- Borel, J.F. Comparative study of in vitro and in vivo drug effects on cell-mediated cytotoxicity. Immunology 1976, 31, 631–641. [Google Scholar] [PubMed]
- Clipstone, N.A.; Crabtree, G.R. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature 1992, 357, 695–697. [Google Scholar] [CrossRef]
- Matsuda, S.; Koyasu, S. Mechanisms of action of cyclosporine. Immunopharmacology 2000, 47, 119–125. [Google Scholar] [CrossRef]
- Green, C.J. Immunosuppression with cyclosporin A: A review. Diagn. Histopathol. 1981, 4, 157–174. [Google Scholar] [PubMed]
- Krishnamurthy, A.; Dasari, A.; Noonan, A.M.; Mehnert, J.M.; Lockhart, A.C.; Leong, S.; Capasso, A.; Stein, M.N.; Sanoff, H.K.; Lee, J.J.; et al. Phase Ib Results of the Rational Combination of Selumetinib and Cyclosporin A in Advanced Solid Tumors with an Expansion Cohort in Metastatic Colorectal Cancer. Cancer Res. 2018, 78, 5398–5407. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Yang, Y.; Zhang, J.; He, H.; Ren, X. Circumvention of cisplatin resistance in ovarian cancer by combination of cyclosporin A and low-intensity ultrasound. Eur. J. Pharm. Biopharm. 2015, 91, 103–110. [Google Scholar] [CrossRef]
- Hojo, M.; Morimoto, T.; Maluccio, M.; Asano, T.; Morimoto, K.; Lagman, M.; Shimbo, T.; Suthanthiran, M. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature 1999, 397, 530–534. [Google Scholar] [CrossRef]
- Guba, M.; Graeb, C.; Jauch, K.W.; Geissler, E.K. Pro- and anti-cancer effects of immunosuppressive agents used in organ transplantation. Transplantation 2004, 77, 1777–1782. [Google Scholar] [CrossRef]
- Dantal, J.; Soulillou, J.P. Immunosuppressive drugs and the risk of cancer after organ transplantation. N. Engl. J. Med. 2005, 352, 1371–1373. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, G.F.; Bouwes Bavinck, J.N.; Euvrard, S. Organ transplantation and skin cancer: Basic problems and new perspectives. Exp. Dermatol. 2010, 19, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Martinez, S.; Redondo, J. Inhibitors of the calcineurin/NFAT pathway. Curr. Med. Chem. 2004, 11, 997–1007. [Google Scholar] [CrossRef]
- Luo, C.; Luo, H.; Zheng, S.; Gui, C.; Yue, L.; Yu, C.; Sun, T.; He, P.; Chen, J.; Shen, J.; et al. Nucleocapsid protein of SARS coronavirus tightly binds to human cyclophilin A. Biochem. Biophys. Res. Commun. 2004, 321, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Laurie, K.; Holcomb, D.; Kames, J.; Komar, A.A.; DiCuccio, M.; Ibla, J.C.; Kimchi-Sarfaty, C. In Silico Evaluation of Cyclophilin Inhibitors as Potential Treatment for SARS-CoV-2. Open Forum Infect. Dis. 2021, 8, ofab189. [Google Scholar] [CrossRef]
- Liu, D.; Ndongwe, T.P.; Ji, J.; Huber, A.D.; Michailidis, E.; Rice, C.M.; Ralston, R.; Tedbury, P.R.; Sarafianos, S.G. Mechanisms of Action of the Host-Targeting Agent Cyclosporin A and Direct-Acting Antiviral Agents against Hepatitis C Virus. Viruses 2023, 15, 981. [Google Scholar] [CrossRef]
- Balasubramanian, D.; Chopra, P.; Ardeshir, F. Cyclolinopeptide—An antamanide analog. FEBS Lett. 1976, 65, 69–72. [Google Scholar] [CrossRef]
- Kessler, H.; Klein, M.; Muller, A.; Wagner, K.; Bats, J.W.; Ziegler, K.; Frimmer, M. Conformational Prerequisites for the in vitro Inhibition of Cholate Uptake in Hepatocytes by Cyclic Analogues of Antamanide and Somatostatin. Angew. Chem. Int. Ed. 1986, 25, 997–999. [Google Scholar] [CrossRef]
- Benedetti, E.; Pedone, C. Cyclolinopeptide A: Inhibitor, immunosuppressor or other? J. Pept. Sci. 2005, 11, 268–272. [Google Scholar] [CrossRef]
- Wieczorek, Z.; Bengtsson, B.; Trojnar, J.; Siemion, I.Z. Immunosuppressive activity of cyclolinopeptide A. Pept. Res. 1991, 4, 275–283. [Google Scholar]
