Structural Characterization and Immunoenhancing Properties of Polysaccharide CPTM-P1 from Taxus media
Abstract
1. Introduction
2. Results and Discussion
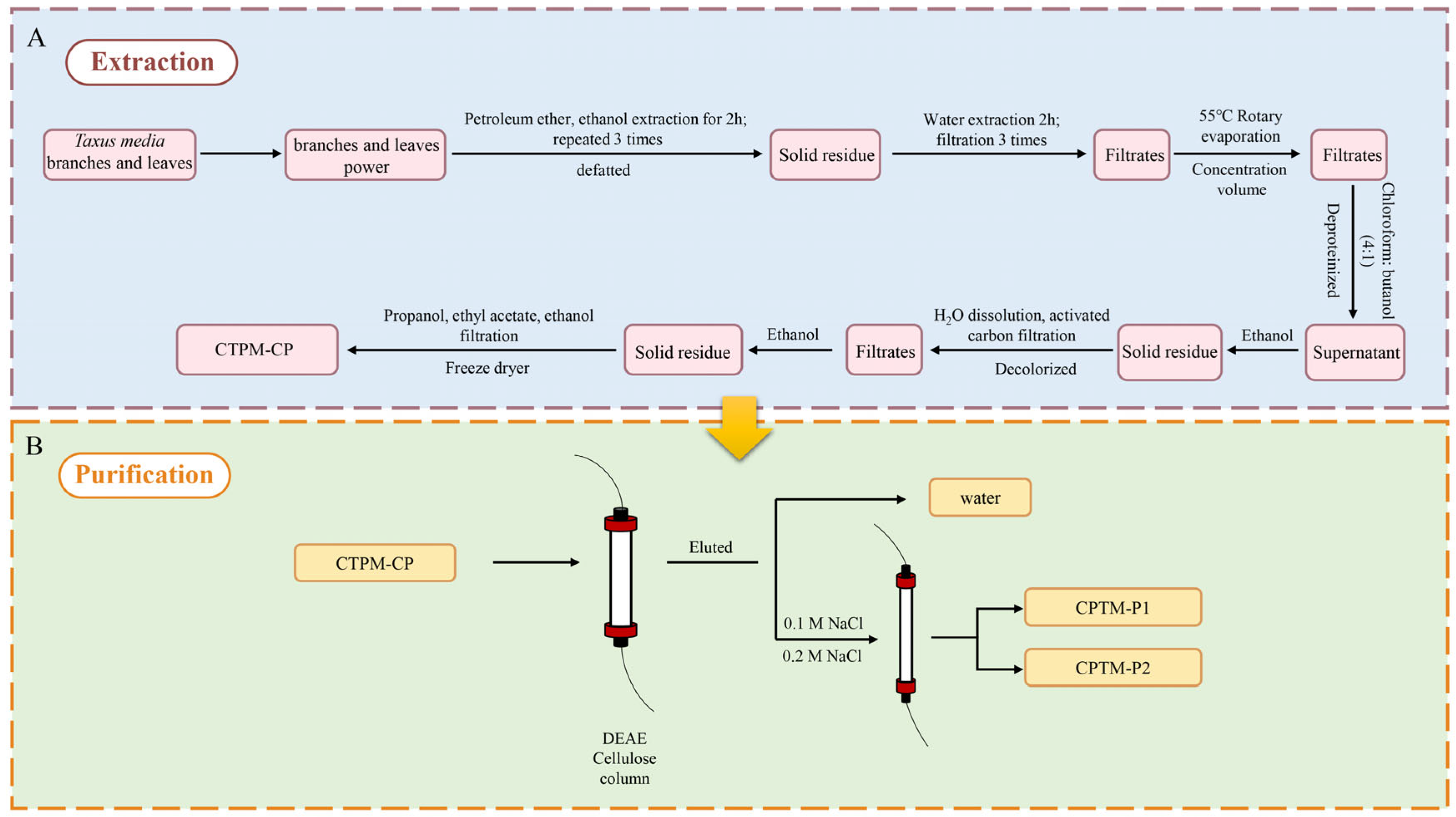
2.1. Isolation and Purification of Polysaccharides from T. media
2.2. Analysis of Structural Characteristics of Polysaccharides
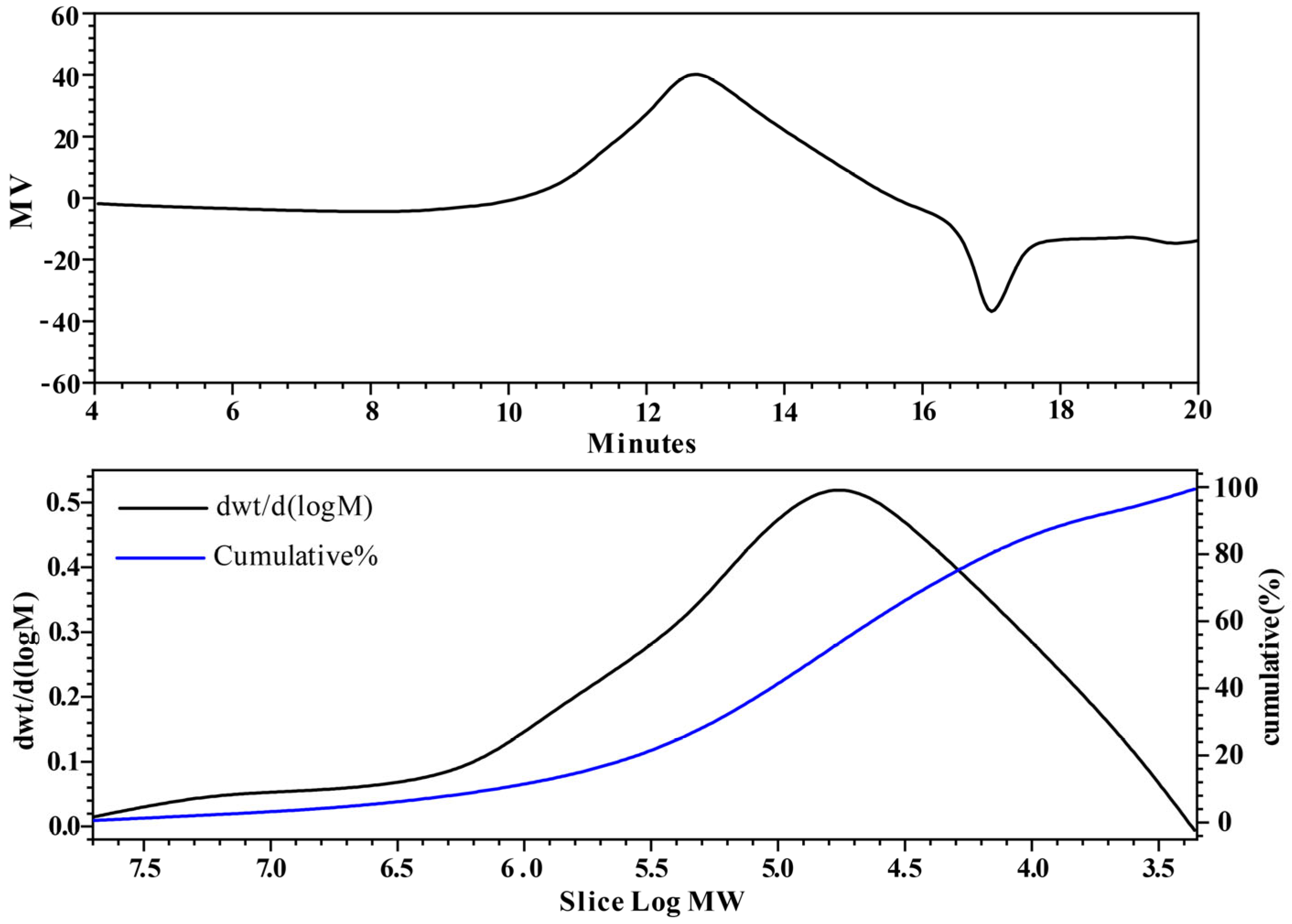
2.2.1. Molecular Weight Analysis
2.2.2. Infrared Spectrum Analysis
2.2.3. Monosaccharide Analysis
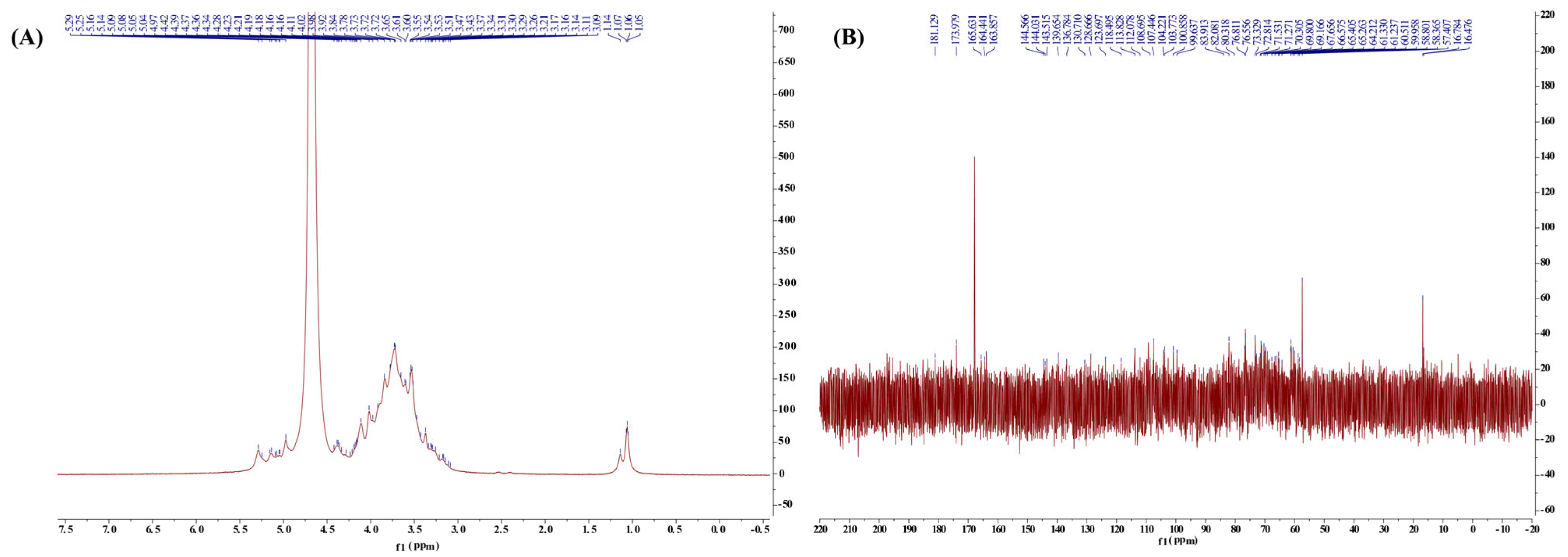
2.2.4. Nuclear Magnetic Resonance (NMR) Analysis
2.2.5. SEM Analysis Results
2.3. Effect of CPTM-P1 on RAW264.7 Cell Immunoreactivity
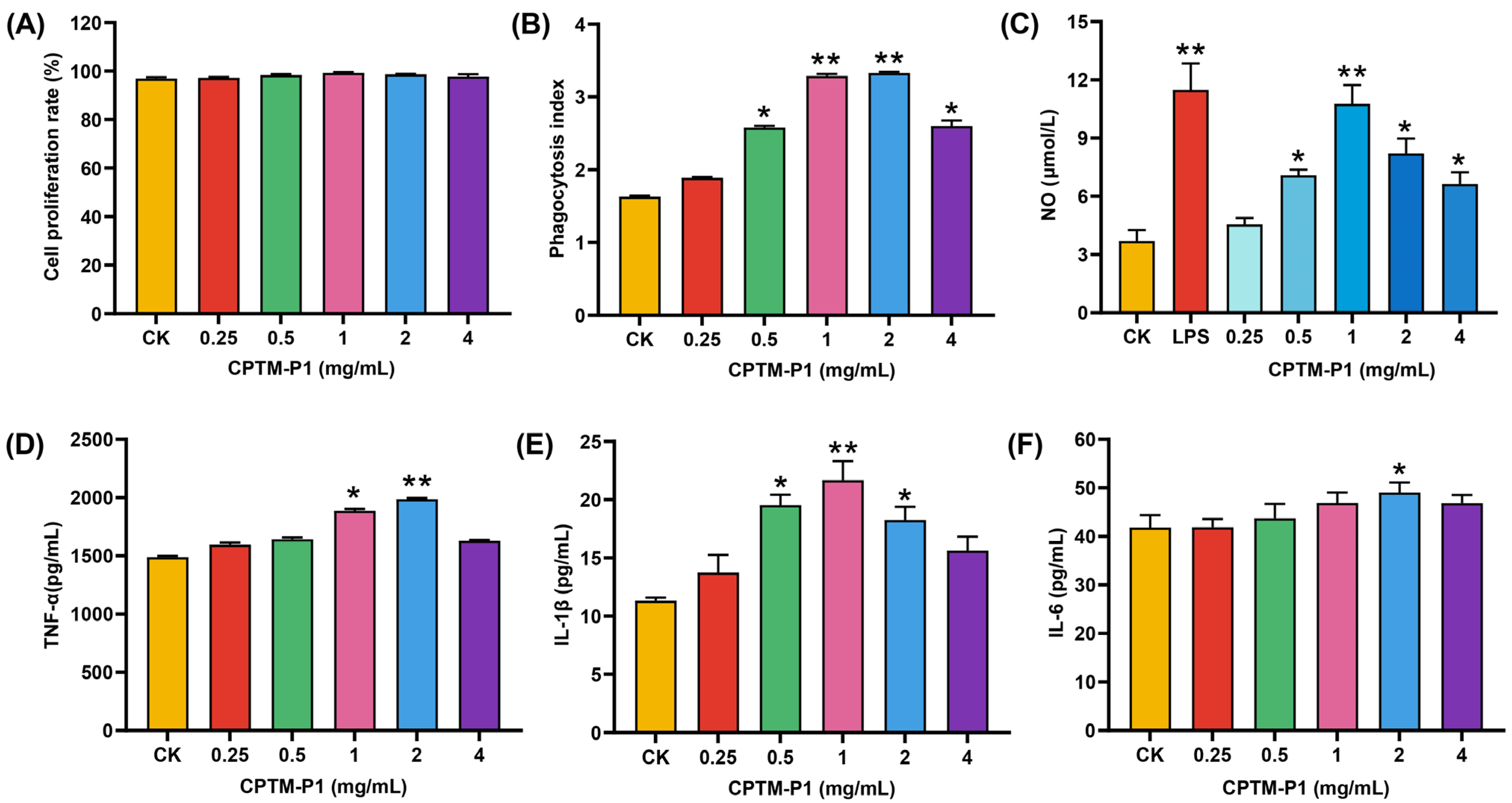
2.3.1. Effect of CPTM-P1 on RAW264.7 Cell Proliferation
2.3.2. Effect of CPTM-P1 on RAW264.7 Cell Phagocytosis
2.3.3. CPTM-P1 Effect on NO, TNF-a, IL-1β, and IL-6 Production
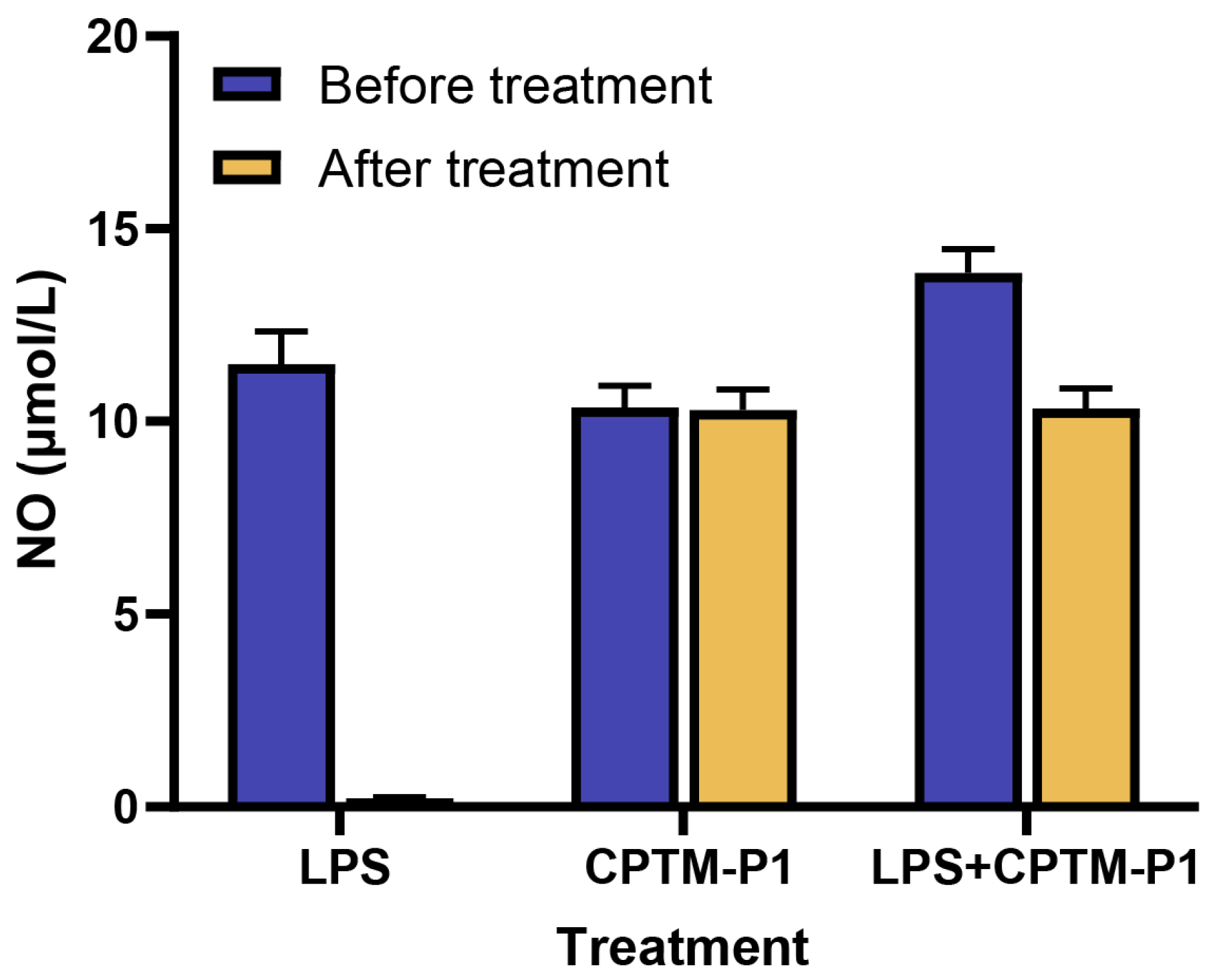
2.4. Endotoxin Contamination
3. Materials and Methods
3.1. Materials and Chemicals
3.2. General Methods
3.3. Extraction, Isolation, and Purification of CPTM-P1
3.4. Analysis of Gel Permeation Chromatograph (GPC)
3.5. Monosaccharide Analysis
3.6. Fourier-Transform Infrared (FT-IR) Analysis
3.7. Nuclear Magnetic Resonance Spectroscopy (NMR) Analysis
3.8. Scanning Electron Microscope (SEM) Analysis
3.9. Measurement of Anti-Inflammatory Activity In Vitro
3.9.1. Culture of RAW264.7 Cells
3.9.2. RAW264.7 Cell Proliferation and Phagocytic Capacity Analysis
3.9.3. Analysis of RAW264.7 Cell’s Secretion Ability of TNF-α, IL-1β, IL-6, and NO
3.10. Removal of Endotoxin
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, F.; Wang, R.; Chen, Y.; Geng, R.; Gao, H.; Wang, F.; Liu, X.; Li, W. Structural characterization of a pectic polysaccharide from laoshan green tea and its inhibitory effects on the production of NO, TNF-α and IL-6. Nat. Prod. Res. 2023, 37, 1797–1805. [Google Scholar] [CrossRef] [PubMed]
