Regulating Effect of Exogenous α-Ketoglutarate on Ammonium Assimilation in Poplar
Abstract
:1. Introduction
2. Results
2.1. Effects of AKG on Poplar Growth under High Ammonium Conditions
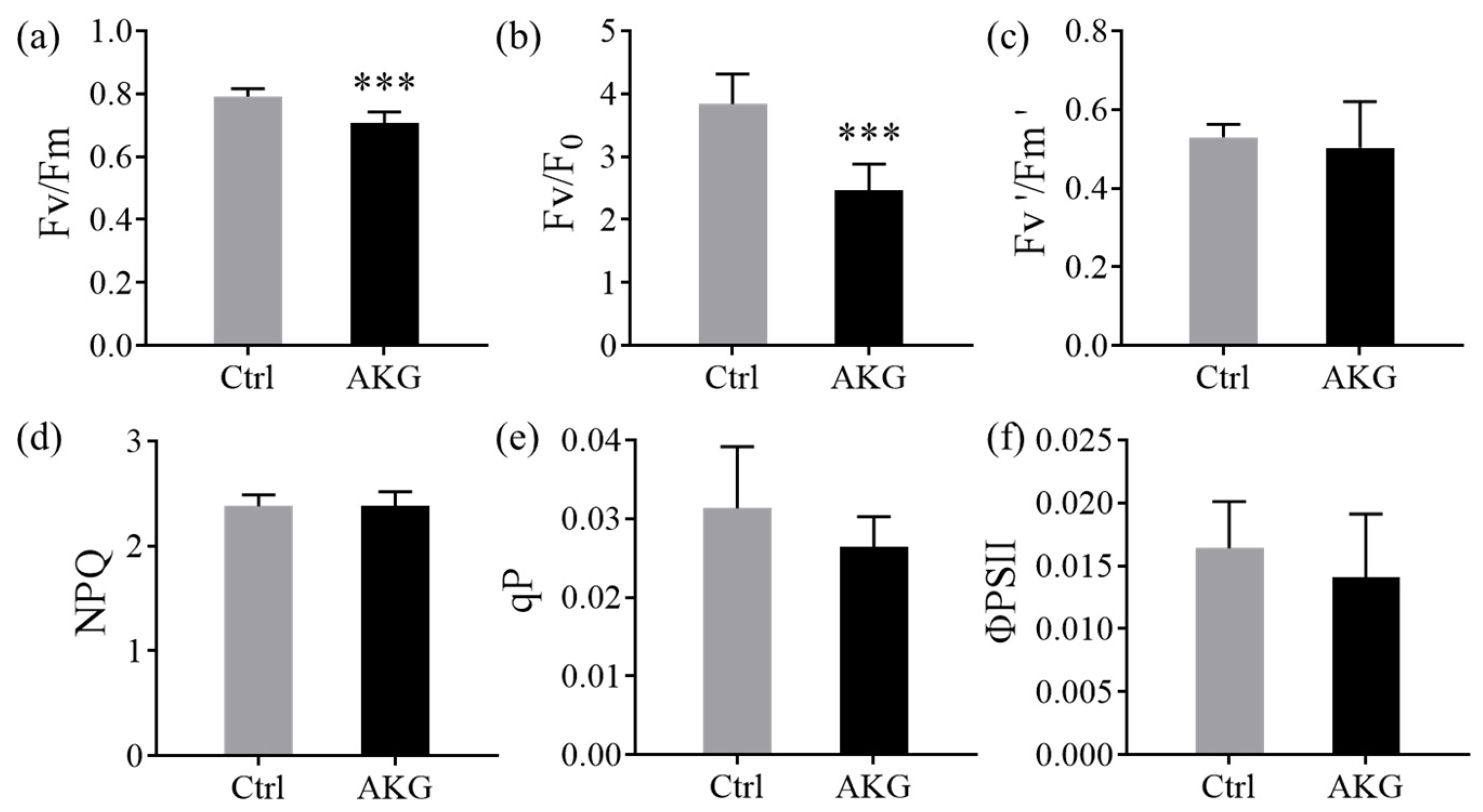
2.2. Effects of AKG on Photosynthetic and Chlorophyll Fluorescence Parameters of Poplars Exposed to High Levels of Ammonium
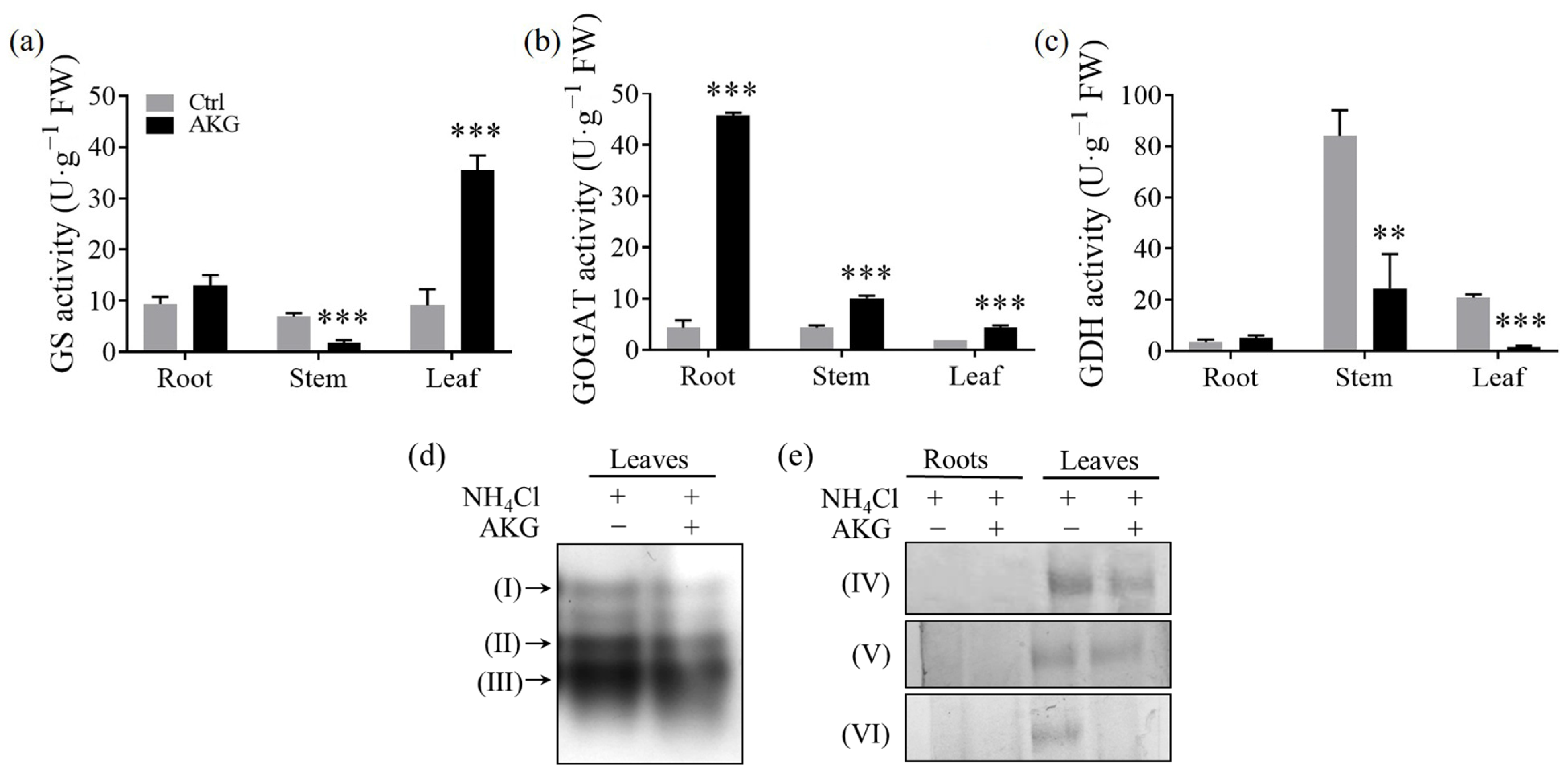
2.3. Effects of AKG on Enzymes Related to Carbon and Nitrogen Metabolism in Poplars Exposed to High Ammonium
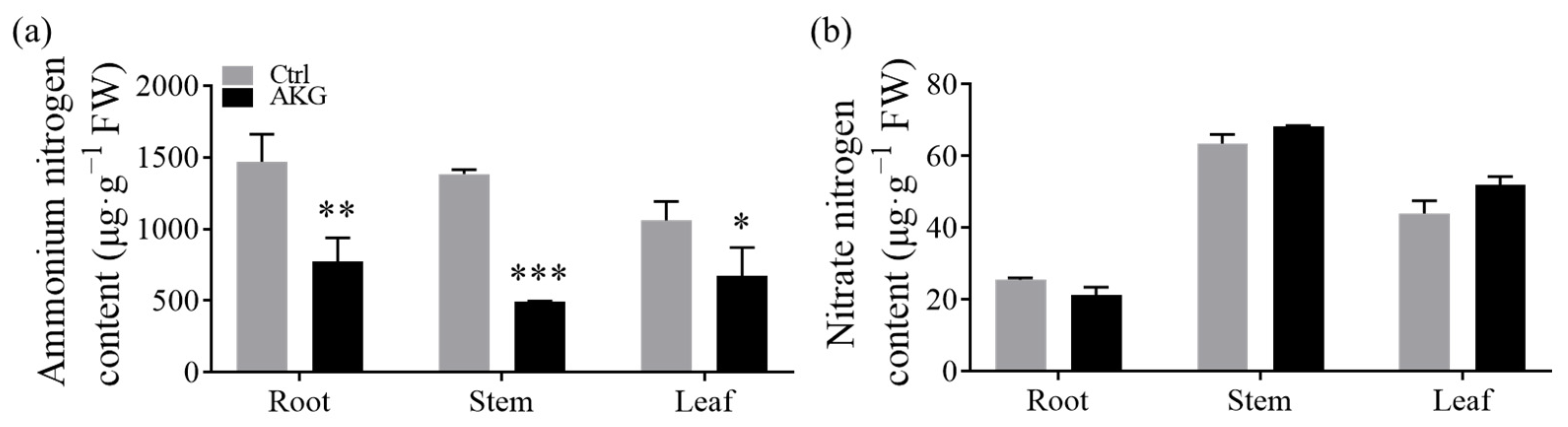
2.4. Effects of AKG on the Inorganic Nitrogen Content in Poplars under High Ammonium Conditions
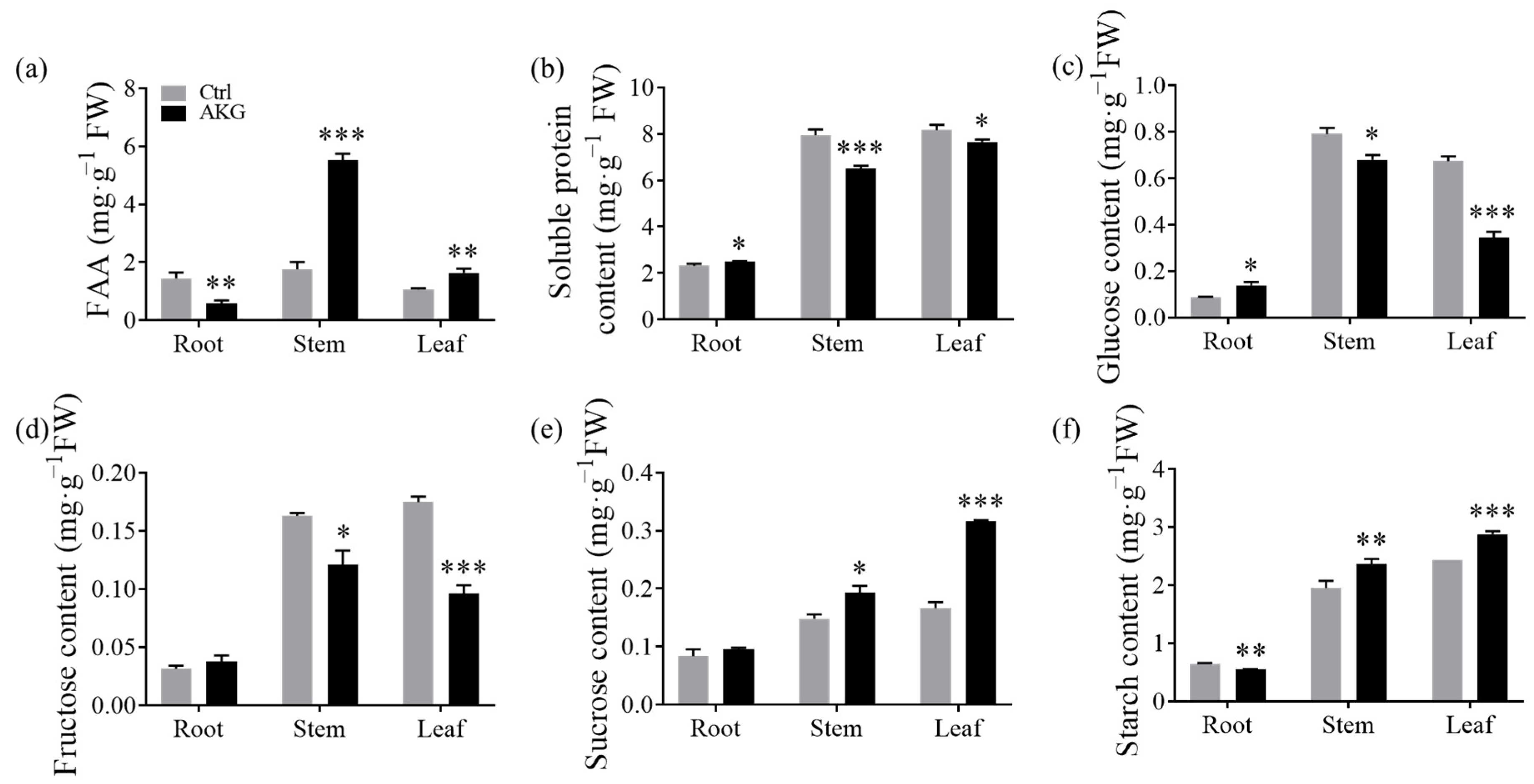
2.5. Impacts of AKG on Nitrogenous Compounds and Carbohydrates in Poplars with High Ammonium Exposure
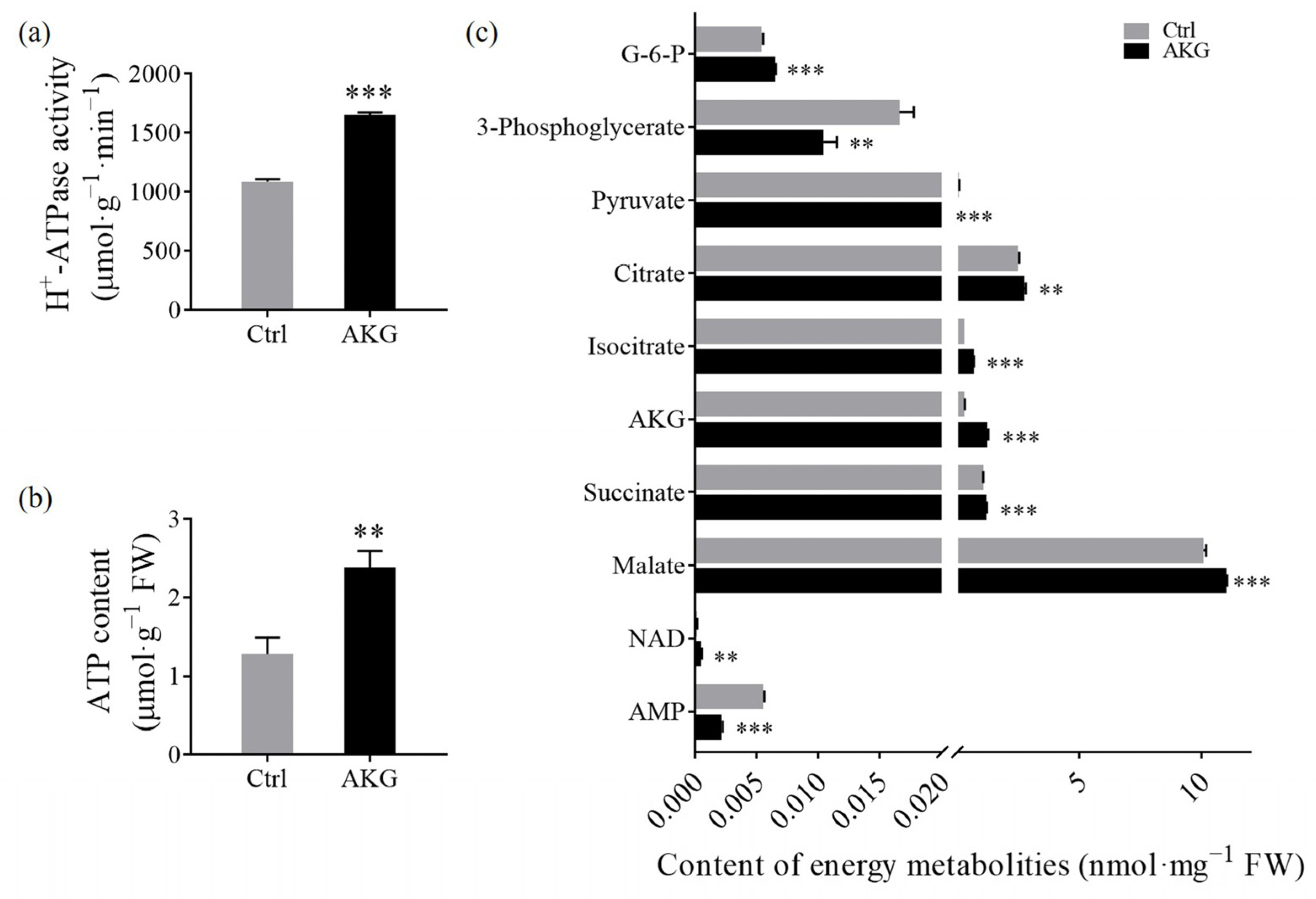
2.6. Effects of AKG on Energy Budget in Poplars with High Ammonium Exposure
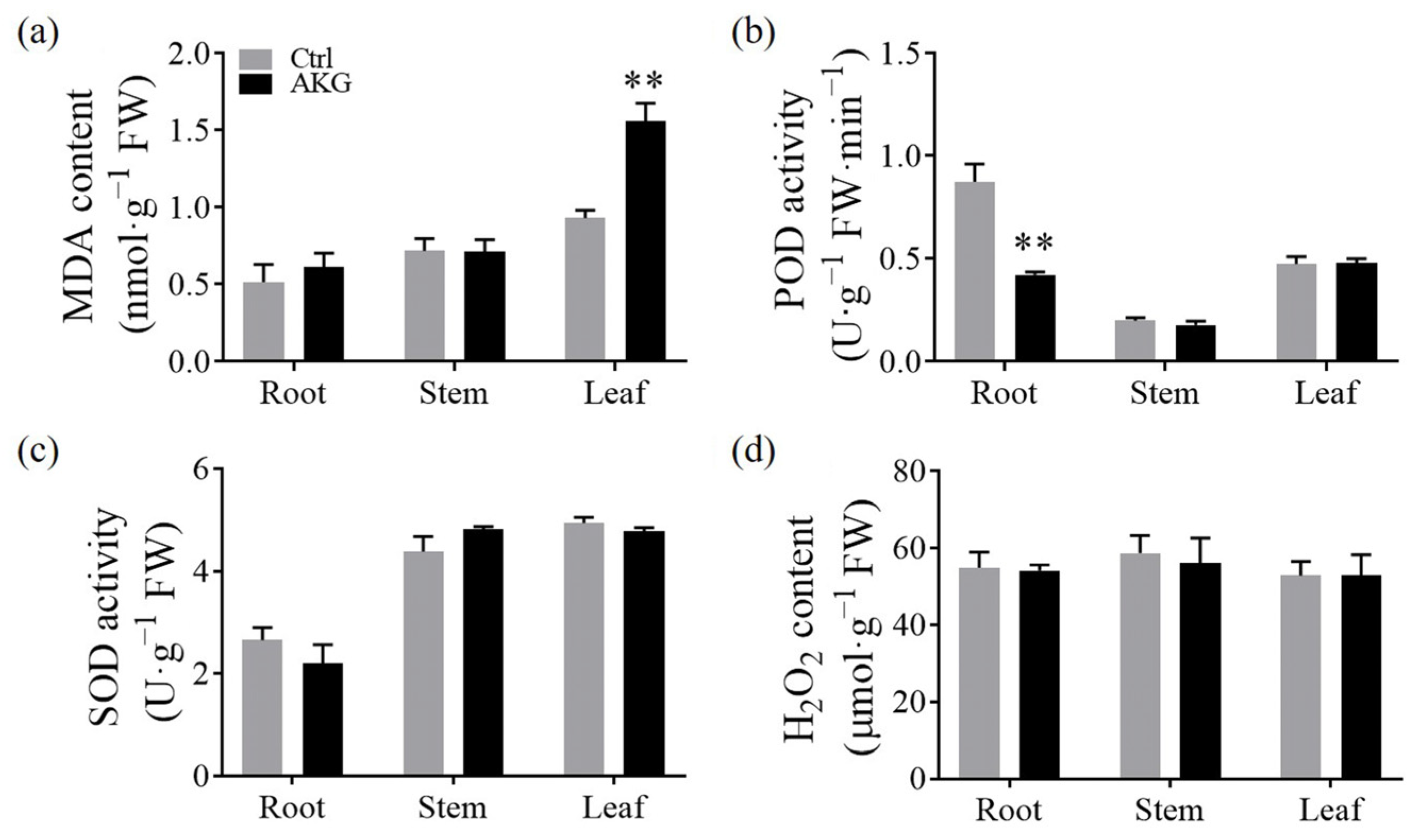
2.7. Effects of AKG on the Enzymatic Antioxidant Capacity of Poplars under High Ammonium Conditions
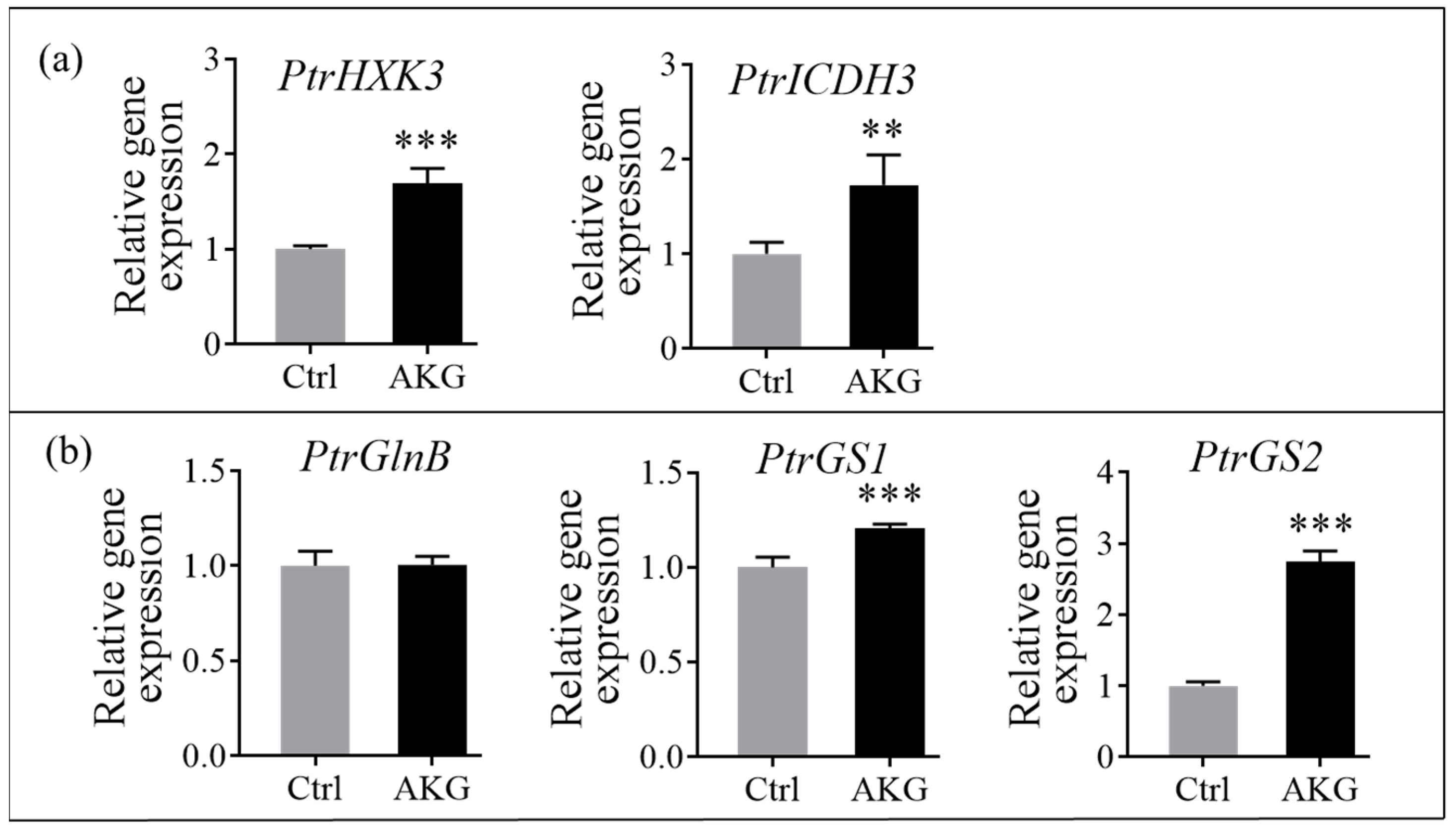
2.8. Effects of AKG on the Carbon and Nitrogen Metabolism-Related Genes in Poplars with High Ammonium Exposure
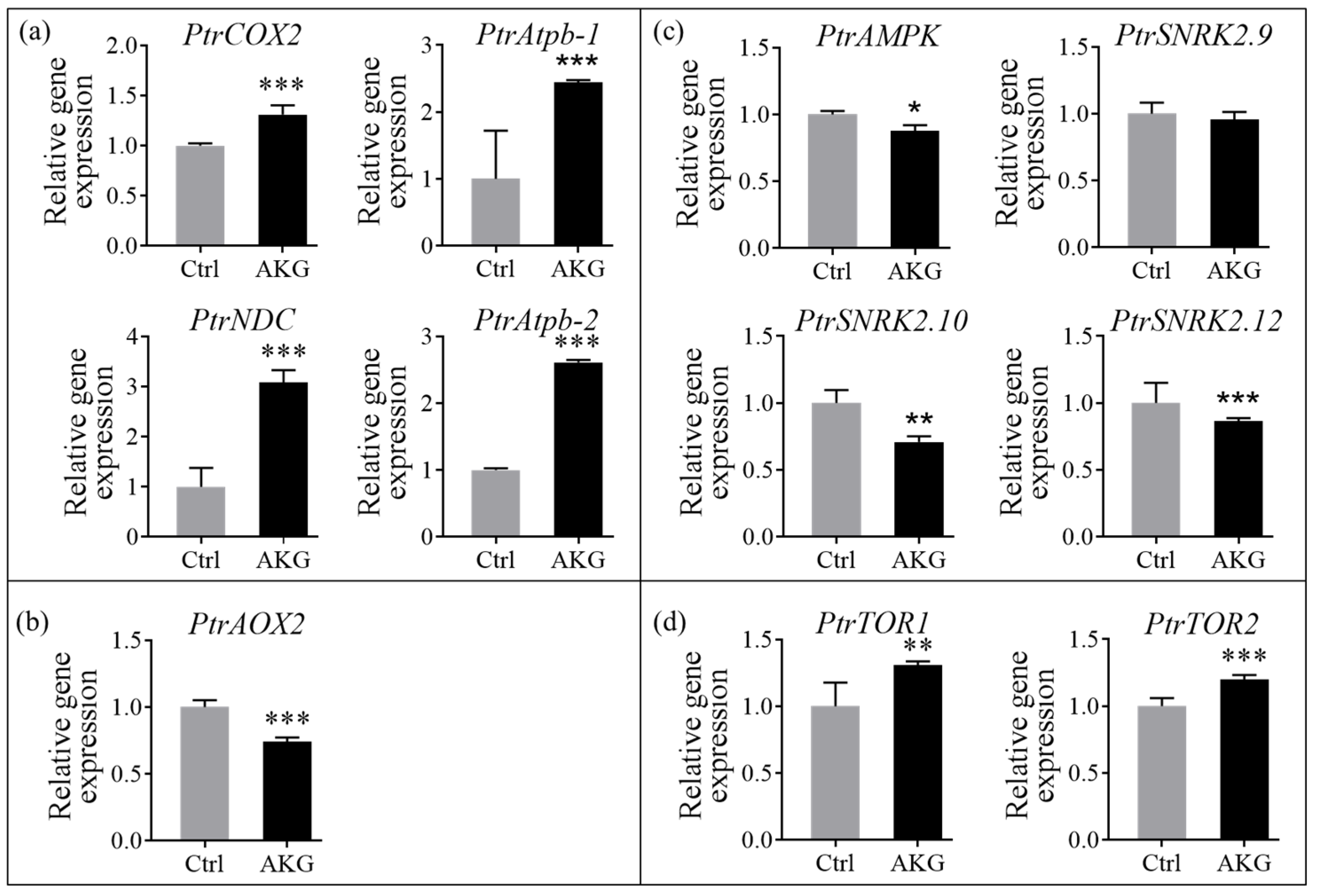
2.9. Effects of AKG on Energy Metabolism-Related Genes in Poplars with High Ammonium Exposure
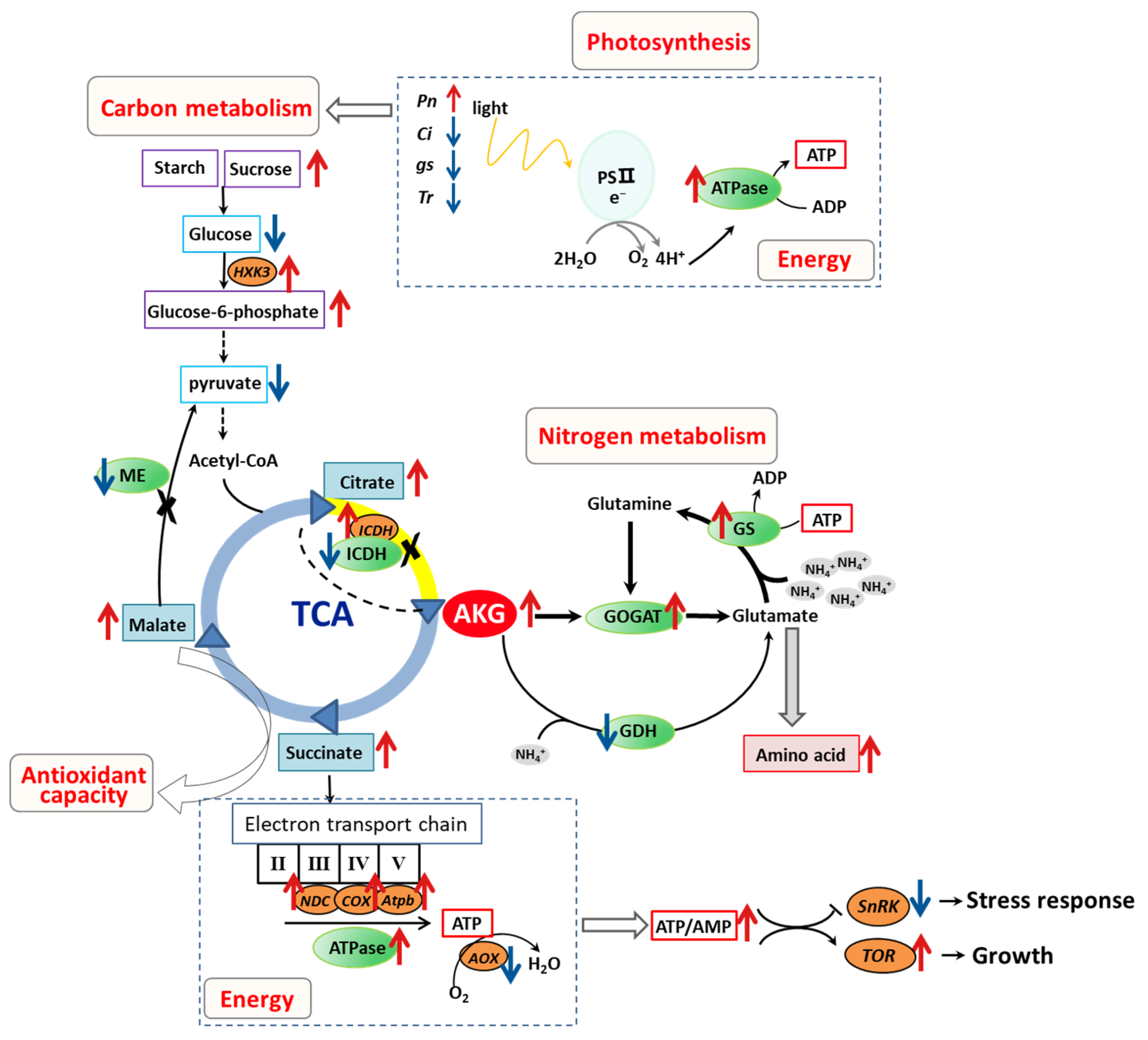
3. Discussion
3.1. Application of AKG Promotes Ammonium Assimilation in Poplars with High Ammonium Exposure
3.2. AKG Promotes Carbon Metabolism in Response to High Ammonium Exposure
3.3. Promotion of Energy Metabolism by AKG in Poplars under High Ammonium Conditions
3.4. Improvement of the Non-Enzymatic Antioxidant Capacity by AKG under High Ammonium Conditions
4. Materials and Methods
4.1. Plant Growth Conditions and Treatment
4.2. Measurement of Chlorophyll Fluorescence and Photosynthetic Parameters
4.3. Determination of Enzyme Activities
4.4. Nitrogen Compounds, Carbohydrates and ATP Content Measurements
4.5. Determination of the Content of Energy Metabolites
4.6. Analysis of Malondialdehyde (MDA), H2O2 Content, and the Antioxidant Enzyme Activities
4.7. Total RNA Extraction and the Quantitative Real-Time PCR (qRT-PCR) Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gong, J.; Zhang, Z.; Wang, B.; Shi, J.; Zhang, W.; Dong, Q.; Song, L.; Li, Y.; Liu, Y. N addition rebalances the carbon and nitrogen metabolisms of Leymus chinensis through leaf N investment. Plant Physiol. Biochem. 2022, 185, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Insausti, M.; Timmis, R.; Kinnersley, R.; Rufino, M.C. Advances in sensing ammonia from agricultural sources. Sci. Total Environ. 2020, 706, 135124. [Google Scholar] [CrossRef] [PubMed]
