Preparation, Characterization, and Bioactivities of Polysaccharide–Nano-Selenium and Selenized Polysaccharides from Acanthopanax senticosus
Abstract
1. Introduction
2. Results and Discussion
2.1. Extraction and Purification of ASPS
2.2. Preparation of A. senticosus Polysaccharide–Nano-Selenium
2.3. Preparation of Selenium Polysaccharides of A. senticosus
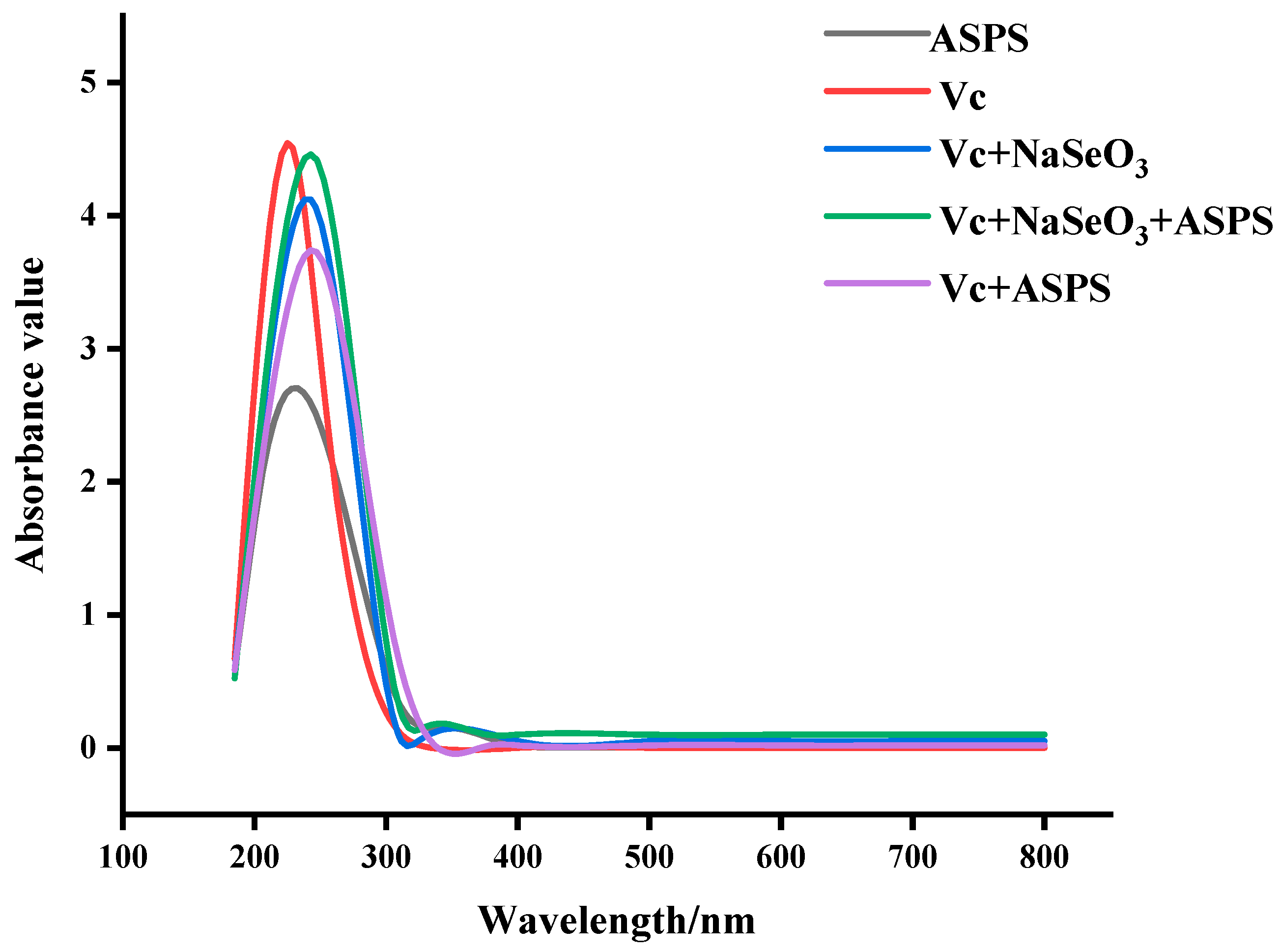
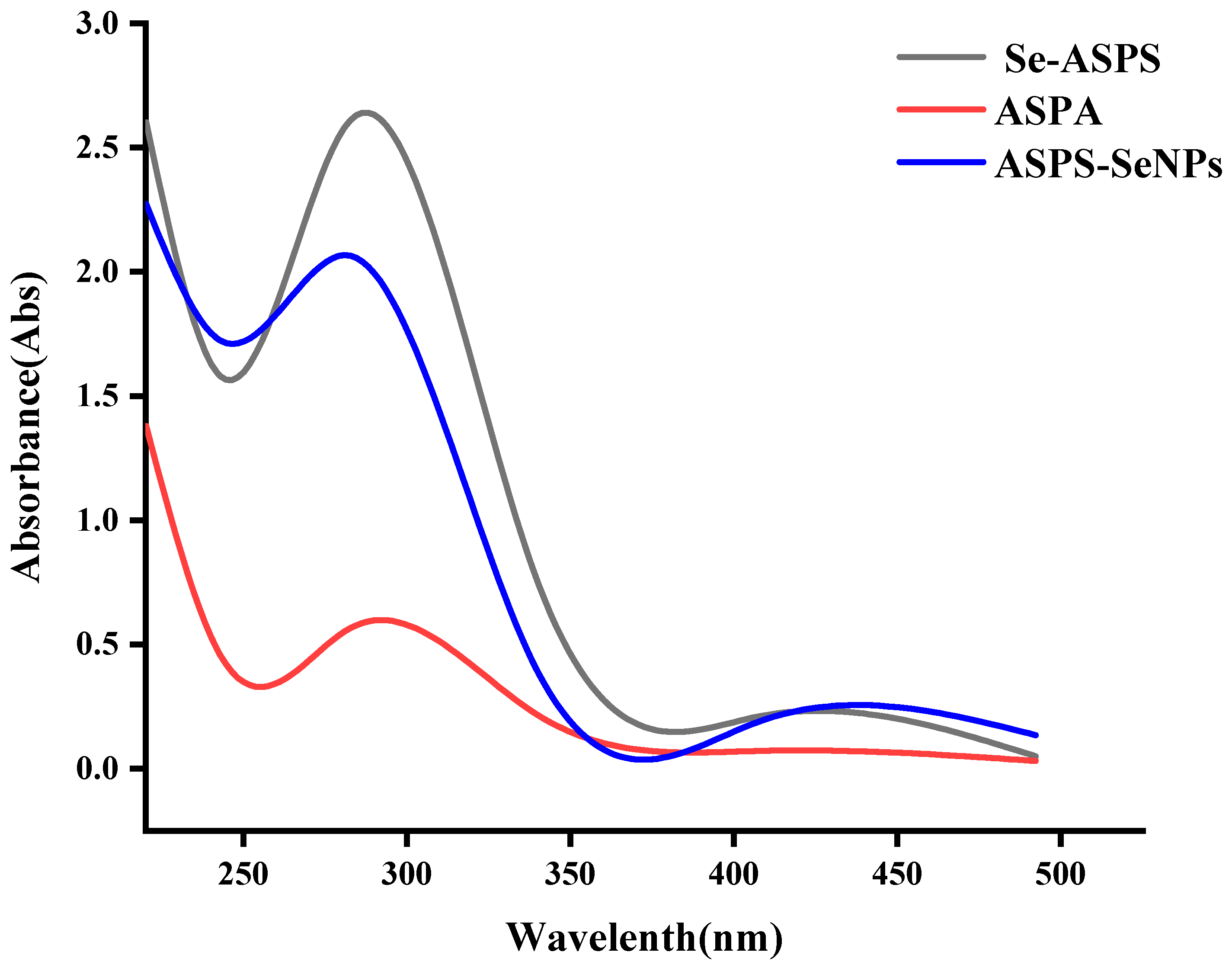
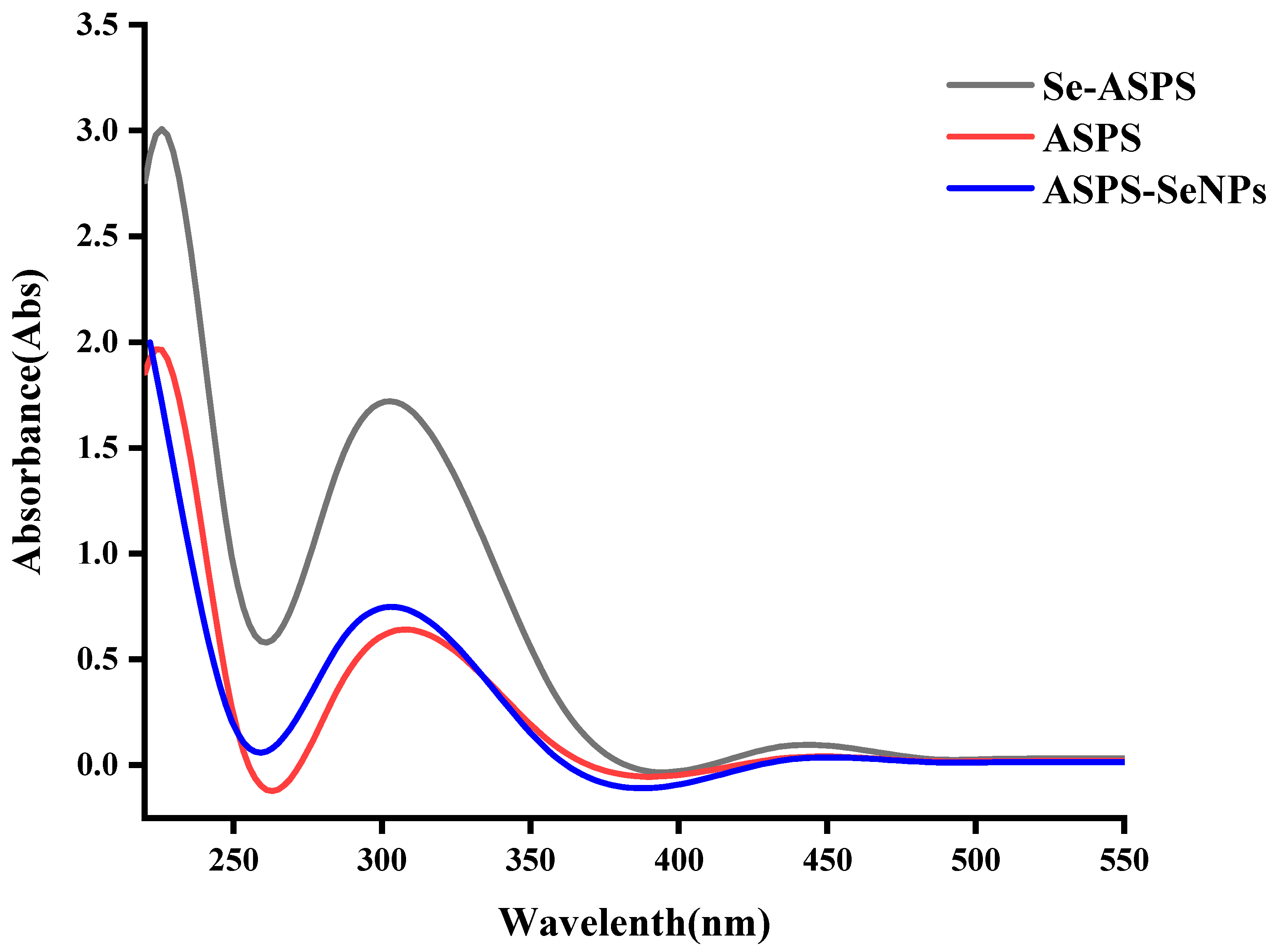
2.4. UV Scan Results
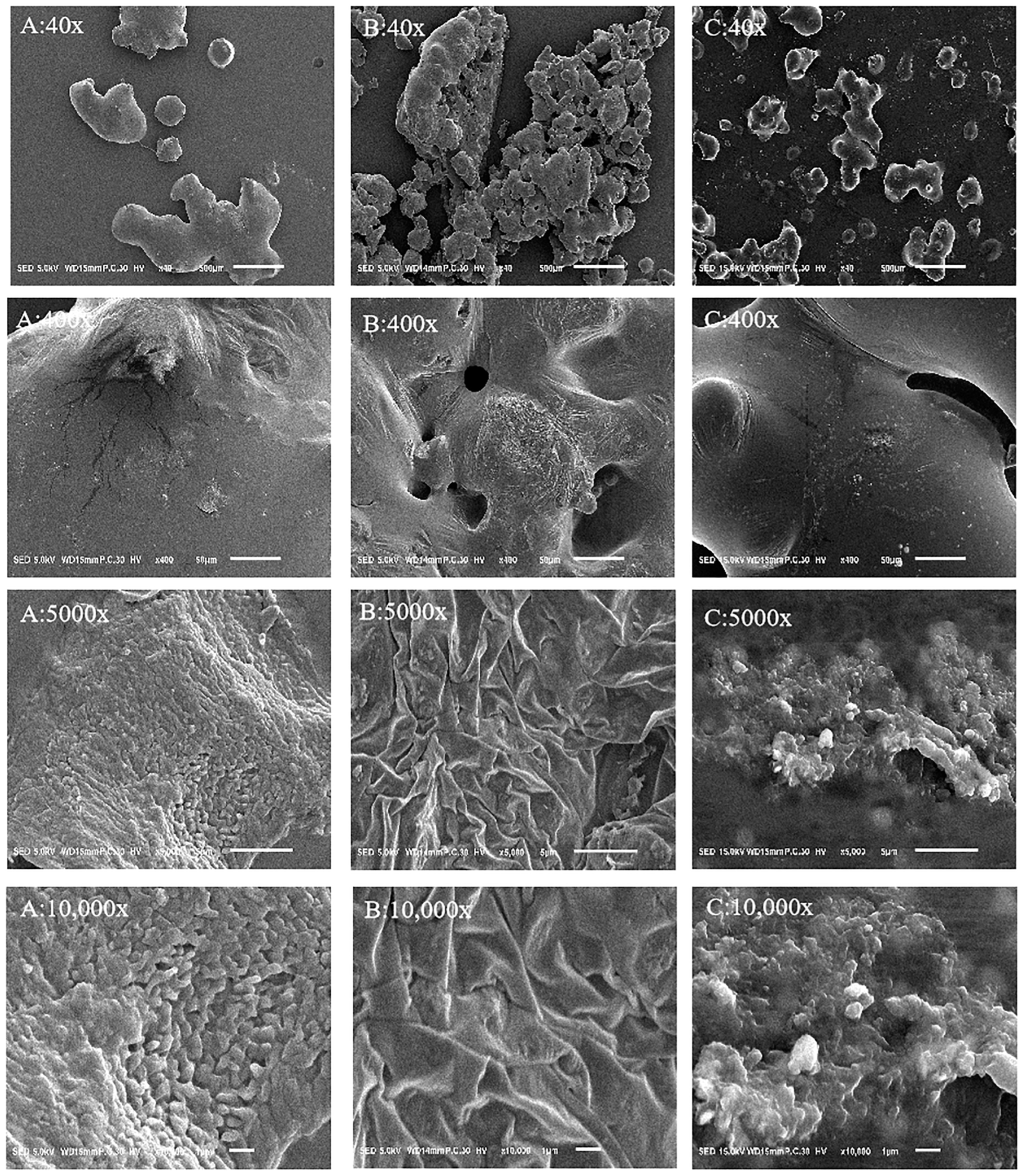
2.5. Electron Microscope Observation
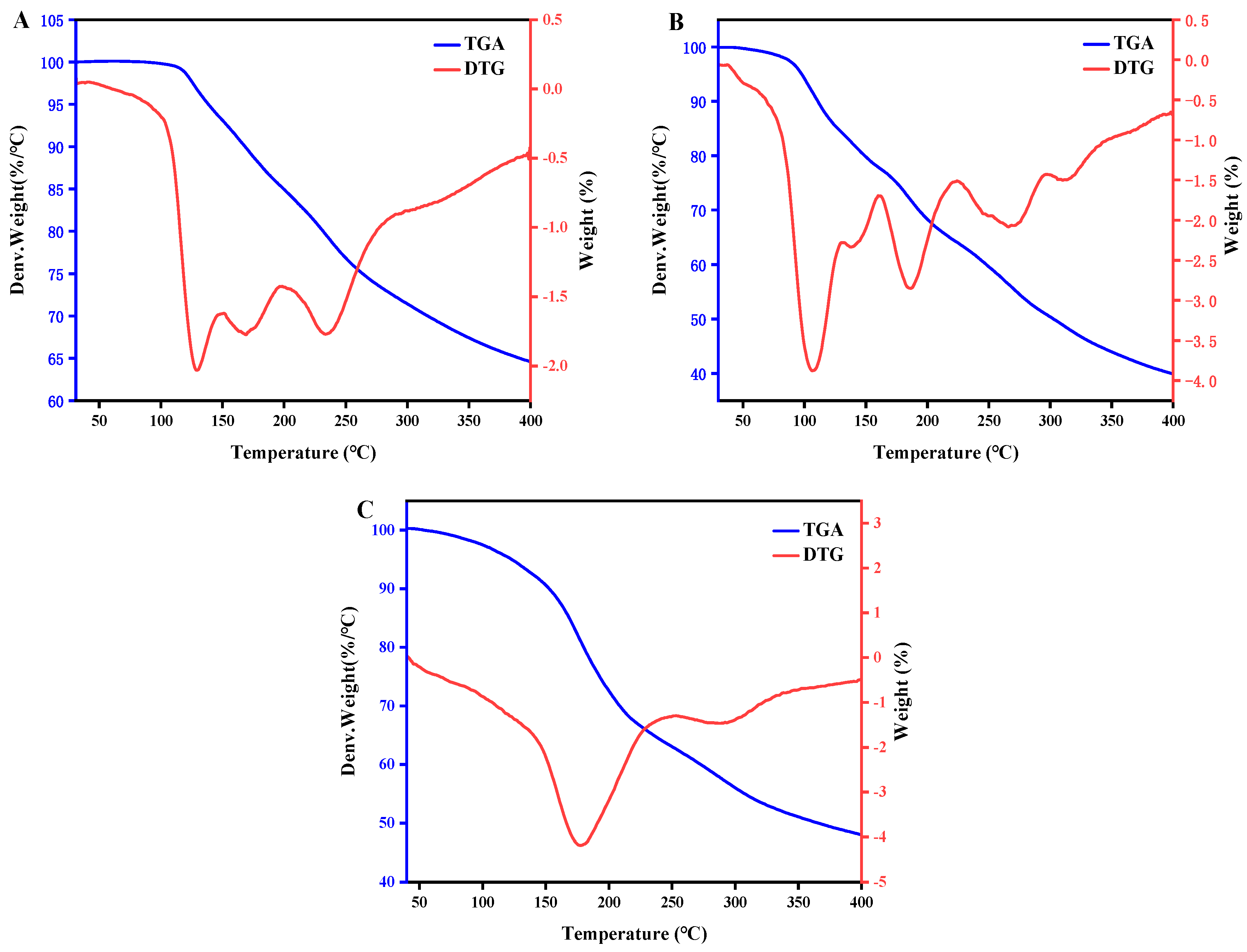
2.6. Thermogravimetric Analysis
2.7. IR Analysis
2.8. Congo Red Test
2.9. I2-KI Assay
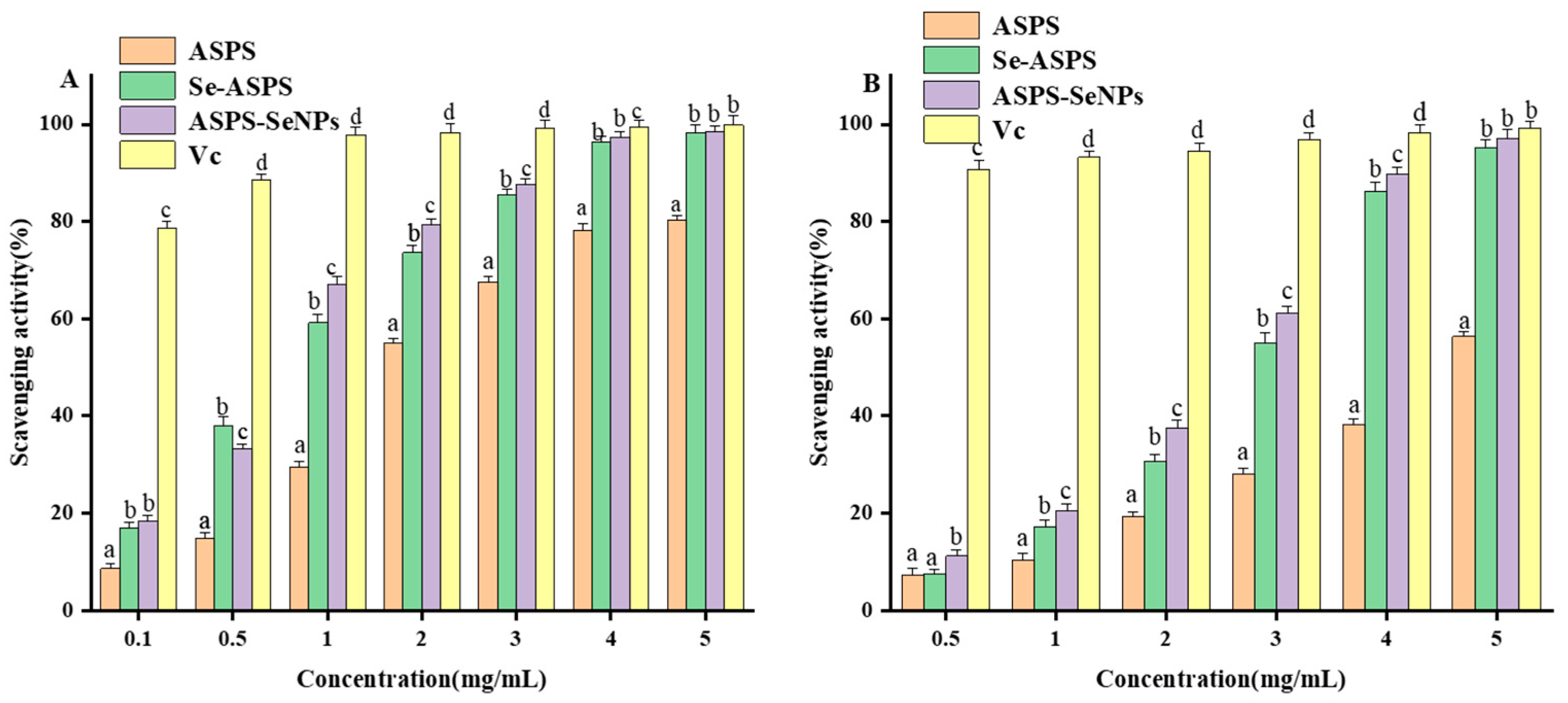
2.10. In Vitro Antioxidant Assay
3. Materials and Methods
3.1. Drugs and Chemical Reagents
3.2. Instruments and Equipment
3.3. Methods
3.3.1. Preparation of Crude Polysaccharides of A. senticosus
Extraction and Purification of ASPS
3.3.2. Preparation of ASPS-SENPS
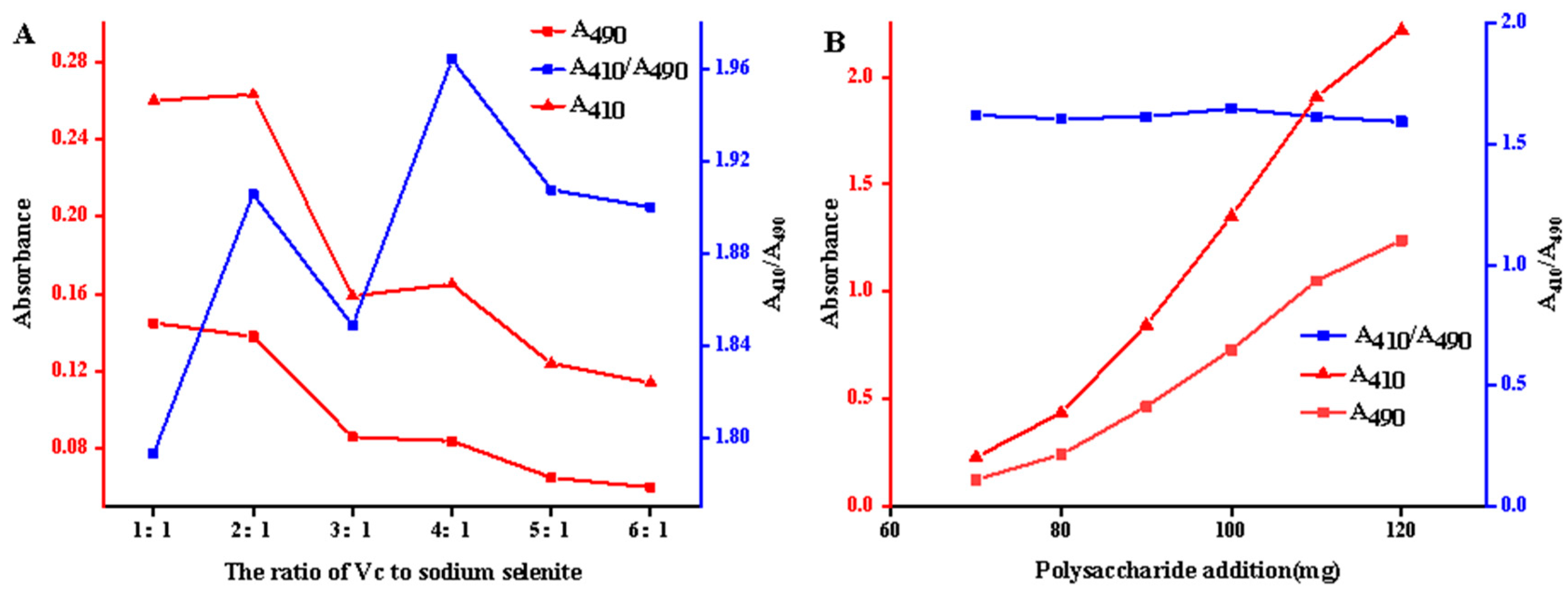
- Optimization of preparation conditions for ASPS-SENPS.
- Effect of ASPS addition
- Effect of reaction temperature
- Effect of reaction time
- Effect of Ascorbic acid–Na2SeO3
3.3.3. Preparation of Se-ASPS
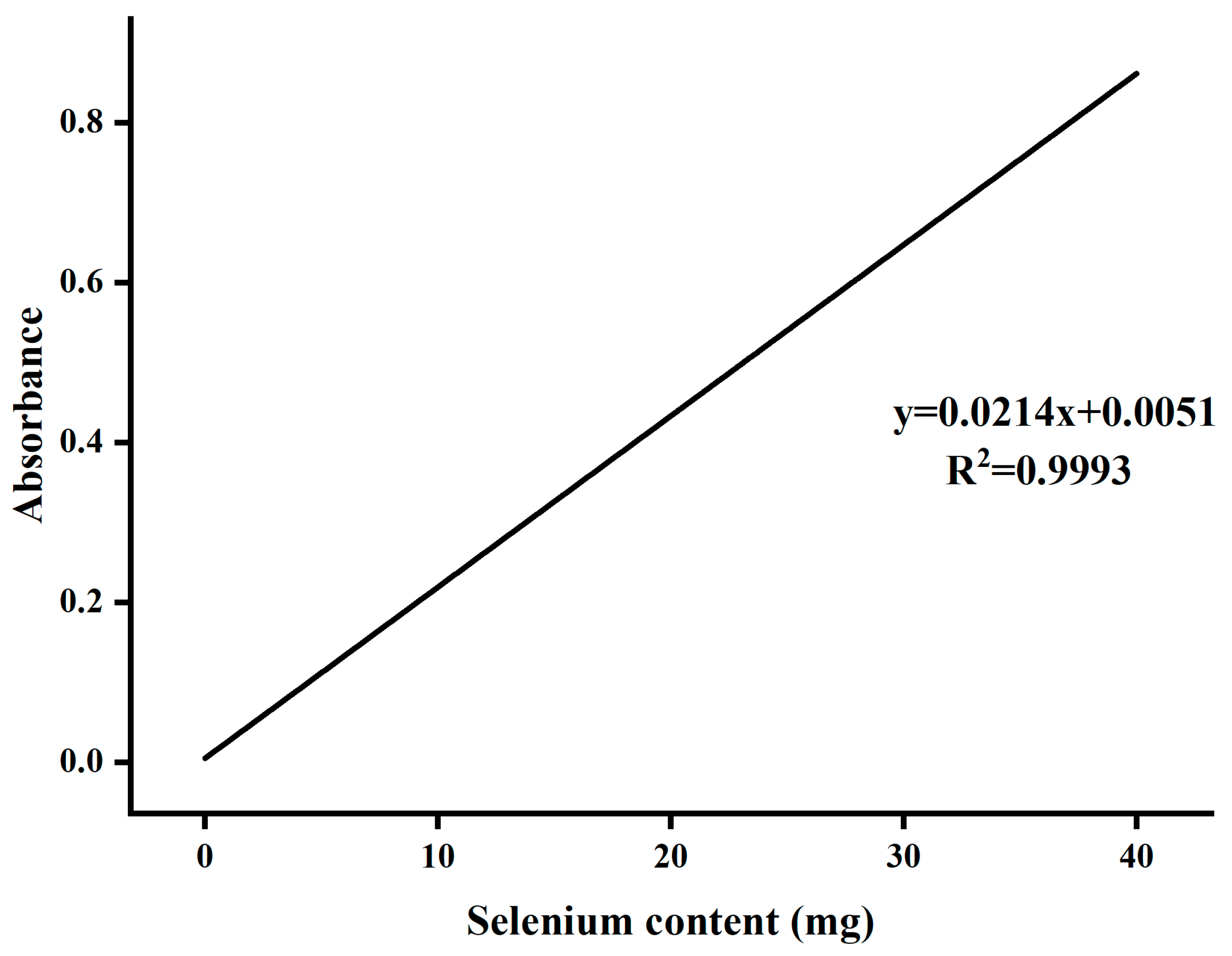
- Determination of Selenium Content
- Preparation of Se-ASPS
- Optimization of Preparation Technology of Selenium Polysaccharide of A. senticosus
- Effect of reaction temperature
- Effect of reaction time
- Effect of sodium selenite dosage
- Orthogonal test of the preparation process of Se-ASPS
3.3.4. Chemical Composition Analysis
3.3.5. Ultraviolet–Visible Spectral Profiling
3.3.6. Characterization
- Scanning Electron Microscopy (SEM)
- Thermogravimetric Analysis (TGA)
- Analysis of Fourier Transform Infrared (FT-IR)
- Congo Red Test
- Potassium Iodide Test (I2-KI)
3.3.7. In Vitro Antioxidant Activity Test