- Xin, T.; Zhang, F.; Jiang, Q.; Chen, C.; Huang, D.; Li, Y.; Shen, W.; Jin, Y. Extraction, purification and antitumor activity of a water-soluble polysaccharide from the roots of Polygala tenuifolia. Carbohydr. Polym. 2012, 90, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xu, H.; Wang, X.; Wan, Y.; Jiang, N.; Qi, H.; Liu, X. Antioxidant and antihyperlipidemic activities of high sulfate content purified polysaccharide from Ulva pertusa. Int. J. Biol. Macromol. 2020, 146, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Huang, G. Antioxidant activity of polysaccharides from different sources of ginseng. Int. J. Biol. Macromol. 2019, 125, 906–908. [Google Scholar] [CrossRef] [PubMed]
- Oka, S.; Okabe, M.; Tsubura, S.; Mikami, M.; Imai, A. Properties of fucoidans beneficial to oral healthcare. Odontology 2020, 108, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Ding, S.; Ai, L.; Deng, K. Antitumor and immunomodulatory activity of water-soluble polysaccharide from Inonotus obliquus. Carbohydr. Polym. 2012, 90, 870–874. [Google Scholar] [CrossRef]
- Hwang, K.C.; Shin, H.Y.; Kim, W.J.; Seo, M.S.; Kim, H. Effects of a high-molecular-weight polysaccharides isolated from korean persimmon on the antioxidant, anti-inflammatory, and antiwrinkle activity. Molecules 2021, 26, 1600. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Danilets, M.G.; Ligacheva, A.A.; Trofimova, E.S.; Selivanova, N.S.; Sherstoboev, E.Y.; Krivoshchekov, S.V.; Gulina, E.I.; Brazovskii, K.S.; Kirpotina, L.N.; et al. Immunomodulatory activity of polysaccharides isolated from Saussurea salicifolia L. and Saussurea frolovii Ledeb. Molecules 2023, 28, 6655. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, X.; Wang, S.; Guo, Q.; Li, Z.; Liu, H.; Wang, C. Structural characterisation and immunomodulatory activity of polysaccharides from white asparagus skin. Carbohydr. Polym. 2020, 227, 115314. [Google Scholar] [CrossRef]
- Lee, J.S.; Kwon, D.S.; Lee, K.R.; Park, J.M.; Ha, S.; Hong, E.K. Mechanism of macrophage activation induced by polysaccharide from Cordyceps militaris culture broth. Carbohydr. Polym. 2015, 120, 29–37. [Google Scholar] [CrossRef]
- Yu, Q.; Nie, S.; Wang, J.; Yin, P.; Huang, D.; Li, W.; Xie, M. Toll-like receptor 4-mediated ROS signaling pathway involved in ganoderma atrum polysaccharide-induced tumor necrosis factor-α secretion during macrophage activation. Food Chem. Toxicol. 2014, 66, 14–22. [Google Scholar] [CrossRef]