- Gaymes, T.J.; Cebrat, M.; Siemion, I.Z.; Kay, J.E. Cyclolinopeptide A (CLA) mediates its immunosuppressive activity through cyclophilin-dependent calcineurin inactivation. FEBS Lett. 1997, 418, 224–227. [Google Scholar] [CrossRef]
- Siemion, I.Z.; Cebrat, M.; Wieczorek, Z. Cyclolinopeptides and their analogs--a new family of peptide immunosuppressants affecting the calcineurin system. Arch. Immunol. Ther. Exp. 1999, 47, 143–153. [Google Scholar]
- Ruchala, P.; Picur, B.; Lisowski, M.; Cierpicki, T.; Wieczorek, Z.; Siemion, I.Z. Synthesis, conformation, and immunosuppressive activity of CLX and its analogues. Biopolymers 2003, 70, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Shishido, A.; Matsumoto, T.; Takeya, K.; Itokawa, H.; Hirano, T.; Oka, K. A New Immunosuppressive Cyclic Nonapeptide, Cyclolinopeptide B from Linum Usitatissimum. Bioorg. Med. Chem. Lett. 1997, 7, 1269–1272. [Google Scholar] [CrossRef]
- Gaymes, T.J.; Carrett, N.J.; Patel, N.; Kay, J.E.; Siemion, I.Z. Effects of cyclolinopeptide A on T lymphocyte activation and peptidyl prolyl isomerase activity. Biochem. Soc. Trans. 1996, 24, 90S. [Google Scholar] [CrossRef]
- Evers, M.; Barrière, J.C.; Bashiardes, G.; Bousseau, A.; Carry, J.C.; Dereu, N.; Filoche, B.; Henin, Y.; Sablé, S.; Vuilhorgne, M.; et al. Synthesis of non-immunosuppressive cyclophilin-Binding cyclosporin A derivatives as potential anti-HIV-1 drugs. Bioorg. Med. Chem. Lett. 2003, 13, 4415–4419. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Steiner, J.P.; Hamilton, G.S.; Wu, Y.Q. Synthesis and neurotrophic activity of nonimmunosuppressant cyclosporin A derivatives. Bioorg. Med. Chem. Lett. 2004, 14, 4549–4551. [Google Scholar] [CrossRef]
- Liu, S.Y.; Zhang, Q.Z.; Hu, M.Q.; Li, F.X.; Fu, J.M.; Zhu, Z.D.; Li, Q.K.; Yang, Z.; Quan, J.M. Targeting Extracellular Cyclophilin A via an Albumin-Binding Cyclosporine A Analogue. ChemMedChem 2021, 16, 3649–3652. [Google Scholar] [CrossRef]
- Hopkins, S.; Scorneaux, B.; Huang, Z.; Murray, M.G.; Wring, S.; Smitley, C.; Harris, R.; Erdmann, F.; Fischer, G.; Ribeill, Y. SCY-635, a novel nonimmunosuppressive analog of cyclosporine that exhibits potent inhibition of hepatitis C virus RNA replication in vitro. Antimicrob. Agents Chemother. 2010, 54, 660–672. [Google Scholar] [CrossRef]
- Hopkins, S.; DiMassimo, B.; Rusnak, P.; Heuman, D.; Lalezari, J.; Sluder, A.; Scorneaux, B.; Mosier, S.; Kowalczyk, P.; Ribeill, Y.; et al. The cyclophilin inhibitor SCY-635 suppresses viral replication and induces endogenous interferons in patients with chronic HCV genotype 1 infection. J. Hepatol. 2012, 57, 47–54. [Google Scholar] [CrossRef]
- Hopkins, S.; Bobardt, M.; Chatterji, U.; Garcia-Rivera, J.A.; Lim, P.; Gallay, P.A. The Cyclophilin Inhibitor SCY-635 Disrupts Hepatitis C Virus NS5A-Cyclophilin A Complexes. Antimicrob. Agents Chemother. 2012, 56, 3888–3897. [Google Scholar] [CrossRef] [PubMed]
- Rosenwirth, B.; Billich, A.; Datema, R.; Donatsch, P.; Hammerschmid, F.; Harrison, R.; Hiestand, P.; Jaksche, H.; Mayer, P.; Peichl, P.; et al. Inhibition of human immunodeficiency virus type 1 replication by SDZ NIM 811, a nonimmunosuppressive cyclosporine analog. Antimicrob. Agents Chemother. 1994, 38, 1763–1772. [Google Scholar] [CrossRef] [PubMed]
- Gaither, L.A.; Borawski, J.; Anderson, L.J.; Balabanis, K.A.; Devay, P.; Joberty, G.; Rau, C.; Schirle, M.; Bouwmeester, T.; Mickanin, C.; et al. Multiple cyclophilins involved in different cellular pathways mediate HCV replication. Virology 2010, 397, 43–55. [Google Scholar] [CrossRef]
- Ma, S.; Boerner, J.E.; TiongYip, C.; Weidmann, B.; Ryder, N.S.; Cooreman, M.P.; Lin, K. NIM811, a cyclophilin inhibitor, exhibits potent in vitro activity against hepatitis C virus alone or in combination with alpha interferon. Antimicrob. Agents Chemother. 2006, 50, 2976–2982. [Google Scholar] [CrossRef]
