Study on the Selectivity of Molecular Imprinting Materials Determined through Hydrogen Bonding on Template Molecular Structures of Flavonoids
Abstract
:1. Introduction
2. Results and Discussion
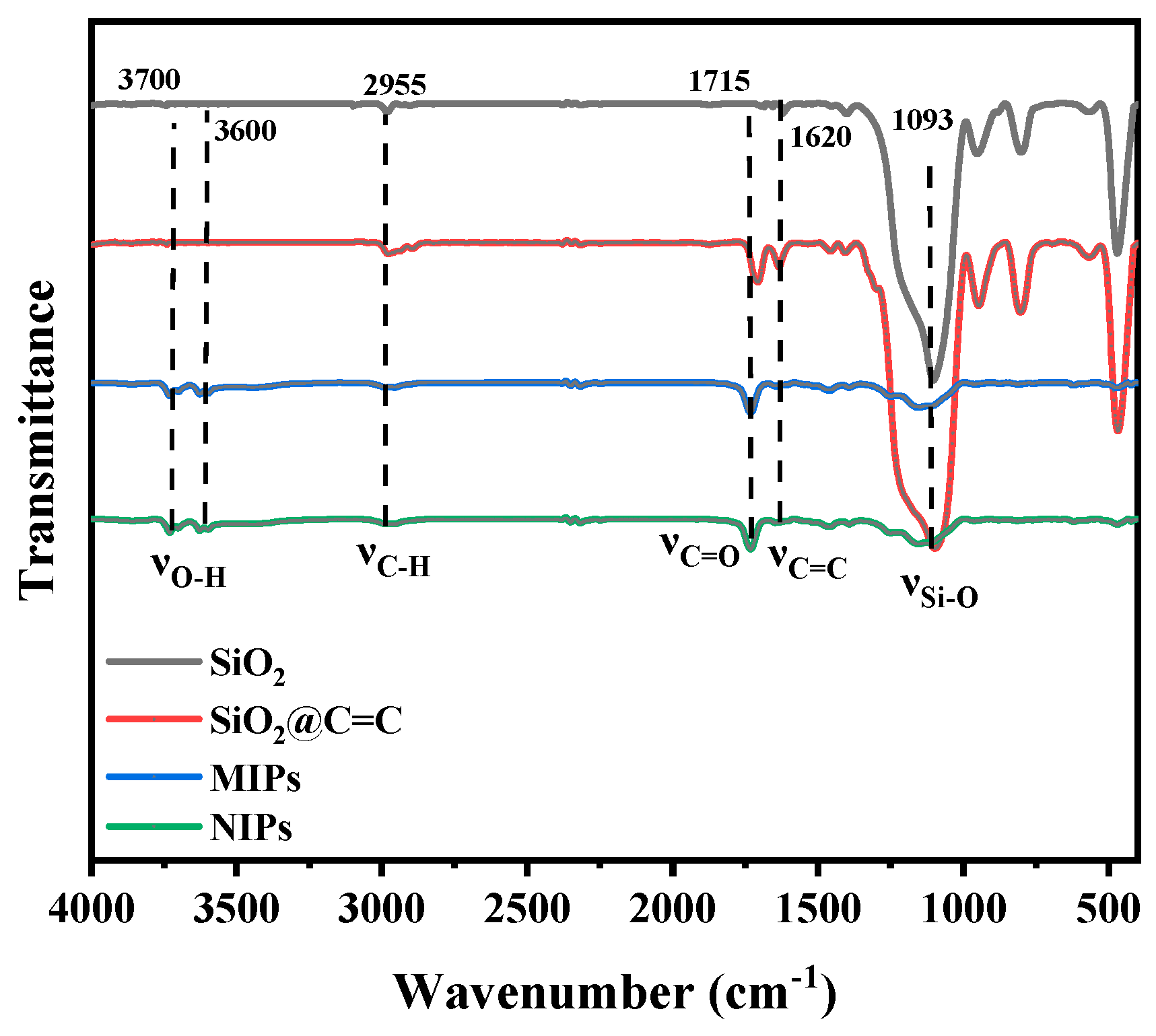
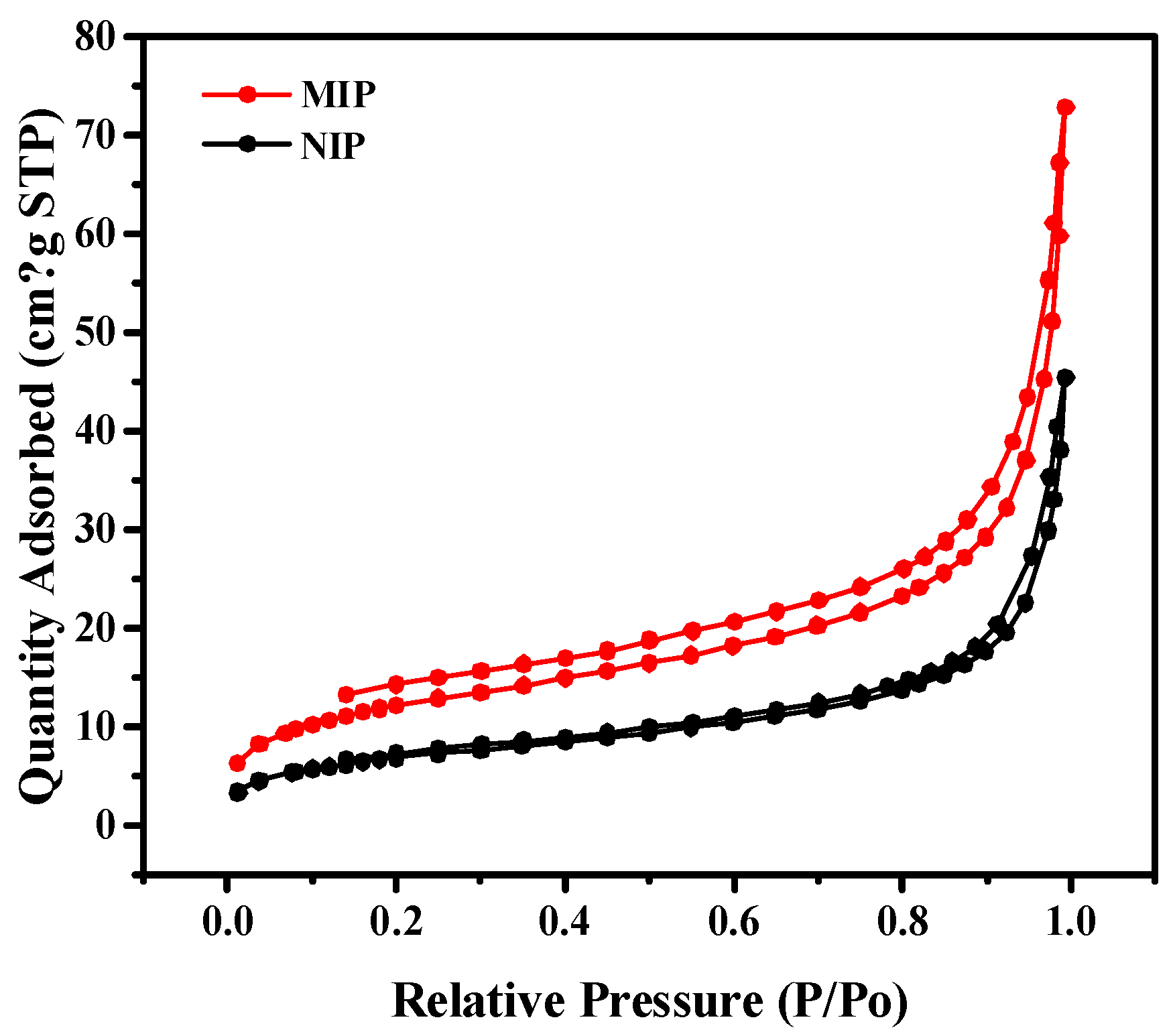
2.1. Preparation and Characterization of MIPs
2.2. Adsorption Properties
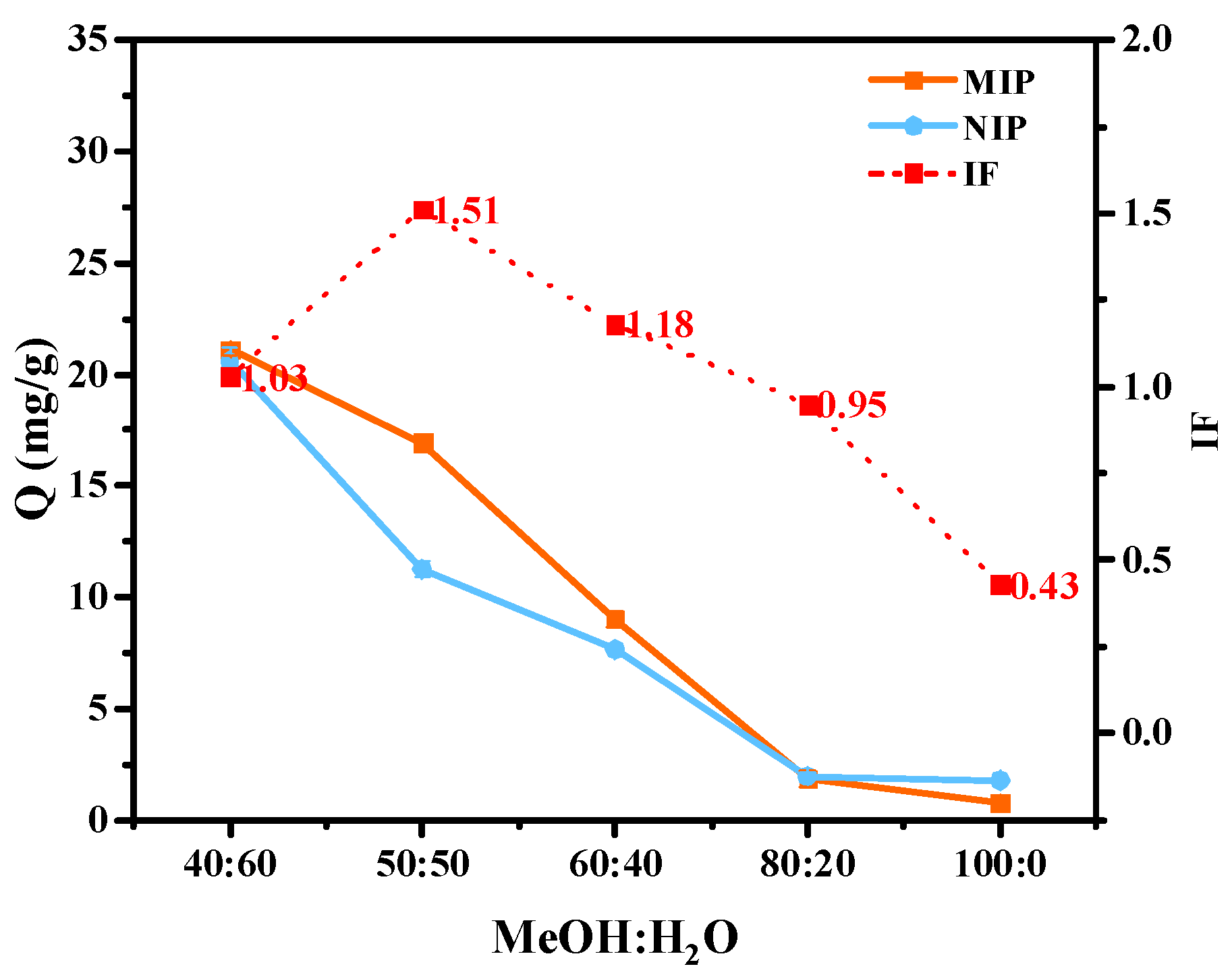
2.2.1. Effect of Solvent Polarity on the Synergistic Action of MAA in MIPs
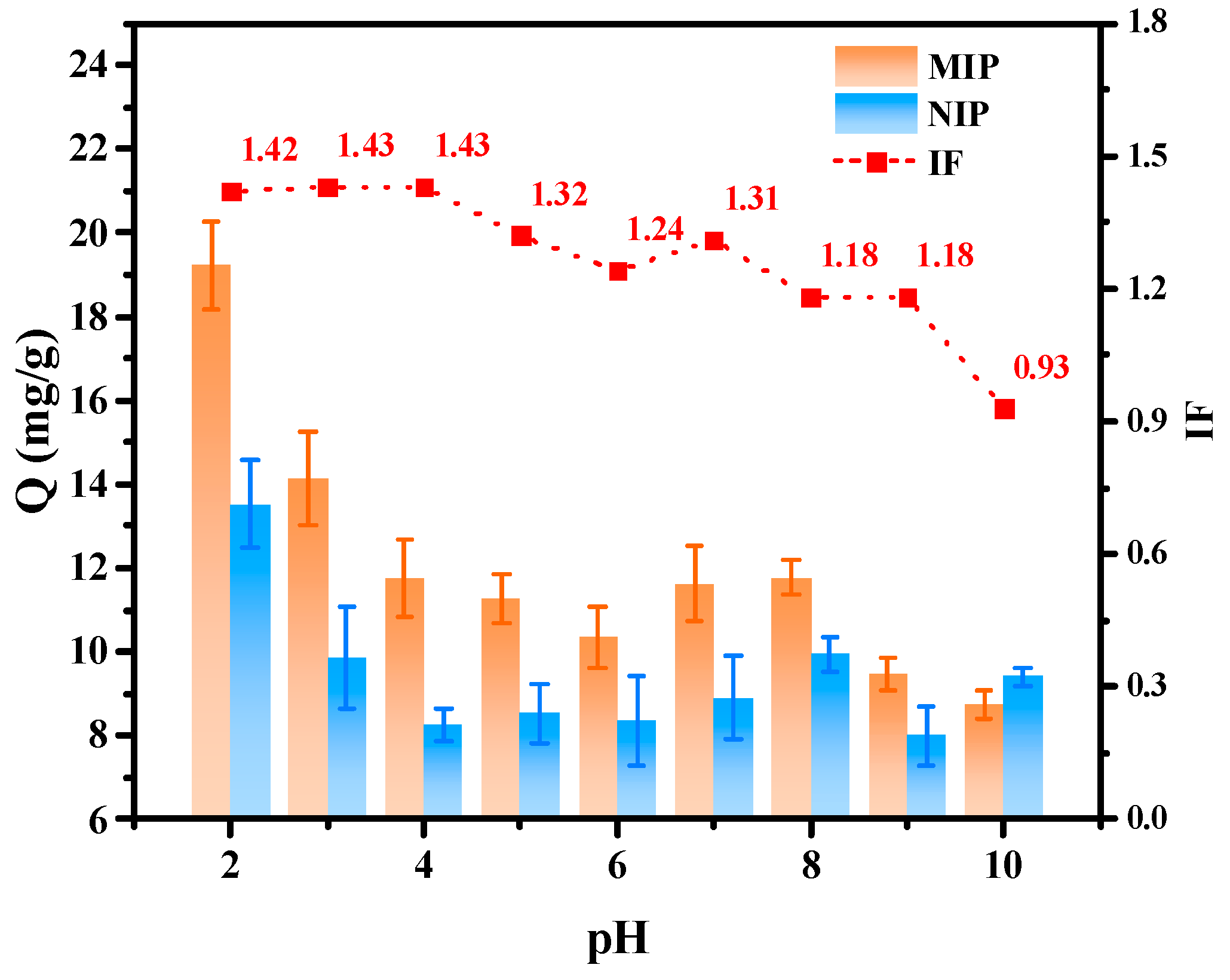
2.2.2. Effect of pH on Hydrogen Bonds
2.2.3. Binding Characteristics of MIPs and NIPs
2.3. Comparison of the MIPs’ Selectivity for the Adsorption of Flavonoids
3. Materials and Methods
3.1. Reagents
3.2. Apparatus and Instrument
3.3. Preparation of SiO2@C=C
3.4. Preparation of Molecularly Imprinted Polymers
3.5. Properties of the Imprinted Material
3.6. Adsorption Experiments for MIPs and NIPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Komiyama, M.; Mori, T.; Ariga, K. Molecular Imprinting: Materials Nanoarchitectonics with Molecular Information. Bull. Chem. Soc. Jpn. 2018, 91, 1075–1111. [Google Scholar] [CrossRef]
- Piletsky, S.; Canfarotta, F.; Poma, A.; Bossi, A.M.; Piletsky, S. Molecularly Imprinted Polymers for Cell Recognition. Trends Biotechnol. 2020, 38, 368–387. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, X.; Lu, W.; Wua, X.; Lia, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef] [PubMed]
