A Six-Year Hydration Evaluation of Cs-Bearing Materials at Room Temperature and 55% Relative Humidity Simulating Radioactive Waste with Different Crystallinities
Abstract
:1. Introduction
2. Results
2.1. Composition, Density, and Specific Surface Areas of the Prepared Materials
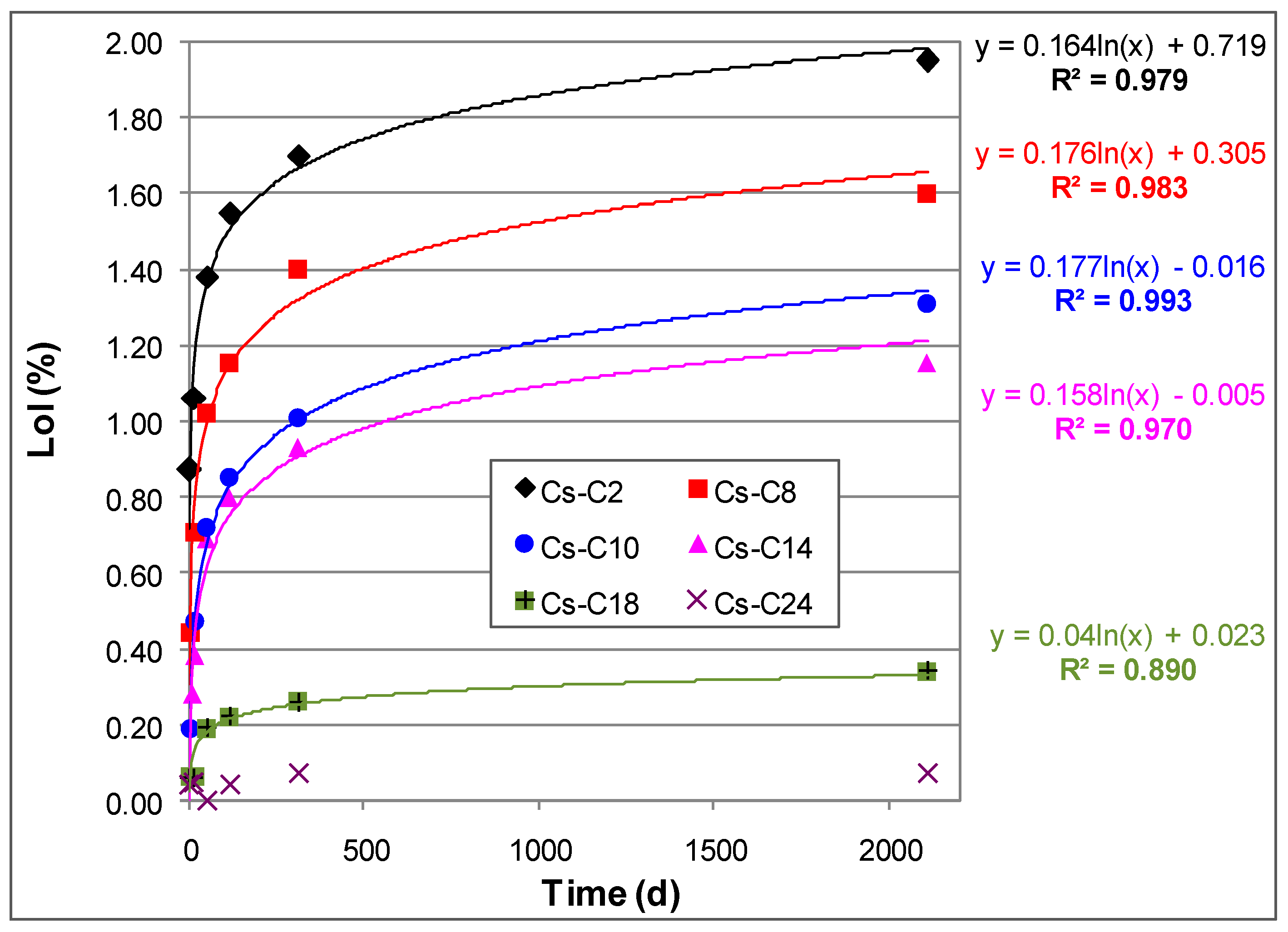
2.2. Hydration over Time of the Prepared Materials
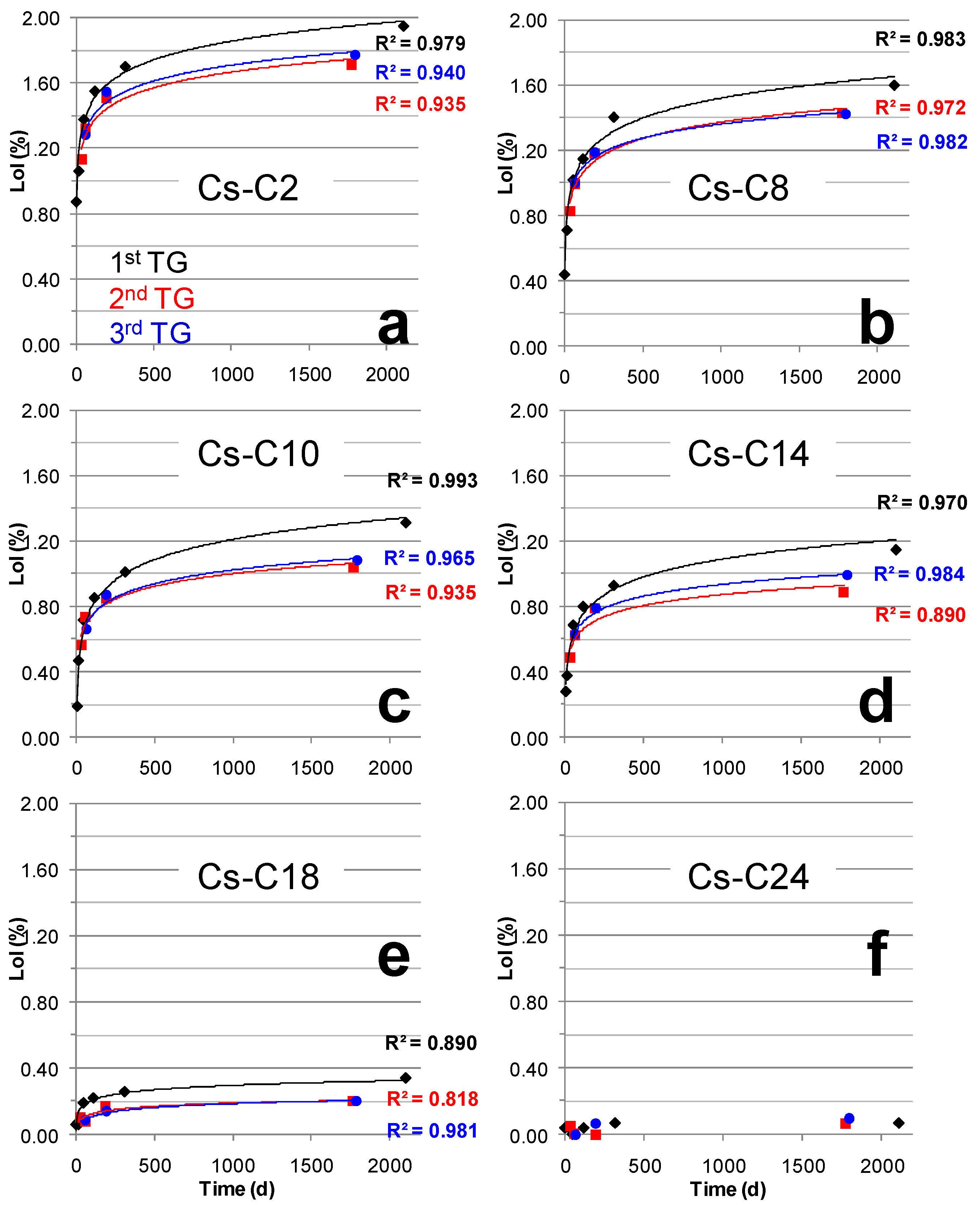
2.3. Hydration over Time of the Samples Submitted to TG
3. Discussion
4. Materials and Methods
4.1. Starting Material
4.2. Preparation of the Materials with Different Contents of AmP and CAS
4.3. XRD Analyses
4.4. Density and Specific Surface Area Analyses
4.5. Storage at Controlled Temperature and RH%
4.6. TG-DTG Analyses
- (a)
- 1 day (Cs-C18 and Cs-C24), 2 days (Cs-C2 and Cs-C8), 4 days (Cs-C10), and 8 days (Cs-C14);
- (b)
- 15 days (all samples);
- (c)
- 50 days (all samples except Cs-C36);
- (d)
- 117 days (all samples except Cs-C36);
- (e)
- 313 days (all samples except Cs-C36);
- (f)
- 2108 days (all samples except Cs-C36).
- (g)
- The aliquots already analyzed in point (b), except Cs-C36, were re-tested after 33 days;
- (h)
- The aliquots already analyzed in point (c) were re-tested after 62 days;
- (i)
- The aliquots already analyzed in point (d) were re-tested after 192 days;
- (l)
- The aliquots already analyzed in point (e) were re-tested after 1772 days.
- (m)
- The aliquots already analyzed in point (g) were re-tested after 63 days;
- (n)
- The aliquots already analyzed in point (h) were re-tested after 194 days;
- (o)
- The aliquots already analyzed in point (i) were re-tested after 1796 days.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, Y.; Zhou, L.; Ren, H.; Zou, H. Determination, Separation and Application of 137Cs: A Review. Int. J. Environ. Res. Public Health 2022, 19, 10183. [Google Scholar] [CrossRef]
- Adenan, M.N.H.; Yazan, L.S.; Christianus, A.; Md Hashim, N.F.; Mohd Noor, S.; Shamsudin, S.; Ahmad Bahri, F.J.; Abdul Rahim, K. Radioprotective Effects of Kelulut Honey in Zebrafish Model. Molecules 2021, 26, 1557. [Google Scholar] [CrossRef]