- Wang, M.; Yang, X.B.; Zhao, J.W.; Lu, C.J.; Zhu, W. Structural characterization and macrophage immunomodulatory activity of a novel polysaccharide from Smilax glabra Roxb. Carbohydr. Polym. 2017, 156, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Jiao, K.; Luo, L.; Xiang, J.; Fan, J.; Zhang, X.; Yi, J.; Zhu, W. Characterization and macrophage immunomodulatory activity of two polysaccharides from the flowers of Paeonia suffruticosa Andr. Int. J. Biol. Macromol. 2019, 124, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure-function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yan, H.; Zhang, X. Structure and immuno-stimulating activities of a new heteropolysaccharide from Lentinula edodes. J. Agr. Food Chem. 2012, 60, 11560–11566. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Luo, Z.; Liu, D.; Ning, Z.; Yang, J.; Ren, J. Structure characterization of a novel polysaccharide from Dictyophora indusiata and its macrophage immunomodulatory activities. J. Agr. Food Chem. 2015, 63, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Xie, G.; Kirpotina, L.N.; Klein, R.A.; Jutila, M.A.; Quinn, M.T. Macrophage immunomodulatory activity of polysaccharides isolated from Opuntia polyacantha. Int. Immunopharmacol. 2008, 8, 1455–1466. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qiu, Z.; Li, L.; Vidyarthi, S.K.; Zheng, Z.; Zhang, R. Structural characterization and antioxidant activities of one neutral polysaccharide and three acid polysaccharides from Ziziphus jujuba cv. Hamidazao: A comparison. Carbohydr. Polym. 2021, 261, 117879. [Google Scholar] [CrossRef]
- Chen, G.; Jiang, N.; Zheng, J.; Hu, H.; Yang, H.; Lin, A.; Hu, B.; Liu, H. Structural characterization and anti-inflammatory activity of polysaccharides from Astragalus membranaceus. Int. J. Biol. Macromol. 2023, 241, 124386. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, F.; Yu, Z.; Lin, J.; Yang, L. Chemical characterization and in vitro antitumor activity of a single-component polysaccharide from Taxus chinensis var. mairei. Carbohydr. Polym. 2015, 133, 294–301. [Google Scholar] [CrossRef]
- Wu, M.; Wu, Y.; Zhou, J.; Pan, Y. Structural characterisation of a water-soluble polysaccharide with high branches from the leaves of Taxus chinensis var. mairei. Food Chem. 2009, 113, 1020–1024. [Google Scholar] [CrossRef]