- Bouvarel, T.; Delaunay, N.; Pichon, V. Molecularly imprinted polymers in miniaturized extraction and separation devices. J. Sep. Sci. 2021, 44, 1727–1751. [Google Scholar] [CrossRef]
- Nematollahzadeh, A.; Shojaei, A.; Abdekhodaie, M.J.; Sellergren, B. Molecularly imprinted polydopamine nano-layer on the pore surface of porous particles for protein capture in HPLC column. J. Colloid Interface Sci. 2013, 404, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Massumi, S.; Ahmadi, E.; Akbari, A.; Gholivand, M.B. Highly sensitive and selective sensor based on molecularly imprinted polymer for voltammetric determination of Nevirapine in biological samples. J. Electroanal. Chem. 2020, 876, 114508. [Google Scholar] [CrossRef]
- Ahmad, O.S.; Bedwell, T.S.; Esen, C.; Garcia-Cruz, A.; Piletsky, S.A. Molecularly Imprinted Polymers in Electrochemical and Optical Sensors. Trends Biotechnol. 2019, 37, 294–309. [Google Scholar] [CrossRef]
- Ostovan, A.; Arabi, M.; Wang, Y.Q.; Li, J.H.; Li, B.W.; Wang, X.Y.; Chen, L.X. Greenificated Molecularly Imprinted Materials for Advanced Applications. Adv. Mater. 2022, 34, 2203154. [Google Scholar] [CrossRef]
- Taniguchi, M.; LaRocca, C.A.; Bernat, J.D.; Lindsey, J.S. Digital Database of Absorption Spectra of Diverse Flavonoids Enables Structural Comparisons and Quantitative Evaluations. J. Nat. Prod. 2023, 86, 1087–1119. [Google Scholar] [CrossRef]
