Synthesis and Spectroscopic Characterization of Selected Water-Soluble Ligands Based on 1,10-Phenanthroline Core
Abstract
1. Introduction
2. Results and Discussion
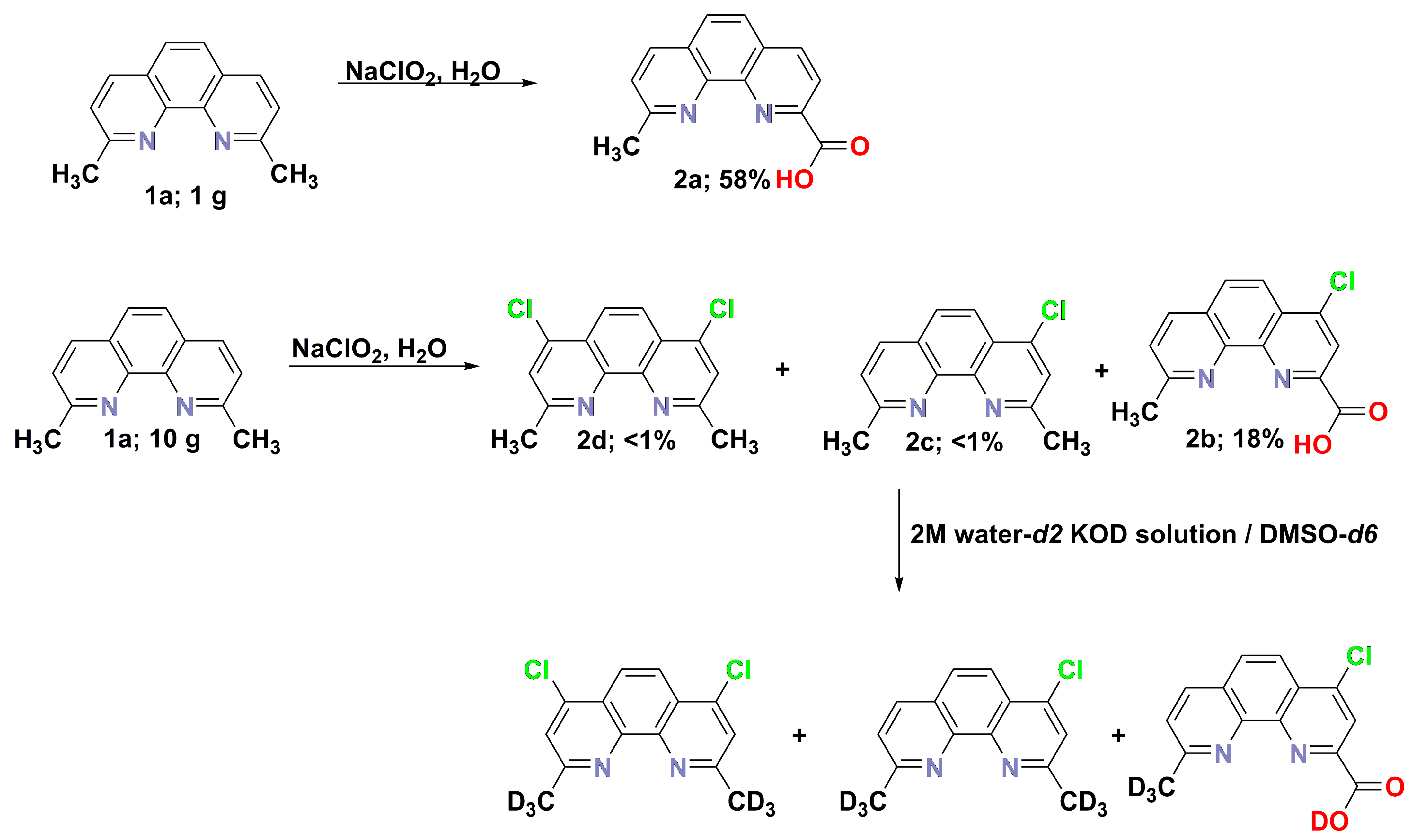
2.1. Synthesis of Carboxylic Acid Products by Oxidation of Methyl Groups
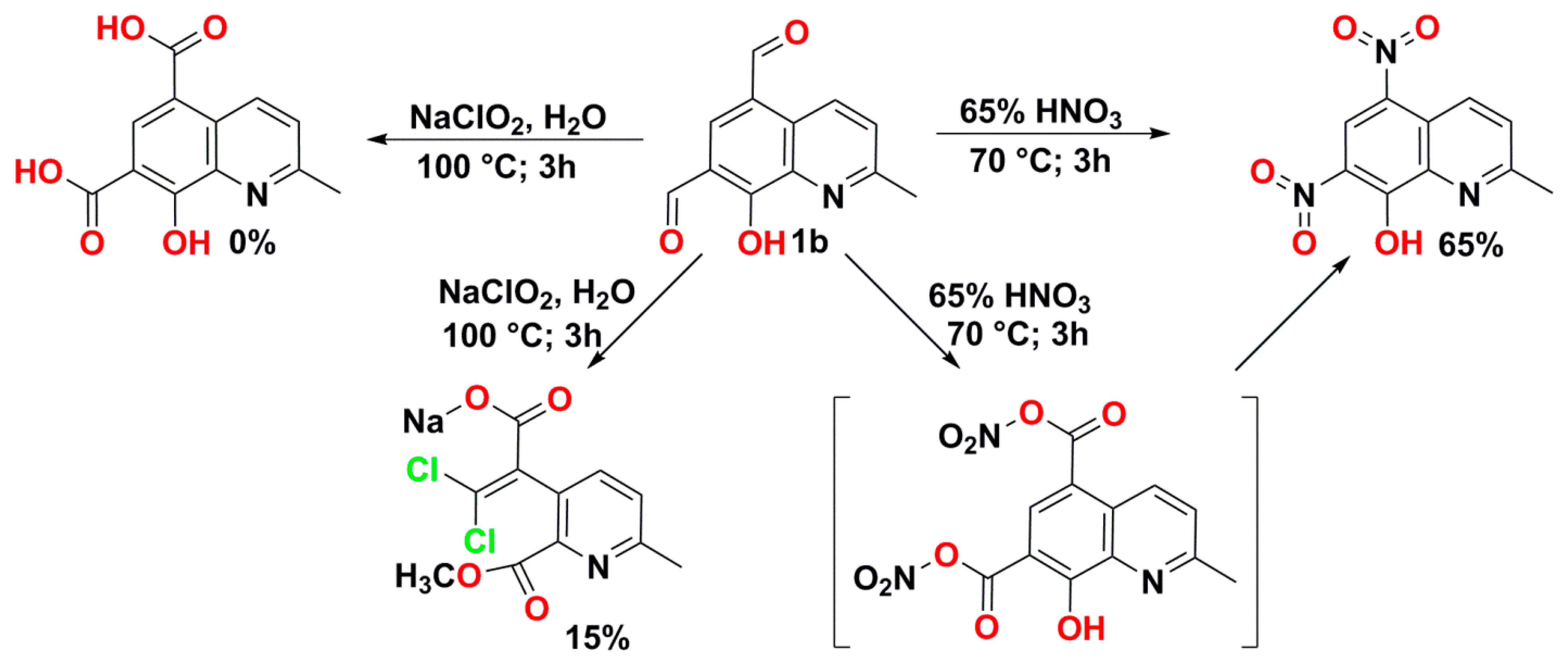
2.2. Oxidation of Aldehydes
2.3. Hydrolysis of Ester- and Nitrile-Substituted Phenanthroline Compounds
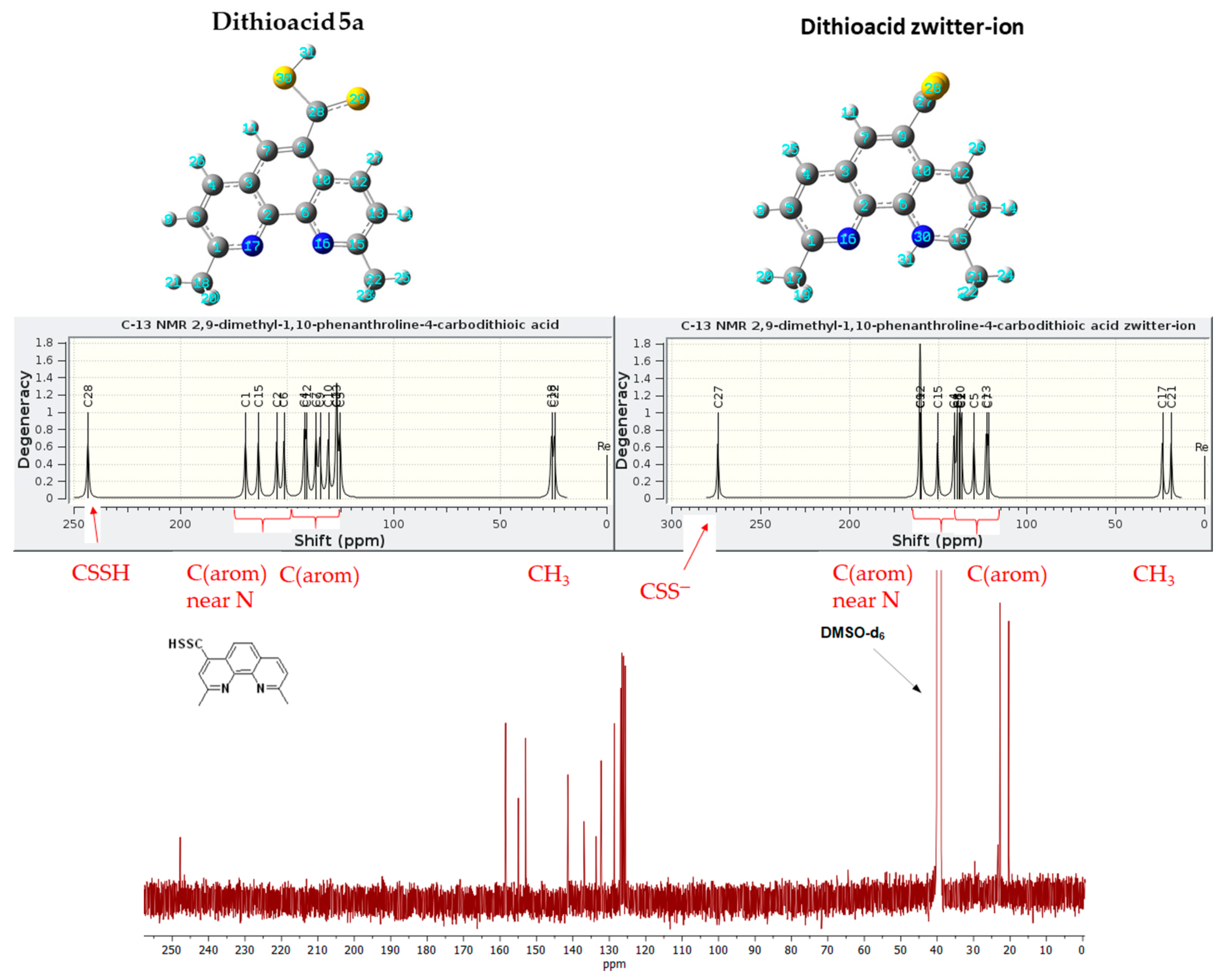
