Antibacterial and Anti-Inflammatory Polysaccharide from Fructus Ligustri Lucidi Incorporated in PVA/Pectin Hydrogels Accelerate Wound Healing
Abstract
1. Introduction
2. Results
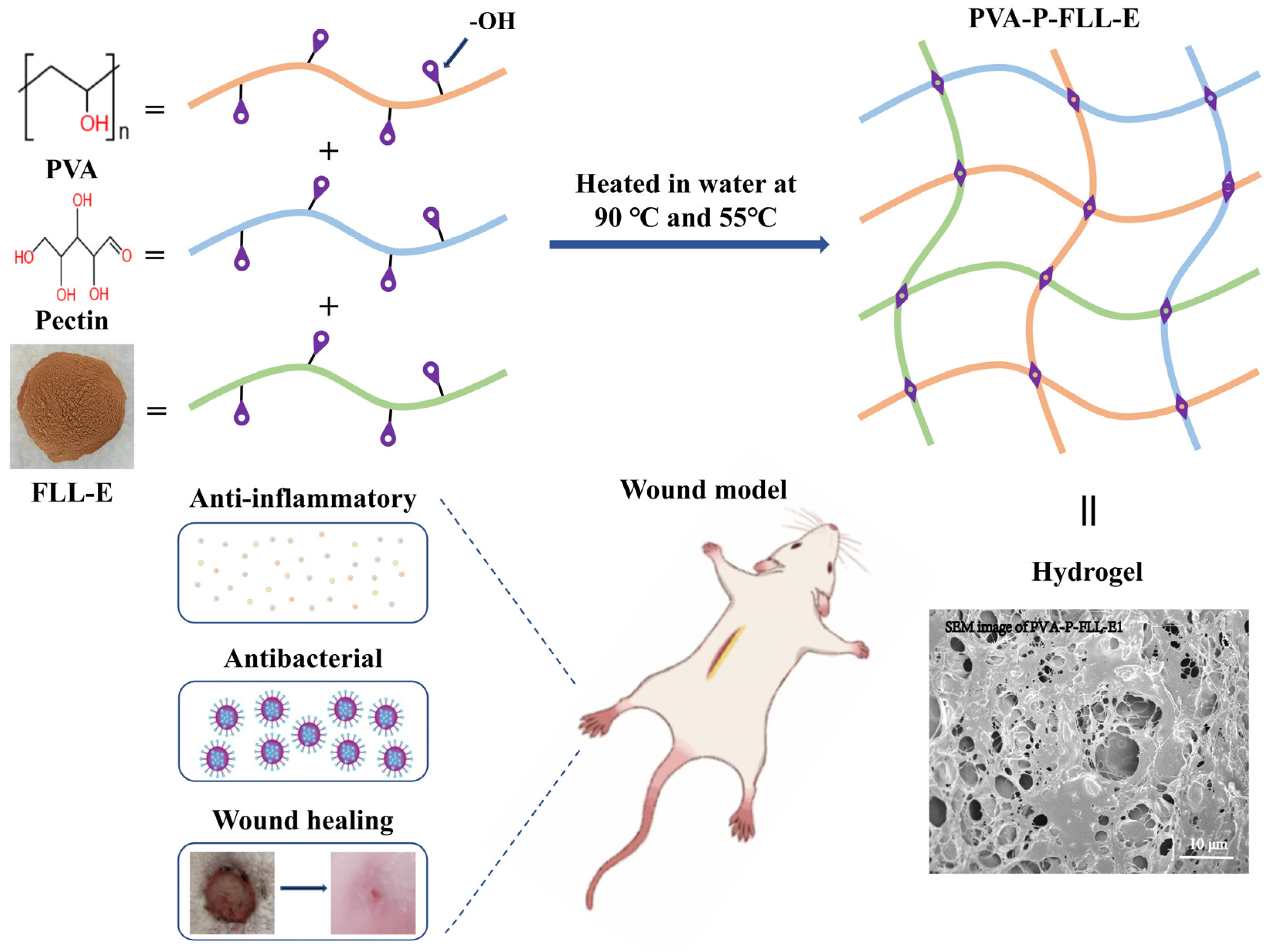
2.1. Evaluation of FLL-E and PVA-P Hydrogel
2.2. Evaluation of the PVA-P-FLL-E Hydrogels
2.3. Antimicrobial Analysis
2.4. PVA-P-FLL-E1 Hydrogels Promoted Skin Wound Healing
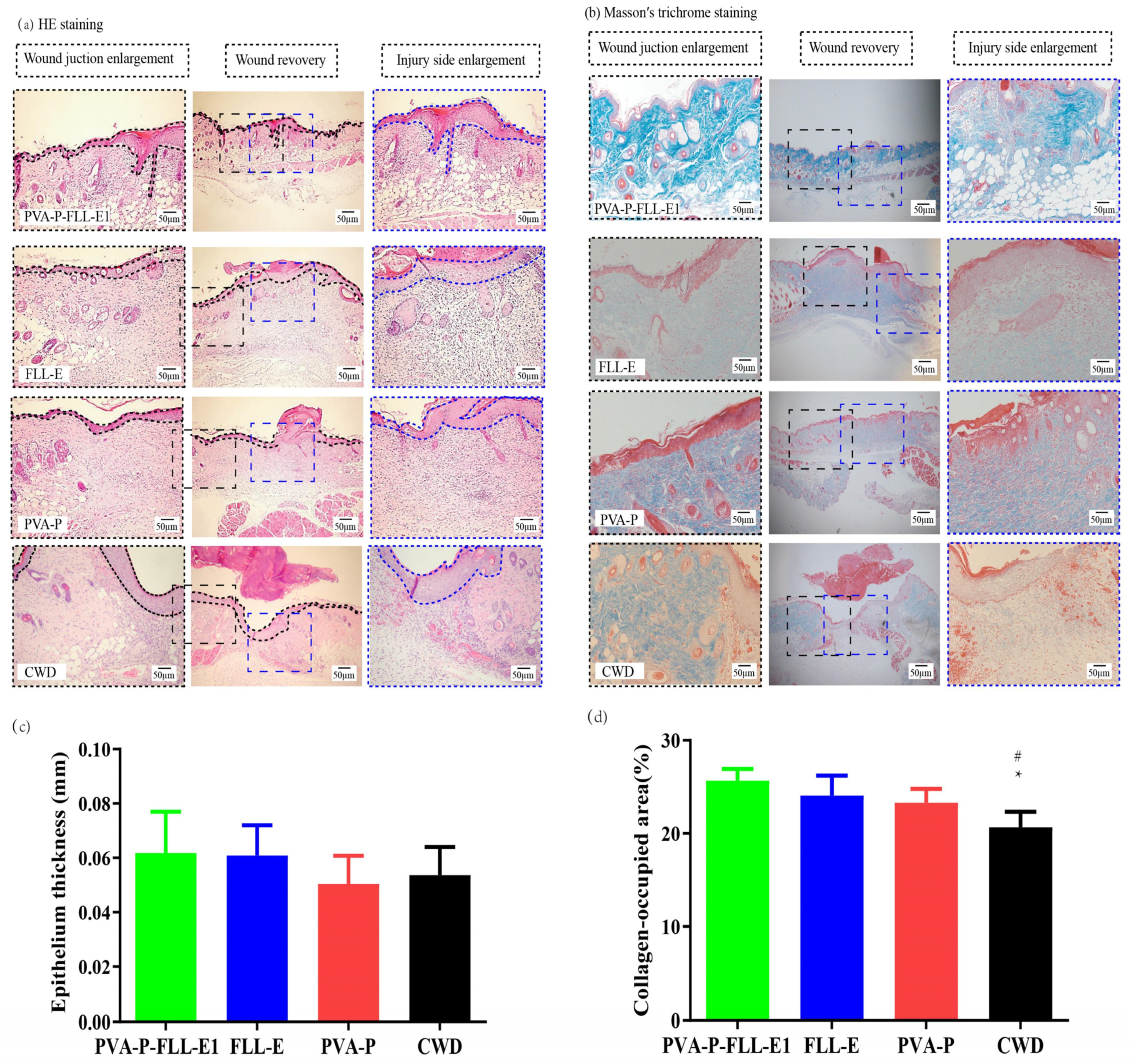
2.5. PVA-P-FLL-E1 Hydrogels Promoted Wound Construction
2.6. PVA-P-FLL-E1 Hydrogels Enhanced the Collagen Deposition
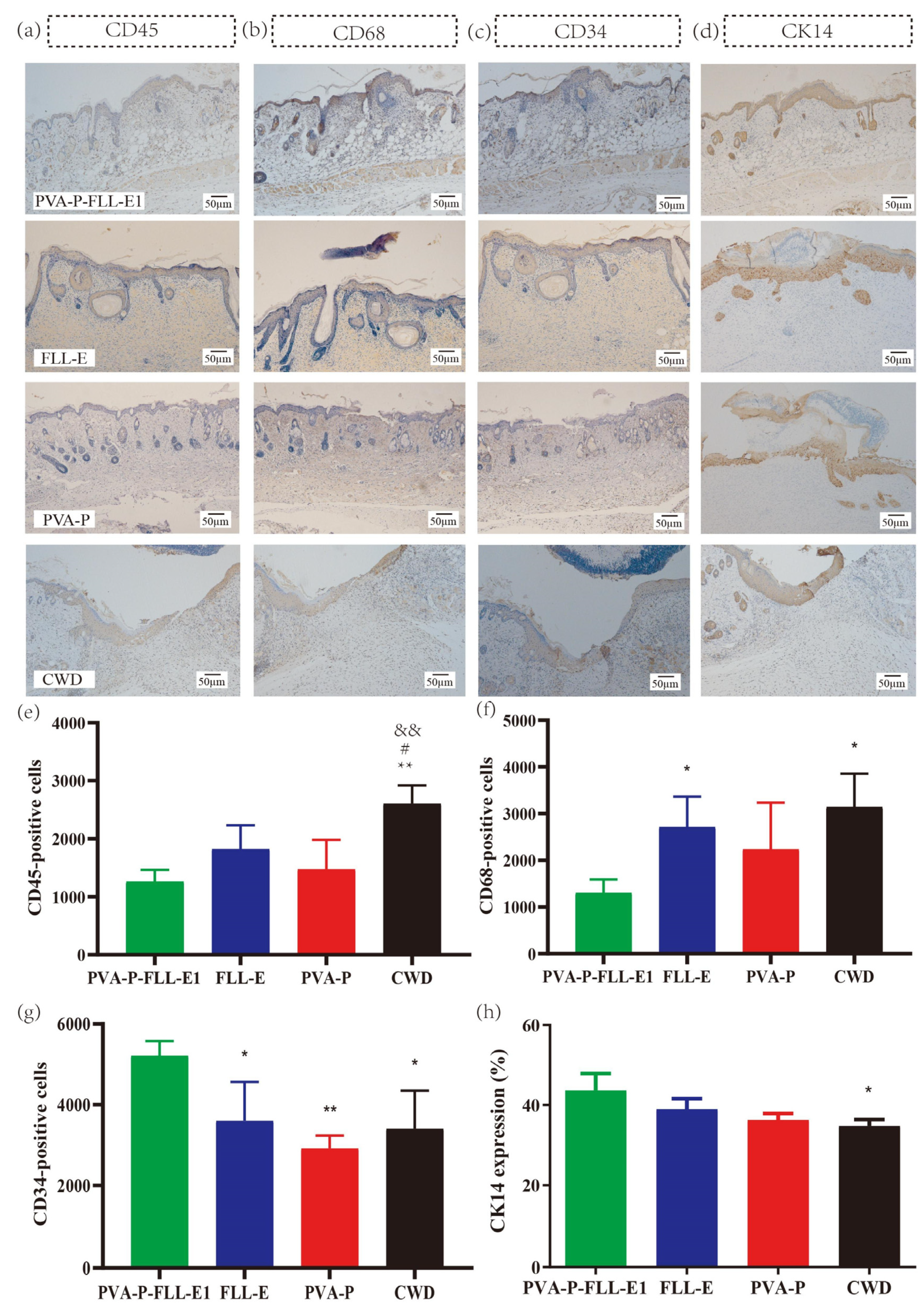
2.7. PVA-P-FLL-E1 Improved the Immune Microenvironment
2.8. PVA-P-FLL-E1 Hydrogels Regulated the Re-Epithelialization Process
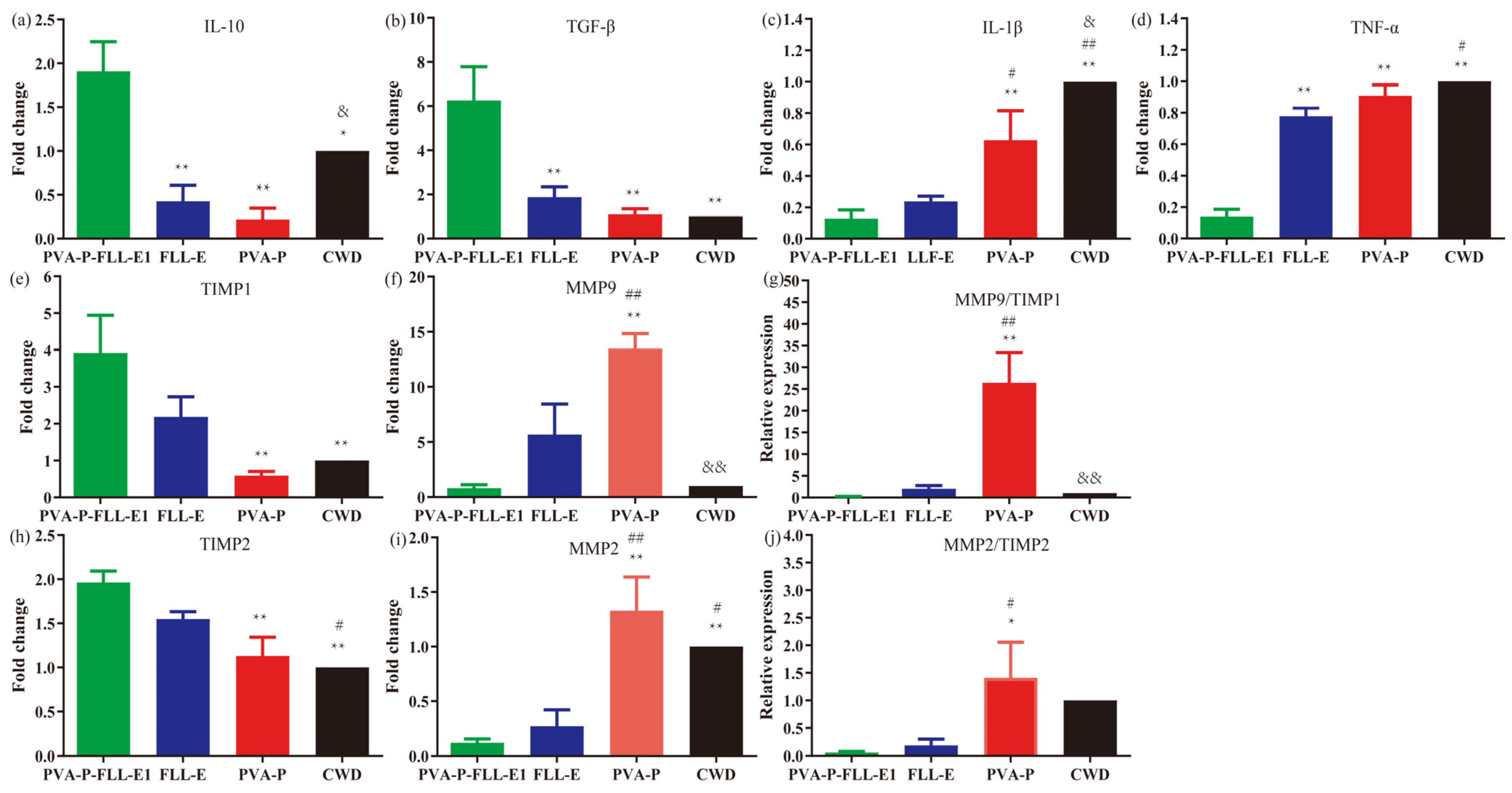
2.9. PVA-P-FLL-E1 Hydrogels Were Involved in Decreasing the Wound Inflammatory Response
2.10. PVA-P-FLL-E1 Hydrogels Promoted ECM Remodeling
3. Materials and Methods
3.1. Chemicals
3.2. Collection and Preparation of Active Ingredients
3.3. Preparation of PVA-P Hydrogels
3.4. Preparation of PVA-P-FLL-E Hydrogels
3.5. Characterization of the Hydrogels
3.6. Drug Release Measurement
3.7. Antimicrobial Analysis
3.8. In Vivo Assay
3.8.1. Animals
3.8.2. Experimental Protocol
3.8.3. Histopathological Studies
3.8.4. Immunohistochemistry (IHC) Evaluation
3.8.5. Analysis of mRNA Expression by qRT-PCR
3.8.6. Statistical Analysis
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kim, J.O.; Choi, J.Y.; Park, J.K.; Kim, J.H.; Jin, S.G.; Chang, S.W.; Li, D.X.; Hwang, M.-R.; Woo, J.S.; Kim, J.-A.; et al. Development of clindamycin-loaded wound dressing with polyvinyl alcohol and sodium alginate. Biol. Pharm. Bull. 2008, 31, 2277–2282. [Google Scholar] [CrossRef] [PubMed]