- Ma, H.; Shen, M.; Tong, Y.; Wang, X. Radioactive Wastewater Treatment Technologies: A Review. Molecules 2023, 28, 1935. [Google Scholar] [CrossRef]
- International Atomic Energy Agency (IAEA). Application of ion exchange processes for the treatment of radioactive waste and management of spent ion exchangers. In Technical Report Series No. 408; IAEA: Vienna, Austria, 2002; 115p. [Google Scholar]
- Eyde, T.H.; Holmes, D.A. Zeolites. In Industrial Minerals and Rocks: Commodities, Markets, and Uses; Kogel, J.E., Trivedi, N.C., Barker, J.M., Krukowsky, S.T., Eds.; Society for Mining, Metallurgy, and Exploration: Englewood, CO, USA, 2006; pp. 1039–1064. [Google Scholar]
- Kulikova, S.A.; Vinokurov, S.E. The Influence of Zeolite (Sokyrnytsya Deposit) on the Physical and Chemical Resistance of a Magnesium Potassium Phosphate Compound for the Immobilization of High-Level Waste. Molecules 2019, 24, 3421. [Google Scholar] [CrossRef]
- A Prajitno, M.Y.; Taufiqurrakhman, M.; Harbottle, D.; Hunter, T.N. Kinetic Studies of Cs+ and Sr2+ Ion Exchange Using Clinoptilolite in Static Columns and an Agitated Tubular Reactor (ATR). ChemEngineering 2021, 5, 9. [Google Scholar] [CrossRef]
- Armbruster, T. Clinoptilotite-heulandite: Applications and basic research. Stud. Surf. Sci. Catal. 2001, 135, 13–27. [Google Scholar] [CrossRef]
- Denton, M.S.; Mertz, J.L.; Bostick, W.D. Fukushima Nuclear Crisis Recovery: A Modular Water Treatment System Deployed in Seven Weeks-12489. In Proceedings of the Waste Management 2012 Conference On Improving the Future in Waste Management, Phoenix, AZ, USA, 26 February–1 March 2012; Volume 7, pp. 3958–3975. [Google Scholar]
- Dyer, A.; Hriljac, J.; Evans, N.; Stokes, I.; Rand, P.; Kellet, S.; Harjula, R.; Moller, T.; Maher, Z.; Heatlie-Branson, R.; et al. The use of columns of the zeolite clinoptilolite in the remediation of aqueous nuclear waste streams. J. Radioanal. Nucl. Chem. 2018, 318, 2473–2491. [Google Scholar] [CrossRef]