- Sutton, M.A.; Erisman, W.; Leip, A.; van Grinsven, H.; Winiwarter, W. Too much of a good thing. Nature 2011, 472, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Zhou, Y.; Qin, J.G.; Wang, W.; Yao, W.; Song, L. Physiological responses of Egeria densa to high ammonium concentration and nitrogen deficiency. Chemosphere 2012, 86, 538–545. [Google Scholar] [CrossRef]
- Schjoerring, J.K.; Husted, S.; Mäck, G.; Nielsen, K.H.; Finnemann, J.; Mattsson, M. Physiological regulation of plant-atmosphere ammonia exchange. Plant Soil 2000, 221, 95–102. [Google Scholar] [CrossRef]
- Hessini, K. Nitrogen form differently modulates growth, metabolite profile, and antioxidant and nitrogen metabolism activities in roots of Spartina alterniflora in response to increasing salinity. Plant Physiol. Biochem. 2022, 174, 35–42. [Google Scholar] [CrossRef]
- Zhou, Q.; Gao, J.; Zhang, R.; Zhang, R. Ammonia stress on nitrogen metabolism in tolerant aquatic plant—Myriophyllum aquaticum. Ecotoxicol. Environ. Saf. 2017, 143, 102–110. [Google Scholar] [CrossRef]
- Vega-Mas, I.; Perez-Delgado, C.M.; Marino, D.; Fuertes-Mendiza’bal, T. Elevated CO2 induces root defensive mechanisms in tomato plants when dealing with ammonium toxicity. Plant Cell Physiol. 2017, 58, 2112–2125. [Google Scholar] [CrossRef]
- Ariz, I.; Asensio, A.C.; Zamarreño, A.M.; García-Mina, J.M.; Aparicio-Tejo, P.M.; Moran, J.F. Changes in the C/N balance caused by increasing external ammonium concentrations are driven by carbon and energy availabilities during ammonium nutrition in pea plants: The key roles of asparagine synthetase and anaplerotic enzymes. Physiol. Plant. 2013, 148, 522–537. [Google Scholar] [CrossRef]
- Ten Hoopen, F.; Cuin, T.A.; Pedas, P.; Hegelund, J.N.; Shabala, S. Competition between uptake of ammonium and potassium in barley and Arabidopsis roots: Molecular mechanisms and physiological consequences. J. Exp. Bot. 2010, 61, 2303–2315. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Li, M.; Becker, A. Rapid ammonia gas transport accounts for futile transmembrane cycling under NH3/NH4+ toxicity in plant roots. Plant Physiol. 2013, 163, 1859–1867. [Google Scholar] [CrossRef]