- DPPH radical scavenging activity
- ABTS radical scavenging activity
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Bian, F.; Yue, L.; Jin, H.; Hong, Z.; Shu, G. Selenium-dependent antitumor immunomodulating activity of polysaccharides from roots of A. membranaceus. Int. J. Biol. Macromol. 2014, 69, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xie, L.; Song, A.; Zhang, C. Selenium Status and Its Antioxidant Role in Metabolic Diseases. Oxid. Med. Cell. Longev. 2022, 2022, 7009863. [Google Scholar] [CrossRef]
- MacFarquhar, J.K.; Broussard, D.L.; Melstrom, P.; Hutchinson, R.; Wolkin, A.; Martin, C.; Burk, R.F.; Dunn, J.R.; Green, A.L.; Hammond, R.; et al. Acute selenium toxicity associated with a dietary supplement. Arch. Intern. Med. 2010, 170, 256–261. [Google Scholar] [CrossRef]
- Huang, S.; Yang, W.; Huang, G. Preparation and activities of selenium polysaccharide from plant such as Grifola frondosa. Carbohydr. Polym. 2020, 242, 116409. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Chen, J.; Chen, X.; Fan, Q.; Zhang, C.; Wang, D.; Li, X.; Chen, X.; Chen, X.; Liu, C.; et al. Optimization of selenylation conditions for lycium barbarum polysaccharide based on antioxidant activity. Carbohydr. Polym. 2014, 103, 148–153. [Google Scholar] [CrossRef]
- Gao, P.; Bian, J.; Xu, S.; Liu, C.; Sun, Y.; Zhang, G.; Li, D.; Liu, X. Structural features, selenization modification, antioxidant and anti-tumor effects of polysaccharides from alfalfa roots. Int. J. Biol. Macromol. 2020, 149, 207–214. [Google Scholar] [CrossRef]
- Gao, Z.; Li, J.; Song, X.; Zhang, J.; Wang, X.; Jing, H.; Ren, Z.; Li, S.; Zhang, C.; Jia, L. Antioxidative, anti-inflammation and lung-protective effects of mycelia selenium polysaccharides from Oudemansiella radicata. Int. J. Biol. Macromol. 2017, 104 Pt A, 1158–1164. [Google Scholar] [CrossRef]