- Ojovan, M.I.; Lee, W.E. Glassy Wasteforms for Nuclear Waste Immobilization. Metall. Mater. Trans. 2011, A42, 837–851. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Wei, Y.; Mimura, H. Development of adsorption and solidification process for decontamination of Cs-contaminated radioactive water in Fukushima through silica-based AMP hybrid adsorbent. Sep. Purif. Technol. 2017, 181, 76–84. [Google Scholar] [CrossRef]
- Lee, K.; Kim, J. Immobilization of 137Cs as a crystalline pollucite surrounded by amorphous aluminosilicate. Environ. Res. 2023, 221, 115309. [Google Scholar] [CrossRef] [PubMed]
- Iucolano, F.; Liguori, B.; Sabová, L.; Chmielewskà, E.; Caputo, D.; Colella, C. Safe trapping of Cs in heat-treated zeolite matrices. Part 2. Stud. Surf. Sci. Catal. 2008, 174A, 537–540. [Google Scholar] [CrossRef]
- Cappelletti, P.; Rapisardo, G.; de Gennaro, B.; Colella, A.; Langella, A.; Graziano, S.F.; Bish, D.L.; de Gennaro, M. Immobilization of Cs and Sr in aluminosilicate matrices derived from natural zeolites. J. Nucl. Mater. 2011, 414, 451–457. [Google Scholar] [CrossRef]
- Brundu, A.; Cerri, G. Thermal transformation of Cs-clinoptilolite to CsAlSi5O12. Micropor. Mesopor. Mat. 2015, 208, 44–49. [Google Scholar] [CrossRef]
- Omerašević, M.; Ružić, J.; Vuković, N.; Jovanović, U.; Mirković, M.; Maksimović, V.; Dondur, V. Removal of Cs Ions from Aqueous Solutions by Using Matrices of Natural Clinoptilolite and its Safe Disposal. Sci. Sinter. 2016, 48, 101–107. [Google Scholar] [CrossRef]
- Omerašević, M.; Ružić, J.; Nedić Vasiljević, B.; Baščarević, Z.; Bučevac, D.; Orlić, J.; Matović, L. Transformation of Cs-exchanged clinoptilolite to CsAlSi5O12 by hot-pressing. Ceram. Int. 2017, 43, 13500–13504. [Google Scholar] [CrossRef]
- Cerri, G.; Sale, E.; Brundu, A. Thermal transformations of (NH4, Cs)-clinoptilolite with compositions in between the end-members. Micropor. Mesopor. Mat. 2018, 258, 122–130. [Google Scholar] [CrossRef]
- Cerri, G.; Brundu, A. On the possibility to modify the CsAlSi5O12/CsAlSi2O6 ratio during the thermal transformation of Cs-clinoptilolite. Micropor. Mesopor. Mat. 2022, 335, 111856. [Google Scholar] [CrossRef]