- Britto, D.T.; Siddiqi, M.Y.; Glass, A.D.M.; Kronzucker, H.J. Futile transmembrane NH4+ cycling: A cellular hypothesis to explain ammonium toxicity in plants. Proc. Natl. Acad. Sci. USA 2001, 98, 4255–4258. [Google Scholar] [CrossRef]
- Wu, N.; Yang, M.; Gaur, U.; Xu, H.; Yao, Y.; Li, D. Alpha-ketoglutarate: Physiological functions and applications. Biomol. Ther. 2016, 24, 1–8. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, C.; Burnap, R.L.; Peng, L. Carbon/Nitrogen Metabolic Balance: Lessons from Cyanobacteria. Trends Plant Sci. 2018, 23, 1116–1130. [Google Scholar] [CrossRef]
- Abla, H.; Sollazzo, M.; Gasparre, G.; Iommarini, L.; Porcelli, A.M. The multifaceted contribution of α-ketoglutarate to tumor progression: An opportunity to exploit? Semin. Cell Dev. Biol. 2020, 98, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Chin, R.M.; Fu, X.; Pai, M.Y.; Vergnes, L.; Hwang, H.; Diep, S.; Lomenick, B.; Meli, V.S.; Monsalve, G.C.; Whelan, S.A.; et al. The metabolite alpha-ketoglutarate extends lifespan by inhibiting the ATP synthase and TOR. Nature 2014, 510, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Velvizhi, S.; Dakshayani, K.B.; Subramanian, P. Effects of α-ketoglutarate on antioxidants and lipid peroxidation products in rats treated with ammonium acetate. Nutrition 2002, 18, 747–750. [Google Scholar] [CrossRef] [PubMed]
- Naeini, S.H.; Mavaddatiyan, L.; Kalkhoran, Z.R.; Taherkhani, S.; Talkhabi, M. Alpha-ketoglutarate as a potent regulator for lifespan and healthspan: Evidences and perspectives. Exp. Gerontol. 2023, 175, 112154. [Google Scholar] [CrossRef] [PubMed]
- Gui, P.; Chen, X.; Liao, Z.; Wang, L. Effect of organic carbon on carbon and nitrogen metabolism and the growth of water spanich as affected by nitrogen levels. Acta Pedol. Sin. 2016, 53, 746–756. [Google Scholar]
- Fu, X.; Gui, R.; Li, W.; Gao, Z.; Ashraf, U.; Tan, J.; Ye, Q.; Chen, J.; Xie, H.; Mo, Z. Nitrogen and α-ketoglutaric acid application modulate grain yield, aroma, nutrient uptake and physiological attributes in fragrant rice. J. Plant Growth Regul. 2021, 40, 1613–1628. [Google Scholar] [CrossRef]
- Magalhães, J.R.; Huber, D.M.; Tsai, C.Y. Evidence of increased 15N-ammonium assimilation in tomato plants with exogenous α-ketoglutarate. Plant Sci. 1992, 85, 135–141. [Google Scholar] [CrossRef]