- Jiao, J.; Yu, J.; Ji, H.; Liu, A. Synthesis of macromolecular Astragalus polysaccharide-nano selenium complex and the inhibitory effects on HepG2 cells. Int. J. Biol. Macromol. 2022, 211, 481–489. [Google Scholar] [CrossRef]
- Stevanović, M.; Filipović, N.; Djurdjević, J.; Lukić, M.; Milenković, M.; Boccaccini, A. 45S5Bioglass®-based scaffolds coated with selenium nanoparticles or with poly(lactide-co-glycolide)/selenium particles: Processing, evaluation and antibacterial activity. Colloids Surf. B Biointerfaces 2015, 132, 208–215. [Google Scholar] [CrossRef]
- Ren, L.; Wu, Z.; Ma, Y.; Jian, W.; Xiong, H.; Zhou, L. Preparation and growth-promoting effect of selenium nanoparticles capped by polysaccharide-protein complexes on tilapia. J. Sci. Food Agric. 2021, 101, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Zhong, J.; Liu, J.; Yang, Y.; Li, M.; Yang, Y.; Xu, Y.; Wang, L. Preparation, characterization and bioactivities of selenized polysaccharides from Lonicera caerulea L. fruits. Int. J. Biol. Macromol. 2023, 225, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yan, H.; Ma, L.; Zhang, H.; Ren, D.F. Preparation and characterization of selenium nanoparticles decorated by Spirulina platensis polysaccharide. J. Food Biochem. 2020, 44, e13363. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Fu, Y.; Zhang, J.; Liu, J.; Liu, X.; Peng, Y.; Kyin, S.L.; Zhang, M.; Zhou, D. Preparation, characterization, and antioxidant and antiapoptotic activities of biosynthesized nano-selenium by yak-derived Bacillus cereus and chitosan-encapsulated chemically synthesized nano-selenium. Int. J. Biol. Macromol. 2023, 242 Pt 1, 124708. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Liu, X.; Cheng, Y.; Wang, Y.; Wang, F. Selenium-enriched Polysaccharide: An Effective and Safe Selenium Source of C57 Mice to Improve Growth Performance, Regulate Selenium Deposition, and Promote Antioxidant Capacity. Biol. Trace Elem. Res. 2022, 200, 2247–2258. [Google Scholar] [CrossRef]