- Gatta, G.D.; Rotiroti, N.; Fisch, M.; Kadiyski, M.; Armbruster, T. Stability at high-pressure, elastic behaviour and pressure-induced structural evolution of CsAlSi5O12, a potential host for nuclear waste. Phys. Chem. Miner. 2008, 35, 521–533. [Google Scholar] [CrossRef]
- Gatta, G.D.; Rotiroti, N.; Boffa Ballaran, T.; Sanchez-Valle, C.; Pavese, A. Elastic behavior and phase stability of pollucite, a potential host for nuclear waste. Am. Mineral. 2009, 94, 1137–1143. [Google Scholar] [CrossRef]
- Gatta, G.D.; Brundu, A.; Cappelletti, P.; Cerri, G.; de’ Gennaro, B.; Farina, M.; Fumagalli, P.; Guaschino, L.; Lotti, P.; Mercurio, M. New insights on pressure, temperature, and chemical stability of CsAlSi5O12, a potential host for nuclear waste. Phys. Chem. Miner. 2016, 43, 639–647. [Google Scholar] [CrossRef]
- Gardner, L.J.; Walling, S.A.; Corkhill, C.L.; Hyatt, N.C. Thermal treatment of Cs-exchanged chabazite by hot isostatic pressing to support decommissioning of Fukushima Daiichi Nuclear Power Plant. J. Hazard. Mat. 2021, 413, 125250. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, C.L.; Neeway, J.J.; Pearce, C.I.; Hand, R.J.; Fisher, A.J.; Walling, S.A.; Hyatt, N.C.; Kruger, A.A.; Schweiger, M.; Kosson, D.S.; et al. Forty years of durability assessment of nuclear waste glass by standard methods. NPJ Mater. Degrad. 2021, 5, 61. [Google Scholar] [CrossRef]
- Shah, J.G.; Wattal, P.K. Development of cesium containing glass for gamma irradiator. IOP Conf. Ser. Mater. Sci. Eng. 2009, 2, 012018. [Google Scholar] [CrossRef]
- Bollin, P.L. Glass Formation in the system Cs2O-Al2O3-SiO2. J. Am. Ceram. Soc. 1972, 55, 483. [Google Scholar] [CrossRef]
- Bouakkaz, R.; Abdelouas, A.; Grambow, B. Kinetic study and structural evolution of SON68 nuclear waste glass altered from 35 to 125 °C under unsaturated H2O and D2O18 vapour conditions. Corros. Sci. 2018, 134, 1–16. [Google Scholar] [CrossRef]
- Narayanasamy, S.; Jollivet, P.; Godon, N.; Angeli, F.; Gin, S.; Cabié, M.; Cambedouzou, J.; Le Guillou, C.; Abdelouas, A. Influence of composition of nuclear waste glasses on vapor phase hydration. J. Nucl. Mater. 2019, 529, 53–71. [Google Scholar] [CrossRef]