- Zhao, C.; Li, Z.; Li, C.; Yang, L.; Yao, L.; Fu, Y.; He, X.; Shi, K.; Lu, Z. Optimized extraction of polysaccharides from Taxus chinensis var. mairei fruits and its antitumor activity. Int. J. Biol. Macromol. 2015, 75, 192–198. [Google Scholar] [CrossRef]
- Yan, C.; Yin, Y.; Zhang, D.; Yang, W.; Yu, R. Structural characterization and invitroantitum or activity of a novel polysaccharide from Taxus yunnanensis. Carbohydr. Polym. 2013, 96, 389–395. [Google Scholar] [CrossRef]
- Yin, Y.; Yu, R.; Yang, W.; Yuan, F.; Yan, C.; Song, L. Structural characterization and anti-tumor activity of a novel heteropolysaccharide isolated from Taxus yunnanensis. Carbohydr. Polym. 2010, 82, 543–548. [Google Scholar] [CrossRef]
- Jiang, P.; Zhang, Q.; Zhao, Y.; Xiong, J.; Wang, F.; Zhang, T.; Zhang, C. Extraction, purification, and biological activities of polysaccharides from branches and leaves of Taxus cuspidata S. et Z. Molecules 2019, 24, 2926. [Google Scholar] [CrossRef]
- Zeng, P.; Li, J.; Chen, Y.; Zhang, L. The structures and biological functions of polysaccharides from traditional Chinese herbs. Prog. Mol. Biol. Transl. Sci. 2019, 163, 423–444. [Google Scholar] [PubMed]
- Wu, N.; Wen, Z.; Xiang, X.; Huang, Y.; Gao, Y.; Qu, Y. Immunostimulative activity of low molecular weight chitosans in RAW264.7 macrophages. Mar. Drugs 2015, 13, 6210–6225. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Katsarov, P.; Nikolova, M.; Iliev, I.; Peychev, L.; Trica, B.; Oancea, F.; Delattre, C.; et al. Immunomodulatory and anti-inflammatory effects of fucoidan: A review. Polymers 2020, 12, 2338. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yao, Y.; Gao, Y.; Hu, Y.; Shi, Z.; Ren, G. Suppressive effects of barley β—Glucans with different molecular weight on 3T3-L1 adipocyte differentiation. J. Food Sci. 2016, 81, H786–H793. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.J.; You, D.; Lee, K. Characterization and immunomodulatory effects of high molecular weight fucoidan fraction from the sporophyll of Undaria pinnatifida in cyclophosphamide-induced immunosuppressed mice. Mar. Drugs 2019, 17, 447. [Google Scholar] [CrossRef] [PubMed]