- Steinkasserer, A.; Harrison, R.; Billich, A.; Hammerschmid, F.; Werner, G.; Wolff, B.; Peichl, P.; Palfi, G.; Schnitzel, W.; Mlynar, E.; et al. Mode of action of SDZ NIM 811, a nonimmunosuppressive cyclosporin A analog with activity against human immunodeficiency virus type 1 (HIV-1): Interference with early and late events in HIV-1 replication. J. Virol. 1995, 69, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Billich, A.; Hammerschmid, F.; Peichl, P.; Wenger, R.; Zenke, G.; Quesniaux, V.; Rosenwirth, B. Mode of action of SDZ NIM 811, a nonimmunosuppressive cyclosporin A analog with activity against human immunodeficiency virus (HIV) type 1: Interference with HIV protein-cyclophilin A interactions. J. Virol. 1995, 69, 2451–2461. [Google Scholar] [CrossRef]
- Dorfman, T.; Göttlinger, H.G. The human immunodeficiency virus type 1 capsid p2 domain confers sensitivity to the cyclophilin-binding drug SDZ NIM 811. J. Virol. 1996, 70, 5751–5757. [Google Scholar] [CrossRef]
- Mlynar, E.; Bevec, D.; Billich, A.; Rosenwirth, B.; Steinkasserer, A. The non-immunosuppressive cyclosporin A analogue SDZ NIM 811 inhibits cyclophilin A incorporation into virions and virus replication in human immunodeficiency virus type 1-infected primary and growth-arrested T cells. J. Gen. Virol. 1997, 78 Pt 4, 825–835. [Google Scholar] [CrossRef]
- Goto, K.; Watashi, K.; Murata, T.; Hishiki, T.; Hijikata, M.; Shimotohno, K. Evaluation of the anti-hepatitis C virus effects of cyclophilin inhibitors, cyclosporin A, and NIM811. Biochem. Biophys. Res. Commun. 2006, 343, 879–884. [Google Scholar] [CrossRef]
- Seizer, P.; Klingel, K.; Sauter, M.; Westermann, D.; Ochmann, C.; Schönberger, T.; Schleicher, R.; Stellos, K.; Schmidt, E.M.; Borst, O.; et al. Cyclophilin A affects inflammation, virus elimination and myocardial fibrosis in coxsackievirus B3-induced myocarditis. J. Mol. Cell. Cardiol. 2012, 53, 6–14. [Google Scholar] [CrossRef]
- Kaufmann, S.H.; Earnshaw, W.C. Induction of apoptosis by cancer chemotherapy. Exp. Cell Res. 2000, 256, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Waldmeier, P.C.; Feldtrauer, J.J.; Qian, T.; Lemasters, J.J. Inhibition of the mitochondrial permeability transition by the nonimmunosuppressive cyclosporin derivative NIM811. Mol. Pharmacol. 2002, 62, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Theruvath, T.P.; Currin, R.T.; Waldmeier, P.C.; Lemasters, J.J. NIM811, a mitochondrial permeability transition inhibitor, prevents mitochondrial depolarization in small-for-size rat liver grafts. Am. J. Transplant. 2007, 7, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Korde, A.S.; Pettigrew, L.C.; Craddock, S.D.; Pocernich, C.B.; Waldmeier, P.C.; Maragos, W.F. Protective effects of NIM811 in transient focal cerebral ischemia suggest involvement of the mitochondrial permeability transition. J. Neurotrauma 2007, 24, 895–908. [Google Scholar] [CrossRef]
- Theruvath, T.P.; Zhong, Z.; Pediaditakis, P.; Ramshesh, V.K.; Currin, R.T.; Tikunov, A.; Holmuhamedov, E.; Lemasters, J.J. Minocycline and N-methyl-4-isoleucine cyclosporin (NIM811) mitigate storage/reperfusion injury after rat liver transplantation through suppression of the mitochondrial permeability transition. Hepatology 2008, 47, 236–246. [Google Scholar] [CrossRef]
- Garbaisz, D.; Turoczi, Z.; Aranyi, P.; Fulop, A.; Rosero, O.; Hermesz, E.; Ferencz, A.; Lotz, G.; Harsanyi, L.; Szijarto, A. Attenuation of skeletal muscle and renal injury to the lower limb following ischemia-reperfusion using mPTP inhibitor NIM-811. PLoS ONE 2014, 9, e101067. [Google Scholar] [CrossRef]