2.4. Synthesis of Dithiocarboxyl Derivatives
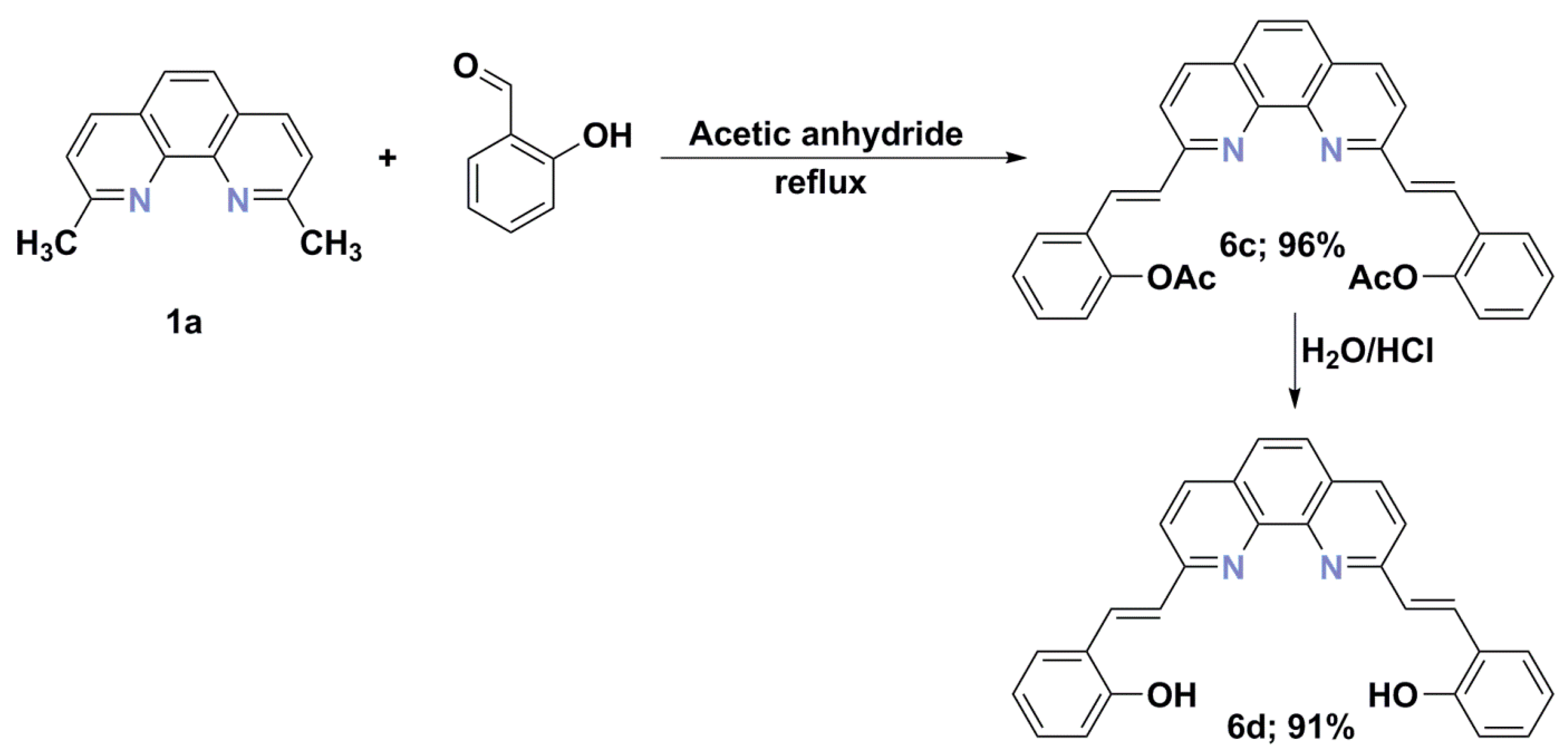
2.5. Synthesis of Vinyl Derivatives
3. Materials and Methods
3.1. Materials
3.2. Instrumentation
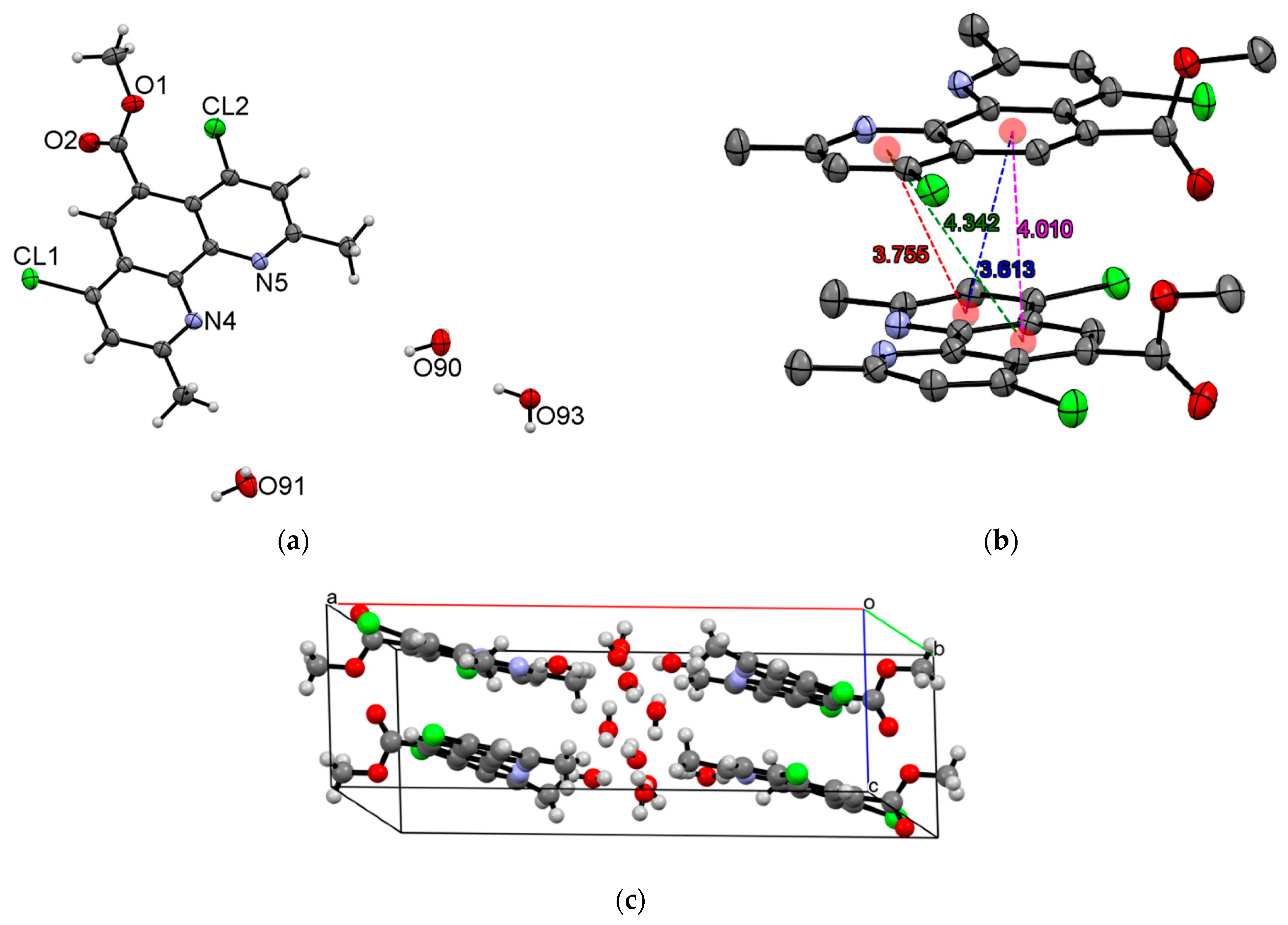
3.3. X-ray Diffraction Experiments
3.4. Synthesis of 9-Methyl-1,10-phenanthroline-2-carboxylic Acid (2a). 1.0 g Scale
- 9-Methyl-1,10-phenanthroline-2-carboxylic acid (2a) 0.66 g (2.8 mmol, 58%); m.p. > 300 °C; 1H NMR (D2O/KOD; 400.2 MHz) δ = 2.46 (s, 3H, CH3), 6.99 (bs, 2H, aromatic), 7.02 (d, 3JH,H = 8.2 Hz, 1H, aromatic), 7.50 (d, 3JH,H = 8.2 Hz, 1H, aromatic), 7.68–7.76 (m, 2H, aromatic), 8.41 (bs, 1H, OH); 13C{1H} NMR (D2O; 100.5 MHz) δ = 23.3, 122.2, 123.7, 124.6, 125.9, 126.8, 128.5, 136.8, 137.0, 142.0, 142.6, 151.2, 158.2, 172.4; HRMS (ESI TOF): m/z Calcd for C14H9N2O2 (M − H)− = 237.0664, Found 237.0662; UV–vis (methanol; λ [nm] (logε)): 314 (3.06), 275 (3.95), 230 (4.10); IR (KBr): = 1628, 1393, 1007, 972, 835 cm−1.