- Zhou, N.; Long, H.; Wang, C.; Zhu, Z.; Yu, L.; Yang, W.; Ren, X.; Liu, X. Characterization of selenium-containing polysaccharide from Spirulina platensis and its protective role against Cd-induced toxicity. Int. J. Biol. Macromol. 2020, 164, 2465–2476. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Liu, H.; Wang, D.; Wang, J.; Liu, M.; Yang, Y.; Zhong, S. A natural selenium polysaccharide from Pleurotus ostreatus: Structural elucidation, anti-gastric cancer and anti-colon cancer activity in vitro. Int. J. Biol. Macromol. 2022, 201, 630–640. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; You, L.; Fu, X.; Liu, R.H. Fractionation, preliminary structural characterization and bioactivities of polysaccharides from Sargassum pallidum. Carbohydr. Polym. 2017, 155, 261–270. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhao, L.; Cha, Q.; Sun, Y.; Ye, H.; Zeng, X. Structural Characterization and Immunostimulatory Activity of a Homogeneous Polysaccharide from Sinonovacula constricta. J. Agric. Food Chem. 2015, 63, 7986–7994. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Huang, Q.; Zheng, Z.; Guan, H.; Liu, S. Construction of a Cordyceps sinensis exopolysaccharide-conjugated selenium nanoparticles and enhancement of their antioxidant activities. Int. J. Biol. Macromol. 2017, 99, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Song, Z.; Shi, L.; Zhou, L.; Zhang, J.; Cui, J.; Li, Y.; Jin, D.Q.; Ohizumi, Y.; Xu, J.; et al. A dandelion polysaccharide and its selenium nanoparticles: Structure features and evaluation of anti-tumor activity in zebrafish models. Carbohydr. Polym. 2021, 270, 118365. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wei, C. Morphology, structure, properties and applications of starch ghost: A review. Int. J. Biol. Macromol. 2020, 163, 2084–2096. [Google Scholar] [CrossRef]