- Rehman, H.; Sun, J.; Shi, Y.; Ramshesh, V.K.; Liu, Q.; Currin, R.T.; Lemasters, J.J.; Zhong, Z. NIM811 prevents mitochondrial dysfunction, attenuates liver injury, and stimulates liver regeneration after massive hepatectomy. Transplantation 2011, 91, 406–412. [Google Scholar] [CrossRef]
- He, L.; Lemasters, J.J. Regulated and unregulated mitochondrial permeability transition pores: A new paradigm of pore structure and function? FEBS Lett. 2002, 512, 1–7. [Google Scholar] [CrossRef]
- Ma-Lauer, Y.; Zheng, Y.; Malešević, M.; von Brunn, B.; Fischer, G.; von Brunn, A. Influences of cyclosporin A and non-immunosuppressive derivatives on cellular cyclophilins and viral nucleocapsid protein during human coronavirus 229E replication. Antivir. Res. 2020, 173, 104620. [Google Scholar] [CrossRef]
- Quarato, G.; D’Aprile, A.; Gavillet, B.; Vuagniaux, G.; Moradpour, D.; Capitanio, N.; Piccoli, C. The cyclophilin inhibitor alisporivir prevents hepatitis C virus–mediated mitochondrial dysfunction. Hepatology 2012, 55, 1333–1343. [Google Scholar] [CrossRef]
- Chatterji, U.; Bobardt, M.; Selvarajah, S.; Yang, F.; Tang, H.; Sakamoto, N.; Vuagniaux, G.; Parkinson, T.; Gallay, P. The isomerase active site of cyclophilin A is critical for hepatitis C virus replication. J. Biol. Chem. 2009, 284, 16998–17005. [Google Scholar] [CrossRef]
- Daelemans, D.; Dumont, J.M.; Rosenwirth, B.; Clercq, E.D.; Pannecouque, C. Debio-025 inhibits HIV-1 by interfering with an early event in the replication cycle. Antivir. Res. 2010, 85, 418–421. [Google Scholar] [CrossRef]
- Landrieu, I.; Hanoulle, X.; Bonachera, F.; Hamel, A.; Sibille, N.; Yin, Y.; Wieruszeski, J.M.; Horvath, D.; Wei, Q.; Vuagniaux, G.; et al. Structural basis for the non-immunosuppressive character of the cyclosporin A analogue Debio 025. Biochemistry 2010, 49, 4679–4686. [Google Scholar] [CrossRef] [PubMed]
- Davra, V.; Saleh, T.; Geng, K.; Kimani, S.; Mehta, D.; Kasikara, C.; Smith, B. Cyclophilin A Inhibitor Debio-025 Targets Crk, Reduces Metastasis, and Induces Tumor Immunogenicity in Breast Cancer. Mol. Cancer Res. 2020, 18, 1189–1201. [Google Scholar] [CrossRef]
- Sanglier, J.J.; Quesniaux, V.; Fehr, T.; Hofmann, H.; Mahnke, M.; Memmert, K.; Schuler, W.; Zenke, G.; Gschwind, L.; Maurer, C.; et al. Sanglifehrins A, B, C and D, novel cyclophilin-binding compounds isolated from Streptomyces sp. A92-308110. I. Taxonomy, fermentation, isolation and biological activity. J. Antibiot. 1999, 52, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Fehr, T.; Kallen, J.; Oberer, L.; Sanglier, J.J.; Schilling, W. Sanglifehrins A, B, C and D, Novel Cyclophilin-binding Compounds Isolated from Streptomyces sp. A92-308110. II. Structure Elucidation, Stereochemistry and Physico-chemical Properties. J. Antibiot. 1999, 52, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Zenke, G.; Strittmatter, U.; Fuchs, S.; Quesniaux, V.F.; Brinkmann, V.; Schuler, W.; Zurini, M.; Enz, A.; Billich, A.; Sanglier, J.J.; et al. Sanglifehrin A, a Novel Cyclophilin-Binding Compound Showing Immunosuppressive Activity with a New Mechanism of Action. J. Immunol. 2001, 166, 7165–7171. [Google Scholar] [CrossRef] [PubMed]
- Kallen, J.; Sedrani, R.; Zenke, G.; Wagner, J. Structure of human cyclophilin A in complex with the novel immunosuppressant sanglifehrin A at 1.6 A resolution. J. Biol. Chem. 2005, 280, 21965–21971. [Google Scholar] [CrossRef] [PubMed]
