Stripping Mechanism of Surfactant System Based on Residual Oil on the Surface of Sand-Conglomerate Rocks with Different Grain Size Mineral Compositions
Abstract
:1. Introduction
2. Results and Discussion
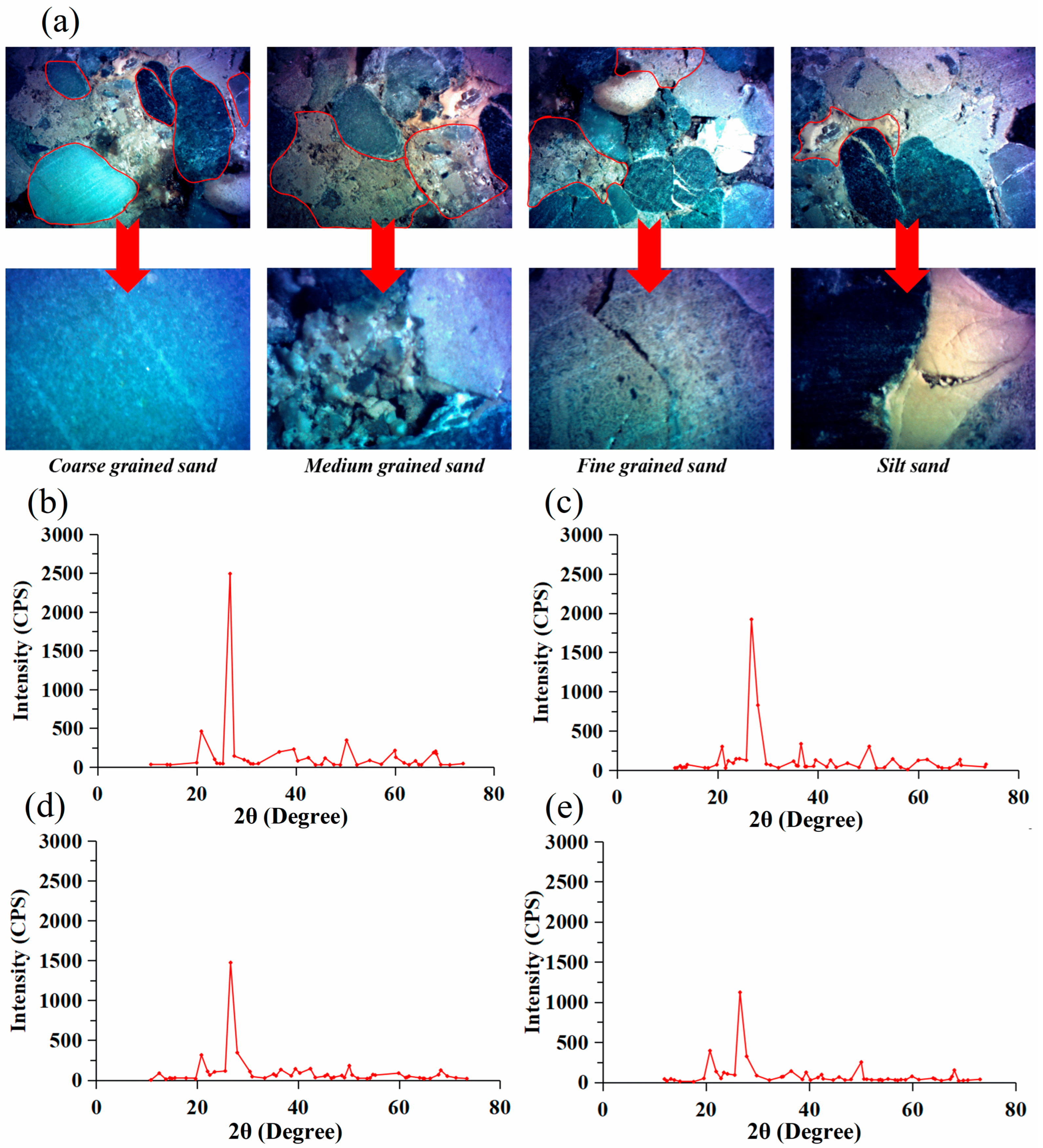
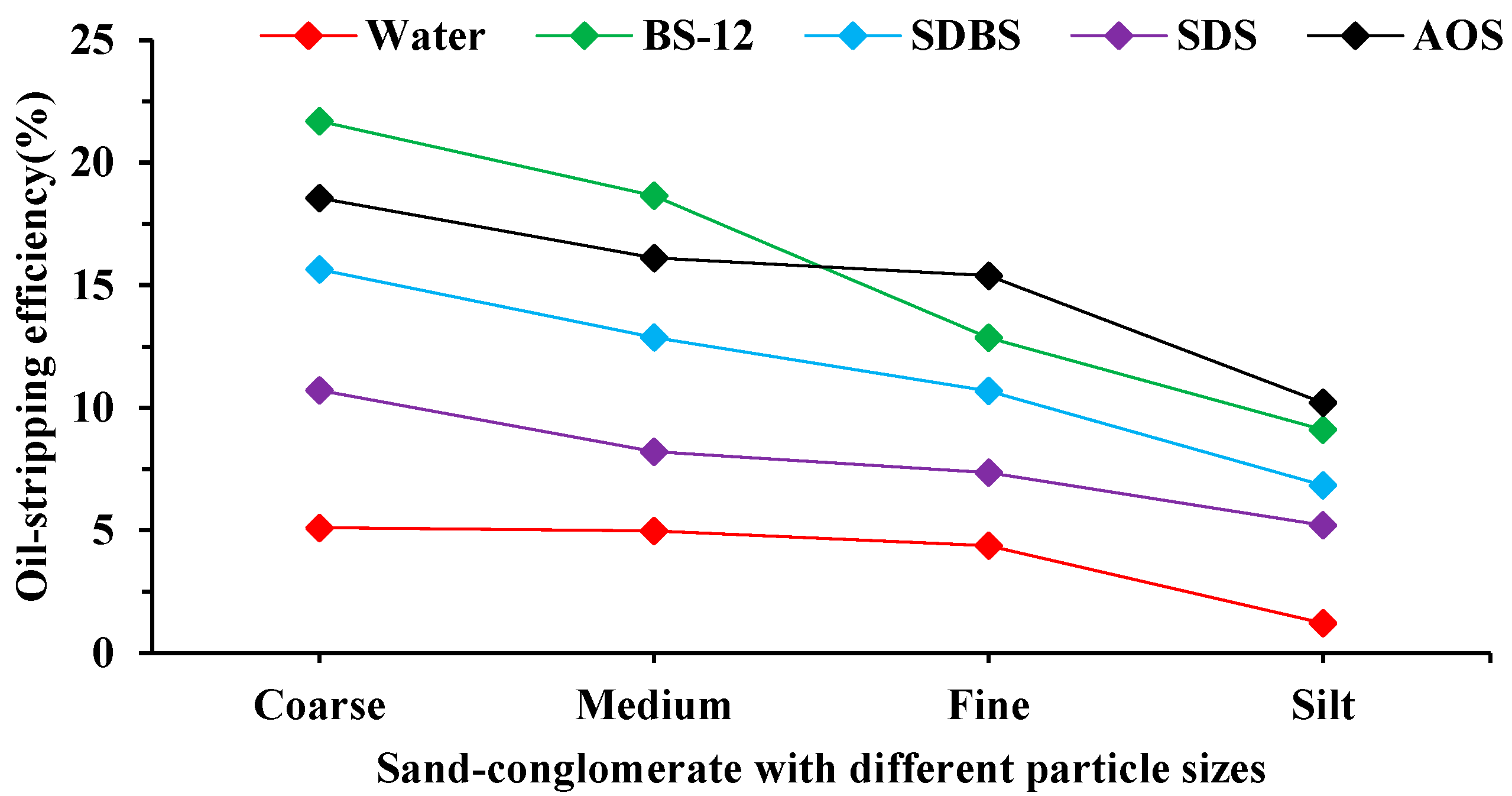
2.1. Wettability and Oil-Stripping Effect of Surfactant Solutions on Minerals with Different Particle Sizes
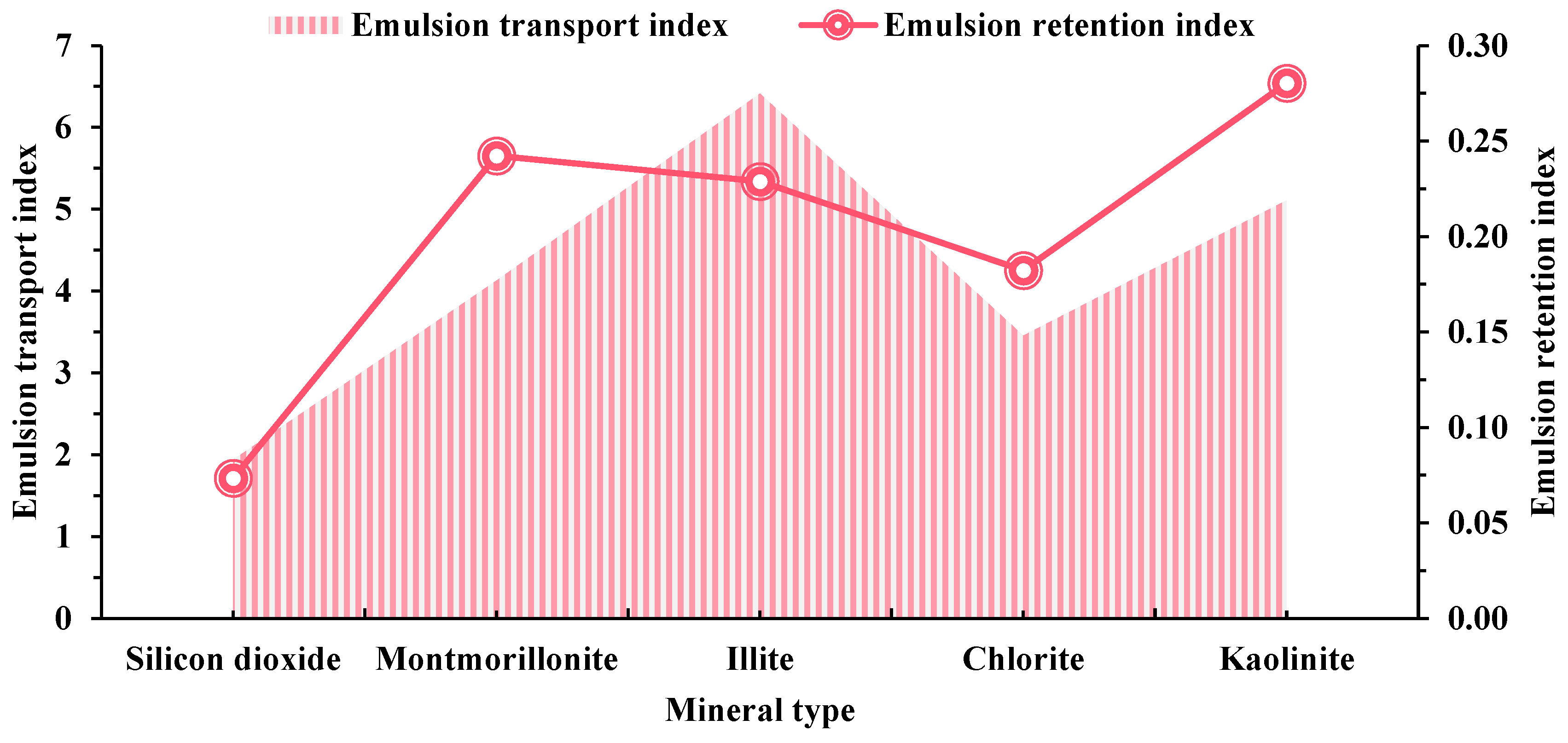
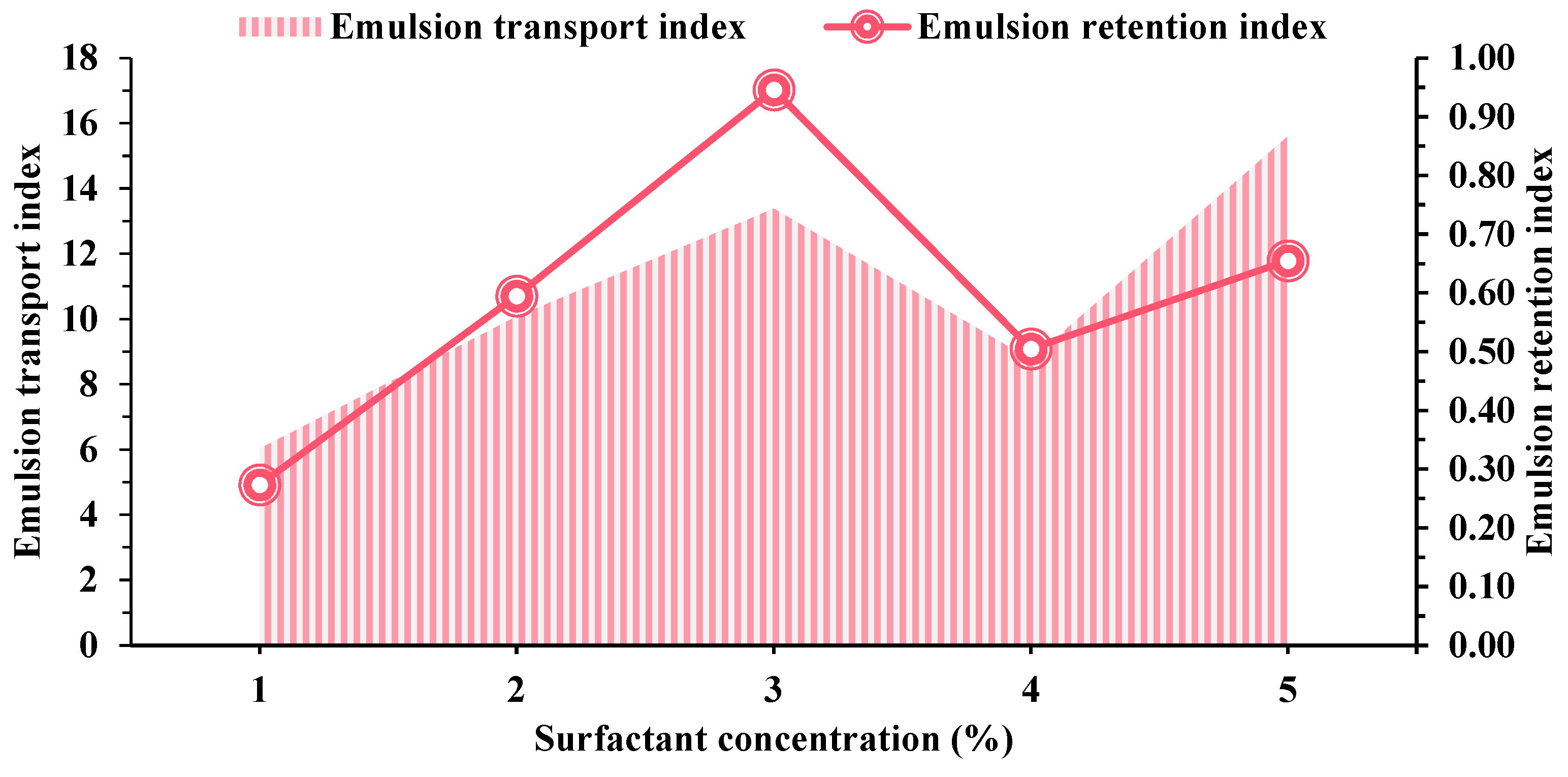
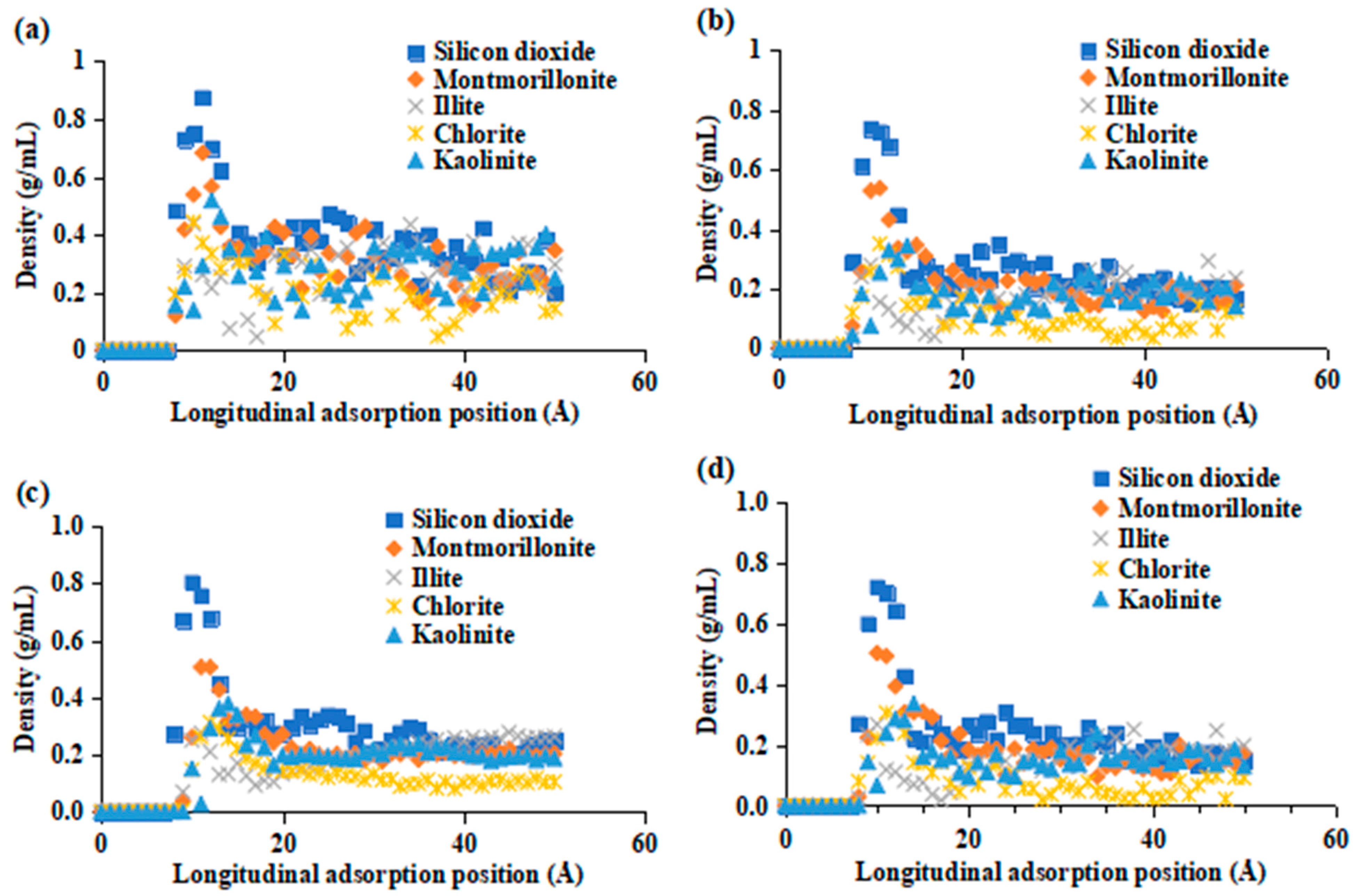
2.2. Adsorption Behavior of Surfactants on Various Minerals
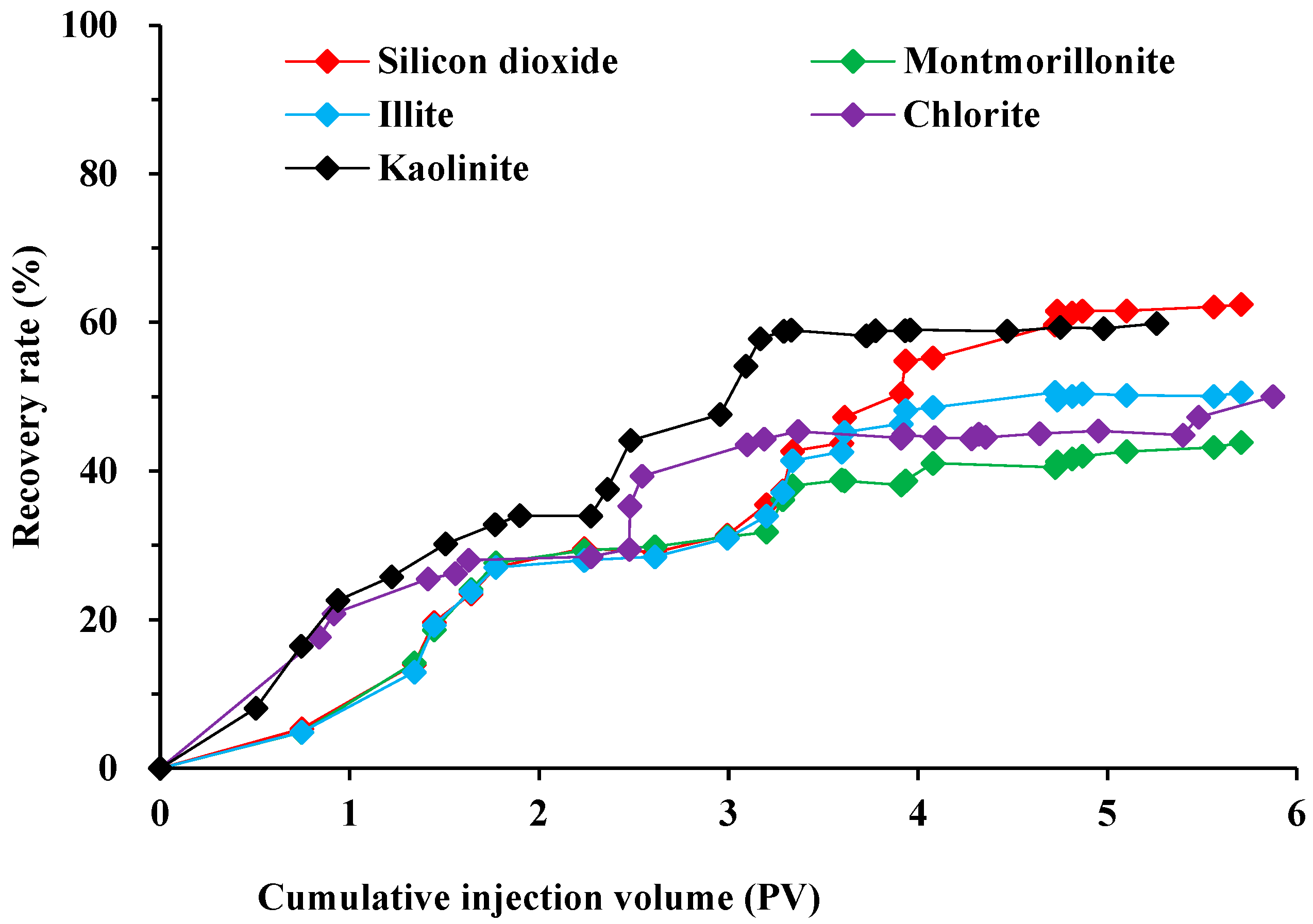