- Leng, Q.; Li, Y.; Pang, X.; Wang, B.; Wu, Z.; Lu, Y.; Xiong, K.; Zhao, L.; Zhou, P.; Fu, S. Curcumin nanoparticles incorporated in PVA/collagen composite films promote wound healing. Drug Deliv. 2020, 27, 1676–1685. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Chang, L.; Chang, R.; Liu, X.; Ma, X.; Chen, D.; Wang, Y.; Li, W.; Qin, J. Self-healing hydrogel based on polyphosphate-conjugated pectin with hemostatic property for wound healing applications. Biomater. Adv. 2022, 139, 212974. [Google Scholar] [CrossRef] [PubMed]
- Alsakhawy, M.A.; Abdelmonsif, D.A.; Haroun, M.; Sabra, S.A. Naringin-loaded Arabic gum/pectin hydrogel as a potential wound healing material. Int. J. Biol. Macromol. 2022, 222 Pt A, 701–714. [Google Scholar] [CrossRef]
- Riyamol; Gada Chengaiyan, J.; Rana, S.S.; Ahmad, F.; Haque, S.; Capanoglu, E. Recent Advances in the Extraction of Pectin from Various Sources and Industrial Applications. ACS Omega 2023, 8, 46309–46324. [Google Scholar] [CrossRef] [PubMed]
- Riccio, B.V.F.; Silvestre, A.L.P.; Meneguin, A.B.; Ribeiro, T.d.C.; Klosowski, A.B.; Ferrari, P.C.; Chorilli, M. Exploiting Polymeric Films as a Multipurpose Drug Delivery System: A Review. AAPS PharmSciTech 2022, 23, 269. [Google Scholar] [CrossRef]
- Jahani-Javanmardi, A.; Sirousazar, M.; Shaabani, Y.; Kheiri, F. Egg white/poly (vinyl alcohol)/MMT nanocomposite hydrogels for wound dressing. J. Biomater. Sci. Polym. Ed. 2016, 27, 1262–1276. [Google Scholar] [CrossRef]
- Chen, W.; Achazi, K.; Schade, B.; Haag, R. Charge-conversional and reduction-sensitive poly(vinyl alcohol) nanogels for enhanced cell uptake and efficient intracellular doxorubicin release. J. Control. Release 2015, 205, 15–24. [Google Scholar] [CrossRef]
- Irvin, C.W.; Satam, C.C.; Liao, J.; Russo, P.S.; Breedveld, V.; Meredith, J.C.; Shofner, M.L. Synergistic Reinforcement of Composite Hydrogels with Nanofiber Mixtures of Cellulose Nanocrystals and Chitin Nanofibers. Biomacromolecules 2020, 22, 340–352. [Google Scholar] [CrossRef]