- Singh, M.; Singh, P.; Prasad, S.M. α-Ketoglutarate enhanced Solanum melongena L. growth: Acceleration of nitrogen assimilating enzymes and antioxidant system under arsenate toxicity. J. Plant Growth Regul. 2022, 41, 1699–1713. [Google Scholar] [CrossRef]
- Alamri, S.; Alsubaie, Q.D.; Al-Amri, A.A.; Al-Munqedi, B.; Ali, H.M. Priming of tomato seedlings with 2-oxoglutarate induces arsenic toxicity alleviatory responses by involving endogenous nitric oxide. Physiol. Plant. 2021, 173, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Huang, B. Metabolic regulation of α-Ketoglutarate associated with heat tolerance in perennial ryegrass. Plant Physiol. Biochem. J. 2022, 190, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; He, L.; Yao, K. The antioxidative function of alpha-ketoglutarate and its applications. BioMed Res. Int. 2018, 1, 3408467. [Google Scholar] [CrossRef] [PubMed]
- Gai, Z.; Liu, J.; Cai, L.; Zhang, J.; Liu, L. Foliar application of alpha-ketoglutarate plus nitrogen improves drought resistance in soybean (Glycine max L. Merr.). Sci. Rep. 2022, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gai, Z.; Zhang, M.; Zhang, P.; Zhang, J.; Liu, J.; Cai, L.; Yang, X.; Zhang, N.; Yan, Z.; Liu, L.; et al. 2-Oxoglutarate contributes to the effect of foliar nitrogen on enhancing drought tolerance during flowering and grain yield of soybean. Sci. Rep. 2023, 13, 7274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, J.; Zhuang, S.; Feng, Z.; Fan, J. Functional characterization of PsAMT1.1 from Populus simonii in ammonium transport and its role in nitrogen uptake and metabolism. Environ. Exp. Bot. 2023, 208, 105255. [Google Scholar] [CrossRef]
- Thakur, A.K.; Kumar, P.; Parmar, N.; Shandil, R.K.; Aggarwal, G.; Gaur, A.; Srivastava, D.K. Achievements and prospects of genetic engineering in poplar: A review. New For. 2021, 52, 889–920. [Google Scholar] [CrossRef]
- Rennenberg, H.; Dannenmann, M. Nitrogen nutrition of trees in temperate forests-the significance of nitrogen availability in the pedosphere and atmosphere. Forests 2015, 6, 2820–2835. [Google Scholar] [CrossRef]
- Krupa, S.V. Effects of atmospheric ammonia (NH3) on terrestrial vegetation: A review. Environ. Pollut. 2003, 124, 179–221. [Google Scholar] [CrossRef]
- Du, Y.D.; Yuan, X.Y.; Feng, Z.Z. Effects of different nitrogen forms on photosynthesis characteristics and growth of poplar. Chin. J. Plant Ecol. 2023, 47, 348–360. [Google Scholar] [CrossRef]