- Li, H.; Yu, W.; Dhital, S.; Gidley, M.J.; Gilbert, R.G. Starch branching enzymes contributing to amylose and amylopectin fine structure in wheat. Carbohydr. Polym. 2019, 224, 115185. [Google Scholar] [CrossRef] [PubMed]
- Kungel, P.; Correa, V.G.; Corrêa, R.C.G.; Peralta, R.A.; Soković, M.; Calhelha, R.C.; Bracht, A.; Ferreira, I.; Peralta, R.M. Antioxidant and antimicrobial activities of a purified polysaccharide from yerba mate (Ilex paraguariensis). Int. J. Biol. Macromol. 2018, 114, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Chen, T.; Yan, M.; Zhao, W.; Li, F.; Cheng, W.; Yuan, L. Synthesis, characterization, antioxidant activity and neuroprotective effects of selenium polysaccharide from Radix hedysari. Carbohydr. Polym. 2015, 125, 161–168. [Google Scholar] [CrossRef]
- Liu, J.; Wu, D.; Leng, Y.; Li, Y.; Li, N. Dietary supplementation with selenium polysaccharide from selenium-enriched Phellinus linteus improves antioxidant capacity, immunity and production performance of laying hens. J. Trace Elem. Med. Biol. 2023, 77, 127140. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Liu, F.; Gao, H.; Sun, H.; Meng, M.; Zhang, Y.M. Synthesis, characterization and antioxidant activity of selenium polysaccharide from Cordyceps militaris. Int. J. Biol. Macromol. 2016, 93 Pt A, 1090–1099. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.; Bao, A.; Liu, X.; Zeng, J.; Yang, X.; Yao, J.; Zhang, J.; Lei, Z. Synthesis of selenium-containing Artemisia sphaerocephala polysaccharides: Solution conformation and anti-tumor activities in vitro. Carbohydr. Polym. 2016, 152, 70–78. [Google Scholar] [CrossRef]
- Wang, S.; Wu, H.; Zhang, X.; Luo, S.; Zhou, S.; Fan, H.; Lv, C. Preparation of nano-selenium from chestnut polysaccharide and characterization of its antioxidant activity. Front. Nutr. 2022, 9, 1054601. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef]