- 4-Chloro-9-methyl-1,10-phenanthroline-2-carboxylic acid (2b) 2.4 g (8.7 mmol, 18%); DSC = 67.4 °C; 1H NMR (DMSO-d6/D2O/KOD; 500.2 MHz) δ = 2.46 (s, 3H, CH3), 7.56 (d, 3JH,H = 8.2 Hz, 1H, aromatic), 7.67 (d, 3JH,H = 8.4 Hz, 1H, aromatic), 7.97 (s, 1H, aromatic), 8.21 (d, 3JH,H = 8.3 Hz, 1H, aromatic), 8.43 (d, 3JH,H = 8.4 Hz, 1H, aromatic); 13C{1H} NMR (DMSO-d6/D2O/KOD; 125.8 MHz) δ = 24.3, 124.6, 124.7, 122.7, 125.3, 126.5, 126.6, 127.9, 128.0, 133.3, 136.2, 136.3, 143.9, 145.4, 159.5, 159.9; HRMS (ESI TOF): m/z Calcd for C14H5D3N2O2Cl (M − H)− = 274.0463, Found 274.0466.
- Sodium (E)-3,3-dichloro-2-(2-(hydroxy(methoxy)methylene)-2,3-dihydropyridin-3-yl)acrylate 0.04 g (0.15 mmol, 15.0%); 1H NMR (400.2 MHz, DMSO-d6) δ = 3.59 (s, 3H), 7.76 (dd, 3JH,H = 4.8, 7.8 Hz, 1H), 8.43 (dd, 3JH,H = 1.7, 4JH,H = 7.9 Hz, 1H), 9.02 (dd, 3JH,H = 1.7, 4JH,H = 4.8 Hz, 1H). 13C{1H} NMR (DMSO–d6; 100.6 MHz) δ = 54.02, 86.56, 90.31, 125.80, 126.89, 134.74, 158.31, 165.55, 168.75, 186.75; HRMS (ESI TOF): m/z Calcd for C10H8Cl2NNaO4 (M)+ = 298.9728, Found 299.9632.
- 10-Hydroxybenzo[h]quinoline-7,9-dicarboxylic acid (3a) yellowish, 0.09 g (0.32 mmol, 32.4%); m.p.dec. = 235–245 °C; 1H NMR (DMSO–d6; 400.2 MHz; 60 °C) δ = 8.13 (t, 3JH,H = 7.0 Hz, 1H, aromatic), 8.24 (d, 3JH,H = 9.5 Hz, 1H, aromatic), 8.94 (s, 1H, aromatic), 9.07 (d, 3JH,H = 8,0 Hz, 1H, aromatic), 9.24 (d, 3JH,H = 5,4 Hz, 1H, aromatic), 9.36 (d, 3JH,H = 9,4 Hz, 1H, aromatic), 16.21 (s, 1H, OH); 13C{1H} NMR (DMSO–d6; 100.6 MHz; 60 °C) δ = 112.2, 113.2, 113.4 122.2, 126.1, 127.6, 128.1, 137.5, 138.3, 141.3, 142.1, 143.1 167.5, 167.8, 172.6; HRMS (ESI TOF): m/z Calcd for C15H9NO5Na (M + Na)+ = 306.0378, Found 306.0388; UV–vis (methanol; λ [nm] (logε)): 322 (3,96), 308 (4,03), 255 (4,49), 232 (4,84), 220 (4,85); IR (KBr): = 2928, 1718, 1678, 1514, 1464, 1245, 846 cm−1.
- 2-Methyl-5,7-dinitroquinolin-8-ol as a pale yellow powder 0.45 g (1.8 mmol, 65.0%); 1H NMR (DMSO-d6; 500.2 MHz) δ = 2.94 (s, 3H, CH3), 8.14 (d, 3JH,H = 9.0 Hz, 1H, aromatic), 9.21 (s, 1H, aromatic), 9.66 (d, 3JH,H = 9.0 Hz, 1H, aromatic); 13C{1H} NMR (DMSO-d6; 125.8 MHz) δ = 19.9, 122.6, 125.0, 129.0, 129.3, 130.8, 137.4, 141.2, 154.9, 161.7; HRMS (ESI TOF): m/z Calcd for C10H6N3O5 (M − H)− = 248.0307, Found 248.0309. IR (KBr): 3510; 3170; 3005; 1594; 1528; 1321; 1260.
- Methyl 4,7-dichloro-2,9-dimethyl-1,10-phenanthroline-5-carboxylate (1d) beige; DSC m.p. = 175.8 °C; 1H-NMR (CDCl3; 500.2 MHz) δ = 2.89 (s, 3H, CH3), 2.89 (s, 3H, CH3), 3.99 (s, 3H, CH3), 7.61 (s, 1H, aromatic), 7.62 (s, 1H, aromatic), 8.29 (s, 1H, aromatic); 13C{1H}-NMR (CDCl3; 125.8 MHz) δ = 25.6, 26.0, 53.2, 121.9, 123.5, 124.7, 125.7, 128.6, 141.3, 143.2, 146.6, 146.9, 160.4, 161.5, 169.3; HRMS (ESI TOF): m/z Calcd for C16H13N2O2Cl2 (M + H)+ = 335.0354, Found 335.0353.
