Characterisation of Modular Polyketide Synthases Designed to Make Pentaene Analogues of Amphotericin B
Abstract
:1. Introduction
2. Results
2.1. Fusion of TE to Module 16
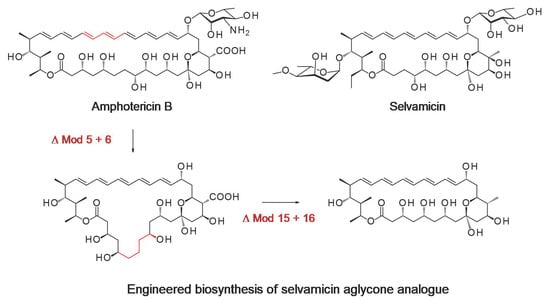
2.2. Deletion of Two Modules from the AmphJ PKS Protein
2.3. Analysis of Pentaenes from S. nodosus M57-16TE and S. nodosus M57-1517
2.4. Analysis of Major Pentaene from S. nodosus M57-1517
2.5. Analysis of M57-1517 Pentaene by NMR Spectroscopy
2.6. Fusion of TE Domain to Module 16 of a Heptaene-Producing Amphotericin PKS
2.7. Attempted Glycosylation Engineering of Pentaenes in S. nodosus M57-1517
3. Discussion
4. Materials and Methods
4.1. DNA Methods
4.2. Extraction of Polyenes
4.3. High Performance Liquid Chromatography (HPLC)
4.4. Mass Spectrometry
4.5. NMR Spectroscopy
4.6. Tests for Antifungal Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caffrey, P.; Hogan, M.; Song, Y. New glycosylated polyene macrolides: Refining the ore from genome mining. Antibiotics 2022, 11, 334. [Google Scholar] [CrossRef] [PubMed]
- Van Arnam, E.B.; Ruzzini, A.C.; Sit, C.S.; Horn, H.; Pinto-Tomás, A.A.; Currie, C.R.; Clardy, J. Selvamicin, an atypical antifungal polyene from two alternative genomic contexts. Proc. Natl. Acad. Sci. USA 2016, 113, 12940–12945. [Google Scholar] [CrossRef] [PubMed]
- Carmody, M.; Murphy, B.; Byrne, B.; Power, P.; Rai, D.; Rawlings, B.; Caffrey, P. Biosynthesis of amphotericin derivatives lacking exocyclic carboxyl groups. J. Biol. Chem. 2005, 280, 34420–34426. [Google Scholar] [CrossRef] [PubMed]
- Bruheim, P.; Borgos, S.E.; Tsan, P.; Sletta, H.; Ellingsen, T.E.; Lancelin, J.-M.; Zotchev, S.B.; Stocker, H.; Kruse, G.; Kreckel, P.; et al. Chemical diversity of polyene macrolides produced by Streptomyces noursei ATCC 11455 and recombinant strain ERD44 with genetically altered polyketide synthase NysC. Antimicrob. Agents Chemother. 2004, 48, 4148–4153. [Google Scholar] [CrossRef] [PubMed]
- Hogan, M.; Song, Y.; Muldoon, J.; Caffrey, P. Generation of new glycoanalogues of polyene antibiotics by synthetic biology—Testing current technical boundaries. SynBio 2024, 2, 31–55. [Google Scholar] [CrossRef]
- Carmody, M.; Byrne, B.; Murphy, B.; Breen, C.; Lynch, S.; Flood, E.; Finnan, S.; Caffrey, P. Analysis and manipulation of amphotericin biosynthetic genes by means of modified phage KC515 transduction techniques. Gene 2004, 343, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.; Anderson, K.; Borissow, C.; Caffrey, P.; Griffith, G.; Hearn, J.; Ibrahim, O.; Khan, N.; Lamburn, N.; Lee, M.; et al. Isolation and characterisation of amphotericin B analogues and truncated polyketide intermediates produced by genetic engineering of Streptomyces nodosus. Org. Biomol. Chem. 2010, 8, 3758–3770. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Hôtel, L.; Paris, C.; Chepkirui, C.; Brachmann, A.O.; Piel, J.; Jacob, C.; Aigle, B.; Weissman, K.J. Engineering the stambomycin modular polyketide synthase yields 37-membered mini-stambomycins. Nat. Commun. 2022, 13, 515. [Google Scholar] [CrossRef]
- Moldenhauer, J.; Chen, X.H.; Borriss, R.; Piel, J. Biosynthesis of the antibiotic bacillaene, the product of a giant polyketide synthase complex of the trans-AT family. Angew. Chem. Int. Ed. Engl. 2007, 46, 8195–8197. [Google Scholar] [CrossRef]
- Li, H.; Han, X.; Dong, Y.; Xu, S.; Chen, C.; Feng, Y.; Cui, Q.; Li, W. Bacillaenes: Decomposition trigger point and biofilm enhancement in Bacillus. ACS Omega 2021, 6, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Gagoś, M.; Czernel, G. Oxidized forms of polyene antibiotic amphotericin B. Chem. Phys. Lett. 2014, 598, 5–9. [Google Scholar] [CrossRef]