- Flórez-Fernández, N.; Torres, M.D.; González-Muñoz, M.J.; Domínguez, H. Potential of intensification techniques for the extraction and depolymerization of fucoidan. Algal Res. 2018, 30, 128–148. [Google Scholar] [CrossRef]
- Fan, Y.; Ma, J.; Wang, G.; Li, X.; Liu, Y.; Xu, E.; Luo, A. Ultrasonic extraction, structural modification and gastric mucosal cells protective activity of a polysaccharide from Dendrobium denneanum. Arab. J. Chem. 2023, 16, 105033. [Google Scholar] [CrossRef]
- Wang, Y.; Han, J.; Yue, Y.; Wu, Y.; Zhang, W.; Xia, W.; Wu, M. Purification, structure identification and immune activity of a neutral polysaccharide from Cynanchum auriculatum. Int. J. Biol. Macromol. 2023, 237, 124142. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhu, J.; Diao, W.; Wang, C. Ultrasound-assisted enzymatic extraction and antioxidant activity of polysaccharides from pumpkin (Cucurbita moschata). Carbohydr. Polym. 2014, 113, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Wang, X.; Wu, Y.; Liu, L.; Zhao, Y.; Zhao, R. Comparative study on anti-inflammatory effect of polysaccharides from vinegar-baked Radix Bupleuri using different methods. ACS Omega 2023, 8, 29253–29261. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Peng, Q.; Yuan, Y.; Shen, J.; Xie, X.; Wang, M. Isolation, structures and bioactivities of the polysaccharides from jujube fruit (Ziziphus Jujuba Mill.): A review. Food Chem. 2017, 227, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Yan, Y.; Hou, C.; Shi, M.; Liu, Y. Structural characterization of a galacturonic acid-rich polysaccharide from Ziziphus jujuba cv. Muzao. Int. J. Biol. Macromol. 2020, 147, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Si, Z.; Jiang, Y.; Ye, M.; Wang, F.; Yang, X.; Yu, J.; Gao, X.; Liu, W. Structure-activity relationship of low molecular weight astragalus membranaceus polysaccharides produced by Bacteroides. Carbohydr. Polym. 2023, 316, 121036. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, C.; Bai, L.; Wu, J.; Bo, R.; Ye, M.; Huang, L.; Chen, H.; Rui, W. Structural differences of polysaccharides from Astragalus before and after honey processing and their effects on colitis mice. Int. J. Biol. Macromol. 2021, 182, 815–824. [Google Scholar] [CrossRef]