- Chaou, A.A.; Abdelouas, A.; El Mendili, Y.; Bouakkaz, R.; Utsunomiya, S.; Martin, C.; Bourbon, X. Vapor hydration of a simulated borosilicate nuclear waste glass in unsaturated conditions at 50 °C and 90 °C. RSC Adv. 2015, 5, 64538–64549. [Google Scholar] [CrossRef]
- Narayanasamy, S.; Jollivet, P.; Sessegolo, L.; Angeli, F.; Abdelouas, A. Influence of temperature and relative humidity on vapor hydration of an AVM nuclear waste glass. J. Nucl. Mater. 2021, 543, 152571. [Google Scholar] [CrossRef]
- Alloteau, F.; Lehuédé, P.; Majérus, O.; Biron, I.; Dervanian, A.; Charpentier, T.; Caurant, D. New insight into atmospheric alteration of alkali-lime silicate glasses. Corros. Sci. 2017, 122, 12–25. [Google Scholar] [CrossRef]
- Rodrigues, A.; Fearn, S.; Palomar, T.; Vilarigues, M. Early stages of surface alteration of soda-rich-silicate glasses in the museum environment. Corros. Sci. 2018, 143, 362–375. [Google Scholar] [CrossRef]
- Alloteau, F.; Majérus, O.; Biron, I.; Lehuédé, P.; Caurant, D.; Charpentier, T.; Seyeux, A. Temperature-dependent mechanisms of the atmospheric alteration of a mixed-alkali lime silicate glass. Corros. Sci. 2019, 159, 108129. [Google Scholar] [CrossRef]
- Rampf, M.; Fisch, M.; Dittmer, M.; Ritzberger, C.; Schweiger, M.; Höland, W. Tailoring the Thermal Expansion of Glass-Ceramics by Controlled Twofold Crystallization of Li2Si2O5 and CsAlSi5O12. Int. J. Appl. Glass Sci. 2016, 7, 285–294. [Google Scholar] [CrossRef]
- Brundu, A.; Cerri, G.; Sale, E. Thermal Transformation of NH4-Clinoptilolite to Mullite and Silica Polymorphs. Minerals 2017, 7, 11. [Google Scholar] [CrossRef]
- Cerri, G.; Sale, E.; Brundu, A. Solid-state transformation of (NH4, Ba)-clinoptilolite to monocelsian, mullite, and cristobalite. Micropor. Mesopor. Mat. 2019, 280, 166–173. [Google Scholar] [CrossRef]
- Park, M.; Kim, S.; Takahashi, Y.; Jeong, H.Y. Thermal stabilization of extraframework Cs+ in zeolite 13X. J. Nucl. Mater. 2022, 572, 154078. [Google Scholar] [CrossRef]