- Guo, H.; Wang, H.; Liu, Q.; An, H.; Liu, C.; Xia, X.; Yin, W. 15N-labeled ammonium nitrogen uptake and physiological responses of poplar exposed to PM2.5 particles. Environ. Sci. Pollut. Res. 2017, 24, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Esteban, R.; Ariz, I.; Cruz, C.; Moran, J.F. Review: Mechanisms of ammonium toxicity and the quest for tolerance. Plant Sci. 2016, 248, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Zhang, Y.; Cheng, S.; Osorio, S.; Sun, Y.; Fernie, A.R.; Cheung, C.Y.M.; Lim, B.L. Impacts of high ATP supply from chloroplasts and mitochondria on the leaf metabolism of Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 922. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, K.; Yoshida, K. Interaction between photosynthesis and respiration in illuminated leaves. Mitochondrion 2008, 8, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Legendre, F.; MacLean, A.; Appanna, V.P.; Appanna, V.D. Biochemical pathways to α-ketoglutarate, a multi-faceted metabolite. World J. Microbiol. Biotechnol. 2020, 36, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Huergo, L.F.; Dixon, R. The emergence of 2-Oxoglutarate as a master regulator metabolite. Microbiol. Mol. Biol. Rev. 2015, 79, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Reitzer, L. Nitrogen assimilation and global regulation in Escherichia coli. Annu. Rev. Microbiol. 2003, 57, 155–176. [Google Scholar] [CrossRef]
- Harper, C.J.; Hayward, D.; Kidd, M.; Wiid, I.; van Helden, P. Glutamate dehydrogenase and glutamine synthetase are regulated in response to nitrogen availability in Myocbacterium smegmatis. BMC Microbiol. 2010, 10, 138. [Google Scholar] [CrossRef]
- Chellamuthu, V.R.; Ermilova, E.; Lapina, T.; Lüddecke, J.; Minaeva, E.; Herrmann, C.; Hartmann, M.D.; Forchhammer, K. A widespread glutamine-sensing mechanism in the plant kingdom. Cell 2014, 159, 1188–1199. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Ninfa, A.J. α-ketoglutarate controls the ability of the Escherichia coli PII signal transduction protein to regulate the activities of NRII (NtrB) but does not control the binding of PII to NRII. Biochemistry 2009, 48, 11514–11521. [Google Scholar] [CrossRef]
- Pérez-Díaz, J.; Batista-Silva, W.; Almada, R.; Medeiros, D.B. Prunus hexokinase 3 genes alter primary c-metabolism and promote drought and salt stress tolerance in Arabidopsis transgenic plants. Sci. Rep. 2021, 11, 7098. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Liang, W.W.; Jia, L.; Wang, Z.Q.; Lin, T.B. Effect of α-ketoglutaric acid on yield-related traits in winter wheat under low water and nitrogen. J. Anhui Agric. Sci. 2014, 42, 671–674, 676. [Google Scholar]