- Anjum, S.; Arora, A.; Alam, M.S.; Gupta, B. Development of antimicrobial and scar preventive chitosan hydrogel wound dressings. Int. J. Pharm. 2016, 508, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.G. Production and Application of Biomaterials Based on Polyvinyl alcohol (PVA) as Wound Dressing. Chem. Asian J. 2022, 17, e202200595. [Google Scholar] [CrossRef]
- Lee, J.S.; Sun, K.H.; Park, Y. Evaluation of Melia azedarach extract-loaded poly (vinyl alcohol)/pectin hydrogel for burn wound healing. PLoS ONE 2022, 17, e0270281. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Cheng, J.; Xu, S.; Cheng, X.; Zhao, J.; Kenny Low, Z.W.; Chee, P.L.; Lu, Z.; Zheng, L.; Kai, D. PVA/pectin composite hydrogels inducing osteogenesis for bone regeneration. Mater. Today Bio 2022, 16, 100431. [Google Scholar] [CrossRef]
- Martinez, Y.N.; Piñuel, L.; Castro, G.R.; Breccia, J.D. Polyvinyl alcohol-pectin cryogel films for controlled release of enrofloxacin. Appl. Biochem. Biotechnol. 2012, 167, 1421–1429. [Google Scholar] [CrossRef]
- Fishman, M.L.; Coffin, D.R. Mechanical, microstructural and solubility properties of pectin/poly(vinyl alcohol) blends. Carbohydr. Polym. 1998, 35, 195–203. [Google Scholar] [CrossRef]
- Sen, S.; Bal, T.; Rajora, A.D. Green nanofiber mat from HLM-PVA-Pectin (Hibiscus leaves mucilage-polyvinyl alcohol-pectin) polymeric blend using electrospinning technique as a novel material in wound-healing process. Appl. Nanosci. 2022, 12, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Jo, C.; Kang, H.; Lee, N.Y.; Kwon, J.H.; Byun, M.W. Pectin-and gelatin-based film: Effect of gamma irradiation on the mechanical properties and biodegradation, Radiat. Phys. Chem. 2005, 72, 745–750. [Google Scholar] [CrossRef]
- Suhasini, M.R.; Rajeshwari, K.M.; Bindya, S.; Hemavathi, A.B.; Vishwanath, P.M.; Syed, A.; Eswaramoorthy, R.; Amachawadi, R.G.; Shivamallu, C.; Chattu, V.K.; et al. Pectin/PVA and pectin-MgO/PVA films: Preparation, characterization and biodegradation studies. Heliyon 2023, 9, e15792. [Google Scholar]