- Sedrani, R.; Kallen, J.; Martin Cabrejas, L.M.; Papageorgiou, C.D.; Senia, F.; Rohrbach, S.; Wagner, D.; Thai, B.; Jutzi Eme, A.M.; France, J.; et al. Sanglifehrin-cyclophilin interaction: Degradation work, synthetic macrocyclic analogues, X-ray crystal structure, and binding data. J. Am. Chem. Soc. 2003, 125, 3849–3859. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.H.; Liu, J.O. Sanglifehrin A, a Novel Cyclophilin-Binding Immunosuppressant, Inhibits IL-2-Dependent T Cell Proliferation at the G1 Phase of the Cell Cycle. J. Immunol. 2001, 166, 5611–5618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.H.; Youn, H.D.; Liu, J.O. Inhibition of cell cycle progression by the novel cyclophilin ligand sanglifehrin A is mediated through the NFkappa B-dependent activation of p53. J. Biol. Chem. 2001, 276, 43534–43540. [Google Scholar] [CrossRef]
- Allen, A.; Zheng, Y.; Gardner, L.; Safford, M.; Horton, M.R.; Powell, J.D. The novel cyclophilin binding compound, sanglifehrin A, disassociates G1 cell cycle arrest from tolerance induction. J. Immunol. 2004, 172, 4797–4803. [Google Scholar] [CrossRef]
- Steinschulte, C.; Taner, T.; Thomson, A.W.; Bein, G.; Hackstein, H. Cutting edge: Sanglifehrin A, a novel cyclophilin-binding immunosuppressant blocks bioactive IL-12 production by human dendritic cells. J. Immunol. 2003, 171, 542–546. [Google Scholar] [CrossRef]
- Woltman, A.M.; Schlagwein, N.; van der Kooij, S.W.; Van Kooten, C. The Novel Cyclophilin-Binding Drug Sanglifehrin A Specifically Affects Antigen Uptake Receptor Expression and Endocytic Capacity of Human Dendritic Cells. J. Immunol. 2004, 172, 6482–6489. [Google Scholar] [CrossRef]
- Chang, C.F.; Flaxman, H.A.; Woo, C.M. Enantioselective Synthesis and Biological Evaluation of Sanglifehrin A and B and Analogs. Angew. Chem. Int. Ed. 2021, 60, 17045–17052. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, S.; Choi, J.; Song, Y.K.; Kim, M.J.; Shin, D.S.; Bae, M.A.; Kim, Y.C. Interplay among Conformation, Intramolecular Hydrogen Bonds, and Chameleonicity in the Membrane Permeability and Cyclophilin A Binding of Macrocyclic Peptide Cyclosporin O Derivatives. J. Med. Chem. 2021, 64, 8272–8286. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, J.; Zhang, L.; Wang, F.; Gui, C.; Zhang, L.; Qin, Y.; Xu, Q.; Liu, H.; Nan, F.; et al. One novel quinoxaline derivative as a potent human cyclophilin A inhibitor shows highly inhibitory activity against mouse spleen cell proliferation. Bioorg. Med. Chem. 2006, 14, 5527–5534. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, J.; Chen, J.; Luo, X.; Zhu, W.; Shen, J.; Liu, H.; Shen, X.; Jiang, H. Strategy for Discovering Chemical Inhibitors of Human Cyclophilin A:? Focused Library Design, Virtual Screening, Chemical Synthesis and Bioassay. J. Comb. Chem. 2006, 8, 326–337. [Google Scholar] [CrossRef]
- Qian, Z.; Zhao, X.; Jiang, M.; Jia, W.; Zhang, C.; Wang, Y.; Li, B.; Yue, W. Downregulation of Cyclophilin A by siRNA diminishes non-small cell lung cancer cell growth and metastasis via the regulation of matrix metallopeptidase 9. BMC Cancer 2012, 12, 442. [Google Scholar] [CrossRef]
- Daum, S.; Schumann, M.; Mathea, S.; Aumüller, T.; Balsley, M.A.; Constant, S.L.; De Lacroix, B.F.A.; Kruska, F.; Braun, M.; Schiene-Fischer, C. Isoform-Specific Inhibition of Cyclophilins. Biochemistry 2009, 48, 6268–6277. [Google Scholar] [CrossRef] [PubMed]
- Sambasivarao, S.V.; Acevedo, O. Computational Insight into Small Molecule Inhibition of Cyclophilins. J. Chem. Inf. Model. 2011, 51, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Moir, E.; Kontopidis, G.; Taylor, P.; Wear, M.A.; Malone, K.; Dunsmore, C.J.; Page, A.P.; Turner, N.J.; Walkinshaw, M.D. Structure-based discovery of a family of synthetic cyclophilin inhibitors showing a cyclosporin-A phenotype in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 2007, 363, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Dunsmore, C.J.; Malone, K.J.; Bailey, K.R.; Wear, M.A.; Florance, H.; Shirran, S.; Barran, P.E.; Page, A.P.; Walkinshaw, M.D.; Turner, N.J. Design and Synthesis of Conformationally Constrained Cyclophilin Inhibitors Showing a Cyclosporin-A Phenotype in C. elegans. ChemBioChem 2011, 12, 802–810. [Google Scholar] [CrossRef]