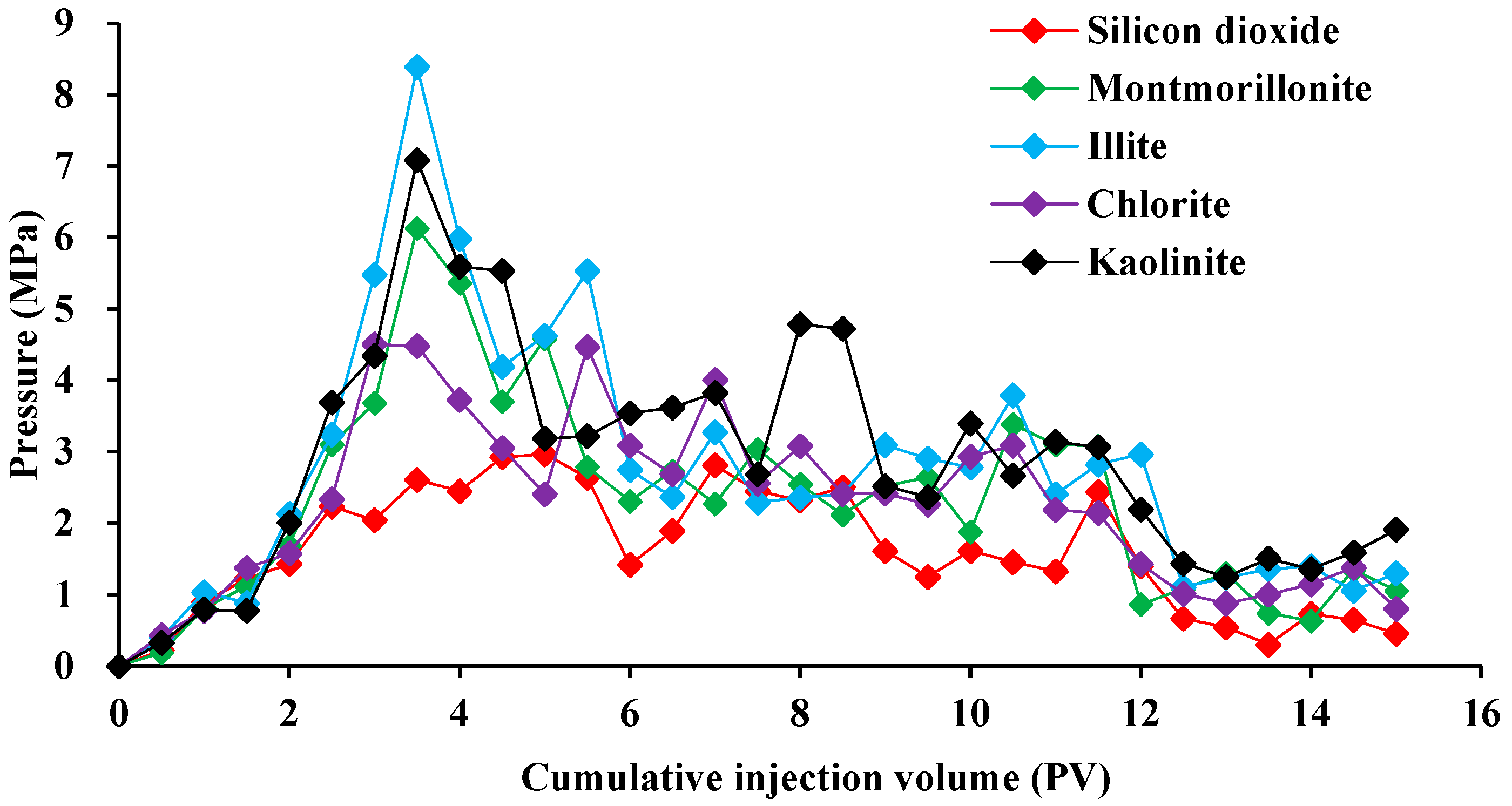
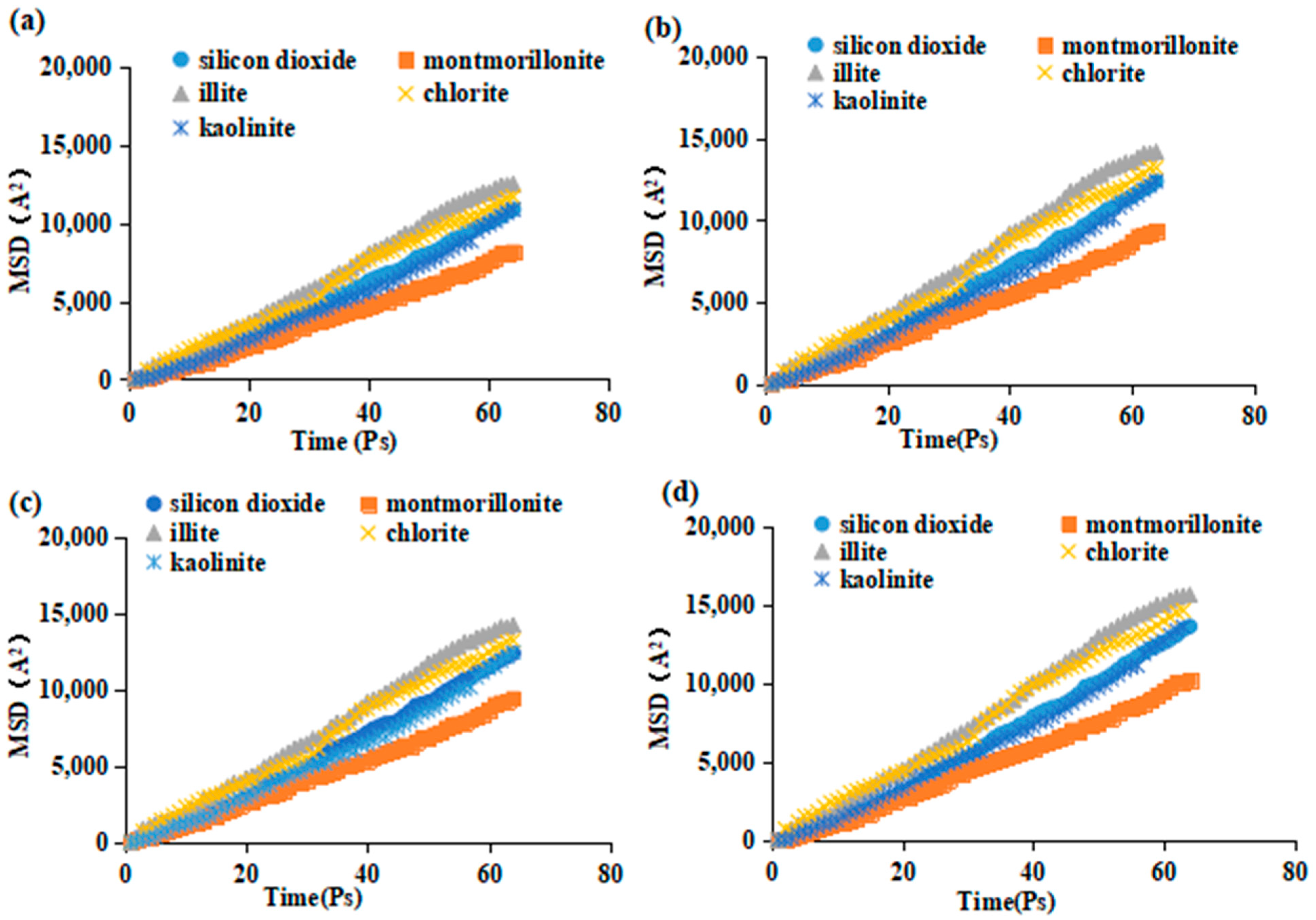
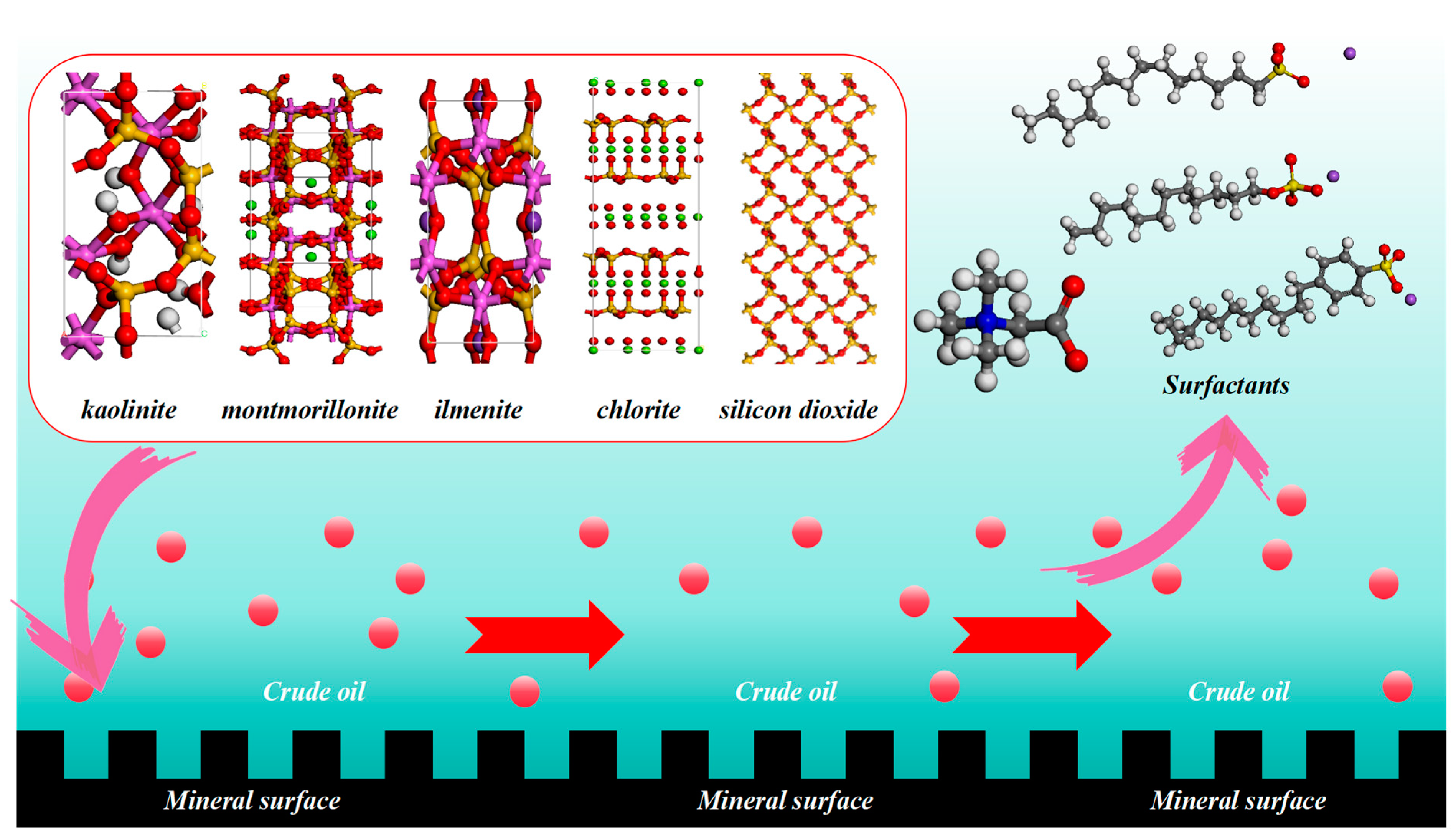
2.3. Mechanism of Oil-Stripping by Surfactants on the Surface of Different Conglomerate Minerals
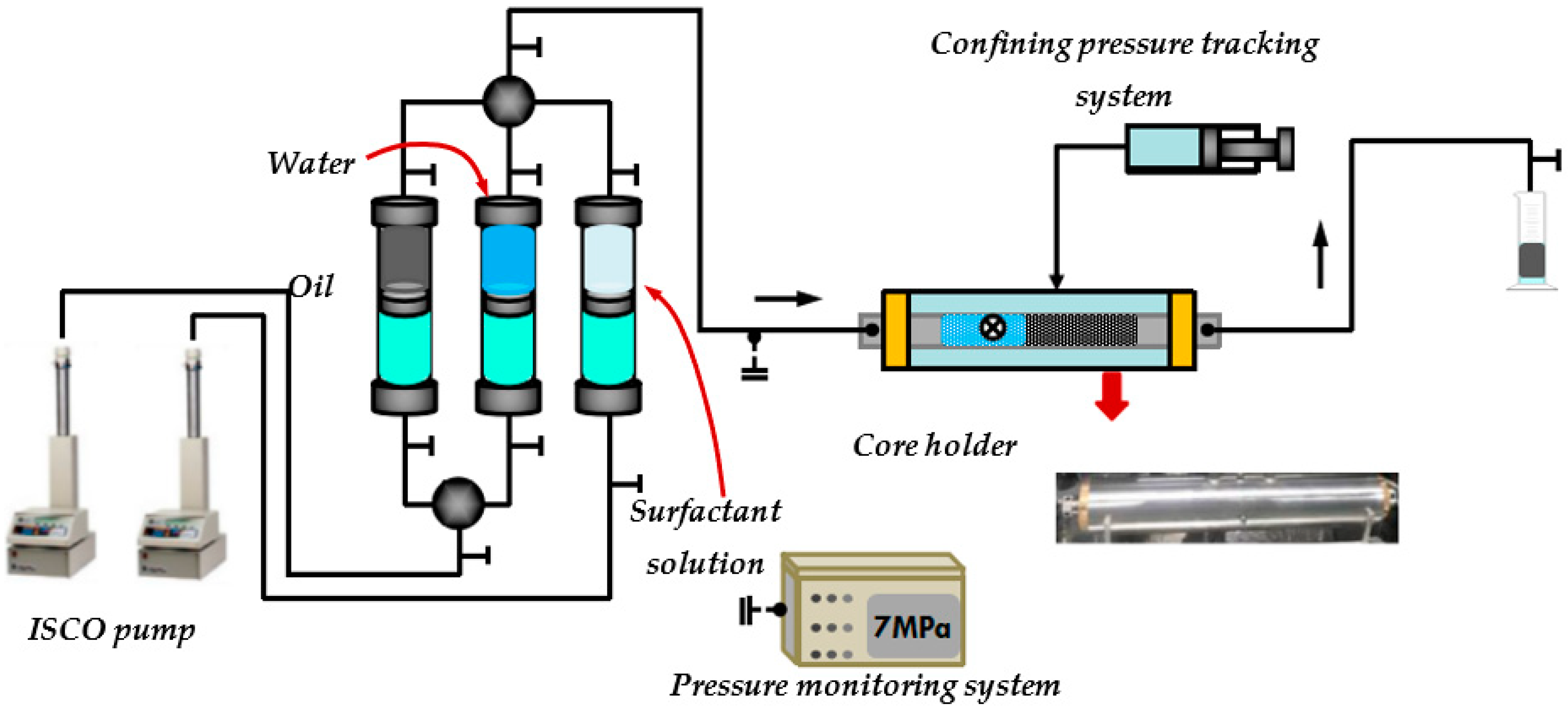
3. Experiment and Methodology
3.1. Materials and Instruments
3.2. Influence of Surfactants on the Wettability and Oil-Stripping Effect of Minerals with Different Particle Sizes
3.2.1. Rock Wettability Experiment
- (1)
- The focal length of the instrument was checked. A specimen with the same thickness as the sample to be tested was