- Abdullah, Z.W.; Dong, Y. Biodegradable and water resistant poly(vinyl) alcohol (PVA)/starch (ST)/glycerol (GL)/halloysite nanotube (HNT) nanocomposite films for sustainable food packaging. Front. Mater. 2019, 6, 58. [Google Scholar] [CrossRef]
- Mao, L.; Imam, S.; Gordon, S.; Cinelli, P.; Chiellini, E. Extruded cornstarch-glycerol-polyvinyl alcohol blends: Mechanical properties, morphology, and biodegradability. J. Polym. Environ. 2000, 8, 205–211. [Google Scholar] [CrossRef]
- Cao, M.; Wu, J.; Peng, Y.; Dong, B.; Jiang, Y.; Hu, C.; Yu, L.; Chen, Z. Ligustri Lucidi Fructus, a traditional Chinese Medicine: Comprehensive review of botany, traditional uses, chemical composition, pharmacology, and toxicity. J. Ethnopharmacol. 2023, 301, 115789. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.Y.; Wang, G.J.; Wang, H.Y.; Yu, W.Y. Research progress on pharmacological action of Ligustrum lucidum. Shanghai J. Tradit. Chin. Med. 2017, 51, 106–108. [Google Scholar]
- Liu, M.H.; Zou, Z.R. Research progress on chemical constituents, pharmacological effects and pharmacokinetics of Ligustrum lucidum. J. Trop. Subtrop. Bot. 2022, 30, 446–460. [Google Scholar]
- Gao, S.; Zhou, X.; Chen, H.G. Research progress on chemical composition and quality control of Ligustrum Lucidum Fructus. Chin. J. Informat Tradit. Chin. Med. 2018, 25, 133–136. [Google Scholar]
- Li, L.; Chen, B.; Zhu, R.; Li, R.; Tian, Y.; Liu, C.; Jia, Q.; Wang, L.; Tang, J.; Zhao, D.; et al. Fructus Ligustri Lucidi preserves bone quality through the regulation of gut microbiota diversity, oxidative stress, TMAO and Sirt6 levels in aging mice. Aging 2019, 11, 9348–9368. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, G.; Liu, P.; Hu, Y.; Chen, Y.; Fang, Y.; Sun, G.; Huang, H.; Wu, J. Hyaluronic acid-based glucose-responsive antioxidant hydrogel platform for enhanced diabetic wound repair. Acta Biomater. 