- Yuan, Y.; Ou, J.; Wang, Z.; Zhang, C.; Zhou, Z.; Lin, Q. Regulation of carbon and nitrogen metabolisms in rice roots by 2-oxoglutarate at the level of hexokinase. Physiol. Plant. 2007, 129, 296–306. [Google Scholar] [CrossRef]
- Suarez, M.F.; Avila, C.; Gallardo, F.; Canton, F.R. Molecular and enzymatic analysis of ammonium assimilation in woody plants. J. Exp. Bot. 2002, 53, 891–904. [Google Scholar] [CrossRef]
- Mullen, A.R.; Hu, Z.; Shi, X.; Jiang, L.; Boroughs, L.K.; Kovacs, Z.; Boriack, R.; Rakheja, D.; Sullivan, L.B.; Linehan, W.M.; et al. Oxidation of alpha-ketoglutarate is required for reductive carboxylation in cancer cells with mitochondrial defects. Cell Rep. 2014, 7, 1679–1690. [Google Scholar] [CrossRef]
- Brugière, N.; Dubois, F.; Limami, A.M.; Lelandais, M.; Roux, Y.; Sangwan, R.S.; Hire, B. Glutamine synthetase in the phloem plays a major role in controlling proline production. Plant Cell 1999, 11, 1995–2011. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Lin, W.; Jacobs-Wagner, C. Connecting single-cell ATP dynamics to overflow metabolism, cell growth, and the cell cycle in Escherichia coli. Curr. Biol. 2022, 32, 3911–3924. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, M.; Yun, Z.; Wang, J.; Feng, G.; Gao, Z.; Shi, X.; Jiang, Y. Effect of tea seed oil treatment on browning of litchi fruit in relation to energy status and metabolism. Postharvest Biol. Technol. J. 2017, 132, 97–104. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, M.; Wang, P. Response of plant plasma membrane H+-ATPase to environmental stress factors: A review. Chin. J. Appl. Environ. Biol. 2021, 27, 485–494. [Google Scholar]
- Erdal, S. Melatonin promotes plant growth by maintaining integration and coordination between carbon and nitrogen metabolisms. Plant Cell Rep. 2019, 38, 1001–1012. [Google Scholar] [CrossRef]
- Rosenberger, C.L.; Chen, J. To grow or not to grow: TOR and SnRK2 coordinate growth and stress response in Arabidopsis. Mol. Cell 2018, 69, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Harrison, A.; Pierzynowski, S.G. Biological effects of 2-oxoglutarate with particular emphasis on the regulation of protein, mineral and lipid absorption/metabolism, muscle performance, kidney function, bone formation and cancerogenesis, all viewed from a healthy ageing perspective state. J. Physiol. Pharmacol. 2008, 59, 91–106. [Google Scholar] [PubMed]