- Gegunde, S.; Alfonso, A.; Alonso, E.; Alvariño, R.; Botana, L.M. Gracilin-Derivatives as Lead Compounds for Anti-inflammatory Effects. Cell Mol. Neurobiol. 2020, 40, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Hamed, A.; Ismail, M.; El-Metwally, M.M.; Frese, M.; Stammler, H.G.; Sewald, N.; Shaaban, M. X-ray, structural assignment and molecular docking study of dihydrogeodin from Aspergillus Terreus TM8. Nat. Prod. Res. 2019, 33, 117–121. [Google Scholar] [CrossRef]
- Li, X.; Han, J.; Lee, H.W.; Yoon, Y.S.; Jin, Y.; Khadka, D.B.; Yang, S.; Kim, M.; Cho, W.J. SAR study of bisamides as cyclophilin a inhibitors for the development of host-targeting therapy for hepatitis C virus infection. Bioorg. Med. Chem. 2020, 28, 115679. [Google Scholar] [CrossRef]
- Nevers, Q.; Ruiz, I.; Ahnou, N.; Donati, F.; Brillet, R.; Softic, L.; Chazal, M.; Jouvenet, N.; Fourati, S.; Baudesson, C.; et al. Characterization of the Anti-Hepatitis C Virus Activity of New Nonpeptidic Small-Molecule Cyclophilin Inhibitors with the Potential for Broad Anti-Flaviviridae Activity. Antimicrob. Agents Chemother. 2018, 62, e00126-18. [Google Scholar] [CrossRef]
- Ahmed-Belkacem, A.; Colliandre, L.; Ahnou, N.; Nevers, Q.; Gelin, M.; Bessin, Y.; Brillet, R.; Cala, O.; Douguet, D.; Bourguet, W.; et al. Fragment-based discovery of a new family of non-peptidic small-molecule cyclophilin inhibitors with potent antiviral activities. Nat. Commun. 2016, 7, 12777. [Google Scholar] [CrossRef]
- Han, J.M.; Kim, S.M.; Kim, H.L.; Cho, H.J.; Jung, H.J. Natural Cyclophilin A Inhibitors Suppress the Growth of Cancer Stem Cells in Non-Small Cell Lung Cancer by Disrupting Crosstalk between CypA/CD147 and EGFR. Int. J. Mol. Sci. 2023, 24, 9437. [Google Scholar] [CrossRef]
- Cho, H.J.; Jung, H.J. Cyclophilin A Inhibitors Suppress Proliferation and Induce Apoptosis of MKN45 Gastric Cancer Stem-like Cells by Regulating CypA/CD147-Mediated Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 4734. [Google Scholar] [CrossRef]
- Li, Q.; Moutiez, M.; Charbonnier, J.B.; Vaudry, K.; Ménez, A.; Quéméneur, E.; Dugave, C. Design of a Gag pentapeptide analogue that binds human cyclophilin A more efficiently than the entire capsid protein: New insights for the development of novel anti-HIV-1 drugs. J. Med. Chem. 2000, 43, 1770–1779. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Huang, X.; Luo, X.; Briggs, J.M.; Ji, R.; Chen, K.; Shen, J.; Jiang, H. Molecular docking and 3D-QSAR studies on gag peptide analogue inhibitors interacting with human cyclophilin A. J. Med. Chem. 2002, 45, 5249–5259. [Google Scholar] [CrossRef]
- Pang, X.; Zhou, L.; Zhang, M.; Xie, F.; Yu, L.; Zhang, L.; Xu, L.; Zhang, X. A mathematical model for peptide inhibitor design. J. Comput. Biol. 2010, 17, 1081–1093. [Google Scholar] [CrossRef]
- Pang, X.; Zhang, M.; Zhou, L.; Xie, F.; Lu, H.; He, W.; Jiang, S.; Yu, L.; Zhang, X. Discovery of a potent peptidic cyclophilin A inhibitor Trp-Gly-Pro. Eur. J. Med. Chem. 2011, 46, 1701–1705. [Google Scholar] [CrossRef]
- Demange, L.; Moutiez, M.; Dugave, C. Synthesis and evaluation of Glyψ(PO2R-N)Pro-containing pseudopeptides as novel inhibitors of the human cyclophilin hCyp-18. J. Med. Chem. 2002, 45, 3928–3933. [Google Scholar] [CrossRef]
- Wang, H.C.; Kim, K.; Bakhtiar, R.; Germanas, J.P. Structure-Activity Studies of Ground- and Transition-State Analogue Inhibitors of Cyclophilin. J. Med. Chem. 2001, 44, 2593–2600. [Google Scholar] [CrossRef]