- Synthesis of dithiocarboxylic acid (5a): A solution of 1a (2.9 g, 13.9 mmol) in THF (50 mL) was cooled (−78 °C), and tBuLi (11.8 mL; 1.7 M solution in pentane) was added dropwise under argon. The resulting violet solution was stirred for 1 h at ca. −78 °C, and then CS2 (5 mL, 6.3 g, 83.1 mmol) was added and stirred for 1 h at ca. −78 °C, and next was stirred for 16 h at r.t. The volatiles were removed in vacuo (16 mmHg). The residue was dissolved in water, acidified by a water solution of hydrochloric acid (10%), and filtered off. The crude product was dissolved in NaOH (10%) water solution and filtered off. The solution obtained was again acidified by a water solution of hydrochloric acid (10%), and the precipitate product was obtained, filtered off, and dried over P4O10 to yield the following:
- 2,9-Dimethyl-1,10-phenanthroline-4-carbodithioic acid (5a) (red) 0.3 g (1.0 mmol, 7%), DSC = 204.5 °C; 1H NMR (DMSO-d6; 500.2 MHz) δ = 2.97 (s, 3H, CH2), 3.01 (s, 3H, CH2), 7.99 (d, 3JH,H = 8.4 Hz, 1H, aromatic), 8.12 (d, 3JH,H = 8.9 Hz, 1H, aromatic), 8.16 (d, 3JH,H = 8.9 Hz, 1H, aromatic), 8.35 (s, 1H, aromatic), 8.84 (d, 3JH,H = 8.3 Hz, 1H, aromatic), 10.47 (s, SH, 1H); 13C{1H} NMR (DMSO-d6; 125.8 MHz) δ = 20.3, 22.7, 125.6, 126.1, 126.5, 126.9, 128.6, 132.2, 133.6, 136.9, 141.4, 153.0, 155.0, 158.5, 247.8; HRMS (ESI TOF): m/z Calcd for C15H12N2S2 (M + H)+ = 285.0520, Found 285.0515;
- 2,9-Bis((E)-2-hydroxystyryl)-5-methyl-1,10-dihydro-1,10-phenanthroline-4,7-dione (6a) yellow 1H-NMR (MeOD/KOD; 500.2 MHz) δ = 2.59 (s, 3H, CH3), 6.35 (d, 3JH,H = 10.0 Hz, 1H, aromatic), 6.49 (dd, 3JH,H = 5.0 Hz, 1H, aromatic), 6.70 (dd, 3JH,H = 8.3 Hz, 4JH,H = 1.2 Hz, 1H, aromatic), 6.84 (s, 1H, aromatic), 7.66 (d, 4JH,H = 1.2 Hz, 1H, aromatic), 7.69 (dd, 3JH,H = 5.5 Hz, 4JH,H = 1.2 Hz, 1H, aromatic), 8.24 (d, 3JH,H = 16.4 Hz, 1H, vinyl), 8.33 (d, 3JH,H = 16.1 Hz, 1H, vinyl), 8.52 (s, 1H, OH), 10.22 (s, 1H, NH); 13C{1H}-NMR (MeOD/KOD; 125.8 MHz) δ = 24.3, 109.5, 111.8, 113.6, 115.1, 120.1, 120.3, 121.6, 122.0, 123.3, 124.0, 125.2, 126.7, 126.9, 131.1, 131.6, 131.7, 132.1, 133.2, 139.0, 152.1, 157.6, 161.3, 167.9, 175.0, 175.6, 175.8, 182.0, 182.1; HRMS (ESI TOF): m/z Calcd for C29H23N2O4 (M + H)+ = 463.1658, Found 463.1656.
- 2,9-Bis((E)-2,4-dihydroxystyryl)-5-methyl-1,10-dihydro-1,10-phenanthroline-4,7-dione (6b) fulvous; DSC m.p. = 65.55 °C; HRMS (ESI TOF): m/z Calcd for C29H23N2O6 (M + H)+ = 495.1556, Found 495.1555.
- ((1E,1′E)-(1,10-Phenanthroline-2,9-diyl)bis(ethene-2,1-diyl))bis(2,1-phenylene) bis(hydrogen carbonate) (6c) beige; DSC m.p. = 213.97 °C; 2.4 g (4.8 mmol, 96%), 1H-NMR (DMSO-d6; 500.2 MHz) δ = 2.45 (s, 6H, CH3), 7.25 (dd, 3JH,H = 8.0 Hz, 4JH,H = 1.4 Hz, 2H, aromatic), 7.39 (td, 3JH,H = 7.0 Hz, 4JH,H = 1.4 Hz, 2H, aromatic), 7.44 (td, 3JH,H = 7.6 Hz, 4JH,H = 1.6 Hz, 2H, aromatic), 7.66 (d, 3JH,H = 16.3 Hz, 2H, vinyl), 7.96 (d, 3JH,H = 17.3 Hz, 2H, vinyl), 8.02 (d, 3JH,H = 8.7 Hz, 3H, aromatic), 8.12 (d, 3JH,H = 8.4 Hz, 2H, aromatic), 8.48 (d, 3JH,H = 8.3 Hz, 2H, aromatic), 10.09 (s, 1H, OH); 13C{1H}-NMR (DMSO-d6; 125.8 MHz) δ = 20.8, 121.7, 123.3, 126.2, 126.4, 126.57, 126.62, 128.0, 128.9, 129.6, 131.1, 136.9, 145.2, 148.6, 154.7, 169.3; HRMS (ESI TOF): m/z Calcd for C32H25N2O4 (M + H)+ = 501.1814, Found 501.1806.
- 2,2′-((1E,1′E)-(1,10-Phenanthroline-2,9-diyl)bis(ethene-2,1-diyl))diphenol (6d) red 1.9 g (4.6 mmol, 91%), DSC m.p. = 183.50 °C; 1H-NMR (DMSO-d6; 500.2 MHz) δ = 6.95 (td, 3JH,H = 7.4 Hz, 4JH,H = 1.2 Hz, 2H, aromatic), 7.09 (dd, 3JH,H = 8.2 Hz, 4JH,H = 1.2 Hz, 2H, aromatic), 7.28 (ddd, 3JH,H = 8.5 Hz, 3JH,H = 7.1 Hz, 4JH,H = 1.7 Hz, 2H, aromatic), 7.70 (dd, 3JH,H = 7.9 Hz, 4JH,H = 1.7 Hz, 2H, aromatic), 8.17 (d, 3JH,H = 16.4 Hz, 2H, vinyl), 8.20 (s, 2H, aromatic), 8.39 (d, 3JH,H = 16.5 Hz, 2H, vinyl), 8.56 (d, 3JH,H = 8.8 Hz, 2H, aromatic), 8.88 (d, 3JH,H = 8.7 Hz, 2H, aromatic); 1H-NMR (DMSO-d6/KOD; 500.2 MHz) δ = 6.22–6.34 (m, 2H, aromatic), 6.45 (d, 3JH,H = 17.2 Hz, 1H, vinyl), 6.46 (d, 3JH,H = 16.8 Hz, 1H, vinyl), 6.85–6.94 (m, 2H, aromatic), 7.37–7.40 (m, 1H, aromatic), 7.41–7.44 (m, 1H, aromatic), 7.49 (dd, 3JH,H = 5.7 Hz, 4JH,H = 2.1 Hz, 2H, aromatic), 7.74 (s, 2H, aromatic), 8.09 (d, 3JH,H = 8.5 Hz, 2H, aromatic), 8.19 (d, 3JH,H = 16.7 Hz, 2H, vinyl), 8.26 (d, 3JH,H = 8.5 Hz, 2H, aromatic); 13C{1H}-NMR (DMSO-d6/KOD; 125.8 MHz) δ = 112.3, 120.5, 122.1, 123.4, 125.3, 126.0, 127.2, 127.7, 131.2, 135.1, 137.3, 145.6, 159.0, 170.2; 13C{1H}-NMR (DMSO-d6; 125.8 MHz) δ = 116.6, 119.6, 122.3, 122.4, 123.6, 126.4, 128.1, 129.1, 131.4, 136.8, 136.9, 140.9, 155.2, 157.1, 169.7; HRMS (ESI TOF): m/z Calcd for C28H21N2O2 (M + H)+ = 417.1603, Found 417.1598.