- Selvakumar, P.; Badgeley, A.; Murphy, P.; Anwar, H.; Sharma, U.; Lawrence, K.; Lakshmikuttyamma, A. Flavonoids and Other Polyphenols Act as Epigenetic Modifiers in Breast Cancer. Nutrients 2020, 12, 761. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.J.; Guan, Y.Y.; Yi, H.Q.; Lai, S.; Sun, Y.F.; Cao, S. Antibacterial activity and mechanism of plant flavonoids to gram-positive bacteria predicted from their lipophilicities. Sci. Rep. 2021, 11, 10471. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.H.; Lin, S.Y.; Chen, L.L.; Ouyang, K.H.; Wang, W.J. The Interaction between Flavonoids and Intestinal Microbes: A Review. Foods 2023, 12, 320. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Bautista, R.J.H.; Sandhu, M.A.; Hussein, O.E. Beneficial Effects of Citrus Flavonoids on Cardiovascular and Metabolic Health. Oxid. Med. Cell Longev. 2019, 2019, 5484138. [Google Scholar] [CrossRef]
- Rathod, N.B.; Elabed, N.; Punia, S.; Ozogul, F.; Kim, S.; Rocha, J.M. Recent Developments in Polyphenol Applications on Human Health: A Review with Current Knowledge. Plants 2023, 12, 1217. [Google Scholar] [CrossRef]
- Yang, Y.; Shen, X. Preparation and Application of Molecularly Imprinted Polymers for Flavonoids: Review and Perspective. Molecules 2022, 27, 7355. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, H.; Chen, H.; Huang, Y.; Bakunina, I.; de Sousa, D.P.; Sun, M.; Zhang, J. Application of molecular imprinting polymers in separation of active compounds from plants. Fitoterapia 2023, 164, 105383. [Google Scholar] [CrossRef]