- Cao, M.X.; Xie, X.D.; Wang, X.R.; Hu, W.Y.; Zhao, Y.; Chen, Q.; Ji, L.; Wei, Y.Y.; Yu, M.L.; Hu, T.J. Separation, Purification, Structure Analysis, In Vitro Antioxidant Activity and circRNA-miRNA-mRNA Regulatory Network on PRV-Infected RAW264.7 Cells of a Polysaccharide Derived from Arthrospira platensis. Antioxidants 2021, 10, 1689. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Chen, J.; Yue, C.; Li, X.; Liu, J.; Gao, Z.; Liu, C.; Lu, Y.; Wang, D.; Li, H.; et al. Modification of lily polysaccharide by selenylation and the immune-enhancing activity. Carbohydr. Polym. 2016, 142, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Qu, Z.; Ji, C.; Yang, X.; Zhang, Y.; Zou, X. Optimum Reaction Conditions for the Synthesis of Selenized Ornithogalum caudatum Ait. (Liliaceae) Polysaccharides and Measurement of Their Antioxidant Activity In Vivo. Molecules 2023, 28, 5929. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.K.; Qiu, W.Y.; Wang, Y.Y.; Wang, W.H.; Yang, Y.; Zhang, H.N. Fabrication and stabilization of biocompatible selenium nanoparticles by carboxylic curdlans with various molecular properties. Carbohydr. Polym. 2018, 179, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Sun, K.; Wang, X.; Wang, D.; Wan, X.; Zhang, J. Protonation of epigallocatechin-3-gallate (EGCG) results in massive aggregation and reduced oral bioavailability of EGCG-dispersed selenium nanoparticles. J. Agric. Food Chem. 2013, 61, 7268–7275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, H.; Bao, Y.; Zhang, L. Nano red elemental selenium has no size effect in the induction of seleno-enzymes in both cultured cells and mice. Life Sci. 2004, 75, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.L.; Dhanya, B.S.; Sukriti; Rani, V.; Thakur, M.; Jeslin, J.; Kushwaha, R. Carbohydrate and protein based biopolymeric nanoparticles: Current status and biotechnological applications. Int. J. Biol. Macromol. 2020, 154, 390–412. [Google Scholar] [CrossRef]