selected, and a droplet of surfactant solution with a volume of 4 μL was dropped on the center of its surface, and the camera system was adjusted so that the needle and droplet contours were clearly imaged in the contact angle measuring instrument.
- (2)
- The surface of the rock sample was made into a thin slice, and the surface of the slice was ground flat. The sample was placed on the test bench of the instrument, the height of the sample bench was adjusted, the droplet flow rate was set to 0.5 μL/s, the droplet contacted with the surface of the sample to be tested and disengaged from the needle (the diameter of the needle was 0.9 mm), and a seated drop was formed on the surface of the sample. The formation of the seated drop and the spreading of the seated drop were photographed with a contact angle meter at a speed of not less than 2 frames/s. The test time was 180 s.
- (3)
- Five photographs with contact times ranging from 55 s to 65 s were identified, and the contact angles were calculated by the spherical calculation method, and the average value of the above contact angles was taken as the static contact angle of the samples.
3.2.2. Oil-Stripping Efficiency Experiment
- (1)
- The washed quartz sand was mixed with crude oil in a ratio of 1:6 to make the oil sand, with the mixture aged for 24 h at 60 °C.
- (2)
- The surfactant solution with a mass concentration of 0.5% was mixed with deionized water.
- (3)
- The oil sand (m1 = 15 g) was placed in the centrifuge tube, and the surfactant was added according to the mass ratio of oil sand/surfactant = 1:2. The samples were put into the centrifuge, which can make the oil sand more compact and achieve the effect of stripping static test.
- (4)
- The mixture in the centrifuge tube of step 3 was filtered, and the filtered oil sand was wrapped and put into a drying oven for 24 h.
- (5)
- The oil sand in step 4 was weighed, and the oil-stripping efficiency was calculated using Equation (1).
3.2.3. Oil Displacement in Different Conglomerate Types by Emulsifier Systems
- (1)
- Artificial cores with physical characteristics resembling those of the reservoirs being studied were collected and deposited in a drying oven. Subsequently, the length and dry weight (m0) of the cores were measured.
- (2)
- The core was soaked with formation water and the weight of the soaked core was measured.
- (3)
- A core full of formation water was put into the core adder. Simulated oil was then pumped into the core at a rate of 0.1 mL/min to push the oil away until the recovered fluid was free of water and the water output was recorded.
- (4)
- Following water-driven dehydration to achieve a water content of 98% at a flow rate of 0.1 mL/min, a surfactant solution with a concentration of 0.3% was injected using a volume of 0.5 PV (pore volume). Subsequent water-driven dehydration was then conducted to observe the variations in oil production, fluid production, and pressure throughout the experiment.
- (5)
- The emulsification regulation system and cores were adjusted.