- Xia, Q.; Yun, Y.; Li, Q.; Huang, Z.; Liang, Z. Preparation and characterization of monodisperse molecularly imprinted polymer microspheres by precipitation polymerization for kaempferol. Des. Monomers Polym. 2017, 20, 201–209. [Google Scholar] [CrossRef]
- Yang, L.; Li, T.; Yang, D.; Li, Y.; He, J.; Zhou, L.; Zhang, Q.; Wang, C.; Yuan, C. Constructing a dummy-template adsorbent using magnetic molecular-imprinted chitosan for rapid enrichment of flavonoids compounds from Penthorum chinense Pursh. Microchem. J. 2023, 191, 108833. [Google Scholar] [CrossRef]
- Liu, S.; Tang, N.; Zhang, X.; Huang, H.; Li, J.; Ou, H. Hydrophilic porous boronate imprinted hydrogels for ultrafast transport and highly specific separation of flavoniods: A interfacial cooperative emulsion imprinted strategy based on microreactors. Chem. Eng. J. 2024, 479, 147502. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, G.; Han, K.; Sun, D.; Zhou, N.; Song, Z.; Liu, H.; Li, J.; Li, G. Applications of Molecular Imprinting Technology in the Study of Traditional Chinese Medicine. Molecules 2022, 28, 301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.M.; Yun, Y.B.; Li, C.L. Preparation and adsorption performance of molecularly imprinted polymers for Kaempferol. Desalination Water Treat. 2013, 51, 3914–3919. [Google Scholar] [CrossRef]