2022, 147, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, G.; Pathak, K. Porous carriers for controlled/modulated drug delivery. Indian J. Pharm. Sci. 2009, 71, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Qiao, Y.; Peng, Q.; Shi, B. Probiotic Properties of Loigolactobacillus coryniformis NA-3 and In Vitro Comparative Evaluation of Live and Heat-Killed Cells for Antioxidant, Anticancer and Immunoregulatory Activities. Foods 2023, 12, 1118. [Google Scholar] [CrossRef]
- Yun, L.; Li, D.; Yang, L.; Zhang, M. Hot water extraction and artificial simulated gastrointestinal digestion of wheat germ polysaccharide. Int. J. Biol. Macromol. 2019, 123, 174–181. [Google Scholar] [CrossRef]
- Tabara, A.; Oneda, H.; Murayama, R.; Matsui, Y.; Hirano, A.; Seguchi, M. Determination of hydrophobicity of dry-heated wheat starch granules using sucrose fatty acid esters (SFAE). Biosci. Biotechnol. Biochem. 2014, 78, 1572–1576. [Google Scholar] [CrossRef]
- Gupta, S.; Mujawdiya, P.; Maheshwari, G.; Sagar, S. Dynamic Role of Oxygen in Wound Healing: A Microbial, Immunological, and Biochemical Perspective. Arch. Razi Inst. 2022, 77, 513–523. [Google Scholar]
- Castellano, J.M.; Ramos-Romero, S.; Perona, J.S. Oleanolic Acid: Extraction, Characterization and Biological Activity. Nutrients 2022, 14, 623. [Google Scholar] [CrossRef]
- Pang, X.; Zhao, J.Y.; Yu, H.Y.; Yu, L.Y.; Wang, T.; Zhang, Y.; Gao, X.M.; Han, L.F. Secoiridoid analogues from the fruits of Ligustrum lucidum and their inhibitory activities against influenza A virus. Bioorg Med. Chem. Lett. 2018, 28, 1516–1519. [Google Scholar] [CrossRef]
- Žiberna, L.; Šamec, D.; Mocan, A.; Nabavi, S.F.; Bishayee, A.; Farooqi, A.A.; Sureda, A.; Nabavi, S.M. Oleanolic Acid Alters Multiple Cell Signaling Pathways: Implication in Cancer Prevention and Therapy. Int. J. Mol. Sci. 2017, 18, 643. [Google Scholar] [CrossRef]