- Li, F.; Zhao, J.; Wei, Y.; Jiao, X.; Li, Q. Holistic review of polysaccharides isolated from pumpkin: Preparation methods, structures and bioactivities. Int. J. Biol. Macromol. 2021, 193, 541–552. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, G. Extraction, structural analysis and antioxidant activity of aloe polysaccharide. J. Mol. Struct. 2023, 1273, 134379. [Google Scholar] [CrossRef]
- Luo, A.; Ge, Z.; Fan, Y.; Luo, A.; Chun, Z.; He, X. In vitro and in vivo antioxidant activity of a water-soluble polysaccharide from Dendrobium denneanum. Molecules 2011, 16, 1579–1592. [Google Scholar] [CrossRef] [PubMed]
- Luo, A.; He, X.; Zhou, S.; Fan, Y.; He, T.; Chun, Z. In vitro antioxidant activities of a water-soluble polysaccharide derived from Dendrobium nobile Lindl. Extracts. Int. J. Biol. Macromol. 2009, 45, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhao, M.; Xie, S.; Peng, D.; An, M.; Chen, Y.; Li, P.; Du, B. Effect of steam explosion pretreatment on polysaccharide isolated from Poria cocos: Structure and immunostimulatory activity. J. Food Biochem. 2022, 46, e14355. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.K.; Cheng, J.J.; Lin, C.Y.; Chang, C.C. Purification, structural elucidation, and anti-inflammatory effect of a water-soluble 1,6-branched 1,3-α-D-galactan from cultured mycelia of Poria cocos. Food Chem. 2010, 118, 349–356. [Google Scholar] [CrossRef]
- Shakhmatov, E.G.; Makarova, E.N. Structure of KON-extractable polysaccharides of tree greenery of from Siberian fir Abies sibirica Ledeb. Carbohydr. Polym. 2022, 276, 118794. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Kamilijiang, M.; Abuduwaili, A.; Zang, D.; Abudukelimu, N.; Liu, G.; Yili, A.; Aisa, H.A. Isolation, structure elucidation, and biological activity of polysaccharides from Saussurea involucrata. Int. J. Biol. Macromol. 2022, 222, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Shang, Z.; Lv, X.; Du, M.; Ma, L.; Hou, G.; Chen, J.; Wang, C.; Zhao, F. Structure elucidation and antitumor activity of a water soluble polysaccharide from Hemicentrotus pulcherrimus. Carbohydr. Polym. 2022, 292, 119718. [Google Scholar] [CrossRef]