- Yan, S.L.; Gao, Z.X.; Fang, Y.J.; Cheng, Y.Y.; Zhou, H.Y. Characterization and quality assessment of binding properties of malachite green molecularly imprinted polymers prepared by precipitation polymerization in acetonitrile. Dyes Pigments 2007, 74, 572–577. [Google Scholar] [CrossRef]
- Fu, X.Y.; Wang, X.; Xia, Z.N.; Huang, Y.K. Preparation of dummy molecularly imprinted polymers for selective extraction of aromatic amine genotoxic impurities. J. Chromatogr. A 2022, 1685, 463617. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Narayanan, N.; Gupta, S. The density function theory calculations showed that the hydrogen bond strength, steric effect, and product energy caused adsorption and separation differences among the different imprinted polymer complexes. Food Chem. 2018, 255, 81–88. [Google Scholar] [CrossRef]
- Ma, X.B.; Lin, H.L.; He, Y.H.; She, Y.X.; Wang, M.; Abd El-Aty, A.M.; Afifi, N.A.; Han, J.C.; Zhou, X.Z.; Wang, J.; et al. Magnetic molecularly imprinted polymers doped with graphene oxide for the selective recognition and extraction of four flavonoids from Rhododendron species. J. Chromatogr. A 2019, 1598, 39–48. [Google Scholar] [CrossRef]
- Ye, K.X.; Xu, S.F.; Zhou, Q.Q.; Wang, S.T.; Xu, Z.G.; Liu, Z.M. Advances in Molecular Imprinting Technology for the Extraction and Detection of Quercetin in Plants. Polymers 2023, 15, 2107. [Google Scholar] [CrossRef]