- Kao, C.L.; Borisova, S.A.; Kim, H.J.; Liu, H.W. Linear aglycones are the substrates for glycosyltransferase DesVII in methymycin biosynthesis: Analysis and implications. J. Am. Chem. Soc. 2006, 128, 5606–5607. [Google Scholar] [CrossRef] [PubMed]
- Borisova, S.A.; Kim, H.J.; Pu, X.; Liu, H.W. Glycosylation of acyclic and cyclic aglycone substrates by macrolide glycosyltransferase DesVII/DesVIII: Analysis and implications. Chembiochem 2008, 9, 1554–1558. [Google Scholar] [CrossRef] [PubMed]
- Lombó, F.; Gibson, M.; Greenwell, L.; Braña, A.F.; Rohr, J.; Salas, J.A.; Méndez, C. Engineering biosynthetic pathways for deoxysugars: Branched-chain sugar pathways and derivatives from the antitumor tetracenomycin. Chem. Biol. 2004, 11, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
- Omura, S.; Tanaka, H. Production, structure and antifungal activity of polyene macrolides. In Macrolide Antibiotics, Chemistry, Biology and Practice; Omura, S., Ed.; Academic Press: New York, NY, USA, 1986; pp. 351–404. [Google Scholar]
- Jeon, B.J.; Kang, J.E.; Do Kim, J.; Seok Kim, B. Pentaene macrolides AB023a and takanawaene C produced by Streptomyces xanthocidicus strain S3 for controlling pepper anthracnose. Appl. Biol. Chem. 2023, 66, 50. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, J.; Li, X.; Xiao, E.; Lange, J.D.; Rienstra, C.M.; Burke, M.D.; Mitchell, D.A. Sterol sponge mechanism is conserved for glycosylated polyene macrolides. ACS Cent. Sci. 2021, 7, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tao, W.; Qi, Z.; Wei, J.; Shi, T.; Kang, Q.; Zheng, J.; Zhao, Y.; Bai, L. Structural and mechanistic insights into chain release of the polyene PKS thioesterase domain. ACS Catal. 2022, 12, 762–776. [Google Scholar] [CrossRef]
- Jiang, C.; Zhou, Y.; Tao, W.; Ji, S.; Mao, Y.; Shi, T.; Zheng, J.; Bai, L.; Zhao, Y.L. Theoretical studies of mutual effects between 6-m-r hemiketalization and 26-m-r lactonization in pimaricin thioesterase. Chem. Asian J. 2023, 18, e202201229. [Google Scholar] [CrossRef] [PubMed]
- Wlodek, A.; Kendrew, S.G.; Coates, N.J.; Hold, A.; Pogwizd, J.; Rudder, S.; Sheehan, L.S.; Higginbotham, S.J.; Stanley-Smith, A.E.; Warneck, T.; et al. Diversity oriented biosynthesis via accelerated evolution of modular gene clusters. Nat. Commun. 2017, 8, 1206. [Google Scholar] [CrossRef] [PubMed]
- Kieser, T.; Bibb, M.J.; Buttner, M.J.; Chater, K.F.; Hopwood, D.A. Practical Streptomyces Genetics: A Laboratory Manual; John Innes Foundation: Norwich, UK, 2000. [Google Scholar]
- Zotchev, S.B.; Caffrey, P. Genetic analysis of nystatin and amphotericin biosynthesis. Methods Enzymol. 2009, 459, 243–258. [Google Scholar] [CrossRef] [PubMed]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Hogan, M.; Muldoon, J.; Evans, P.; Caffrey, P. Characterisation of Modular Polyketide Synthases Designed to Make Pentaene Analogues of Amphotericin B. Molecules 2024, 29, 1396. https://doi.org/10.3390/molecules29061396
Song Y, Hogan M, Muldoon J, Evans P, Caffrey P. Characterisation of Modular Polyketide Synthases Designed to Make Pentaene Analogues of Amphotericin B. Molecules. 2024; 29(6):1396. https://doi.org/10.3390/molecules29061396
Chicago/Turabian StyleSong, Yuhao, Mark Hogan, Jimmy Muldoon, Paul Evans, and Patrick Caffrey. 2024. "Characterisation of Modular Polyketide Synthases Designed to Make Pentaene Analogues of Amphotericin B" Molecules 29, no. 6: 1396. https://doi.org/10.3390/molecules29061396
APA StyleSong, Y., Hogan, M., Muldoon, J., Evans, P., & Caffrey, P. (2024). Characterisation of Modular Polyketide Synthases Designed to Make Pentaene Analogues of Amphotericin B. Molecules, 29(6), 1396. https://doi.org/10.3390/molecules29061396