3.5. Computational Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DFT | density functional theory |
| MS | mass spectrometry |
| ESI | electrospray ionization |
| HRMS | high-resolution mass spectrometry |
| THF | tetrahydrofuran |
| DMSO | dimethyl sulfoxide |
| DSS (NMR standard) | sodium trimethylsilylpropanesulfonate |
References
- Jackson, M.N.; Kaminsky, C.J.; Oh, S.; Melville, J.F.; Surendranath, Y. Graphite Conjugation Eliminates Redox Intermediates in Molecular Electrocatalysis. J. Am. Chem. Soc. 2019, 141, 14160–14167. [Google Scholar] [CrossRef] [PubMed]
- ten Brink, G.-J.; Arends, I.W.C.E.; Sheldon, R.A. Catalytic Conversions in Water. Part 21: Mechanistic Investigations on the Palladium-Catalysed Aerobic Oxidation of Alcohols in Water. Adv. Synth. Catal. 2002, 344, 365–369. [Google Scholar] [CrossRef]
- Ru, T.; Liang, G.; Zhang, L.; Ning, Y.; Chen, F.-E. Linear Selective Hydroformylation of 2-Arylpropenes Using Water-Soluble Rh-PNP Complex: Straightforward Access to 3-Aryl-Butyraldehydes. ChemCatChem 2021, 13, 5073–5077. [Google Scholar] [CrossRef]
- Terhorst, M.; Plass, C.; Hinzmann, A.; Guntermann, A.; Jolmes, T.; Rösler, J.; Panke, D.; Gröger, H.; Vogt, D.; Vorholt, A.J.; et al. One-pot synthesis of aldoximes from alkenes via Rh-catalysed hydroformylation in an aqueous solvent system. Green Chem. 2020, 22, 7974–7982. [Google Scholar] [CrossRef]
- Camerel, F.; Strauch, P.; Antonietti, M.; Faul, C.F.J. Copper—Metallomesogen Structures Obtained by Ionic Self-Assembly (ISA): Molecular Electromechanical Switching Driven by Cooperativity. Chem. Eur. J. 2003, 9, 3764–3771. [Google Scholar] [CrossRef] [PubMed]
- De Pascali, S.A.; Migoni, D.; Papadia, P.; Muscella, A.; Marsigliante, S.; Ciccaresea, A.; Fanizzi, F.P. New water-soluble platinum(II) phenanthroline complexes tested as cisplatin analogues: First-time comparison of cytotoxic activity between analogous four- and five-coordinate species. Dalton Trans. 2006, 42, 5077–5087. [Google Scholar] [CrossRef] [PubMed]
- Eberhart, M.S.; Phelan, B.T.; Niklas, J.; Sprague-Klein, E.A.; Kaphan, D.M.; Gosztola, D.J.; Chen, L.X.; Tiede, D.M.; Poluektov, O.G.; Mulfort, K.L. Surface immobilized copper(I) diimine photosensitizers as molecular probes for elucidating the effects of confinement at interfaces for solar energy conversion. Chem. Commun. 2020, 56, 12130. [Google Scholar] [CrossRef]
- Herrmann, W.A.; Kohlpaintner, C.W. Water-Soluble Ligands, Metal Complexes, and Catalysts: Synergism of Homogeneous and Heterogeneous Catalysis. Angew. Chem. Int. Ed. Engl. 1993, 32, 1524–1544. [Google Scholar] [CrossRef]
- Joó, F.; Beck, M.T. Formation and catalytic properties of water-soluble phosphine complexes. React. Kinet. Catal. Lett. 1975, 2, 257–263. [Google Scholar] [CrossRef]
- Dror, Y.; Manassen, J. Hydrogenation of olefins with rhodium-phosphine complexes, having substrate and catalyst in two different immiscible phases. An alternative method for the heterogenization of a homogeneous catalyst. J. Mol. Catal. 1977, 2, 219–222. [Google Scholar] [CrossRef]
- Joó, F.; Tóth, Z.; Beck, M.T. Homogeneous hydrogenations in aqueous solutions catalyzed by transition metal phosphine complexes. Inorg. Chim. Acta 1977, 25, L61–L62. [Google Scholar] [CrossRef]
- Tóth, Z.; Joó, F.; Beck, M.T. Homogeneous hydrogenations in aqueous solution catalyzed by ruthenium-phospine complexes. Inorg. Chim. Acta 1980, 42, 153–161. [Google Scholar] [CrossRef]
- Canivet, J.; Süss-Fink, G.; Štěpnička, P. Water-Soluble Phenanthroline Complexes of Rhodium, Iridium and Ruthenium for the Regeneration of NADH in the Enzymatic Reduction of Ketones. Eur. J. Inorg. Chem. 2007, 2007, 4736–4742. [Google Scholar] [CrossRef]
- Koning, B.; de Boer, J.H.; Meetsma, A.; Kellogg, R.M. Synthesis and complexation characteristics of phenanthroline and bipyridine diols. Arkivoc 2004, 2, 189–205. [Google Scholar] [CrossRef]
- Janiak, C. A critical account on π–π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc. Dalton Trans. 2000, 21, 3885–3896. [Google Scholar] [CrossRef]
- Bhaskar, C.; Chandrasekar, S.; Mohandas, T.; Butcher, R.J. Dibromidobis(3-bromobenzyl-κC)(4,7-diphenyl-1,10-phenanthroline-κ2N,N′)tin(IV). IUCrData 2019, 4, 190336. [Google Scholar] [CrossRef]
- Zhong, K.-L.; Cao, G.Q.; Song, W.; Ni, C. Tris(1,10-phenanthroline-κ2N,N′)cobalt(II) bis(2,4,5-tricarboxybenzoate) monohydrate. IUCrData 2019, 4, 190059. [Google Scholar] [CrossRef]