| TM (mml) | FM (mmol) | Crosslinking Agent (mmol) | Pore-Forming Agent (mL) | IF | |||
|---|---|---|---|---|---|---|---|
| MIPs | Myricetin | MAA | EDGMA | MeOH | THF | ACN | QMIP/QNIP |
| MIP 1 | 0.04 | 0.04 | 1.20 | 30 | 1.03 | ||
| MIP 2 | 0.04 | 0.20 | 1.20 | 30 | 1.85 | ||
| MIP 3 | 0.04 | 0.40 | 1.20 | 30 | 1.43 | ||
| MIP 4 | 0.04 | 0.60 | 1.20 | 30 | 1.08 | ||
| MIP 5 | 0.04 | 0.20 | 0.40 | 30 | 0.49 | ||
| MIP 6 | 0.04 | 0.20 | 0.80 | 30 | 1.02 | ||
| MIP 7 | 0.04 | 0.20 | 1.60 | 30 | 1.37 | ||
| MIP 8 | 0.04 | 0.20 | 1.20 | 30 | 1.26 | ||
| MIP 9 | 0.04 | 0.20 | 1.20 | 30 | 1.14 | ||
| Low-Affinity Sites | High-Affinity Sites | |||
|---|---|---|---|---|
| KD (mg/L) | Qmax (mg/g) | KD (mg/L) | Qmax (mg/g) | |
| MIP | 990.10 | 268.49 | 140.84 | 59.08 |
| NIP | 122.70 | 42.39 | / | / |
| Flavonoids | Chemical Structure | Hydroxyl Number | Hydroxyl Site | Q (mg/g) | Rs a | Imprinting Factor |
|---|---|---|---|---|---|---|
| Chrysin | 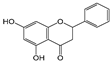 | 2 | R2, R4 | 7.42 | 1.22 | 1.78 |
| Baicalin | 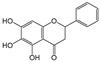 | 3 | R2, R3, R4 | 4.17 | 2.17 | 2.50 |
| Fisetin |  | 4 | R1, R4, R5, R6 | 8.78 | 1.03 | 3.37 |
| Kaempferol | 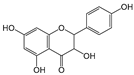 | 4 | R1, R2, R4, R6 | 6.79 | 1.33 | 2.65 |