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Punia, S.; Sharma, A.K. Ursolic Acid and Oleanolic Acid: Pentacyclic Terpenoids with Promising Anti-Inflammatory Activities. Recent Pat. Inflamm. Allergy Drug Discov. 2016, 10, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.T.; Shi, S.C.; Chen, C.K. Sandwich-Structured, Hydrophobic, Nanocellulose-Reinforced Polyvinyl Alcohol as an Alternative Straw Material. Polymers 2021, 13, 4447. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.M.; Nguyen, T.H.; Dang, T.M.; Tran, T.X.; Rachtanapun, P. Characteristics and Antimicrobial Properties of Active Edible Films Based on Pectin and Nanochitosan. Int. J. Mol. Sci. 2020, 21, 2224. [Google Scholar] [CrossRef]
- Fathollahipour, S.; Koosha, M.; Tavakoli, J.; Maziarfar, S.; Mehrabadi, J.F. Erythromycin Releasing PVA/sucrose and PVA/honey Hydrogels as Wound Dressings with Antibacterial Activity and Enhanced Bio-adhesion. Iran. J. Pharm. Res. 2020, 19, 448–464. [Google Scholar] [PubMed]
- Shanmugapriya, K.; Kim, H.; Saravana, P.S.; Chun, B.S.; Kang, H.W. Fabrication of multifunctional chitosan-based nanocomposite film with rapid healing and antibacterial effect for wound management. Int. J. Biol. Macromol. 2018, 118, 1713–1725. [Google Scholar] [CrossRef]
- Low, Z.W.K.; Luo, Y.; Zhang, K.; Lin, Q.; Owh, C.; Chen, X.; Loh, X.J. Tough hydrogel module towards an implantable remote and controlled release device. Biomater. Sci. 2020, 8, 960–972. [Google Scholar] [CrossRef] [PubMed]
- Zoratto, N.; Matricardi, P. Semi-IPN- and IPN-Based Hydrogels. Adv. Exp. Med. Biol. 2018, 1059, 155–188. [Google Scholar] [PubMed]
- Hwang, M.R.; Kim, J.O.; Lee, J.H.; Kim, Y.I.; Kim, J.H.; Chang, S.W.; Jin, S.G.; Kim, J.A.; Lyoo, W.S.; Han, S.S.; et al. Gentamicin-loaded wound dressing with polyvinyl alcohol/dextran hydrogel: Gel characterization and in vivo healing evaluation. AAPS PharmSciTech 2010, 11, 1092–1103. [Google Scholar] [CrossRef] [PubMed]