- Yuan, Q.; Yuan, Y.; Zheng, Y.; Sheng, R.; Liu, L.; Xie, F.; Tan, J. Anti-cerebral ischemia reperfusion injury of polysaccharides: A review of the mechanisms. Biomed. Pharmacother. 2021, 137, 111303. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, S.; Liu, Y.; Wu, W.; Shen, Y.; Zhang, L.; Li, C.; Chen, H.; Liu, A.; Shen, L.; et al. Synthesis and antidiabetic activity of selenium nanoparticles in the presence of polysaccharides from Catathelasma ventricosum. Int. J. Biol. Macromol. 2018, 114, 632–639. [Google Scholar] [CrossRef]
- Dong, Z.; Dong, G.; Lai, F.; Wu, H.; Zhan, Q. Purification and comparative study of bioactivities of a natural selenized polysaccharide from Ganoderma Lucidum mycelia. Int. J. Biol. Macromol. 2021, 190, 101–112. [Google Scholar] [CrossRef]
- Gu, Y.; Qiu, Y.; Wei, X.; Li, Z.; Hu, Z.; Gu, Y.; Zhao, Y.; Wang, Y.; Yue, T.; Yuan, Y. Characterization of selenium-containing polysaccharides isolated from selenium-enriched tea and its bioactivities. Food Chem. 2020, 316, 126371. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, S.; Ding, X.; Yue, D.; Bian, J.; Zhang, X.; Zhang, G.; Gao, P. Structural characteristics of Medicago sativa L. Polysaccharides and Se-modified polysaccharides as well as their antioxidant and neuroprotective activities. Int. J. Biol. Macromol. 2020, 147, 1099–1106. [Google Scholar] [CrossRef] [PubMed]




| Na2SeO3 (mg) | Temperature (°C) | Time (h) | Selenium Content (mg/g) | Carbohydrate Content (%) | |
|---|---|---|---|---|---|
| sASPS1 | 600 | 60 | 9 | 145.78 | 69.53 |
| sASPS2 | 400 | 50 | 11 | 140.92 | 61.21 |
| sASPS3 | 600 | 40 | 11 | 138.3 | 58.01 |
| sASPS4 | 400 | 60 | 10 | 110.63 | 61.21 |
| sASPS5 | 500 | 60 | 11 | 121.48 | 75.61 |
| sASPS6 | 600 | 50 | 10 | 99.98 | 72.09 |
| sASPS7 | 500 | 50 | 9 | 91.76 | 80.73 |
| sASPS8 | 500 | 40 | 10 | 107.83 | 73.69 |