- Lin, P.; Chen, L.; Huang, X.; Xiao, F.; Fu, L.; Jing, D.; Wang, J.; Zhang, H.; Sun, L.; Wu, Y. Structural characteristics of polysaccharide GP2a in Gardenia jasminoides and its immunomodulatory effect on macrophages. Int. J. Mol. Sci. 2022, 23, 11279. [Google Scholar] [CrossRef]
- Cavaillon, J. Exotoxins and endotoxins: Inducers of inflammatory cytokines. Toxicon 2018, 149, 45–53. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, J.; Wei, Y.; Yu, G.; Li, F.; Li, Q. Structural characterization and mechanisms of macrophage immunomodulatory activity of a pectic polysaccharide from Cucurbita moschata Duch. Carbohydr. Polym. 2021, 269, 118288. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, Y.N.; Paulsen, B.S.; Kiyohara, H.; Ciz, M.; Ognyanov, M.H.; Vasicek, O.; Rise, F.; Denev, P.N.; Yamada, H.; Lojek, A.; et al. The common lavender (Lavandula angustifolia Mill.) Pectic polysaccharides modulate phagocytic leukocytes and intestinal Peyer’s patch cells. Carbohydr. Polym. 2017, 174, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Tang, D.; Wang, Y.; Li, X.; Hong, L.; Sun, C. Characteristics and immune-enhancing activity of pectic polysaccharides from sweet cherry (Prunus avium). Food Chem. 2018, 254, 47–54. [Google Scholar] [CrossRef]
- Wang, H.; Bi, H.; Gao, T.; Zhao, B.; Ni, W.; Liu, J. A homogalacturonan from Hippophae rhamnoides L. Berries enhance immunomodulatory activity through TLR4/MyD88 pathway mediated activation of macrophages. Int. J. Biol. Macromol. 2018, 107, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Pugh, N.D.; Tamta, H.; Balachandran, P.; Wu, X.; Howell, J.; Dayan, F.E.; Pasco, D.S. The majority of in vitro macrophage activation exhibited by extracts of some immune enhancing botanicals is due to bacterial lipoproteins and lipopolysaccharides. Int. Immunopharmacol. 2008, 8, 1023–1032. [Google Scholar] [CrossRef][Green Version]
- Gertsch, J.; Viveros-Paredes, J.M.; Taylor, P. Plant immunostimulants—Scientific paradigm or myth? J. Ethnopharmacol. 2011, 136, 385–391. [Google Scholar] [CrossRef]



| Monosaccharide | Content (mg/g) | Percentage (%) |
|---|---|---|
| Gal | 36.42 | 30.53 |
| Ara | 26.24 | 22.00 |
| Rha | 14.23 | 5.63 |