- Katsuda, S.; Okada, Y.; Minamoto, T.; Oda, Y.; Matsui, Y.; Nakanishi, I. Collagens in human atherosclerosis. Immunohistochemical analysis using collagen type-specific antibodies. Arterioscler. Thromb. 1992, 12, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Dab, H.; Kacem, K.; Hachani, R.; Dhaouadi, N.; Hodroj, W.; Sakly, M.; Randon, J.; Bricca, G. Physiological regulation of extracellular matrix collagen and elastin in the arterial wall of rats by noradrenergic tone and angiotensin II. J. Renin Angiotensin Aldosterone Syst. 2012, 13, 19–28. [Google Scholar] [CrossRef]
- Singh, D.; Rai, V.; Agrawal, D.K. Regulation of Collagen I and Collagen III in Tissue Injury and Regeneration. Cardiol. Cardiovasc. Med. 2023, 7, 5–16. [Google Scholar] [CrossRef]
- Mukherjee, A.; Wagner, W.L.; Zheng, Y.; Pierce, A.; Ackermann, M.; Horstmann, H.; Kuner, T.; Ronchi, P.; Schwab, Y.; Konietzke, P.; et al. Mesopolysaccharides: The extracellular surface layer of visceral organs. PLoS ONE 2020, 15, e0238798. [Google Scholar]
- Pierce, A.; Zheng, Y.; Wagner, W.L.; Scheller, H.V.; Mohnen, D.; Ackermann, M.; Mentzer, S.J. Visualizing pectin polymer-polymer entanglement produced by interfacial water movement. Carbohydr. Polym. 2020, 246, 116618. [Google Scholar] [CrossRef]
- Servais, A.B.; Kienzle, A.; Valenzuela, C.D.; Ysasi, A.B.; Wagner, W.L.; Tsuda, A.; Ackermann, M.; Mentzer, S.J. Structural Heteropolysaccharide Adhesion to the Glycocalyx of Visceral Mesothelium. Tissue Eng. Part A 2018, 24, 199–206. [Google Scholar] [CrossRef]
- Charron, P.N.; Braddish, T.A.; Oldinski, R.A. PVA-gelatin hydrogels formed using combined theta-gel and cryo-gel fabrication techniques. J. Mech. Behav. Biomed. Mater. 2019, 92, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Zarei, H.; Tamri, P.; Asl, S.S.; Soleimani, M.; Moradkhani, S. Hydroalcoholic Extract of Scrophularia striata Attenuates Hypertrophic Scar, Suppresses Collagen Synthesis, and Stimulates MMP2 and 9 Gene Expression in Rabbit Ear Model. J. Pharmacopunct. 2022, 25, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Cook, H.; Stephens, P.; Davies, K.J.; Thomas, D.W.; Harding, K.G. Defective extracellular matrix reorganization by chronic wound fibroblasts is associated with alterations in TIMP-1, TIMP-2, and MMP-2 activity. J. Investig. Dermatol. 2000, 115, 225–233. [Google Scholar] [CrossRef]