- Starosta, R.; Puchalska, M.; Cybińska, J.; Barys, M.; Mudring, A.V. Structures, electronic properties and solid state luminescence of Cu(I) iodide complexes with 2,9-dimethyl-1,10-phenanthroline and aliphatic aminomethylphosphines or triphenylphosphine. Dalton Trans. 2011, 40, 2459–2468. [Google Scholar] [CrossRef]
- Viganor, L.; Howe, O.; McCarron, P.; McCann, M.; Devereux, M. The Antibacterial Activity of Metal Complexes Containing 1,10- phenanthroline: Potential as Alternative Therapeutics in the Era of Antibiotic Resistance. Curr. Top. Med. Chem. 2017, 17, 1280–1302. [Google Scholar] [CrossRef]
- Zhou, M.; Yu, L. Electronically Neutral Metal Complexes as Biological Labels. U.S. Patent 2,016,146,826, 12 February 2019. [Google Scholar]
- Moraleda, G.O.; Fresnadillo, D.G.; De Oteyza, P.V.G.; Aranda, J.G.; Pobes, J.M.S.; Martínez, F.Á.; Quevedo, A.R. Luminescent Pigments and Their Use in Security Applications. EP2673335A1, 10 February 2012. [Google Scholar]
- Blondel, M.; Voisset, C.; Lista, M.-J.; Fahraeus, R.; Teulade-Fichou, M.-P. Bisquinolium Derivatives for Preventing or Treating EBV-Related Cancers. WO2018211148A1, 22 May 2018. [Google Scholar]
- Mitrofanov, A.; Brandès, S.; Herbst, F.; Rigolet, S.; Bessmertnykh-Lemeune, A.; Beletskaya, I. Immobilization of copper complexes with (1,10-phenanthrolinyl)phosphonates on titania supports for sustainable catalysis. J. Mater. Chem. A 2017, 5, 12216–12235. [Google Scholar] [CrossRef]
- Yapi, A.-D.; Valentin, A.; Chezal, J.-M.; Chavignon, O.; Chaillot, B.; Gerhardt, R.; Teulade, J.-C.; Blache, Y. In Vitro and in Vivo Antimalarial Activity of Derivatives of 1,10-Phenanthroline Framework. Arch. Pharm. Chem. Life Sci. 2006, 339, 201–206. [Google Scholar] [CrossRef]
- De Cian, A.; DeLemos, E.; Mergny, J.-L.; Teulade-Fichou, M.-P.; Monchaud, D. Highly Efficient G-Quadruplex Recognition by Bisquinolinium Compounds. J. Am. Chem. Soc. 2007, 129, 1856–1857. [Google Scholar] [CrossRef] [PubMed]
- Miron, C.E.; Petitjean, A. An Improved Route for the Synthesis of Guanine Quadruplex Ligand Phen-DC3. Synlett 2018, 29, 1362–1366. [Google Scholar]
- Larsen, A.F.; Ulven, T. Efficient synthesis of 4,7-diamino substituted 1,10-phenanthroline-2,9-dicarboxamides. Org. Lett. 2011, 13, 3546–3548. [Google Scholar] [CrossRef]
- Meng, Z.; Yang, F.; Wang, X.; Shan, W.-L.; Liu, D.; Zhang, L.; Yuan, G. Trefoil-Shaped Metal−Organic Cages as Fluorescent Chemosensors for Multiple Detection of Fe3+, Cr2O72−, and Antibiotics. Inorg. Chem. 2023, 62, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Prasad, B.; Doimo, M.; Andréasson, M.; ĽHôte, V.; Chorell, E.; Wanrooij, S. A complementary chemical probe approach towards customized studies of G-quadruplex DNA structures in live cells. Chem. Sci. 2022, 13, 2347–2354. [Google Scholar] [CrossRef] [PubMed]
- Corey, E.J.; Borror, A.L.; Foglia, T. Transformations in the 1,10-Phenanthroline Series. J. Org. Chem. 1965, 30, 288–290. [Google Scholar] [CrossRef]
- Drommi, M.; Rulmont, C.; Esmieu, C.; Hureau, C. Hybrid Bis-Histidine Phenanthroline-Based Ligands to Lessen Aβ-Bound Cu ROS Production: An Illustration of Cu(I) Significance. Molecules 2021, 26, 7630. [Google Scholar] [CrossRef] [PubMed]
- Nycz, J.E.; Wantulok, J.; Sokolova, R.; Pajchel, L.; Stankevič, M.; Szala, M.; Malecki, J.G.; Swoboda, D. Synthesis and electrochemical and spectroscopic characterization of 4,7-diamino-1,10-phenanthrolines and their precursors. Molecules 2019, 24, 4102. [Google Scholar] [CrossRef]
- Hardy, G.; King-Underwood, J.; Hardy, G.; Murray, P.J.; Williams, G.J.; Onions, T.S.; Gareth, J. Pyrazole p38 Map Kinase Inhibitors. U.S. Patent 201,302,999,0A1, 31 January 2013. [Google Scholar]
- Nycz, J.E.; Szala, M.; Malecki, G.J.; Nowak, M.; Kusz, J. Synthesis, spectroscopy and computational studies of selected hydroxyquinolines and their analogues. Spectrochim. Acta A 2014, 117, 351–359. [Google Scholar] [CrossRef]
- Nycz, J.E.; Malecki, G.J. Synthesis, spectroscopy and computational studies of selected hydroxyquinoline carboxylic acids and their selected fluoro-, thio-, and dithioanalogues. J. Mol. Struct. 2013, 1032, 159–168. [Google Scholar] [CrossRef]
- Kathirgamanathan, P.; Surendrakumar, S.; Vanga, R.R.; Ravichandran, S.; Antipan-Lara, J.; Ganeshamurugan, S.; Kumaraverl, M.; Paramaswara, G.; Arkley, V. Arylvinylene phenanthroline derivatives for electron transport in blue organic light emitting diodes. Org. Electron. 2011, 12, 666–676. [Google Scholar] [CrossRef]