| Glc | 13.92 | 11.67 |
| Gal-UA | 27.42 | 11.93 |
| Xyl | 2.01 | 1.69 |
| Fuc | 1.47 | 8.5 |
| Man | 10.14 | 1.23 |
| Rib | 1.4 | 5.63 |
| Glc-UA | 6.72 | 1.17 |
| Glycosyl Residues | Chemical Shift H/C (ppm) | |||||
|---|---|---|---|---|---|---|
| H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6/C6 | |
| α-D-Galp-(1→ | 5.12/99.52 | 3.80/68.68 | 3.89/69.37 | 4.024/70.36 | 4.12/70.69 | 3.71/61.36 |
| →6)-α-D-Glcp-(1→ | 5.12/99.52 | 3.62/76.71 | 3.71/73.72 | 3.55/70.36 | 3.96/70.69 | 4.07/67.01 |
| →1-(-α-L-Rhap-(2→ | 5.30/95.47 | 4.14/76.71 | 3.96/69.37 | 3.40/73.37 | 3.80/68.88 | 1.14/16.78 |
| →1)-α-L-Araf-(5→ | 5.12/107.54 | 4.14/78.74 | 4.07/76.71 | 4.18/82.08 | 3.96/68.88 | - |
| α-D-Manp-(1→ | 5.12/99.52 | 4.07/70.36 | 3.87/70.69 | 3.71/68.68 | 3.78/73.72 | 4.02/61.33 |
| →2)-α-D-Manp-(1→ | 5.30/99.52 | 4.07/78.74 | 4.00/70.69 | 3.67/68.68 | 3.71/73.72 | 3.80/61.33 |
| →4)-α-GalpA-(1→ | 4.99/99.52 | 3.89/70.69 | 4.12/73.37 | 4.36/78.74 | 4.68/73.77 | 177.848 |
| α-D-GlcAp-(-1→ | 5.30/99.52 | 3.62/73.72 | 3.71/76.71 | 3.55/73.72 | 4.02/73.37 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, J.; Huang, X.; Dou, M.; Tang, S.; Wang, G.; Fan, Y.; Luo, A.; Wang, G.; Wang, Y. Structural Characterization and Immunoenhancing Properties of Polysaccharide CPTM-P1 from Taxus media. Molecules 2024, 29, 1370. https://doi.org/10.3390/molecules29061370
Fan J, Huang X, Dou M, Tang S, Wang G, Fan Y, Luo A, Wang G, Wang Y. Structural Characterization and Immunoenhancing Properties of Polysaccharide CPTM-P1 from Taxus media. Molecules. 2024; 29(6):1370. https://doi.org/10.3390/molecules29061370
Chicago/Turabian StyleFan, Jiangtao, Xiong Huang, Mengke Dou, Shuqin Tang, Gang Wang, Yijun Fan, Aoxue Luo, Gang Wang, and Yong Wang. 2024. "Structural Characterization and Immunoenhancing Properties of Polysaccharide CPTM-P1 from Taxus media" Molecules 29, no. 6: 1370. https://doi.org/10.3390/molecules29061370
APA StyleFan, J., Huang, X., Dou, M., Tang, S., Wang, G., Fan, Y., Luo, A., Wang, G., & Wang, Y. (2024). Structural Characterization and Immunoenhancing Properties of Polysaccharide CPTM-P1 from Taxus media. Molecules, 29(6), 1370. https://doi.org/10.3390/molecules29061370