- Rebiscoul, D.; Frugier, P.; Gin, S.; Ayral, A. Protective properties and dissolution ability of the gel formed during nuclear glass alteration. J. Nucl. Mater. 2005, 342, 26–34. [Google Scholar] [CrossRef]
- Fisch, M.; Armbruster, T.; Libowitzky, E. Microporous CsAlSi5O12 at non-ambient conditions: Partial Na exchange at 100 °C. In Topics in Chemistry and Materials Science: Advanced Micro- and Mesoporous Materials-09; Hadjiivanov, K., Valtchev, V., Mintova, S., Vayssilov, G., Eds.; Heron Press: Sofia, Bulgaria, 2010; Volume 4, pp. 61–70. [Google Scholar]
- Bunker, B.C. Molecular mechanisms for corrosion of silica and silicate glasses. J. Non Cryst. Solids 1994, 179, 300–308. [Google Scholar] [CrossRef]
- Cerri, G.; Brundu, A. Solid-state transformations of Zn-clinoptilolite through heating. J. Solid State Chem. 2020, 283, 121165. [Google Scholar] [CrossRef]
- Abrajano, T.A.; Bates, J.K.; Mazer, J.J. Aqueous corrosion of natural and nuclear waste glasses II. Mechanisms of vapor hydration of nuclear waste glasses. J. Non Cryst. Solids 1989, 108, 269–288. [Google Scholar] [CrossRef]
- Warring, S.L.; Beattie, D.A.; McQuillan, A.J. Surficial Siloxane-to-Silanol Interconversion during Room-Temperature Hydration/Dehydration of Amorphous Silica Films Observed by ATR-IR and TIR-Raman Spectroscopy. Langmuir 2016, 32, 1568–1576. [Google Scholar] [CrossRef]
- Cerri, G.; Farina, M.; Brundu, A.; Daković, A.; Giunchedi, P.; Gavini, E.; Rassu, G. Natural zeolites for pharmaceutical formulations: Preparation and evaluation of a clinoptilolite-based material. Micropor. Mesopor. Mater. 2016, 223, 58–67. [Google Scholar] [CrossRef]
- Hagopian-Babikian, S.; Hamilton, R.F.; Iyengar, S.S.; Jenkins, R.; Renault, J. Grinding for X-ray diffraction application. In A Practical Guide for the Preparation of Specimens for X-ray Diffraction Analysis; Burke, V.E., Jenkins, R., Smith, D.K., Eds.; John Wiley & Sons Inc.: New York, NY, USA, 1998; pp. 22–26. [Google Scholar]