| sASPS9 | 400 | 40 | 9 | 114.56 | 80.41 |
| Item | Selenium Content (mg/g) | Protein Content (%) | Glucuronic Acid Content (%) | Polysaccharide Content (mg/g) | Reducing Sugar Content (%) |
|---|---|---|---|---|---|
| Se-ASPS | 145.78 ± 3.20 | 1.60 ± 0.08 | 2.31 ± 0.49 | 17.12 ± 1.01 | 49.36 ± 1.51 |
| ASPS | 5.34 ± 0.12 | 4.30 ± 0.51 | 3.04 ± 0.16 | 11.47 ± 1.82 | 13.60 ± 1.82 |
| ASPS-SENPS | 21.92 ± 2.01 | 7.91 ± 1.15 | 3.50 ± 1.04 | 14.29 ± 1.38 | 42.13 ± 0.53 |
| Level | Dosage of Sodium Selenite (mg) | Temperature of the Reaction (°C) | Duration of the Reaction (h) |
|---|---|---|---|
| 1 | 400 | 40 | 9 |
| 2 | 500 | 50 | 10 |
| 3 | 600 | 60 | 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Li, Y.; Wang, X.; Zhang, R.; Xue, J.; Ding, Y.; Chu, X.; Su, J. Preparation, Characterization, and Bioactivities of Polysaccharide–Nano-Selenium and Selenized Polysaccharides from Acanthopanax senticosus. Molecules 2024, 29, 1418. https://doi.org/10.3390/molecules29071418
Li X, Li Y, Wang X, Zhang R, Xue J, Ding Y, Chu X, Su J. Preparation, Characterization, and Bioactivities of Polysaccharide–Nano-Selenium and Selenized Polysaccharides from Acanthopanax senticosus. Molecules. 2024; 29(7):1418. https://doi.org/10.3390/molecules29071418
Chicago/Turabian StyleLi, Xiaoli, Ying Li, Xueyan Wang, Rui Zhang, Jiaojiao Xue, Yi Ding, Xiuling Chu, and Jianqing Su. 2024. "Preparation, Characterization, and Bioactivities of Polysaccharide–Nano-Selenium and Selenized Polysaccharides from Acanthopanax senticosus" Molecules 29, no. 7: 1418. https://doi.org/10.3390/molecules29071418
APA StyleLi, X., Li, Y., Wang, X., Zhang, R., Xue, J., Ding, Y., Chu, X., & Su, J. (2024). Preparation, Characterization, and Bioactivities of Polysaccharide–Nano-Selenium and Selenized Polysaccharides from Acanthopanax senticosus. Molecules, 29(7), 1418. https://doi.org/10.3390/molecules29071418