- Dantal, J.; Hourmant, M.; Cantarovich, D.; Giral, M.; Blancho, G.; Dreno, B.; Soulillou, J.P. Effect of long-term immunosuppression in kidney-graft recipients on cancer incidence: Randomised comparison of two cyclosporin regimen. Lancet 1998, 351, 623–628. [Google Scholar] [CrossRef]
- Muellenhoff, M.W. KJ Cyclosporine and skin cancer: An international dermatologic perspective over 25 years of experience. A comprehensive review and pursuit to define safe use of cyclosporine in dermatology. J. Dermatol. Treat. 2012, 23, 290–304. [Google Scholar] [CrossRef] [PubMed]
- Euvrard, S.; Kanitakis, J.; Claudy, A. Skin cancers after organ transplantation. N. Engl. J. Med. 2003, 348, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.J.; Piao, Y.J.; Lim, M.J.; Kim, J.H.; Ha, J.; Choe, W.; Kim, S.S. Overexpressed cyclophilin A in cancer cells renders resistance to hypoxia- and cisplatin-induced cell death. Cancer Res. 2007, 67, 3654–3662. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, M.; Ma, H.; Saiyin, H.; Shen, S.; Xi, J.; Wan, B.; Yu, L. Oligo-microarray analysis reveals the role of cyclophilin A in drug resistance. Cancer Chemother. Pharmacol. 2008, 61, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhou, K.; Jing, L.; Zhang, L.; Peng, G.; Li, Y.; Ye, L.; Li, J.; Fan, H.; Li, Y.; et al. Treatment of T-cell large granular lymphocyte leukemia with cyclosporine A: Experience in a Chinese single institution. Leuk. Res. 2013, 37, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Middleton, G.; Brown, S.; Lowe, C.; Maughan, T.; Gwyther, S.; Oliver, A.; Richman, S.; Blake, D.; Napp, V.; Marshall, H.; et al. A randomised phase III trial of the pharmacokinetic biomodulation of irinotecan using oral ciclosporin in advanced colorectal cancer: Results of the Panitumumab, Irinotecan & Ciclosporin in COLOrectal cancer therapy trial (PICCOLO). Eur. J. Cancer 2013, 49, 3507–3516. [Google Scholar]
- Murren, J.R.; Ganpule, S.; Sarris, A.; Durivage, H.; Davis, C.; Makuch, R.; Handschumacher, R.E.; Marsh, J.C. A phase II trial of cyclosporin A in the treatment of refractory metastatic colorectal cancer. Am. J. Clin. Oncol. 1991, 14, 208–210. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zheng, C.; Wang, H.; Zhang, L.; Liu, Z.; Xu, P. The state of the art of PROTAC technologies for drug discovery. Eur. J. Med. Chem. 2022, 235, 114290. [Google Scholar] [CrossRef]
- Rodriguez-Bussey, I.G.; Doshi, U.; Hamelberg, D. Enhanced molecular dynamics sampling of drug target conformations. Biopolymers 2016, 105, 35–42. [Google Scholar] [CrossRef]
- Zhang, M.; Dai, C.; Zhu, H.; Chen, S.; Wu, Y.; Li, Q.; Zeng, X.; Wang, W.; Zuo, J.; Zhou, M.; et al. Cyclophilin A promotes human hepatocellular carcinoma cell metastasis via regulation of MMP3 and MMP9. Mol. Cell Biochem. 2011, 357, 387–395. [Google Scholar] [CrossRef]
- Hakim, S.; Craig, J.M.; Koblinski, J.E.; Clevenger, C.V. Inhibition of the Activity of Cyclophilin A Impedes Prolactin Receptor-Mediated Signaling, Mammary Tumorigenesis, and Metastases. iScience 2020, 23, 101581. [Google Scholar] [CrossRef]

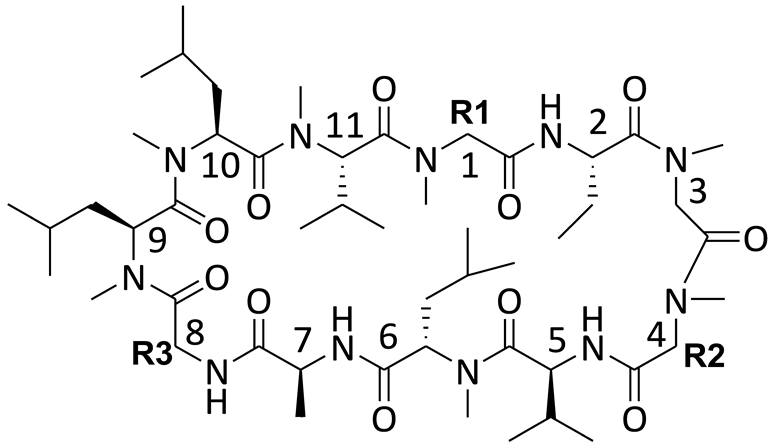 Cyclosporin A (CsA, C1) | |||
| R1 | R2 | R3 | |
| CsA | 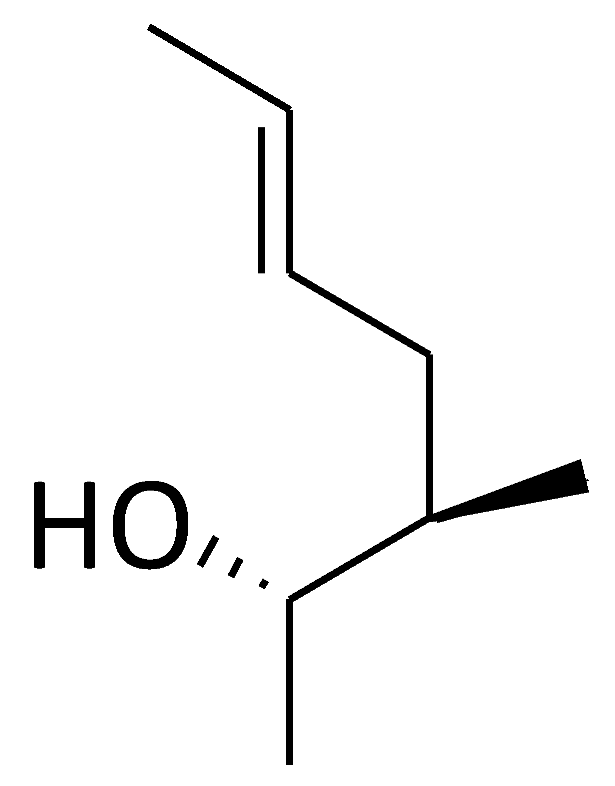 |  | 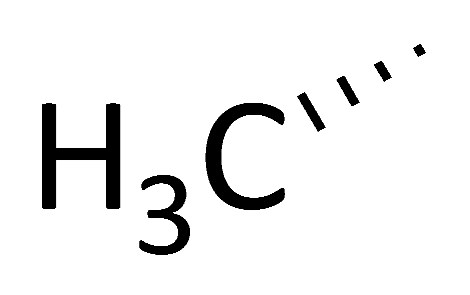 |
| C8 (Dihydro-CsA) | 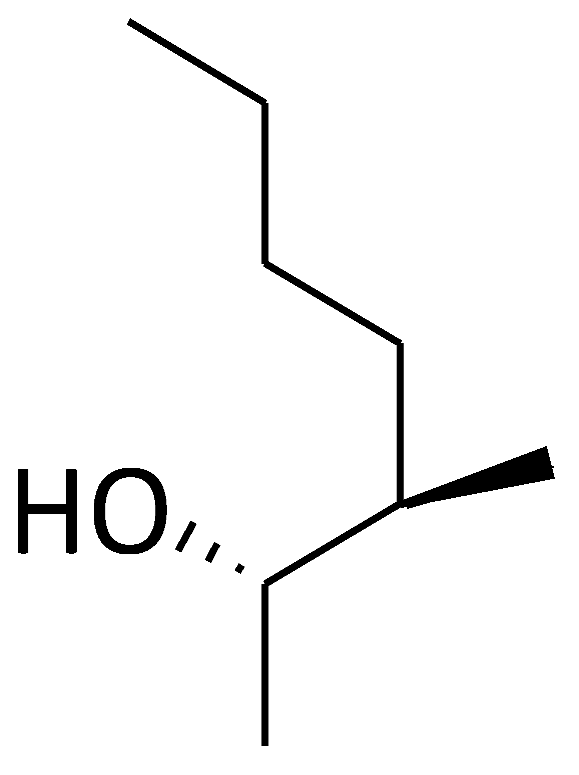 | 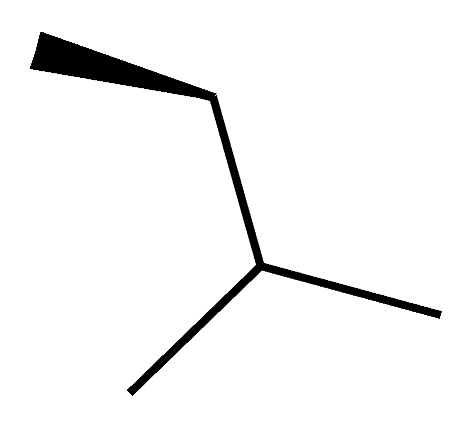 | 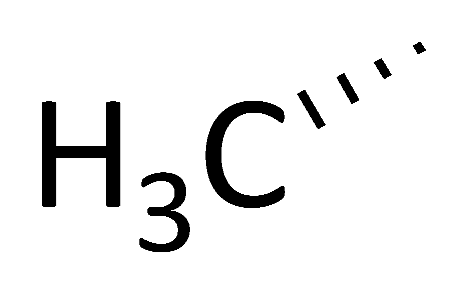 |
| C9 ([DehydroAla]8-CsA) |  | 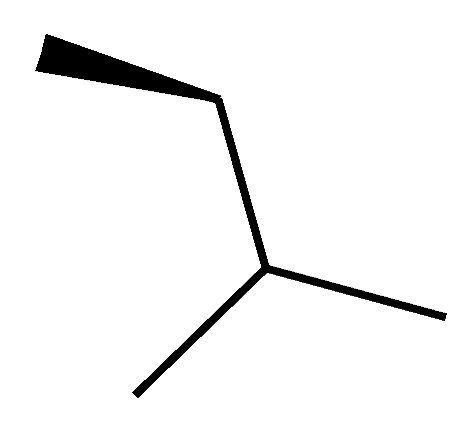 |  |
| C10 ([MeVal]4-CsA) |  |  |  |
| C11 ([MeAbu]4-CsA) | 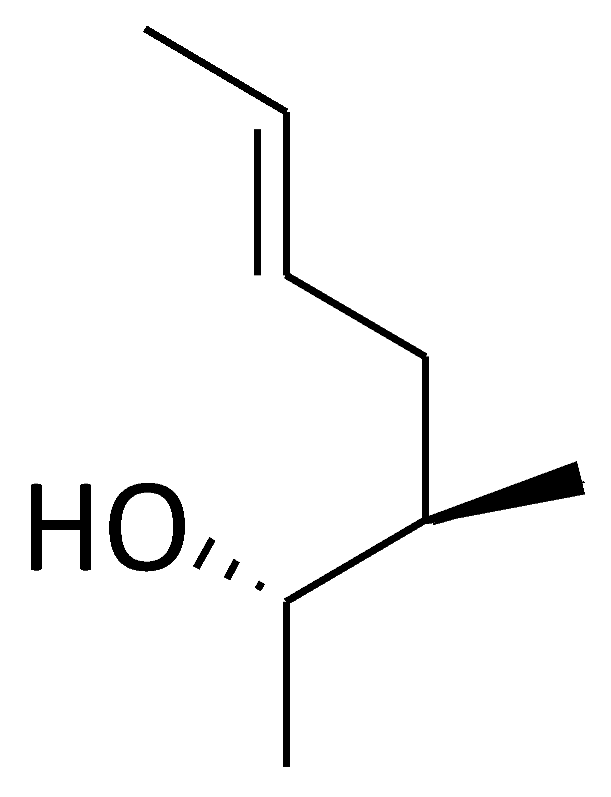 |  |  |
| C12 ([Me(α-methyl)Thr]4-CsA) | 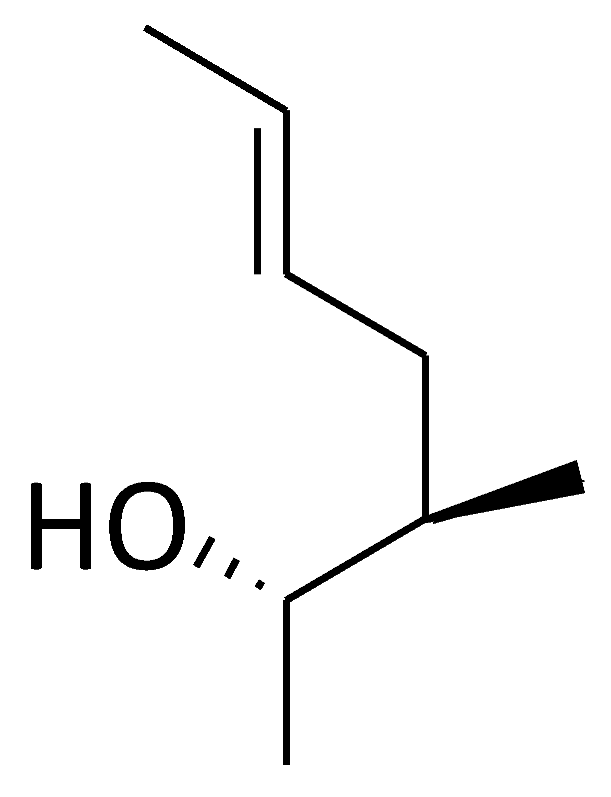 | 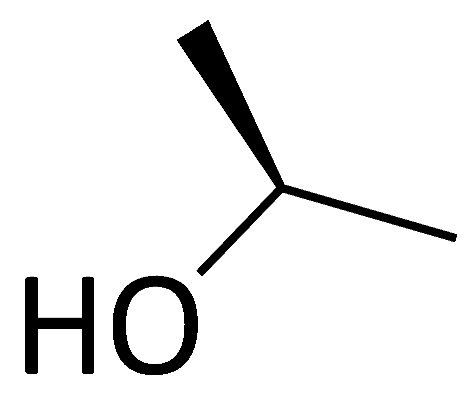 | 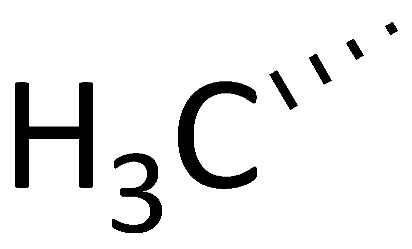 |
| Inhibitor Number | Inhibitor Name | NCT Number | Alone or in Combination | Sponsors | Diseases | Status |