- Mimura, H.; Iijima, K.; Akiba, K. Leaching Behavior and Surface Alteration of Cesium Aluminum Silicate under Static and Dynamic Conditions. J. Nucl. Sci. Technol. 1997, 34, 269–276. [Google Scholar] [CrossRef]
- Mimura, H.; Akiba, K. Preparation of Ceramic Solid Forms Immobilizing Cesium and/or Strontium and Evaluation of their Physical and Chemical Properties. In Proceedings of the International Conference Nuclear Energy for New Europe, Kranjska Gora, Slovenia, 9–12 September 2002; pp. 1105.1–1105.8. [Google Scholar]
- International Crystal Structure Database, ICSD CD-Rom; FIZ Karlsruhe: Germany, 2010. Available online: https://icsd.products.fiz-karlsruhe.de/ (accessed on 29 January 2024).
- Farina, M.; Brundu, A.; Bonferoni, M.C.; Juliano, C.; Rassu, G.; Gavini, E.; Cerri, G. Antibacterial activity of Na-clinoptilolite against Helicobacter pylori: In-vitro tests, synergistic effect with amoxicillin and stability of the antibiotic formulated with the zeolite. Micropor. Mesopor. Mat. 2019, 288, 109592. [Google Scholar] [CrossRef]

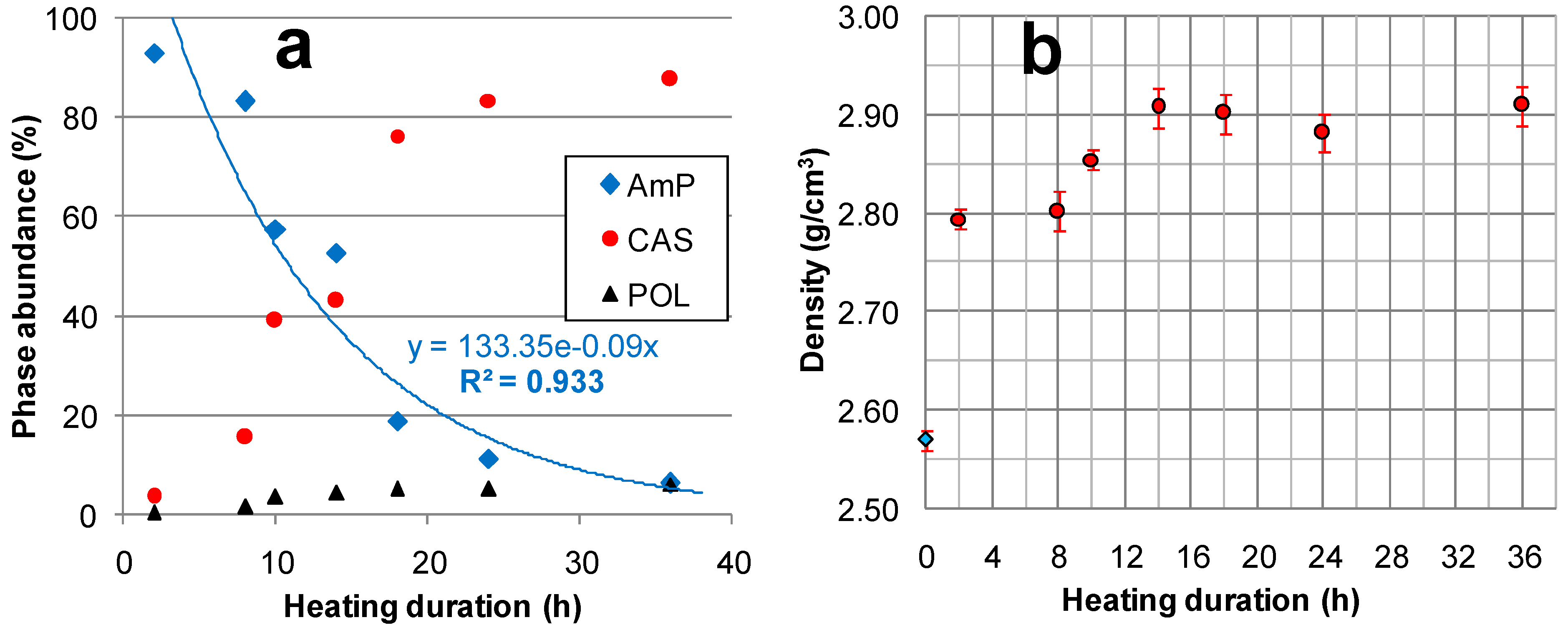

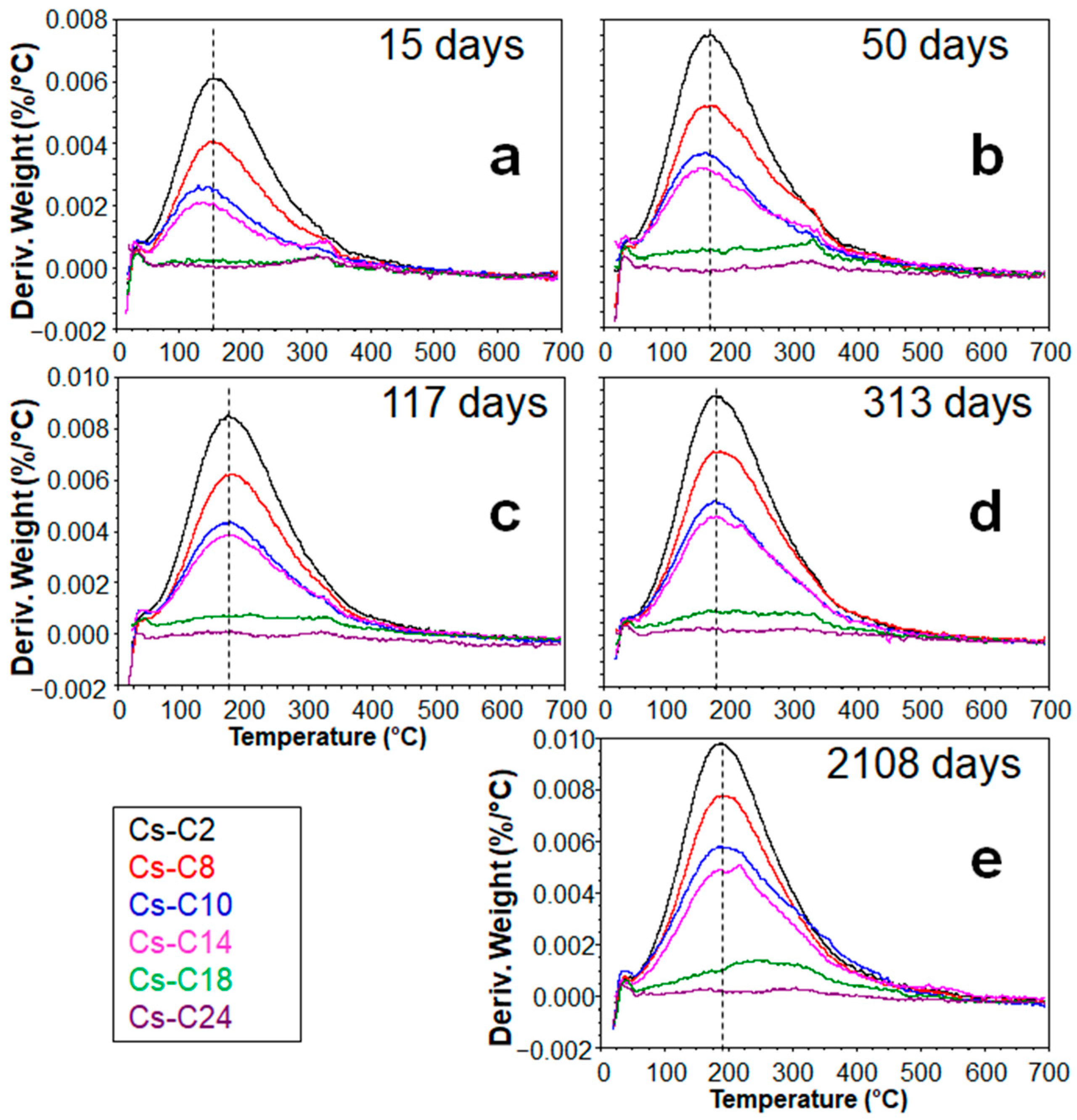
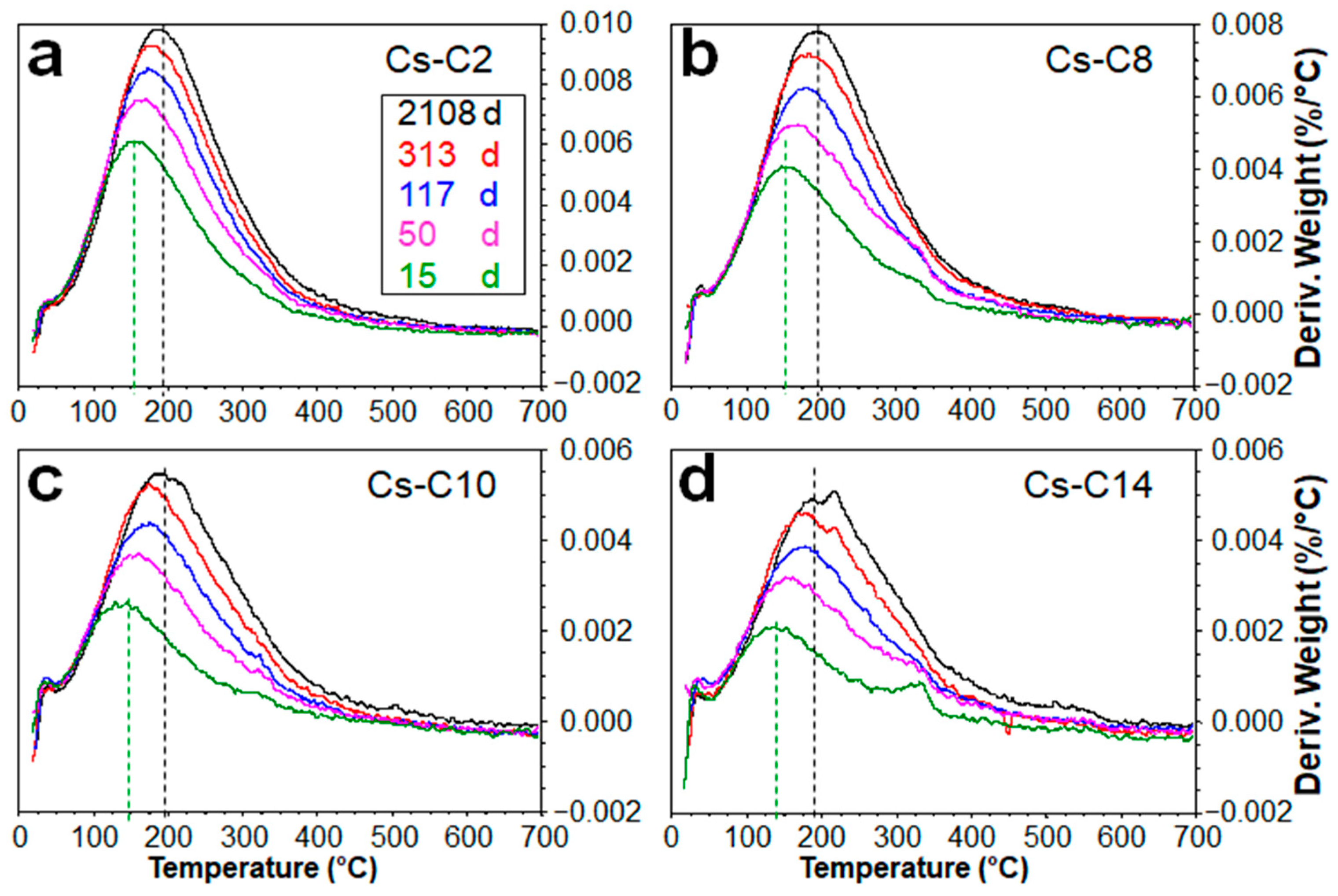
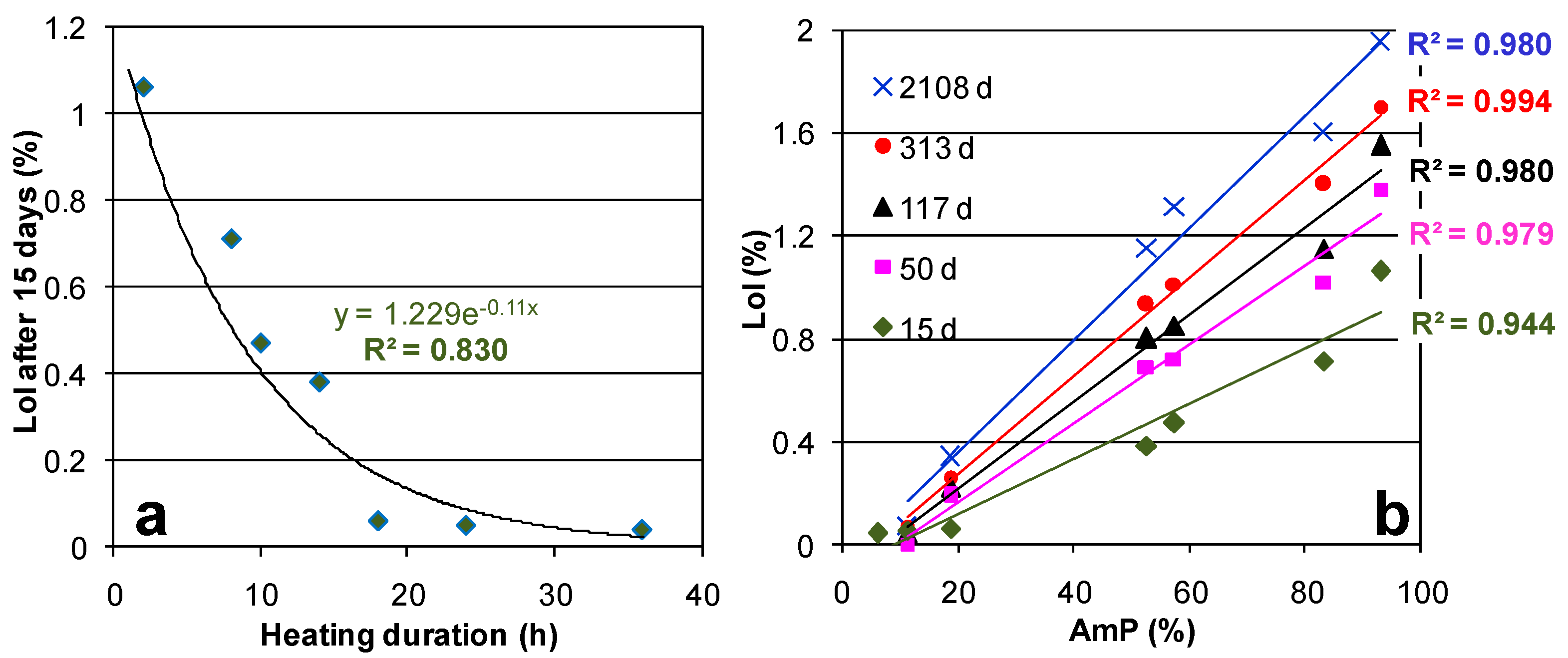
| Sample | CAS | POL | Feld. * | AmP | AmP + CAS | AmP/CAS | Cs-Occup. | Cs Phase/Cs Sample (%) | Cs2O% | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| in CAS | CAS | POL | AmP | in AmP | |||||||
| Cs-C2 | 3.5 | 0.4 | 3.2 | 92.9 | 96.4 | 26.5 | 1.00 | 3.8 | 0.7 | 95.5 | 26.9 |
| e.s.d. ** | ±0.8 | ±0.1 | ±0.6 | ±3.0 | |||||||
| Cs-C8 | 15.4 | 1.4 | - | 83.2 | 98.6 | 5.4 | 0.92 | 15.9 | 2.4 | 81.7 | 25.7 |
| e.s.d. | ±2.6 | ±0.2 | ±2.8 | ||||||||
| Cs-C10 | 39.1 | 3.5 | - | 57.4 | 96.5 | 1.5 | 0.93 | 40.6 | 6.0 | 53.4 | 24.4 |
| e.s.d. | ±3.5 | ±0.5 | ±2.5 | ||||||||
| Cs-C14 | 43.1 | 4.2 | - | 52.7 | 95.8 | 1.2 | 0.93 | 44.7 | 7.2 | 48.1 | 23.9 |
| e.s.d. | ±3.8. | ±0.7 | ±2.3 | ||||||||
| Cs-C18 | 76.3 | 5.0 | 18.7 | 95.0 | 0.2 | 0.92 | 78.1 | 8.6 | 13.3 | 18.7 | |
| e.s.d. | ±4.2 | ±0.8 | ±2.0 | ||||||||
| Cs-C24 | 83.5 | 5.2 | - | 11.3 | 94.8 | 0.1 | 0.91 | 85.0 | 9.0 | 6.0 | 14.0 |
| e.s.d. | ±4.4 | ±0.8 | ±1.6 | ||||||||