- Tcherkez, G.; Carroll, A.; Abadie, C.; Mainguet, S.; Davanture, M.; Zivy, M. Protein synthesis increases with photosynthesis via the stimulation of translation initiation. Plant Sci. 2020, 291, 110352. [Google Scholar] [CrossRef]
- Zdzisińska, B.; Żurek, A.; Kandefer-Szerszeń, M. Alpha-ketoglutarate as a molecule with pleiotropic activity: Well-known and novel possibilities of therapeutic use. Arch. Immunol. Ther. Exp. 2017, 65, 21–36. [Google Scholar] [CrossRef]
- Chen, Q.; Vazquez, E.J.; Moghaddas, S.; Hoppel, C.L. Production of reactive oxygen species by mitochondria. J. Biol. Chem. 2003, 278, 36027–36031. [Google Scholar] [CrossRef]
- Puntel, R.L.; Roos, D.H.; Grotto, D.; Garcia, S.C.; Nogueira, C.W.; Batista Teixeira Rocha, J. Antioxidant properties of Krebs cycle intermediates against malonate pro-oxidant activity in vitro: A comparative study using the colorimetric method and HPLC analysis to determine malondialdehyde in rat brain homogenates. Life Sci. 2007, 81, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhang, Z.; Xiang, Z.; Yang, Z. Exogenous application of citric acid ameliorates the adverse effect of heat stress in tall fescue (Lolium arundinaceum). Front. Plant Sci. 2016, 7, 179. [Google Scholar] [CrossRef]
- Han, M.; Xu, M.Y.; Wang, S.Z.; Wu, L.D.; Sun, S.Y.; Su, T. Effects of exogenous L-Glutamine as a sole nitrogen source on physiological characteristics and nitrogen use efficiency of poplar. Plant Physiol. Biochem. 2022, 172, 1–13. [Google Scholar] [CrossRef]
- Gong, L.; Zhang, S.; Chen, D.; Liu, K.; Lu, J. Response of biofilms-leaves of two submerged macrophytes to high ammonium. Chemosphere 2018, 192, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gu, W.; Li, J.; Li, C.; Xie, T.; Qu, D.; Meng, Y.; Li, C.; Wei, S. Exogenously applied spermidine alleviates photosynthetic inhibition under drought stress in maize (Zea mays L.) seedlings associated with changes in endogenous polyamines and phytohormones. Plant Physiol. Biochem. 2018, 129, 35–55. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Han, M.; Min, J.; Cao, D.; Zhai, G.; Zhou, H.; Li, N.; Li, M. Genome-wide characterization of AspATs in Populus: Gene expression variation and enzyme activities in response to nitrogen perturbations. Forests 2019, 10, 449. [Google Scholar] [CrossRef]