|---|---|---|---|---|---|---|
| C1 | Cyclosporin A (CsA) | ~381 cancer-related clinical trials in early phase 1 and phases 1, 2, 3 or 4 b | Alone or in combination | Virginia G. Kaklamani, Novartis, Allergan, NCI, MD Anderson Cancer Center, and others | Breast cancer, colon cancer, melanoma, nonmelanoma skin cancer, hematologic cancer, colorectal cancer, and others | Active, recruiting, completed, or terminated |
| NCT00983424 (phase 1) | CsA Nab-paclitaxel | Northwestern University, Avon Foundation | Metastatic breast cancer | Completed | ||
| NCT00003950 (phase 2) | CsA CPT-11 | NCI, University of Chicago | Metastatic, advanced, or locally recurrent colorectal cancer | Completed | ||
| NCT00389870 (phase 3) | CsA plus Irinotecan | University of Leeds | Colorectal cancer | Completed | ||
| NCT04979884 (phase 3) | Alone | Alexandria University | COVID-19 | Completed | ||
| NCT04392531 (phase 4) | CsA plus SOC c | Instituto de Investigación Sanitaria de la Fundación Jiménez Díaz | COVID-19 | Completed | ||
| NCT00979706 (phase 4) | CsA plus HAART c | Hospital Clinic of Barcelona | HIV | Completed | ||
| NCT00866684 (phase 4) | CsA as Comparator | Charite University, Berlin, Germany | Skin cancer | Completed | ||
| C13 | SCY-635 | NCT01290965 (phase 1) | Alone | Scynexis | Hepatitis C infection | Completed |
| NCT01265511 (phase 2) | Alone | Scynexis | Hepatitis C infection | Completed | ||
| C14 | NIM811 | NCT00983060 (phase 2) | Alone | Novartis | Chronic hepatitis C Genotype-1 relapse | Completed |
| C15 | Alisporivir (Deb 025) | NCT01975337 (phase 1) | Alone | Debiopharm International SA | Kidney failure | Completed |
| NCT02173574 (phase 1) | Deb 025, EDP239 | Enanta Pharmaceuticals | Hepatitis C infection | Completed | ||
| NCT01860326 (phase 1) | Alone | Debiopharm International SA | Hepatitis C | Completed | ||
| NCT01183169 (phase 2) | Deb 025, Peginterferon alfa-2a, Ribavirin | Debiopharm International SA | Hepatitis C infection | Completed | ||
| NCT00537407 (phase 2) | Deb 025, Peginterferon alfa-2a, Ribavirin | Debiopharm International SA | Chronic hepatitis C | Completed | ||
| NCT02094443 (phase 2) | Deb 025, Ribavirin | Debiopharm International SA | Hepatitis C infection | Completed | ||
| NCT01215643 (phase 2) | Deb 025, Peginterferon alfa-2a, Ribavirin | Debiopharm International SA | Hepatitis C infection | Completed | ||
| NCT04608214 (phase 2) | Alone | Assistance Publique—Hôpitaux de Paris | SARS-CoV-2 | Completed | ||
| NCT02753699 (phase 3) | Alone | DebiopharmInternational SA | Hepatitis C infection | Completed | ||
| NCT01318694 (phase 3) | Deb 025, Peginterferon alfa-2a, Ribavirin | Enanta Pharmaceuticals | Hepatitis C infection | Completed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Zhao, X.; Di, W.; Wang, C. Inhibitors of Cyclophilin A: Current and Anticipated Pharmaceutical Agents for Inflammatory Diseases and Cancers. Molecules 2024, 29, 1235. https://doi.org/10.3390/molecules29061235
Zhao X, Zhao X, Di W, Wang C. Inhibitors of Cyclophilin A: Current and Anticipated Pharmaceutical Agents for Inflammatory Diseases and Cancers. Molecules. 2024; 29(6):1235. https://doi.org/10.3390/molecules29061235
Chicago/Turabian StyleZhao, Xuemei, Xin Zhao, Weihua Di, and Chang Wang. 2024. "Inhibitors of Cyclophilin A: Current and Anticipated Pharmaceutical Agents for Inflammatory Diseases and Cancers" Molecules 29, no. 6: 1235. https://doi.org/10.3390/molecules29061235
APA StyleZhao, X., Zhao, X., Di, W., & Wang, C. (2024). Inhibitors of Cyclophilin A: Current and Anticipated Pharmaceutical Agents for Inflammatory Diseases and Cancers. Molecules, 29(6), 1235. https://doi.org/10.3390/molecules29061235