- Gibran, N.S.; Heimbach, D.M.; Holbrook, K.A. Immunolocalization of FXIIIa+ dendritic cells in human burn wounds. J. Surg. Res. 1995, 59, 378–386. [Google Scholar] [CrossRef]
- Balqis, U.; Darmawi; Iskandar, C.D.; Salim, M.N. Angiogenesis activity of Jatropha curcas L. latex in cream formulation on wound healing in mice. Vet. World 2018, 11, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, I.; Mizutani, H.; Kusumoto, K.; Nishijima, S.; Tsujita-Kyutoku, M.; Shikata, N.; Tsubura, A. Cytokeratin, filaggrin, and p63 expression in reepithelialization during human cutaneous wound healing. Wound Repair. Regen. 2006, 14, 38. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, Y.; Hu, L.; Chen, X.; Zuo, L.; Bai, X.; Du, W.; Xu, N. Antibacterial and Anti-Inflammatory Polysaccharide from Fructus Ligustri Lucidi Incorporated in PVA/Pectin Hydrogels Accelerate Wound Healing. Molecules 2024, 29, 1423. https://doi.org/10.3390/molecules29071423
Xi Y, Hu L, Chen X, Zuo L, Bai X, Du W, Xu N. Antibacterial and Anti-Inflammatory Polysaccharide from Fructus Ligustri Lucidi Incorporated in PVA/Pectin Hydrogels Accelerate Wound Healing. Molecules. 2024; 29(7):1423. https://doi.org/10.3390/molecules29071423
Chicago/Turabian StyleXi, Yanli, Lianxin Hu, Xiang Chen, Lili Zuo, Xuesong Bai, Weijie Du, and Na Xu. 2024. "Antibacterial and Anti-Inflammatory Polysaccharide from Fructus Ligustri Lucidi Incorporated in PVA/Pectin Hydrogels Accelerate Wound Healing" Molecules 29, no. 7: 1423. https://doi.org/10.3390/molecules29071423
APA StyleXi, Y., Hu, L., Chen, X., Zuo, L., Bai, X., Du, W., & Xu, N. (2024). Antibacterial and Anti-Inflammatory Polysaccharide from Fructus Ligustri Lucidi Incorporated in PVA/Pectin Hydrogels Accelerate Wound Healing. Molecules, 29(7), 1423. https://doi.org/10.3390/molecules29071423