- Nycz, J.E.; Czyż, K.; Szala, M.; Malecki, J.G.; Shaw, G.; Gilmore, B.; Jon, M. Synthesis, spectroscopy and computational studies of some novel π-conjugated vinyl N-alkylated quinolinium salts and their precursor’s. J. Mol. Struct. 2016, 1106, 416–423. [Google Scholar] [CrossRef]
- Gudat, D.; Nycz, J.E.; Polanski, J. A solid state and solution NMR study of the tautomerism in hydroxyquinoline carboxylic acids. Magn. Reson. Chem. 2008, 46, S115–S119. [Google Scholar] [CrossRef] [PubMed]
- Wantulok, J.; Szala, M.; Quinto, A.; Nycz, J.E.; Giannarelli, S.; Sokolova, R.; Książek, M.; Kusz, J. Synthesis and electrochemical and spectroscopic characterization of selected quinolinecarbaldehydes and Schiff bases as their derivatives. Molecules 2020, 25, 2053. [Google Scholar] [CrossRef] [PubMed]
- Rigaku Oxford Diffraction. CrysAlisPro Software System, Version 1.171.38.41q; Rigaku Corporation: Wroclaw, Poland, 2015. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.T.; Yang, W.T.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, Version 6; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]


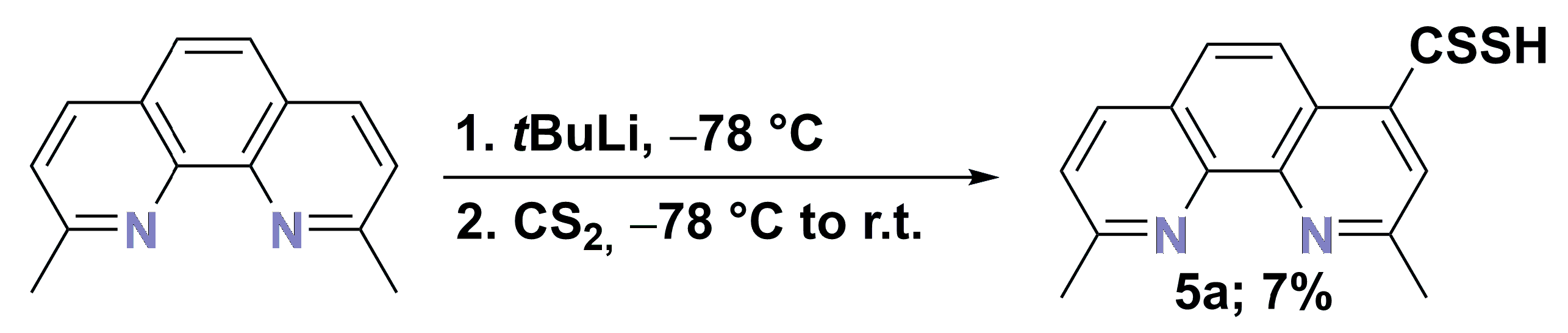

| Molecule | Properties | Sources |
|---|---|---|
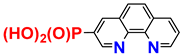 | 1H; 31P NMR; EA 1; procedure | [23] |
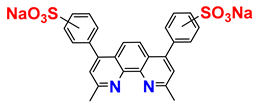 | a mixture of regioisomers | commercially available |
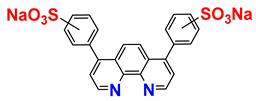 | a mixture of regioisomers | commercially available |
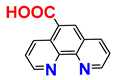 | commercially available | |
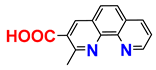 | 1H; 13C NMR; EA; procedure | [24] |
 | 1H; 13C NMR; EA; procedure | [24] |
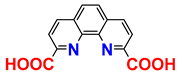 | 1H; 31C NMR; IR; mp 2; procedure | [25] |
| 1H NMR | [26] | |
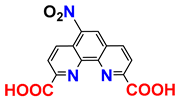 | 1H NMR; MS; procedure | [24] |
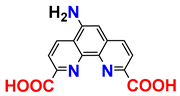 | patent claims | [20] |
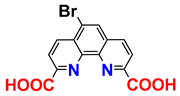 | 1H; 31C NMR; MS; procedure | 22 |
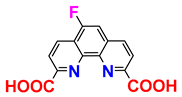 | [22], commercially available | |
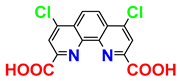 | 1H; 31C NMR; MS; mp; procedure | [27] |
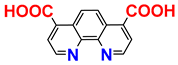 | 1H NMR; procedure | [7] |
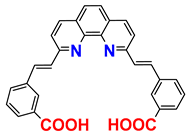 | 1H NMR; procedure | [28] |
| T/K | 200 |
| CCDC number | 2298150 |
| Chemical formula | C16H18Cl2N2O5 |
| Formula mass | 389.22 |
| Crystal system | Monoclinic |
| Space group | P 21/c |
| Z | 4 |
| Unit cell dimensions | |
| a/Å | 21.0882(6) |
| b/Å | 11.6270(3) |
| c/Å | 7.0683(2) |
| β/° | 94.101(3) |
| Unit cell volume/Å3 | 1728.66(8) |
| F(000) | 808 |
| Dx (Mg m−3) | 1.496 |
| μ (mm−1) | 0.406 |
| Theta range for data collection/° | 3.379 to 36.495 |
| Completeness | theta = 25.242° 99.8% |
| Range of h, k, l | |
| −35 ≤ h ≤ 34 | |
| −19 ≤ k ≤ 19 | |
| −11 ≤ l ≤ 6 | |
| No. of measured reflections | 26,199 |
| No. of independent reflections | 7930 |
| Rint | 0.0344 |
| Data/restraints/parameters | 7930/0/239 |
| Goodness-of-fit on F2 | 1.046 |
| Final R1 values (I > 2σ(I)) | 0.0425 |
| Final wR(F2) values (I > 2σ(I)) | 0.1040 |
| Final R1 values (all data) | 0.0692 |
| Final wR(F2) values (all data) | 0.1210 |
| Largest diff. peak and hole/eÅ3 | 0.438 and −0.294 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nycz, J.E.; Martsinovich, N.; Wantulok, J.; Chen, T.; Książek, M.; Kusz, J. Synthesis and Spectroscopic Characterization of Selected Water-Soluble Ligands Based on 1,10-Phenanthroline Core. Molecules 2024, 29, 1341. https://doi.org/10.3390/molecules29061341
Nycz JE, Martsinovich N, Wantulok J, Chen T, Książek M, Kusz J. Synthesis and Spectroscopic Characterization of Selected Water-Soluble Ligands Based on 1,10-Phenanthroline Core. Molecules. 2024; 29(6):1341. https://doi.org/10.3390/molecules29061341
Chicago/Turabian StyleNycz, Jacek E., Natalia Martsinovich, Jakub Wantulok, Tieqiao Chen, Maria Książek, and Joachim Kusz. 2024. "Synthesis and Spectroscopic Characterization of Selected Water-Soluble Ligands Based on 1,10-Phenanthroline Core" Molecules 29, no. 6: 1341. https://doi.org/10.3390/molecules29061341
APA StyleNycz, J. E., Martsinovich, N., Wantulok, J., Chen, T., Książek, M., & Kusz, J. (2024). Synthesis and Spectroscopic Characterization of Selected Water-Soluble Ligands Based on 1,10-Phenanthroline Core. Molecules, 29(6), 1341. https://doi.org/10.3390/molecules29061341