| Cs-C36 | 87.7 | 6.1 | - | 6.2 | 93.9 | 0.1 | 0.91 | 89.6 | 10.4 | 0 | 0 |
| e.s.d. | ±4.7 | ±1.1 | ±1.4 | ||||||||
| Time (Days) | Cs-C2 | Cs-C8 | Cs-C10 | Cs-C14 | Cs-C18 | Cs-C24 | Cs-C36 |
|---|---|---|---|---|---|---|---|
| 1 | - | - | - | - | 0.06 | 0.04 | - |
| 2 | 0.87 | 0.44 | - | - | - | - | - |
| 4 | - | - | 0.19 | - | - | - | - |
| 8 | - | - | - | 0.28 | - | - | - |
| 15 | 1.06 | 0.71 | 0.47 | 0.38 | 0.06 | 0.05 | 0.04 |
| 50 | 1.38 | 1.02 | 0.72 | 0.69 | 0.19 | 0.00 | - |
| 117 | 1.55 | 1.15 | 0.85 | 0.80 | 0.22 | 0.04 | - |
| 313 | 1.70 | 1.40 | 1.01 | 0.93 | 0.26 | 0.07 | - |
| 2108 | 1.95 | 1.60 | 1.31 | 1.15 | 0.34 | 0.07 | - |
| LoI15d/LoI2108d | 0.54 | 0.44 | 0.36 | 0.33 | 0.18 | 0.71 | - |
| Time (Days) | Cs-C2 | Cs-C8 | Cs-C10 | Cs-C14 | Cs-C18 | Cs-C24 |
|---|---|---|---|---|---|---|
| 33 | 1.13 | 0.83 | 0.56 | 0.49 | 0.10 | 0.05 |
| 62 | 1.32 | 0.99 | 0.74 | 0.62 | 0.08 | 0.01 |
| 192 | 1.51 | 1.18 | 0.85 | 0.79 | 0.17 | 0.00 |
| 1772 | 1.71 | 1.43 | 1.04 | 0.89 | 0.20 | 0.07 |
| Time (Days) | Cs-C2 | Cs-C8 | Cs-C10 | Cs-C14 | Cs-C18 | Cs-C24 |
|---|---|---|---|---|---|---|
| 63 | 1.28 | 1.00 | 0.66 | 0.63 | 0.09 | 0.00 |
| 194 | 1.55 | 1.19 | 0.87 | 0.79 | 0.14 | 0.07 |
| 1796 | 1.77 | 1.42 | 1.08 | 0.99 | 0.20 | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerri, G.; Brundu, A. A Six-Year Hydration Evaluation of Cs-Bearing Materials at Room Temperature and 55% Relative Humidity Simulating Radioactive Waste with Different Crystallinities. Molecules 2024, 29, 1302. https://doi.org/10.3390/molecules29061302
Cerri G, Brundu A. A Six-Year Hydration Evaluation of Cs-Bearing Materials at Room Temperature and 55% Relative Humidity Simulating Radioactive Waste with Different Crystallinities. Molecules. 2024; 29(6):1302. https://doi.org/10.3390/molecules29061302
Chicago/Turabian StyleCerri, Guido, and Antonio Brundu. 2024. "A Six-Year Hydration Evaluation of Cs-Bearing Materials at Room Temperature and 55% Relative Humidity Simulating Radioactive Waste with Different Crystallinities" Molecules 29, no. 6: 1302. https://doi.org/10.3390/molecules29061302