- Singh, R.; Chénier, D.; Bériault, R.; Mailloux, R.; Hamel, R.D.; Appanna, V.D. Blue native polyacrylamide gel electrophoresis and the monitoring of malate- and oxaloacetate-producing enzymes. J. Biochem. Biophys. Methods 2005, 64, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Lin, Y.; Lin, H.; Ritenour, M.A.; Shi, J.; Zhang, S.; Chen, Y.; Wang, H. Hydrogen peroxide-induced pericarp browning of harvested longan fruit in association with energy metabolism. Food Chem. 2017, 225, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.; Cakmak, T.; Cooper, A.; Lager, I.; Rasmusson, A.G.; Escobar, M.A. Distinct signalling pathways and transcriptome response signatures differentiate ammonium- and nitrate-supplied plants. Plant. Cell Environ. 2010, 33, 1486–1501. [Google Scholar] [CrossRef]
- Bräutigam, A.; Gagneul, D.; Weber, A.P.M. High-throughput colorimetric method for the parallel assay of glyoxylic acid and ammonium in a single extract. Anal. Biochem. 2007, 362, 151–153. [Google Scholar] [CrossRef]
- Liao, Y.; Cui, R.; Yuan, T.; Xie, Y.; Gao, Y. Cysteine and methionine contribute differentially to regulate alternative oxidase in leaves of poplar (Populus deltoides x Populus euramericana ‘Nanlin 895’) seedlings exposed to different salinity. J. Plant Physiol. 2019, 240, 153017. [Google Scholar] [CrossRef]
- Zhang, T.; Cao, Y.; Chen, Y.; Liu, G. Non-structural carbohydrate dynamics in Robinia pseudoacacia saplings under three levels of continuous drought stress. Trees 2015, 29, 1837–1849. [Google Scholar] [CrossRef]
- Patterson, B.D.; Payne, L.A.; Chen, Y.-Z.; Graham, D. An inhibitor of Catalase induced by cold in chilling-sensitive plants. Plant Physiol. 1984, 76, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Wu, L.; Si, Y.; Zhai, Y.; Niu, M.; Han, M.; Su, T. Regulating Effect of Exogenous α-Ketoglutarate on Ammonium Assimilation in Poplar. Molecules 2024, 29, 1425. https://doi.org/10.3390/molecules29071425
Liu X, Wu L, Si Y, Zhai Y, Niu M, Han M, Su T. Regulating Effect of Exogenous α-Ketoglutarate on Ammonium Assimilation in Poplar. Molecules. 2024; 29(7):1425. https://doi.org/10.3390/molecules29071425
Chicago/Turabian StyleLiu, Xiaoning, Liangdan Wu, Yujia Si, Yujie Zhai, Mingyi Niu, Mei Han, and Tao Su. 2024. "Regulating Effect of Exogenous α-Ketoglutarate on Ammonium Assimilation in Poplar" Molecules 29, no. 7: 1425. https://doi.org/10.3390/molecules29071425