| Quercelin | 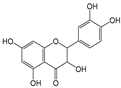 | 5 | R1, R2, R4, R5, R6 | 3.12 | 2.90 | 2.00 |
| Myricetin | 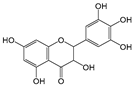 | 6 | R1, R2, R4 R5, R6, R7 | 9.04 | 1 | 1.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, S.; Wang, Y.; Hu, T.; Che, L.; Wang, X.; Huang, Y.; Xia, Z. Study on the Selectivity of Molecular Imprinting Materials Determined through Hydrogen Bonding on Template Molecular Structures of Flavonoids. Molecules 2024, 29, 1292. https://doi.org/10.3390/molecules29061292
Guan S, Wang Y, Hu T, Che L, Wang X, Huang Y, Xia Z. Study on the Selectivity of Molecular Imprinting Materials Determined through Hydrogen Bonding on Template Molecular Structures of Flavonoids. Molecules. 2024; 29(6):1292. https://doi.org/10.3390/molecules29061292
Chicago/Turabian StyleGuan, Siyue, Yue Wang, Ting Hu, Lingling Che, Xiaoqiao Wang, Yike Huang, and Zhining Xia. 2024. "Study on the Selectivity of Molecular Imprinting Materials Determined through Hydrogen Bonding on Template Molecular Structures of Flavonoids" Molecules 29, no. 6: 1292. https://doi.org/10.3390/molecules29061292
APA StyleGuan, S., Wang, Y., Hu, T., Che, L., Wang, X., Huang, Y., & Xia, Z. (2024). Study on the Selectivity of Molecular Imprinting Materials Determined through Hydrogen Bonding on Template Molecular Structures of Flavonoids. Molecules, 29(6), 1292. https://doi.org/10.3390/molecules29061292