3.3. Adsorption Retention Experiments of Surfactants on Minerals with Different Particle Sizes
3.4. Molecular Modeling of Surfactants with Different Conglomerate Minerals
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, X.; Liu, H.; Wang, C.; Lu, C.; Yan, F. The polymer-enhanced foam injection process: An improved-oil-recovery technique for light oil reservoirs after polymer flooding. Energy Sources Part A 2016, 38, 354–361. [Google Scholar] [CrossRef]
- Yang, E.L. The Application of Fuzzy Clustering Method in the Division of Reservoir Flow Unit. Adv. Mater. Res. 2011, 219, 1670–1674. [Google Scholar] [CrossRef]
- Thakur, G. Enhanced recovery technologies for unconventional oil reservoirs. J. Pet. Technol. 2019, 71, 66–69. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, S.; Li, Z.; Zhu, R.; Xie, S.; Jing, C.; Lei, L. Multifractal study of three-dimensional pore structure of sand-conglomerate reservoir based on CT images. Energy Fuels 2018, 32, 4797–4807. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, Z.; Fan, W.; Wang, J.; Li, Z. Evaluating the sedimentological and diagenetic impacts on terrestrial lacustrine fan delta sandy conglomerates reservoir quality: Insights from the Triassic Baikouquan Formation in the Mahu sag, Junggar Basin, Western China. Mar. Pet. Geol. 2022, 146, 105973. [Google Scholar] [CrossRef]
- Yin, S.; Chen, Y.; Wu, X. Different pore structure modalities in sandy conglomerate reservoirs and their forming mechanisms. Arabian J. Geosci. 2018, 11, 654. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, S.; Li, Z.; Zhu, R.; Xie, S.; Zhai, X.; Lei, L. Investigation of microscopic pore structure and permeability prediction in sand-conglomerate reservoirs. J. Earth Sci. 2021, 32, 818–827. [Google Scholar] [CrossRef]
- Gong, Q.; Liu, Z.; Zhu, C.; Wang, B.; Jin, Y.; Shi, Z.; Wu, J. Heterogeneity of a Sandy Conglomerate Reservoir in Qie12 Block, Qaidam Basin, Northwest China and Its Influence on Remaining Oil Distribution. Energies 2023, 16, 2972. [Google Scholar] [CrossRef]
- Ge, X.; Fan, Y.; Zhu, X.; Chen, Y.; Li, R. Determination of nuclear magnetic resonance T2 cutoff value based on multifractal theory—An application in sandstone with complex pore structure. Geophysics 2015, 80, 11–21. [Google Scholar] [CrossRef]
- Ding, L.; Wu, Q.; Zhang, L.; Guérillot, D. Application of Fractional Flow Theory for Analytical Modeling of Surfactant Flooding, Polymer Flooding, and Surfactant/Polymer Flooding for Chemical Enhanced Oil Recovery. Water 2020, 12, 2195. [Google Scholar] [CrossRef]
- Mohsenatabar Firozjaii, A.; Derakhshan, A.; Shadizadeh, S.R. An investigation into surfactant flooding and alkaline-surfactant-polymer flooding for enhancing oil recovery from carbonate reservoirs: Experimental study and simulation. Energy Sources Part A 2018, 40, 2974–2985. [Google Scholar] [CrossRef]
- Demirbas, A.; Alsulami, H.E.; Hassanein, W.S. Utilization of surfactant flooding processes for enhanced oil recovery (EOR). Pet. Sci. Technol. 2015, 33, 1331–1339. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Y.; Liu, S.; Wang, K.; Jiang, Y. Pore structure characterization of coal by NMR cryoporometry. Fuel 2017, 190, 359–369. [Google Scholar] [CrossRef]
- Lu, J.; Weerasooriya, U.P.; Pope, G.A. Investigation of gravity-stable surfactant floods. Fuel 2014, 124, 76–84. [Google Scholar] [CrossRef]
- Bagrezaie, M.A.; Pourafshary, P. Improvement of surfactant flooding performance by application of nanoparticles in sandstone reservoirs. J. Jpn. Pet. Inst. 2015, 58, 97–102. [Google Scholar] [CrossRef]
- Jouenne, S. Polymer flooding in high temperature, high salinity conditions: Selection of polymer type and polymer chemistry, thermal stability. J. Pet. Sci. Eng. 2020, 195, 107545. [Google Scholar] [CrossRef]
- Cheng, Q.; Cao, G.; Bai, Y.; Zhu, Z.; Zhang, N.; Li, D. Probing the demulsification mechanism of emulsion with SPAN series based on the effect of solid phase particles. Molecules 2023, 28, 3261. [Google Scholar] [CrossRef] [PubMed]
- Raffa, P.; Broekhuis, A.A.; Picchioni, F. Polymeric surfactants for enhanced oil recovery: A review. J. Pet. Sci. Eng. 2016, 145, 723–733. [Google Scholar] [CrossRef]
- Cao, G.; Du, T.; Bai, Y.; Yang, T.; Zuo, J. Effects of surfactant molecular structure on the stability of water in oil emulsion. J. Pet. Sci. Eng. 2021, 196, 107695. [Google Scholar] [CrossRef]
- Afolabi, F.; Mahmood, S.M.; Yekeen, N.; Akbari, S.; Sharifigaliuk, H. Polymeric surfactants for enhanced oil recovery: A review of recent progress. J. Pet. Sci. Eng. 2022, 208, 109358. [Google Scholar] [CrossRef]
- Cao, G.; Cheng, Q.; Liu, Y.; Bu, R.; Zhang, N.; Wang, P. Influencing factors of surfactant stripping crude oil and spontaneous imbibition mechanism of surfactants in a tight reservoir. ACS Omega 2022, 7, 19010–19020. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, D.; Ding, C.; Li, J.; Jiang, S. Stripping Mechanism of Surfactant System Based on Residual Oil on the Surface of Sand-Conglomerate Rocks with Different Grain Size Mineral Compositions. Molecules 2024, 29, 1278. https://doi.org/10.3390/molecules29061278
Wang Y, Wang D, Ding C, Li J, Jiang S. Stripping Mechanism of Surfactant System Based on Residual Oil on the Surface of Sand-Conglomerate Rocks with Different Grain Size Mineral Compositions. Molecules. 2024; 29(6):1278. https://doi.org/10.3390/molecules29061278
Chicago/Turabian StyleWang, Yuanyuan, Daigang Wang, Chao Ding, Jing Li, and Shengdong Jiang. 2024. "Stripping Mechanism of Surfactant System Based on Residual Oil on the Surface of Sand-Conglomerate Rocks with Different Grain Size Mineral Compositions" Molecules 29, no. 6: 1278. https://doi.org/10.3390/molecules29061278
APA StyleWang, Y., Wang, D., Ding, C., Li, J., & Jiang, S. (2024). Stripping Mechanism of Surfactant System Based on Residual Oil on the Surface of Sand-Conglomerate Rocks with Different Grain Size Mineral Compositions. Molecules, 29(6), 1278. https://doi.org/10.3390/molecules29061278





